Abstract
Plasminogen activation has been proposed to play a critical role in cancer invasion and metastasis. The effects of complete ablation of plasminogen activation in cancer was studied by inoculation of a metastatic Lewis lung carcinoma expressing high levels of plasminogen activator into plasminogen-deficient (Plg−/−) mice and matched control mice. Primary tumors developed in all mice with no difference in the rate of appearance between Plg−/− and control mice. However, the primary tumors in Plg−/− mice were smaller and less hemorrhagic and displayed reduced skin ulceration. In addition, dissemination of the tumor to regional lymph nodes was delayed in Plg−/− mice. Surprisingly, no quantitative differences were observed in lung metastasis between Plg−/− and control mice. In addition, Plg deficiency was compatible with metastasis of the primary tumor to a variety of other organs. Nevertheless, Plg−/− mice displayed a moderately increased survival after primary tumor resection. These findings suggest that plasmin-mediated proteolysis contributes to the morbidity and mortality of Lewis lung carcinoma in mice, but sufficient proteolytic activity is generated in Plg−/− mice for efficient tumor development and metastasis.
THE PLASMINOGEN activation (PA) system is a carefully regulated system of serine proteases, protease receptors, and protease inhibitors that governs the conversion of the inactive protease zymogen plasminogen (Plg) to the active protease, plasmin.1-5 Plasmin efficiently degrades fibrin and has a well-established role in thrombolysis.6 In addition, plasmin has a critical role in extravascular fibrinolysis in the degradation of fibrin-rich extracellular matrices in the context of tissue remodeling and cell migration.7,8 Plasmin has a relatively broad substrate specificity in vitro and can degrade several common extracellular matrix glycoproteins in addition to fibrin.3,4 Plasmin has also been proposed to facilitate matrix degradation indirectly via the activation of certain latent matrix metalloproteases involved in collagenolysis.9 Furthermore, in cell culture, plasmin can activate several latent growth factors associated with tumor growth, including latent transforming growth factor β and latent basic fibroblast growth factor.10 11
Over the past two decades, extensive indirect evidence has accumulated for the involvement of the PA system in tumor invasion and metastasis. Histologic analysis of both human tumors and experimental mouse tumors has consistently demonstrated the expression of the components of the PA system at sites of active invasion, implying an important role in extracellular matrix degradation by malignant tumors.12-15 Furthermore, epidemiologic studies have shown that high expression of PA, PA receptor, or PA inhibitors in human breast, colorectal, lung, and other types of cancer is associated with poor prognosis.16-25 In addition to these correlative studies, a number of experiments have been undertaken to directly address the causal relationship between PA expression and tumor invasion and metastasis. Most significantly, antisense RNA, anticatalytic PA antibodies, natural and synthetic PA inhibitors, and PA receptor antagonists have been used to inhibit the expression or activity of PA components in conjunction with transplantation of malignant tumors into experimental animals. Most, but not all, of these studies found a significant reduction in tumor dissemination when PA inhibitors were experimentally introduced.26-38
The recent generation of Plg-deficient mice (Plg−/−)39,40 provides a unique new opportunity to directly define the role of plasmin-mediated proteolysis in tumor progression in vivo. The mice are viable and generally healthy until young adulthood, although a variety of phenotypic abnormalities associated with impaired fibrinolysis develop as the mice age.8 The advantages of using Plg−/− mice to address the role of PA in tumor invasion and metastasis include the following: (1) tumor growth and metastasis can be studied at the level of the intact organism, (2) Plg−/− mice have an absolute and lifelong deficiency in Plg, (3) Plg−/− mice have no known alterations in other enzyme systems, and (4) tumors can easily be induced in Plg−/− mice by breeding to transgenic mouse lines carrying activated oncogenes or lacking tumor suppressor genes, by tumor transplantation, or by carcinogen treatment.
In this study, we have used a well-established transplantable tumor model, the Lewis lung carcinoma, to directly test the hypothesis that plasmin-mediated proteolysis is critical for tumor invasion and metastasis. We have chosen this tumor because it is highly metastatic in immunocompetent mice and produces copious amounts of PA and because previous studies have shown dramatic effects of anticatalytic PA antibodies and PA receptor antagonists on spontaneous metastasis of this tumor in the mouse.15,32 33 We report that tumors develop, invade local tissues, and metastasize in the complete absence of Plg, but display decreased growth, morbidity, and mortality.
MATERIALS AND METHODS
Mice and genotype analysis. Plg gene-targeted mice of a mixed 129/Black Swiss background39 were backcrossed for 6 generations to C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) to produce mice with a genetic background close to C57BL/6, although not formally congenic. Genotypes of offspring were determined by polymerase chain reaction analysis, as described previously.8 Experimental mice were 6 to 14 weeks old at the initiation of experiments. For survival studies in tumor-bearing mice, animals were less than 11 weeks of age. For each experiment, cohorts of age-matched Plg−/− and littermate control mice (Plg+/+ and Plg+/−) were used. Before each experiment, the mice were housed singly and coded, and all subsequent manipulations and analyses were performed by an investigator unaware of animal genotype.
Tumor cell inoculation. A line of Lewis lung carcinoma41 that was propagated by sequential subcutaneous transplantation in C57BL/6 mice was kindly provided by Dr Michael S. O'Reilly (Boston, MA). A single-cell suspension of tumor cells was prepared by mincing the tumor, followed by passage of the suspension first through a stainless steel mesh and then through a series of hypodermic needles of increasing gauge. This suspension of cells was aliquoted and frozen. For each series of injections, an aliquot of frozen tumor cells was thawed and expanded by culturing for 3 passages in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum, penicillin, and streptomycin. Before injection, the tumor cells were detached by trypsinization, washed once in cold DMEM containing 10% fetal calf serum, and once in cold serum-free medium. Cell viability was usually greater than 85%, as determined by trypan blue exclusion. The dorsal skin of mice was shaved 1 day before injection. The mice were anesthetized by Metofane (Mallinckrodt Veterinary, Mundelein, IL) inhalation, and tumor cells (0.5 × 106 trypan blue-excluding cells in 100 μL) were injected into the dorsal subcutis using a 27-gauge needle.
Estimation of primary tumor weight in live mice. The weight of the primary tumors was estimated by the standard method of calipation used routinely to measure the growth of subcutaneous tumors.42 Briefly, the long and short diameters of the tumors were measured with calipers, and the tumor weight calculated using the following formula: grams = (length × [width]2)/2, assuming a hemiellipsoid shape.
Resection of primary tumors. Tumor-bearing mice were anesthetized by inhalation of 2% Isoflurane (Ohmeda PPD, Liberty Corner, NJ). The tumor was carefully excised, and wound edges were joined using surgical staples. Mortality from the procedure was less than 2% (1 control and 1 Plg−/− mouse dying from a total of 110 mice having tumors resected).
Quantitation of lung metastasis. Mice were killed using CO2 narcosis, and the lungs were removed, rinsed in phosphate-buffered saline (PBS), and placed in Bouin's fixative for at least 24 hours. The fixed lungs were carefully separated into individual lobes with forceps, and the number of surface metastases (appearing as white foci against a yellow background) was counted for each lobe using a dissecting microscope at 4× magnification (total metastatic foci) or by the naked eye (large metastatic foci).
Histologic analysis. Mice were killed under ketamine anesthesia as described previously.8 The lungs of the mice were either placed directly into Bouin's fixative, or the mice were first perfused under ketamine anesthesia with cold PBS, followed by perfusion with cold 4% (wt/vol) paraformaldehyde in PBS, and the lungs fixed for 24 hours in 4% (wt/vol) paraformaldehyde. Primary tumors were either obtained from mice perfused with paraformaldehyde or resected from mice under Isoflurane anesthesia and fixed in paraformaldehyde or 10% neutral-buffered formalin. Fixed tissues were processed into paraffin, sectioned, and stained with hematoxylin/eosin or Mason's trichrome.
Immunohistochemical analysis. Immunohistochemistry was performed with a Vector Elite ABC kit (Vector Laboratories, Burlingame, CA) using a peroxidase detection system and 3-amino-9-ethyl-carbazole as a chromogen (Biomeda Corp, Foster City, CA). Endothelial cell staining was performed on frozen sections of paraformaldehyde-perfused tissue with a rat antimouse platelet-endothelial cell adhesion molecule-1 (PECAM) monoclonal antibody. Fibrin(ogen) immunostaining was performed using a rabbit antimouse polyclonal antiserum, as described previously.43
In situ hybridization. Fixed tissues were cryosectioned and hybridized with 35S-labeled RNA probes complementary to mouse urokinase PA (uPA) and PA inhibitor-1 (PAI-1) mRNA as described.44 The template plasmid for sense and antisense mouse uPA probes contained a Xba I-Pst I fragment of the mouse uPA cDNA (nucleotides 37 to 428 in the numbering system of Belin et al45 ). The template plasmid for mouse PAI-1 probes46 contained a Pst I fragment of the mouse PAI-1 cDNA (nucleotides 6 to 341 in the numbering system of Prendergast et al47 ).
Northern blot analysis. Total RNA was isolated as described previously39 from primary tumors excised at day 10 after tumor cell injection and from liver samples. Samples of RNA (40 μg) were electrophoretically fractionated on denaturing agarose gels, transferred to BA85 nitrocellulose filters, and hybridized with a 32P-labeled 900-bp DNA fragment from the 3′-end of the mouse Plg cDNA.39 Hybridized material was detected by a Molecular Dynamics PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
RESULTS
Lewis lung carcinoma expresses uPA and PAI-1, but not Plg. To establish that the Lewis lung carcinoma line used in this study expressed PA, we injected tumor cells into the subcutis of Plg−/− and littermate control mice. Six and 11 days after injection, we killed the mice, excised the tumors, and studied the expression of uPA and PAI-1 mRNA using in situ hybridization (Fig 1). Consistent with results published previously,15 all subcutaneous tumor nodules showed expression of uPA mRNA in the tumor cells. uPA mRNA was detected in many of the infiltrating tumor cells, although there was cell to cell variability in the level of expression (Fig 1A and B). Only areas of necrotic tumor showed complete absence of detectable signal, and expression was not observed in the infiltrating inflammatory cells in these areas of necrosis. The dermal connective tissue and overlying epidermal layers at the periphery of the tumor nodules showed minimal to no expression. The tumors also expressed PAI-1 mRNA. The PAI-1 mRNA expression was most intense at the periphery of the tumor nodule, where both tumor cells and nearby connective tissue cells frequently showed intense signal (data not shown).
Expression of uPA but not Plg by Lewis lung carcinoma. (A) Darkfield section of a subcutaneous tumor nodule that was hybridized with an antisense probe for uPA. Many of the tumor cells show a strong signal (bright white grains), indicating uPA mRNA expression. (B) Brightfield image of a higher magnification of the tumor cells shown in (A). Although there is cell to cell variability in the expression level of uPA, as shown in (A), the expression of uPA is localized to many of the tumor cells (arrows) forming the tumor nodule. Original magnifications: (A) ×100 and (B) ×650. (C) Northern blot of total RNA from subcutaneous tumors of control mice (lanes 2 and 3) and Plg−/− mice (lanes 5 and 6) inoculated with Lewis lung carcinoma. Lanes 1 and 4 are total liver RNA purified in parallel from a control mouse and a Plg−/− mouse, respectively. The positions of Plg mRNA, 28 S, and 18 S RNA are indicated. (D) Ethidium bromide staining of the agarose gel before blotting.
Expression of uPA but not Plg by Lewis lung carcinoma. (A) Darkfield section of a subcutaneous tumor nodule that was hybridized with an antisense probe for uPA. Many of the tumor cells show a strong signal (bright white grains), indicating uPA mRNA expression. (B) Brightfield image of a higher magnification of the tumor cells shown in (A). Although there is cell to cell variability in the expression level of uPA, as shown in (A), the expression of uPA is localized to many of the tumor cells (arrows) forming the tumor nodule. Original magnifications: (A) ×100 and (B) ×650. (C) Northern blot of total RNA from subcutaneous tumors of control mice (lanes 2 and 3) and Plg−/− mice (lanes 5 and 6) inoculated with Lewis lung carcinoma. Lanes 1 and 4 are total liver RNA purified in parallel from a control mouse and a Plg−/− mouse, respectively. The positions of Plg mRNA, 28 S, and 18 S RNA are indicated. (D) Ethidium bromide staining of the agarose gel before blotting.
To determine if the tumor cells express Plg, we injected tumor cells into 2 Plg−/− and 2 control mice. Tumors were excised at day 10, and total RNA was extracted from the tumors and analyzed for expression of Plg mRNA by a sensitive Northern blot assay developed previously (Fig 1C and D).39 No hybridizing transcript was detected in RNA preparations from any of the tumors, even with very long exposures of the blots to PhosphorImaging screens. In contrast, Plg mRNA was easily detected in liver RNA purified in parallel from a control mouse. The expression of PA, but not Plg, by the Lewis lung tumor cells thus permitted a rigorous analysis of the importance of Plg in tumor cell dissemination, by examining the growth and invasion of the tumor cells in Plg−/− and littermate control mice.
Primary Tumor Development in Control and Plg−/− Mice
| Experiment No. . | Mice Developing Tumors . | 1st Tumor (day)* (mean ± SD) . | Tumor Size (g)† (mean ± SD) . | |||||
|---|---|---|---|---|---|---|---|---|
| . | Control . | Plg−/− . | Control . | Plg−/− . | Day . | Control . | Plg−/− . | P Value‡ . |
| 1 | 13/13 | 17/17 | 1.9 ± 0.9 | 2.4 ± 0.9 NS | 2 | 0.03 ± 0.03 (13) | 0.03 ± 0.05 (17) | NS |
| 4 | 0.07 ± 0.04 (13) | 0.04 ± 0.02 (17) | <.04 | |||||
| 6 | 0.22 ± 0.07 (13) | 0.13 ± 0.07 (17) | <.004 | |||||
| 7 | 0.26 ± 0.09ρ (4) | 0.18 ± 0.08ρ (10) | <.05 | |||||
| 8 | 0.48 ± 0.18 (9) | 0.25 ± 0.13 (7) | <.02 | |||||
| 10 | 0.83 ± 0.30 (9) | 0.44 ± 0.14 (7) | <.008 | |||||
| 12 | 1.2 ± 0.5 (9) | 0.76 ± 0.25 (7) | <.05 | |||||
| 14 | 2.1 ± 0.8 (9) | 1.3 ± 0.5 (7) | <.04 | |||||
| 16 | 3.1 ± 0.8 (9) | 1.9 ± 0.8 (7) | <.02 | |||||
| 18 | 3.6 ± 1.2 (9) | 2.2 ± 0.6 (7) | <.03 | |||||
| 2 | 14/14 | 14/14 | 2.2 ± 0.6 | 2.5 ± 0.7 NS | 2 | 0.02 ± 0.02 (13) | 0.01 ± 0.01 (13) | NS |
| 4 | 0.06 ± 0.01 (13) | 0.03 ± 0.01 (13) | <.0001 | |||||
| 6 | 0.26 ± 0.05 (13) | 0.17 ± 0.07 (13) | <.001 | |||||
| 8 | 0.42 ± 0.10 (13) | 0.28 ± 0.10 (12) | <.001 | |||||
| 10 | 0.86 ± 0.24 (14) | 0.58 ± 0.21 (13) | <.005 | |||||
| 12 | 1.4 ± 0.5 (14) | 1.0 ± 0.4 (13) | <.05 | |||||
| 14 | 2.2 ± 0.8 (14) | 1.7 ± 0.7 (13) | NS | |||||
| 16 | 3.1 ± 1.1 (14) | 2.8 ± 1.0 (13) | NS | |||||
| 3 | 12/12 | 12/12 | 2.3 ± 0.5 | 2.6 ± 1.2 NS | 2 | 0.01 ± 0.01 (8) | 0.00 ± 0.01 (8) | NS |
| 4 | 0.05 ± 0.02 (9) | 0.03 ± 0.01 (8) | <.02 | |||||
| 6 | 0.23 ± 0.06ρ (3) | 0.10 ± 0.02ρ (3) | <.03 | |||||
| 4 | 15/15 | 15/15 | ND | ND | 6 | 0.18 ± 0.05 (4) | 0.09 ± 0.05 (4) | <.04 |
| Experiment No. . | Mice Developing Tumors . | 1st Tumor (day)* (mean ± SD) . | Tumor Size (g)† (mean ± SD) . | |||||
|---|---|---|---|---|---|---|---|---|
| . | Control . | Plg−/− . | Control . | Plg−/− . | Day . | Control . | Plg−/− . | P Value‡ . |
| 1 | 13/13 | 17/17 | 1.9 ± 0.9 | 2.4 ± 0.9 NS | 2 | 0.03 ± 0.03 (13) | 0.03 ± 0.05 (17) | NS |
| 4 | 0.07 ± 0.04 (13) | 0.04 ± 0.02 (17) | <.04 | |||||
| 6 | 0.22 ± 0.07 (13) | 0.13 ± 0.07 (17) | <.004 | |||||
| 7 | 0.26 ± 0.09ρ (4) | 0.18 ± 0.08ρ (10) | <.05 | |||||
| 8 | 0.48 ± 0.18 (9) | 0.25 ± 0.13 (7) | <.02 | |||||
| 10 | 0.83 ± 0.30 (9) | 0.44 ± 0.14 (7) | <.008 | |||||
| 12 | 1.2 ± 0.5 (9) | 0.76 ± 0.25 (7) | <.05 | |||||
| 14 | 2.1 ± 0.8 (9) | 1.3 ± 0.5 (7) | <.04 | |||||
| 16 | 3.1 ± 0.8 (9) | 1.9 ± 0.8 (7) | <.02 | |||||
| 18 | 3.6 ± 1.2 (9) | 2.2 ± 0.6 (7) | <.03 | |||||
| 2 | 14/14 | 14/14 | 2.2 ± 0.6 | 2.5 ± 0.7 NS | 2 | 0.02 ± 0.02 (13) | 0.01 ± 0.01 (13) | NS |
| 4 | 0.06 ± 0.01 (13) | 0.03 ± 0.01 (13) | <.0001 | |||||
| 6 | 0.26 ± 0.05 (13) | 0.17 ± 0.07 (13) | <.001 | |||||
| 8 | 0.42 ± 0.10 (13) | 0.28 ± 0.10 (12) | <.001 | |||||
| 10 | 0.86 ± 0.24 (14) | 0.58 ± 0.21 (13) | <.005 | |||||
| 12 | 1.4 ± 0.5 (14) | 1.0 ± 0.4 (13) | <.05 | |||||
| 14 | 2.2 ± 0.8 (14) | 1.7 ± 0.7 (13) | NS | |||||
| 16 | 3.1 ± 1.1 (14) | 2.8 ± 1.0 (13) | NS | |||||
| 3 | 12/12 | 12/12 | 2.3 ± 0.5 | 2.6 ± 1.2 NS | 2 | 0.01 ± 0.01 (8) | 0.00 ± 0.01 (8) | NS |
| 4 | 0.05 ± 0.02 (9) | 0.03 ± 0.01 (8) | <.02 | |||||
| 6 | 0.23 ± 0.06ρ (3) | 0.10 ± 0.02ρ (3) | <.03 | |||||
| 4 | 15/15 | 15/15 | ND | ND | 6 | 0.18 ± 0.05 (4) | 0.09 ± 0.05 (4) | <.04 |
Abbreviations: ND, not done; SD, standard deviation; NS, not significant (P > .05).
Determined by visual inspection of mice.
Tumor size was determined by calipation as described in Material and Methods or by direct measurement as indicated. Numbers in parentheses indicate the number of mice included in measurements.
P values were determined by the Student's t-test (two-tailed).
ρ Direct determination of the weight of excised tumors.
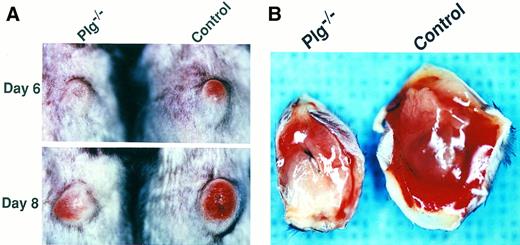
Plg deficiency reduces primary tumor size, skin ulceration, and hemorrhaging of Lewis lung carcinoma. (A) Representative examples of the macroscopic appearance of primary tumors in a Plg−/− mouse (left panels) and a control mouse (right panels) at day 6 (upper panel) and day 8 (lower panel) after tumor cell injection. Note that the tumor in the Plg−/− mouse is markedly smaller and displays reduced skin redness. (B) Representative examples of subcutaneous hemorrhaging in a Plg−/− mouse (left) and a control mouse (right). Mice were killed at day 11 and perfusion fixed with paraformaldehyde as described in Materials and Methods, and the primary tumors were excised and photographed with the base of the tumor facing up. Note the marked reduction in free blood in the Plg−/− mouse.
Plg deficiency reduces primary tumor size, skin ulceration, and hemorrhaging of Lewis lung carcinoma. (A) Representative examples of the macroscopic appearance of primary tumors in a Plg−/− mouse (left panels) and a control mouse (right panels) at day 6 (upper panel) and day 8 (lower panel) after tumor cell injection. Note that the tumor in the Plg−/− mouse is markedly smaller and displays reduced skin redness. (B) Representative examples of subcutaneous hemorrhaging in a Plg−/− mouse (left) and a control mouse (right). Mice were killed at day 11 and perfusion fixed with paraformaldehyde as described in Materials and Methods, and the primary tumors were excised and photographed with the base of the tumor facing up. Note the marked reduction in free blood in the Plg−/− mouse.
Primary tumors develop in the absence of Plg, but are smaller, less ulcerating, and less hemorrhagic. The C57BL/6-inbred mice raised for this study were histocompatible with and supported the growth and dissemination of transplanted Lewis lung carcinoma. Primary tumors developed and disseminated in all 55 Plg+/+ and 52 Plg+/− mice used in the study (see below and data not shown). No differences were observed between Plg+/+ and Plg+/− mice with respect to the rate of primary tumor formation, tumor growth, number and size of lung metastasis, or survival of tumor-bearing mice (data not shown). All Plg−/− mice injected with tumor cells formed a primary tumor in six independent experiments involving a total of 100 Plg−/− mice, showing that Plg is not essential for tumor development (Table 1 and data not shown). Furthermore, no significant difference was observed between Plg−/− and littermate control mice in the rate of primary tumor development as evidenced by the time of appearance of a visible tumor (Table 1). Although the frequency and kinetics of primary tumor formation was unchanged in Plg−/− mice, a closer examination of primary tumors showed obvious quantitative and qualitative differences between tumors developing in the absence and presence of Plg (Fig 2 and data not shown). Four and 10 days after tumor cell inoculation, when primary tumor growth was vigorous, the tumors of Plg−/− mice were significantly smaller, with average tumor masses in Plg−/− mice ranging from 50% to 69% of average tumor masses of littermate control mice. At later time points, the difference in tumor mass was less dramatic (Plg−/− tumors were 62% to 90% of control mice) and the variation in tumor size was larger (Table 1). Proliferation of the primary tumor in both control and Plg−/− mice was associated with ulceration of the overlying skin, initially manifested as progressive skin redness (Fig 2A). However, this process was significantly delayed in Plg−/− mice. For example, in two independent experiments in which skin ulceration was examined, 7 of 13 (54%) and 5 of 9 (56%) of the control mice demonstrated evidence of skin ulceration 5 days after injection, compared with 0 of 13 (0%; P < .002) and 1 of 8 (13%; P < .063) of the Plg−/− mice, respectively. Moreover, the skin involvement in most control mice progressed to hemorrhaging ulcers, whereas this was observed infrequently in Plg−/− mice. Thus, in control mice, hemorrhagic ulcers developed in 11 of 14 mice (79%) within an observation period of 18 days, compared with just 2 of 14 Plg−/− mice (14%) within an observation period of 19 days (P < .001, log-rank test). Furthermore, tumors grown in control mice and Plg−/− mice were immediately distinguishable after excision from perfused animals. Tumors grown in control mice uniformly displayed massive basal hemorrhaging/edema, contributing substantially to the tumor volume. In contrast, tumors excised from Plg−/− mice displayed limited hemorrhaging (Fig 2B). This difference was not related to the size of the primary tumors, because even the smallest control tumors displayed prominent hemorrhaging.
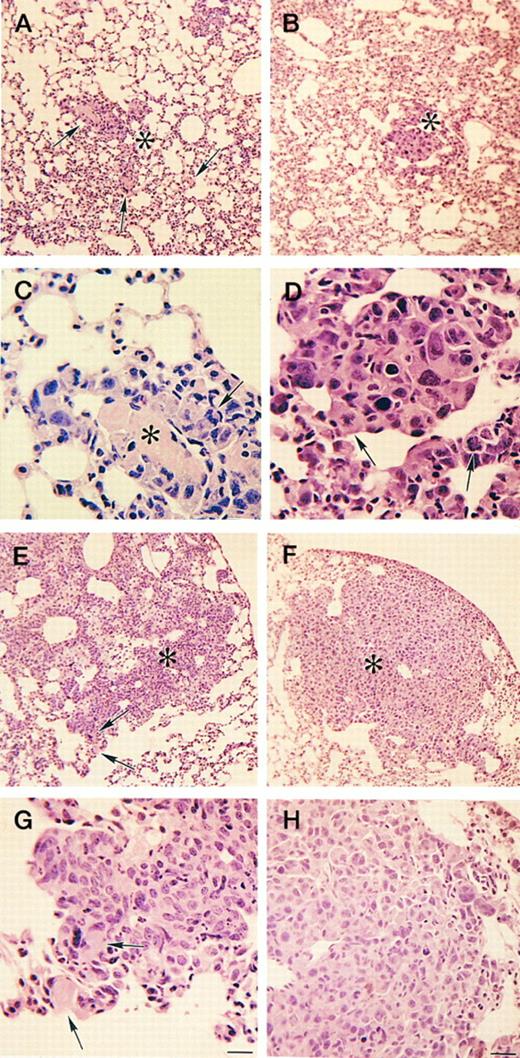
Histologic appearance of primary tumors in Plg−/− and control mice. Hematoxylin/eosin stain (A through F ) and PECAM immunostain (G and H) of primary tumors 11 days after tumor inoculation. (A and B) Typical appearance of skin overlying the primary tumor in a Plg−/− mouse (A) and a control mouse (B). Both tumors exhibit skin breakdown with surface accumulation of serum proteins, exudate, and dead cell debris (escar). The arrow indicates intact skin. Arrowheads indicate escar. The asterisk indicates tumor. Bar = 100 μm. (C and D) Tumor invasion of dermal muscle in a Plg−/− mouse (C) and a control mouse (D). Both tumors display diffuse invasion with separation and destruction of myocytes. The arrow indicates intact muscle. Arrowheads indicate muscle invaded by tumor. Bar = 100 μm. (E and F ) High magnification micrographs of the tumor in a Plg−/− mouse (E) and a control mouse (F ). The micrographs show similar cytologic features with dense cellularity and severe anaplasia characterized by variation in cell size and shape and numerous mitoses (arrowheads), with some being bizarre (asterisk). Capillaries and endothelial cells are frequent in each tumor (arrows). Bar = 22 μm. (G and H) Immunostain for endothelial cells (PECAM) confirms the presence of numerous vessels in both Plg−/− mice (G) and control mice (H). Bar = 100 μm.
Histologic appearance of primary tumors in Plg−/− and control mice. Hematoxylin/eosin stain (A through F ) and PECAM immunostain (G and H) of primary tumors 11 days after tumor inoculation. (A and B) Typical appearance of skin overlying the primary tumor in a Plg−/− mouse (A) and a control mouse (B). Both tumors exhibit skin breakdown with surface accumulation of serum proteins, exudate, and dead cell debris (escar). The arrow indicates intact skin. Arrowheads indicate escar. The asterisk indicates tumor. Bar = 100 μm. (C and D) Tumor invasion of dermal muscle in a Plg−/− mouse (C) and a control mouse (D). Both tumors display diffuse invasion with separation and destruction of myocytes. The arrow indicates intact muscle. Arrowheads indicate muscle invaded by tumor. Bar = 100 μm. (E and F ) High magnification micrographs of the tumor in a Plg−/− mouse (E) and a control mouse (F ). The micrographs show similar cytologic features with dense cellularity and severe anaplasia characterized by variation in cell size and shape and numerous mitoses (arrowheads), with some being bizarre (asterisk). Capillaries and endothelial cells are frequent in each tumor (arrows). Bar = 22 μm. (G and H) Immunostain for endothelial cells (PECAM) confirms the presence of numerous vessels in both Plg−/− mice (G) and control mice (H). Bar = 100 μm.
Histologic appearance of lung metastases in Plg−/− and control mice. Representative examples of lung sections of Plg−/− mice (A, C, E, and G) and control mice (B, D, F, and H) 15 days after tumor cell inoculation stained with hematoxylin/eosin. (A and B) Clusters of small metastatic foci (asterisk) are numerous in the lungs of both Plg−/− mice (A) and control mice (B). Small fibrin thrombi are numerous in Plg−/− mice (arrows). Bar = 100 μm. (C and D) Higher magnification of these metastatic foci show cytologic features similar to the primary tumor in both Plg−/− mice (C) and control mice (D). Metastatic foci are often associated with fibrin thrombi in Plg−/− mice (asterisk) but rarely in control mice. Abnormal mitoses are indicated with arrows. Bar = 22 μm. (E and F ) Examples of large metastatic foci (asterisk) of Plg−/− mice (E) and control mice (F ). Large metastatic foci in Plg−/− mice tend to incorporate thrombi (arrows), whereas this is rare in control mice. (G and H) Higher magnification of large metastatic foci shows similar cytologic features in Plg−/− mice (G) and control mice (H). Fibrin deposits are indicated with arrows. Bar = 22 μm.
Histologic appearance of lung metastases in Plg−/− and control mice. Representative examples of lung sections of Plg−/− mice (A, C, E, and G) and control mice (B, D, F, and H) 15 days after tumor cell inoculation stained with hematoxylin/eosin. (A and B) Clusters of small metastatic foci (asterisk) are numerous in the lungs of both Plg−/− mice (A) and control mice (B). Small fibrin thrombi are numerous in Plg−/− mice (arrows). Bar = 100 μm. (C and D) Higher magnification of these metastatic foci show cytologic features similar to the primary tumor in both Plg−/− mice (C) and control mice (D). Metastatic foci are often associated with fibrin thrombi in Plg−/− mice (asterisk) but rarely in control mice. Abnormal mitoses are indicated with arrows. Bar = 22 μm. (E and F ) Examples of large metastatic foci (asterisk) of Plg−/− mice (E) and control mice (F ). Large metastatic foci in Plg−/− mice tend to incorporate thrombi (arrows), whereas this is rare in control mice. (G and H) Higher magnification of large metastatic foci shows similar cytologic features in Plg−/− mice (G) and control mice (H). Fibrin deposits are indicated with arrows. Bar = 22 μm.
Histologic characterization of primary tumors. A detailed microscopic analysis of primary tumors from Plg−/− and control mice killed 6, 7, 11, and 15 days after tumor cell injection did not show striking histologic differences between tumors developing in the presence or absence of Plg (Fig 3). In both Plg−/− and control mice, the tumor nodules were composed of anaplastic appearing cells, which varied in size and shape with relatively scant cytoplasm, and large angulated nuclei with prominent nucleoli. There were numerous mitoses, with some abnormal in configuration (Fig 3E and F ). All tumors were highly vascularized, demonstrating that Plg is not critical for tumor angiogenesis (Fig 3E and F ). Immunostaining for endothelial cells confirmed the presence of numerous vessels, with no obvious qualitative differences between tumors from control and Plg−/− mice (Fig 3G and H). Also, no major differences could be detected in the invasion of adjacent tissues by the primary tumor in the presence or absence of Plg. In all histologic sections from tumors developing in Plg−/− mice, the tumor cells displayed diffuse infiltration of the skin (example in Fig 3A and B) and dermal muscle (example in Fig 3C and D) with ensuing tissue destruction and necrosis. Taken together, the histologic analyses clearly shows that the invasive phenotype of Lewis lung carcinoma is retained in the absence of Plg.
In light of the physiologic role of Plg in fibrin clearance, fibrin(ogen) deposition was explored in both primary tumors and metastasis by immunohistochemistry. Deposition of fibrin(ogen) was evident within tumors of both control and Plg−/− mice and was particularly prominent along the deep margin of tumor nodules and in central necrotic areas (data not shown). Fibrin(ogen) was also frequently observed in small occluded vessels within the tumor. No staining was observed in parallel sections from a fibrinogen-deficient mouse carrying the Lewis lung carcinoma, demonstrating the specificity of the antibody. No obvious differences in extent of fibrin(ogen) deposition were evident between Plg−/− mice and littermate control mice, although more quantitative studies are needed to definitively address this issue.
Delayed regional lymph node metastasis and tumor regrowth in Plg−/− mice. To examine the kinetics of Lewis lung carcinoma metastasis to regional lymph nodes in the presence or absence of Plg, we observed a prospective cohort consisting of control mice and Plg−/− mice with the primary tumor resected at day 10 and determined the appearance of overt lymph node metastasis by palpation and visual inspection. Lymphatic spread of the tumor was apparent macroscopically in 9 of 15 (60%) control mice within a 12-day observation period after primary tumor resection, as opposed to just 2 of 15 (13%) of the Plg−/− mice within an observation period of 14 days (P < .006, log-rank test). Similar results were obtained without resection of the primary tumor, although quantitation of the frequency of lymph node metastasis was complicated by the high mortality of mice with nonresected tumors during the observation period (data not shown). Plg deficiency was also associated with a reduction of regrowth of the tumor below the area of resection. Six of 15 control mice (40%) showed visible tumor growth in this area between days 11 and 14 after resection, whereas regrowth was only observed in 1 of 15 Plg−/− mice (7%) within the same period (P = .03, χ2 analysis; data not shown).
Metastasis to lungs and other organs in the absence of Plg. To determine the impact of Plg deficiency on distant metastasis of Lewis lung carcinoma after the formation of primary skin tumors, we performed a detailed quantitative analysis of spontaneous lung metastasis in Plg−/− and control mice. The data are summarized in Table 2. Based on simple macroscopic inspection, large metastatic foci were apparent on the surface of the lungs of all the Plg−/− mice examined at day 21, showing that plasminogen activation is not essential for successful metastasis to the lung. Moreover, quantitation of the number of surface metastatic foci did not show a significant difference between Plg−/− and littermate control mice, suggesting that the lack of plasmin-mediated proteolysis does not markedly affect the overall efficiency of lung colonization by this tumor (Table 2). Resection of the primary tumor at either day 7 or 10 reduced the number of surface metastatic foci in the lungs of both Plg−/− and control mice by a factor of 25 and 3, respectively (Table 2). Thus, under the experimental conditions used, leaving the primary tumor intact greatly increased the number of metastatic foci regardless of mouse genotype. However, no significant differences were observed between Plg−/− and littermate control mice in the number of surface metastatic foci when compared within study protocols, with and without primary tumor resection (Table 2).
Lung Metastasis in Control and Plg−/− Mice
| . | Metastatic Foci in Lungs at Day 21* . | |
|---|---|---|
| . | (median; range) . | |
| . | Control . | Plg−/− . |
| Primary tumor intact | Total† 350; 13->400‡ (8) | 361; 243->400‡ (7) |
| Primary tumor resected | ||
| Day 7 | Total† 14; 6-22 (4) | 13; 2-61 (10) |
| Day 10 | Total† 120; 43-251 (9) | 103; 57-182 (10) |
| Largeρ 38; 14-93 (10) | 43; 16-76 (10) | |
| . | Metastatic Foci in Lungs at Day 21* . | |
|---|---|---|
| . | (median; range) . | |
| . | Control . | Plg−/− . |
| Primary tumor intact | Total† 350; 13->400‡ (8) | 361; 243->400‡ (7) |
| Primary tumor resected | ||
| Day 7 | Total† 14; 6-22 (4) | 13; 2-61 (10) |
| Day 10 | Total† 120; 43-251 (9) | 103; 57-182 (10) |
| Largeρ 38; 14-93 (10) | 43; 16-76 (10) | |
Numbers in parentheses indicate number of mice used for determination of the value.
Lungs were fixed in Bouin's fixative and mechanically separated into individual lobes and metastatic foci were counted on each lobe.
Number of metastatic foci determined by counting under a stereo microscope at 4× magnification.
The lungs of 3 control mice and of 3 Plg−/− mice presented with too many metastatic foci for precise quantitation. The number of metastatic foci in these lungs was estimated to be between 400 and 500 and was assigned the value 400 in the determination of the median value.
ρ Number of metastatic foci determined by counting by naked eye.
The possibility that Plg deficiency altered metastatic lung tumor mass was explored by measuring lung weights in Plg−/− mice and control mice 28 days after tumor inoculation (primary tumor resected at day 10). At this time point, the metastatic tumor mass can be determined by the increase in total lung weight (normal lung weight, ∼0.20 g). Consistent with earlier data comparing the number of metastatic lung foci, total lung tumor burden in Plg−/− mice (lung weight, 0.38 ± 0.08 g; n = 5) was comparable to that observed in control animals (lung weight, 0.39 ± 0.02 g; n = 6). However, it should be recognized that differences in the survival of tumor-bearing Plg−/− and littermate control mice (see below) could bias the comparison at this late time point; in the cohort used for lung weight determination, 6 of 12 (50%) of the control mice died before day 28, as opposed to just 1 of 6 (17%) of the Plg−/− mice. No meaningful comparison of metastasis burden could be made between mice with intact and resected tumors at this time point, because more than 75% of both Plg−/− and control mice with intact tumors died before day 28 (see below and data not shown).
In addition to lungs and lymph nodes, metastatic dissemination of the tumor to numerous other sites was documented in both control and Plg−/− mice by macroscopic and microscopic examination of tissues between days 21 and 31 (data not shown). These included liver, pleural cavity, diaphragm, pericardium, cardiac muscle, pancreas, adipose tissue, and esophagus. Further studies are needed to determine if the rate of metastasis to these sites is quantitatively affected by Plg deficiency.
Histologic characterization of lung metastasis in control and Plg−/− mice. A detailed microscopic analysis of lung tissue from Plg−/− and control mice at days 15, 21, and 28 (Fig 4 and data not shown) did not show any striking differences in the histology of metastases developing in the absence or presence of Plg. At early time points, multiple tiny metastases were apparent in both Plg−/− mice and control mice, usually concentric or eccentric to a vessel and often associated with a thrombus in Plg−/− mice (Fig 4A through D). In larger tumor nodules, the cells grew in confluent sheets without patterning and with a substructure of fine, thin-walled capillaries most numerous near the surface (Fig 4E through H and data not shown). As the nodules expanded, they invaded and separated adjacent tissue structures eventually leading to tissue degeneration. Large tumor masses underwent necrosis, sometimes accompanied by local hemorrhage, and, rarely, acute inflammation (data not shown).
Effect of Plg deficiency on survival of tumor-bearing mice. The survival characteristics were compared in a prospective cohort of matched Plg−/− and littermate control mice with and without surgical resection of the primary tumor. With the primary tumor intact, the lack of Plg did not significantly alter the survival of tumor-bearing mice. Control mice succumbed to their tumors within 19 to 32 days after tumor cell injection, with a median survival of 24 days, and Plg−/− mice died within 21 to 32 days after tumor cell injection, with a median survival of 26 days. However, with the primary tumors resected at day 10, Plg deficiency was associated with a small but significant increase in survival, with the median survival after tumor resection being increased from 17 days in control mice to 21 days in Plg−/− mice (P = .011; Fig 5). A gross necroscopic analysis of animals showed widespread dissemination of the tumor in all mice irrespective of genotype, making a precise determination and comparison of the cause of death of the mice in the two groups difficult (data not shown).
Lack of Plg increases the survival of mice with Lewis lung carcinoma after surgical resection of the primary tumor. Plot of the percentage of surviving mice versus time after resection of primary tumors. Tumor cells were injected into 15 control mice and 14 Plg−/− mice, and the primary tumor was surgically resected at day 10. The P value was determined by the log-rank test. One control mouse did not die within the observation period and was alive and healthy more than 5 months after tumor cell inoculation, indicating that the tumor had spontaneously regressed. This mouse was included in the determination of the P value.
Lack of Plg increases the survival of mice with Lewis lung carcinoma after surgical resection of the primary tumor. Plot of the percentage of surviving mice versus time after resection of primary tumors. Tumor cells were injected into 15 control mice and 14 Plg−/− mice, and the primary tumor was surgically resected at day 10. The P value was determined by the log-rank test. One control mouse did not die within the observation period and was alive and healthy more than 5 months after tumor cell inoculation, indicating that the tumor had spontaneously regressed. This mouse was included in the determination of the P value.
DISCUSSION
The findings presented in this report show that all the steps involved in the growth and dissemination of a malignant tumor can take place in the complete absence of plasmin-mediated proteolysis. Lewis lung carcinoma cells inoculated into the subcutis of Plg−/− mice proliferated vigorously to form well-vascularized primary tumors. Furthermore, these tumor cells both invaded local tissue and spread to numerous distant sites. Thus, cancer cells can successfully clear all the major hurdles previously associated with metastatic tumor growth (eg, vasation, arrest, extravasation, growth, development of a supporting neovasculature) without any benefit of plasminogen activation. These findings are consistent with the previous findings that Plg is not strictly required for many physiologic processes involving extensive tissue remodeling and/or cell migration, including embryonic development and growth to adulthood.39 40
Although not essential for tumor growth and dissemination, the findings in this report show that Plg is, nevertheless, relevant to tumor biology. Relative to control mice, the primary tumors in Plg−/− mice were consistently smaller, less hemorrhagic, and slower to ulcerate the overlying skin. Plg−/− mice also showed reduced tumor growth in regional lymph nodes and delayed or absent tumor regrowth after surgical resection of the primary tumor. Finally, after resection of the primary tumor, Plg−/− mice generally survived longer than control mice. Although this study clearly demonstrates that Plg contributes to tumor dissemination, the exact mechanism(s) by which Plg modulates tumor pathobiology is unclear. Plasmin could activate latent growth factors or collagen-degrading matrix metalloproteases, directly degrade extracellular matrix components, or contribute by a combination of these activities.
We have recently shown that plasmin-mediated proteolysis is important for migration of dermal keratinocytes into the provisional matrix during the healing of skin wounds, a physiologic process with many similarities to tumor invasion.7,48 However, the need for plasmin-mediated proteolysis in these processes appears to be restricted to fibrinolysis, because loss of fibrinogen in Plg−/− mice restores normal healing times.8 Fibrin is a prominent component of the stroma of both human and experimental mouse tumors, including Lewis lung carcinoma.49-54 In the absence of plasmin-mediated fibrinolysis, fibrin may constitute a significant physical barrier slowing, but not preventing, tumor dissemination. This model would be consistent with the profound delay, but not prevention, of keratinocyte migration into wound fields in Plg−/− mice.7 8 Nevertheless, the relevance of reduced fibrinolysis to the impairment of tumor cell dissemination in Plg−/− mice remains to be established. A detailed evaluation of tumor biology in mice deficient in both Plg and fibrinogen should settle this question, and these studies are in progress.
The lack of a stronger effect of Plg deficiency on tumor metastasis was unexpected in light of previous reports that systemic administration of neutralizing uPA antibodies, uPA receptor antagonists, or other inhibitors of the PA system dramatically suppressed spontaneous metastasis of Lewis lung carcinoma32,33 and many other tumor types.26-31,34 36-38 The importance of the PA system may vary from critical to irrelevant in individual tumor types with distinct origins and biochemical properties. The site of initial tumor development and the pattern of expression of adhesion molecules, matrix proteins, matrix-degrading proteases, and procoagulants may be factors that determine the relative importance of Plg activation in tumor growth and metastasis. Thus, earlier findings suggesting that Plg activation is important in tumor metastasis and our finding that Plg is not required for tumor dissemination may reflect tumor-specific differences in the relative importance of plasmin-mediated proteolysis. It will be critical to compare the behavior of many distinct classes of tumors in control and Plg−/− mice, including adenomas, hemangiomas, carcinomas, ascites tumors, and leukemia, to rigorously determine the general importance of Plg in tumor biology. However, the present findings clearly document that there are settings, or classes of tumors, in which the loss of Plg does not present a fundamental impediment to tumor cell metastasis.
Loss of the Plg-derived inhibitor of angiogenesis, angiostatin,41 was not associated with an obvious increase in tumor neovascularization within primary tumors and metastases, as assessed by qualitative microscopic analysis using an endothelial cell marker, or on the rate of growth of primary tumors or metastases. However, more detailed studies need to be performed to detect any possible quantitative differences in the angiogenic potential of Lewis lung carcinoma in a Plg-free environment.
A spectrum of serine, metallo, aspartic, and cysteine proteases have been implicated in tumor-associated matrix degradation.1,2,55-61 The involvement of these proteases in tumor dissemination in most cases has been inferred indirectly from their expression pattern within tumors and from their value as prognostic markers. The recent development of mice with targeted mutations in matrix-degrading proteases, as well as the development of specific and efficient synthetic matrix metalloprotease inhibitors, has provided powerful tools to directly dissect the individual roles of matrix-degrading proteases in cancer invasion and metastasis in vivo.39,40,42,62-69 A recent study showed that intestinal cancer was reduced, but not abolished, in mice deficient in the matrix metalloprotease matrilysin.64 Systemic administration of a synthetic gelatinase inhibitor demonstrated very similar effects on the dissemination of Lewis lung carcinoma as those of Plg deficiency, ie, a moderate reduction in primary tumor growth and metastasis, and slightly increased survival.69 It remains to be established whether the combined elimination of several cancer-associated matrix-degrading proteases will seriously impede tumor dissemination. Such studies will be most important for evaluating the prospects for efficient cancer therapy directed against matrix-degrading proteases.
ACKNOWLEDGMENT
The authors are indebted to Drs Zhongyun Dong and Isaiah J. Fidler for their invaluable help and support, Drs Judah Folkman and Michael S. O'Reilly for providing the Lewis lung tumor cells, Dr Leif R. Lund for uPA and PAI-1 plasmids, and Dr Peter Gartside for help with statistical analysis. We thank Drs Keld Danø and Morten Johnsen for helpful suggestions and Drs Mary Jo S. Danton and Angela Drew for critically reading the manuscript.
Supported by National Institutes of Health Grant No. HL47826 (to J.L.D.). Additional support was provided by the National American Heart Association (with funds contributed by the American Heart Association Ohio Affiliate; 92-1103; to J.L.D.). T.H.B. was supported by the Danish Medical Research Council.
Address reprint requests to Jay L. Degen, PhD, Children's Hospital Research Foundation, IDR-NRB Room 2025, 3333 Burnet Ave, Cincinnati, OH 45229.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal