Abstract
Dilazep, an antiplatelet agent, is generally used as an antithrombotic drug in clinical practice. Dilazep is also known to exert cytoprotective and antioxidant effects on endothelial cells. However, its effect on the endothelial or monocyte procoagulant activity is unknown. In the current study, the effect of dilazep on the expression of tissue factor (TF ) in human umbilical vein endothelial cells (HUVECs) after the stimulation with tumor necrosis factor-α (TNF ), thrombin, or phorbol 12-myristate 13-acetate (PMA) was evaluated. We also evaluated the effect of dilazep on TNF (1,000 U/mL)-induced TF expression on monocytes. Dilazep inhibited TF activity induced on HUVECs by each stimulant, TNF (1000 U/mL), thrombin (25 nmol/L), or PMA (5 nmol/L) in a dose-dependent fashion (1 to 100 μg/mL). TF activity decreased to approximately 10% after treating with 100 μg/mL of dilazep. Dilazep also blocked the expression of TF antigen induced by each stimulant on the surface of HUVECs as determined by flow cytometric analysis. In addition, in HUVECs, it significantly decreased the expression of TF mRNA and the total TF antigen induced by thrombin or PMA, but not those induced by TNF, suggesting that dilazep blocks the TF expression induced by PMA or thrombin at a transcriptional level and that induced by TNF at a posttranscriptional level. Western blot analysis showed that dilazep reduces the accumulation of native TF but increases that in lower molecular weight TF derivatives. The adenosine receptor antagonist, 8-(p-sulfophenyl) theophylline, partially counteracted the anticoagulant activity of dilazep on HUVECs, thereby suggesting that the inhibitory effect of dilazep on TF expression in HUVECs depends, at least in part, on its adenosine potentiating activity. Dilazep also inhibited TNF-induced TF expression on monocytes in a dose-dependent fashion (0.1 to 100 μg/mL). In brief, the current study showed for the first time that dilazep, a commonly used antiplatelet drug, strongly inhibits the TF expression in HUVECs and monocytes. Dilazep may have a potent therapeutic value in patients with hypercoagulable state for its inhibitory property on the procoagulant activity of endothelial cells and monocytes.
TISSUE FACTOR (TF ) is a membrane-bound glycoprotein that activates blood coagulation via the extrinsic clotting pathway.1 Abnormal expression of TF on endothelial cells, monocytes, macrophages, and other intravascular cells may be associated with the occurrence of coronary disease, strokes, and arterial or venous thrombosis.2-4 Upregulation of TF on endothelial cells is induced in vitro by cytokines (tumor necrosis factor-α [TNF ] and interleukin-1 [IL-1]), mitogens (tumor-promoting phorbol esters such as phorbol 12-myristate 13-acetate [PMA]), thrombin, and endotoxin.5-7 Downregulation of TF expression on endothelial cells can be induced by various anti-inflammatory cytokines (IL-4, IL-10, and IL-13), retinoic acid, and antioxidants.8-11 Antiplatelet agents of the prostanoid family have also been shown to inhibit the procoagulant activity of endothelial cells by increasing the intracellular concentration of cAMP.12
Dilazep, an antiplatelet agent widely used in European countries and Japan, exerts its antiplatelet activity by increasing the adenosine levels in the extracellular space fluid.13,14 Dilazep is also known to have a vasodilating activity on coronary, cerebral, and renal vessels,15,16 and it is often used in patients with ischemic heart disease, cerebral ischemic, or renal dysfunction to improve tissue blood irrigation.13,14 Previous studies suggested that dilazep also exerts cytoprotective and antioxidant effects on endothelial cells17 and antithrombotic effect in experimental animal models.18 Furthermore, in preliminary clinical studies, we found that dilazep decreased the plasma levels of various hemostatic markers in patients with hypercoagulable state. However, whether it affects the procoagulant activity of endothelial cells or monocytes remains unclear. We then evaluated the effect of dilazep on the TF expression in human umbilical vein endothelial cells (HUVECs) stimulated with TNF, thrombin, or PMA and in TNF-stimulated monocytes. The results of the present study showed that dilazep inhibits TF expression in HUVECs and monocytes, that dilazep blocks TF expression in HUVECs at both transcriptional and posttranscriptional levels, and that its anticoagulant activity depends, in part, on the stimulation of adenosine receptors.
MATERIALS AND METHODS
Materials.Citrated human plasma was obtained from the local Red Cross blood center. Human factor VIIa and factor X were provided by Dr Tomohiro Nakagaki (Chemo-Sera Therapeutic Institute, Kumamoto, Japan). Antithrombin,19 protein C,20 and thrombin21 were prepared from human plasma as described. Recombinant human TNF was donated by the Asahi Chemical Industry (Fuji, Shizuoka, Japan). The synthetic peptide substrate for factor Xa, N-b e n z o y l - L - i s o l e u c y l - L - g l u t a m y l - g l y c y l - L - a r g i n i n e - p - n i t r o a n i l i d e -hydrochloride (S2222), was purchased from Chromogenix (Mölndal, Sweden). The fluorogenic substrate for activated protein C, Boc-Leu-Ser-Thr-Arg-methylcoumarylamide (MCA), was obtained from the Protein Research Foundation (Osaka, Japan). Dulbecco's phosphate-buffered saline (PBS), gentamycin, bovine serum albumin (BSA), heparin (174 U/mg), and adenosine were from Sigma (St Louis, MO). Nick column was obtained from Pharmacia-LKB (Uppsala, Sweden). Triton X-100 and aprotinin were from Boehringer Mannheim GmbH (Mannheim, Germany). Glyceraldehyde-3 phosphate dehydrogenase (GAPDH) cDNA probe and 8-(p-sulfophenyl) theophylline (8-SPT) was purchased from Clontech Labs (Palo Alto, CA) and Research Biochemicals International (Natick, MA), respectively. Horseradish peroxidase (HRP)-conjugated antimouse IgG-goat antibody and antigoat IgG-rabbit antibody were obtained from Bio-Rad (Richmond, CA). The reagents to check the viability of the cells, WST-1 [2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl )-2H-tetrazolium-Na] and 1-methoxy PMS (1-methoxy-5-methyl-phenazinium methylsulphate), were purchased from Dojindo (Kumamoto, Japan).
Patients.Twenty inoperable patients with malignant disease (adenocarcinoma, 12; squamous cell carcinoma, 2; small-cell carcinoma, 2; hepatoma, 2; melanoma, 1; and myeloma, 1) and 8 with benign disorder (dissecting aneurysm of the aorta, 6; chronic enteritis, 1; and chronic viral infection, 1) were studied. All of these patients were in the hypercoagulable state and were treated with dilazep (300 mg/d) for 1 month before the initiation of any kind of therapy. Blood samples were collected from the patients before and after 1 month of starting the therapy with dilazep. Plasma levels of thrombin-antithrombin complex (TAT) and fibrin degradation product (FDP)-D-dimer were determined using Enzygnost-TAT kit (Behringwerke AG, Marburg, Germany; normal range, 1.8 ± 0.7 ng/mL22 ) and Felisa D-dimer (Agen, Brisbane, Australia; normal range, 48 ± 34 ng/mL22 ), respectively. Plasma fibrinogen level was determined using the clotting time method (normal range, 2.5 ± 0.4 mg/mL22 ). The plasma level of TF antigen was determined by TF enzyme-linked immunosorbent assay (ELISA) kit (Chemo-Sera Therapeutic Institute; normal range, 196.8 ± 54.3 pg/mL). Data are expressed as the mean ± SD. The statistical difference between variables was evaluated by the Student's t-test or the Wilcoxon rank test according to the Gaussian distribution of the data.
Culture of HUVECs.HUVECs were purchased from Kurabo (Osaka, Japan). The cells were cultured in modified MCDB medium (Chlorella Industry, Tokyo, Japan) supplemented with 2% fetal bovine serum (GIBCO BRL, Grand Island, NY), 50 μg/mL gentamycin, 0.01 μg/mL epithelial growth factor (Becton Dickinson, Bedford, MA), 10 μg/mL endothelial cell growth factor (Becton Dickinson), and 10 μg/mL unfractionated heparin (Sigma) under an atmosphere of 95% air and 5% CO2.
Preparation of peripheral blood mononuclear cells.Peripheral blood cells were obtained from healthy donors by vein puncture using EDTA as anticoagulant. Mononuclear cells were isolated by the Lymphoprep Tube (Nycorned Pharma Diagnostica, Oslo, Norway). The mononuclear cell phase, comprising monocytes and lymphocytes, was harvested, washed twice with RPMI (RPMI 1640 medium supplemented with 2 nmol/L L-glutamine, 100 μg/mL streptomycin, 100 μg/mL penicillin, 10% fetal bovine serum), and resuspended in RPMI.
Determination of HUVEC surface TF activity.TF activity was determined as factor X activation by factor VIIa/TF complex on HUVECs after stimulation with TNF, thrombin, or PMA. After incubation of the cells (24-well plate, 5 × 105 cells/well) with each stimulant in the presence or absence of dilazep or adenosine, the cells were washed twice with HEPES-buffered saline (20 mmol/L HEPES, 150 mmol/L NaCl, pH 7.5) containing 5 mmol/L CaCl2 . In separate experiments, 1 mmol/L 8-SPT was incubated with HUVECs for 15 minutes before the addition of dilazep. Aliquots of a mixture of 50 μL of 3.76 nmol/L factor VIIa, 50 μL of 980 nmol/L factor X, and 150 μL of HEPES-buffered saline containing 5 mmol/L CaCl2 were incubated in wells for 30 minutes at room temperature. The reaction was stopped by adding 10 μL of 100 mmol/L EDTA and the generated factor Xa was determined using 100 μmol/L S2222 after incubating for 20 minutes at room temperature. The reaction was stopped by the addition of 10 μL of 20% acetic acid, and color development was determined by measuring the absorbance at 405 nm with an EAR 340 microplate reader (SLT-Lab Instruments, Salzburg, Austria).
Flow cytometric analysis of HUVEC surface TF antigen.HUVECs were incubated with or without TNF, thrombin, or PMA in the presence or absence of dilazep for 5 hours and harvested using trypsin. The cells were then washed with cold Dulbecco's PBS and resuspended (2 × 106 cells) in 100 μL PBS. The cells were incubated with mouse monoclonal antihuman TF IgG (5 μg/mL)23 for 30 minutes at 4°C and subsequently washed three times with PBS. The cells were then incubated with the F(ab′)2 fraction of a fluorescein isothiocyanate (FITC)-labeled antimouse IgG-goat antibody (1/20 dilution) for 30 minutes at 4°C. The cells were then washed three times with PBS and fixed in fluorescence-activated cell sorting (FACS) lysing solution (Becton Dickinson) according to the manufacturer's instructions. Flow cytometric analysis was performed using a FACScan analyzer (Becton Dickinson) equipped with a 15-mW, 488-nm argon-ion laser. Green fluorescence emission was recorded at 530 nm. A total of 10,000 events were analyzed per sample. Median channel fluorescence in each sample was determined using a logarithmic scale. Logarithmic channel was converted into linear channel fluorescence units to facilitate comparison of results.
Measurement of total TF antigen level in HUVECs.The total cell TF antigen levels in lysates of HUVECs were measured by ELISA.24 Briefly, after stimulation of HUVECs with TNF, thrombin, or PMA in the presence or absence of dilazep, the cells were washed twice with cold Dulbecco's PBS and harvested. Subsequently, harvested cells were centrifuged (2,000 rpm for 5 minutes) and the precipitate was dissolved in Tris-buffered saline (TBS; 50 mmol/L Tris/HCl, 150 mmol/L NaCl, pH 7.5) containing, 0.6% (wt/vol) Triton X-100 and aprotinin (100 U/mL). The cellular extraction was then diluted 1,000-fold with TBS containing 0.1% BSA and 0.02% thimerosal and applied to wells coated with rabbit antihuman TF antibodies (American Diagnostica, Greenwich, CT). The wells were blocked with 100 mmol/L phosphate buffer, pH 8.0, containing 5% BSA, and then washed three times with TBS containing 0.1% BSA and 0.02% thimerosal. Then, biotynyl-monoclonal antibody against human TF (MSTF-3)23 was added and incubated for 2 hours. After washing the wells, HRP-labeled streptavidin was added and incubated for 1 hour. After the addition of H2O2 and peroxidase substrate, color development was measured at 492 nm using an EAR microplate reader.
Western blotting analysis of TF antigen in HUVECs.TF expression in HUVECs was determined by Western blotting using polyclonal goat antihuman TF antibodies (American Diagnostica). Sodium dodecyl sulphate-polyacrylamide gel electrophoresis of HUVEC lysates was performed, followed by blotting onto nitrocellulose membrane. The membrane was incubated with blocking solution (100 mmol/L PBS, pH 7.5, containing 5% BSA and 0.5% Tween 20) and then exposed to a 1/2,000 dilution of goat anti-TF antibodies for 1 hour while shaking at room temperature. After washing the membrane with PBS containing 0.1% BSA and 0.5% Tween 20, the membrane was then incubated with a 1/20,000 dilution of antigoat IgG antibody conjugated to HRP. The membrane was washed and quantitated using an enhanced chemiluminescence detection system (ECL; Amersham, Buckinghamshire, UK) as described previously.25
Analysis of TF mRNA with reverse transcription and polymerase chain reaction (RT-PCR).Total RNA of HUVECs was prepared using the RNA zol (Cinna/Biotex Laboratories, Houston, TX) after stimulating the cells with TNF, thrombin, or PMA in the presence or absence of dilazep. RT-PCR was performed as described previously.26,27 Briefly, total RNA (1 μg) was reverse transcripted by superscript preamplification system (GIBCO BRL) with random hexamer primers, and the reverse-transcripted cDNA was then amplified by PCR using the primers prepared based on the mRNA of TF or GAPDH, a housekeeping gene12 that was not affected by the stimulants used in this study. The sequences of the primers used for TF mRNA amplification were 5′ ACTACTGTTTCAGTGTTCAAGCAGTGATTC 3′ corresponding to 722-751 nucleotides and the 5′ ATTCAGTGGGGAGTTCTCCTTCCCAGCTCTG 3′ corresponding to 925-954 nucleotides.12,28 The sequences of the primers used for GAPDH mRNA amplification were 5′ CCACCCATGGCAAATTCCATGGCA 3′ corresponding to 150-169 nucleotides and 5′ TCTAGACGGCAGGTCAGGTCCACC 3′ corresponding to 720-743 nucleotides.12 PCR was performed with 22 cycles of 1 minute of denaturation at 94°C, 1 minute of hybridization at 52°C, and 2 minutes of elongation at 73°C. An aliquot of sample was loaded onto a 2% agarose gel. Amplified DNA of TF or GAPDH was blotted on a nylon membrane and hybridized with the TF or GAPDH cDNA probe labeled with a [32P]dCTP (3,000 Ci/mmol) using a DNA labeling kit (TaKaRa Shuzo, Osaka, Japan). The membrane was then exposed to x-ray film at −70°C. The intensity of the hybridization signals was densitometrically quantified by a Fujix BAS-2000 Bio-Image analyzer (Fuji Photo Film, Tokyo, Japan). The amount of TF mRNA was normalized against the GAPDH mRNA.
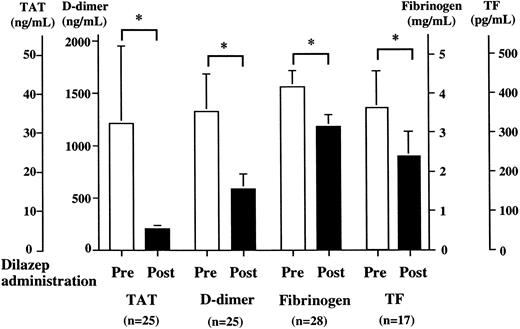
Effect of dilazep in patients with hypercoagulable state. Twenty-eight patients with hypercoagulable state were treated with dilazep (300 mg/d) for 1 month. Plasma levels of TAT, FDP-D-dimer, fibrinogen, and TF antigen were determined by ELISA or the clotting time method before and after treatment with dilazep as described in the Materials and Methods. Values are expressed as the mean ± SD. *P < .001.
Effect of dilazep in patients with hypercoagulable state. Twenty-eight patients with hypercoagulable state were treated with dilazep (300 mg/d) for 1 month. Plasma levels of TAT, FDP-D-dimer, fibrinogen, and TF antigen were determined by ELISA or the clotting time method before and after treatment with dilazep as described in the Materials and Methods. Values are expressed as the mean ± SD. *P < .001.
Determination of HUVEC surface thrombomodulin (TM) activity and TM antigen in HUVECs.TM activity on the surface of HUVECs was determined as a cofactor activity of TM for thrombin-catalyzed protein C activation using 48-well collagen-coated plates, as described previously.29 After washing the HUVECs (2 × 105 cells) in the wells three times, 50 μL of TBS, pH 8.0, containing 0.1% BSA and 5 mmol/L CaCl2 , 25 μL of 0.5 μg/mL thrombin, and 50 μL of 50 μg/mL protein C was added to each well. After 1 hour of incubation at 37°C, antithrombin (5 μg/mL, final concentration) and heparin (2 U/mL, final concentration) were added to the wells. The amount of activated protein C in the reaction mixture (100 μL) was assayed by incubating with 2 mL of 200 μmol/L Boc-Leu-Ser-Thr-Arg-MCA in TBS. The fluorescence of the released amino-methylcoumarin was determined by using a fluorescence spectrophotometer (Shimadzu, Kyoto, Japan) with excitation at 380 nm and emission at 440 nm.
The amount of total TM antigen in HUVECs was determined by an ELISA using two murine monoclonal antihuman TM IgGs as reported previously.29 Briefly, HUVECs were incubated with 100 μg/mL dilazep, followed by appropriate washing with cold Dulbecco's PBS and harvesting of the cells. Subsequently, the cells were centrifuged (2,000 rpm for 5 minutes) and the precipitate was dissolved in 50 μL TBS containing 0.6% (wt/vol) Triton X-100 and 0.2 U/mL aprotinin. The cell extract was then applied to the wells coated with a monoclonal anti-TM IgG (MFTM-5).29 The wells were blocked with PBS, pH 8.0, containing 5% BSA and then washed three times with TBS containing 0.1% BSA and 0.02% thimerosal. Thereafter, HRP-labeled monoclonal anti-TM IgG (MFTM-6)29 was added and incubated for 1 hour. After the addition of H2O2 and peroxidase substrate, color development was measured at 492 nm using an EAR microplate reader.
Determination of monocytic cell surface TF activity.Mononuclear cells (monocytes plus lymphocytes) suspended in RPMI were incubated with or without 100 μg/mL dilazep for 15 minutes and stimulated with TNF for 5 hours. Procoagulant activity (PCA) on the surface of intact monocytes was determined by a single-stage clotting assay as described previously.30 Briefly, 50 μL of mononuclear cell suspension was mixed with 50 μL of 25 mmol/L CaCl2 . Clotting was initiated by the addition of 50 μL of normal human plasma, and the clotting time at 37°C was recorded using KC 10 coagulometer (Heinrich Amelung, GmbH, Lemgo, Germany). The PCA was extrapolated from a standard curve drawn using rabbit brain thromboplastin standard (Ortho, Raritan, NJ), as described previously.30 Cell surface-associated PCA was linear over the range of 12 × 106 mononuclear cells/mL. PCA of monocytes was confirmed to depend on TF, because it was specifically inhibited by polyclonal anti-TF antibody.
RESULTS
Effect of dilazep in patients with hypercoagulable state.We evaluated the effect of dilazep on hemostatic markers in patients with hypercoagulable state. The plasma levels of TAT (32.3 ± 19.7 v 5.4 ± 0.9 ng/mL), FDP-D-dimer (1,411.1 ± 384.4 v 617.6 ± 127.3 ng/mL), fibrinogen (4.1 ± 0.4 v 3.1 ± 0.3 mg/mL), and TF antigen (355.5 ± 94.0 v 234.9 ± 62.2 pg/mL) significantly (P < .001) decreased after treatment with dilazep (300 mg/d), as shown in Fig 1. These hemostatic parameters also decreased significantly in each patient group (malignant and benign disease) after treatment with dilazep (P < .001) as compared with baseline values (data not shown). This finding suggests that dilazep has a potent anticoagulant activity on endothelial cells or intravascular leukocytes. This preliminary clinical study prompted us to evaluate the effect of dilazep on endothelial cell procoagulant activity.
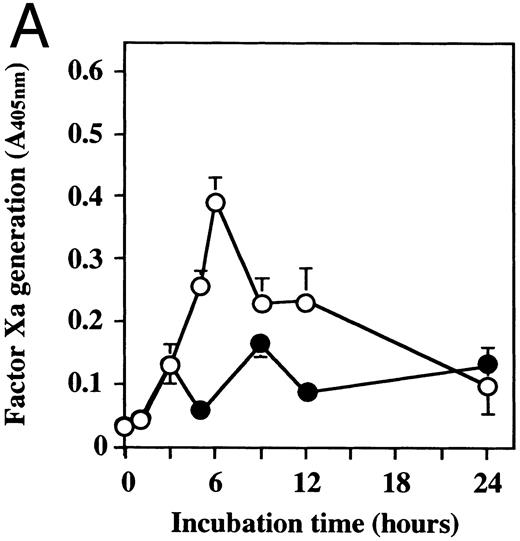
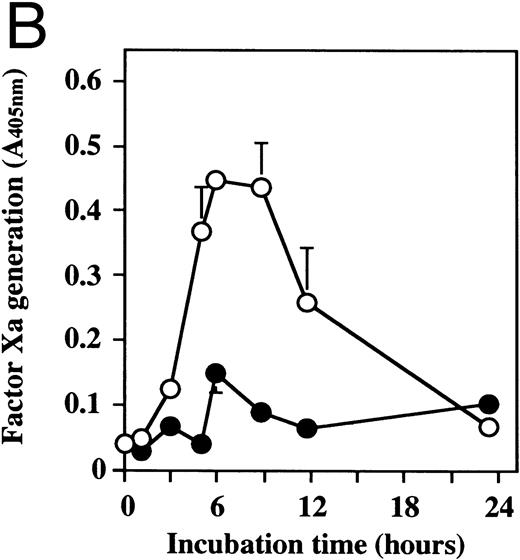
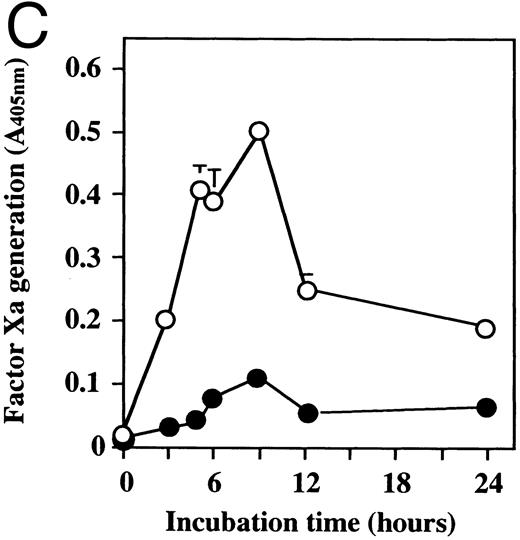
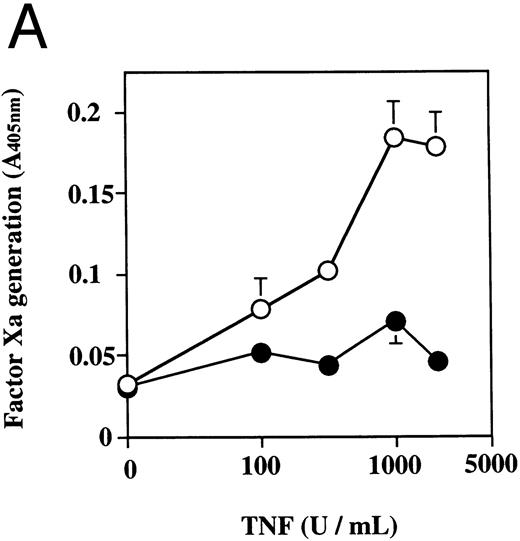
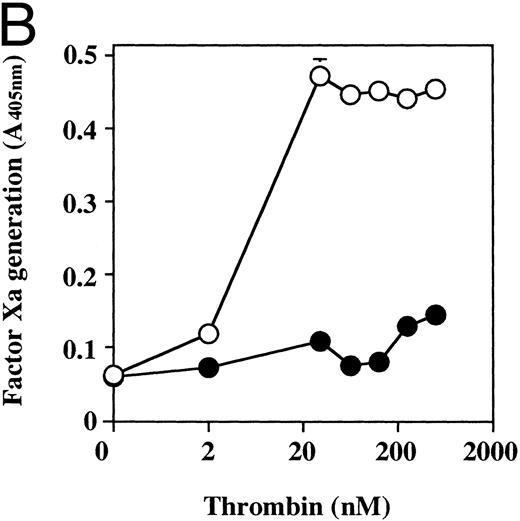
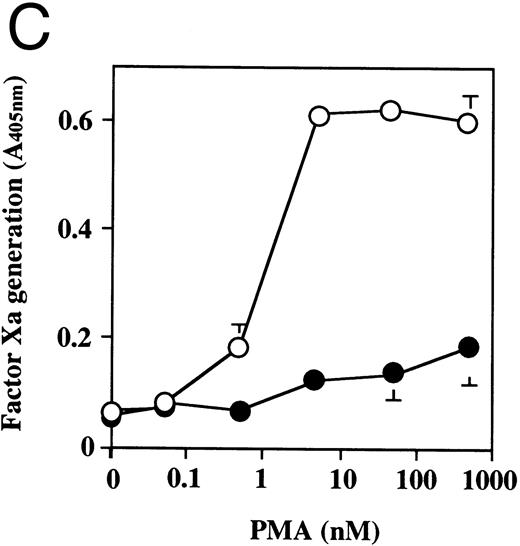
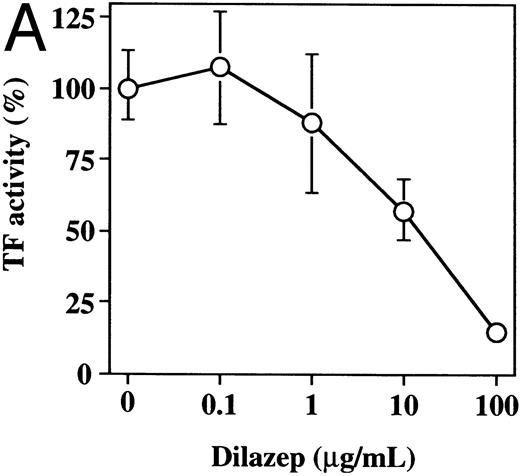
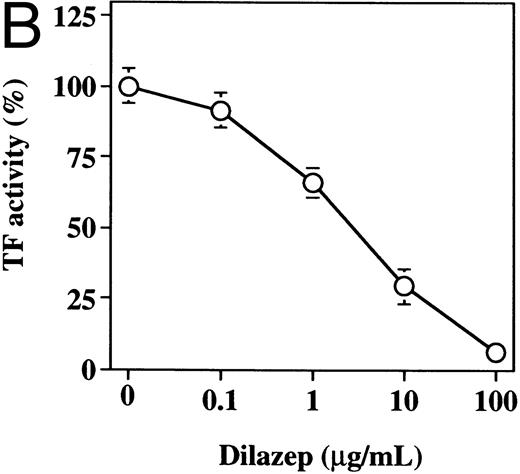
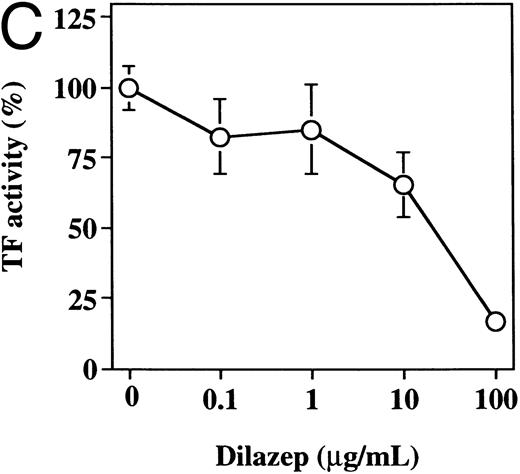
Effect of dilazep on TF activity of HUVECs stimulated with TNF, thrombin, or PMA.We evaluated the effect of dilazep on TF expression in HUVECs stimulated with the inflammatory mediators TNF, thrombin, or PMA. A gradual increase in TF activity was observed on HUVECs after stimulating with TNF, thrombin, or PMA, reaching a maximum level after 5 to 6 hours. As shown in Fig 2, 100 μg/mL dilazep inhibited this stimulatory effect of TNF, thrombin, or PMA on TF activity of HUVECs. TF activity on stimulated HUVECs for 5 hours depended on the dose of the stimulant as described previously,5-7 and the activity reached maximal levels at concentrations of 1,000 U/mL TNF, 25 nmol/L thrombin, and 5 nmol/L PMA, respectively. Coincubation with 100 μg/mL dilazep decreased approximately 90% of the TF activity induced by each stimulant (Fig 3). Increasing concentrations of dilazep (0 to 100 μg/mL) decreased the cell surface TF activity in a dose-dependent fashion (Fig 4). The minimal concentration of dilazep required for inhibiting the TNF-stimulated TF expression was 1 μg/mL, but that for the thrombin- and PMA-stimulated TF expression was 0.1 μg/mL. The concentrations of dilazep required for 50% inhibition of the expression of TF activity on HUVECs induced by 1,000 U/mL TNF, 25 nmol/L thrombin, and 5 nmol/L PMA were approximately 20, 2.5, and 20 μg/mL, respectively. The viability of HUVECs as determined by using WST-1 and 1-methoxy PMS was not affected by dilazep at any concentration used in this experiment.
Time course of TF activity expression on HUVECs stimulated with TNF, thrombin, or PMA and the effect of dilazep on the TF expression. HUVECs (5 × 105/well) were stimulated with (A) 1,000 U/mL TNF, (B) 25 nmol/L thrombin, or (C) 5 nmol/L PMA in the presence (•) or absence (○) of 100 μg/mL dilazep. The TF activity on HUVEC surface was determined at 0, 3, 5, 6, 9, 12, and 24 hours after incubation with each stimulant as factor Xa generation (A405nm /20 minutes) using S2222 (see the Materials and Methods). The values represent the mean ± SD of four independent experiments.
Time course of TF activity expression on HUVECs stimulated with TNF, thrombin, or PMA and the effect of dilazep on the TF expression. HUVECs (5 × 105/well) were stimulated with (A) 1,000 U/mL TNF, (B) 25 nmol/L thrombin, or (C) 5 nmol/L PMA in the presence (•) or absence (○) of 100 μg/mL dilazep. The TF activity on HUVEC surface was determined at 0, 3, 5, 6, 9, 12, and 24 hours after incubation with each stimulant as factor Xa generation (A405nm /20 minutes) using S2222 (see the Materials and Methods). The values represent the mean ± SD of four independent experiments.
The effect of dilazep on TF activity on HUVECs stimulated with varying concentrations of TNF, thrombin, or PMA. HUVECs were treated for 5 hours with varying concentrations of each stimulant. (A) TNF (0 to 2,500 U/mL), (B) thrombin (0 to 250 U/mL), and (C) PMA (0 to 250 nmol/L) in the presence (•) or absence (○) of 100 μg/mL dilazep. TF activity on HUVEC surface was determined as factor Xa generation (A405nm /20 minutes) using S2222 (see the Materials and Methods). The values represent the mean ± SD of four independent experiments.
The effect of dilazep on TF activity on HUVECs stimulated with varying concentrations of TNF, thrombin, or PMA. HUVECs were treated for 5 hours with varying concentrations of each stimulant. (A) TNF (0 to 2,500 U/mL), (B) thrombin (0 to 250 U/mL), and (C) PMA (0 to 250 nmol/L) in the presence (•) or absence (○) of 100 μg/mL dilazep. TF activity on HUVEC surface was determined as factor Xa generation (A405nm /20 minutes) using S2222 (see the Materials and Methods). The values represent the mean ± SD of four independent experiments.
The effect of varying concentrations of dilazep on TF activity expression on HUVECs stimulated with TNF, thrombin, or PMA. HUVECs were treated for 5 hours with each stimulant. (A) 1,000 U /mL TNF, (B) 25 nmol/L thrombin, and (C) 5 nmol/L PMA in the presence of varying concentrations of dilazep (0 to 100 μg/mL). TF activity on HUVEC surface was determined as factor Xa generation using the S2222 (see the Materials and Methods). The data are expressed as the percentage of the control (each stimulant induced TF activity in the absence of dilazep). The values represent the mean ± SD of four independent experiments.
The effect of varying concentrations of dilazep on TF activity expression on HUVECs stimulated with TNF, thrombin, or PMA. HUVECs were treated for 5 hours with each stimulant. (A) 1,000 U /mL TNF, (B) 25 nmol/L thrombin, and (C) 5 nmol/L PMA in the presence of varying concentrations of dilazep (0 to 100 μg/mL). TF activity on HUVEC surface was determined as factor Xa generation using the S2222 (see the Materials and Methods). The data are expressed as the percentage of the control (each stimulant induced TF activity in the absence of dilazep). The values represent the mean ± SD of four independent experiments.
To discern whether the inhibitory effect of dilazep on TF expression is caused by a global effect on protein synthesis, the effect of dilazep on TM, which is constitutively expressed on resting HUVECs, was also investigated. The time course of the levels of TM activity and the total TM antigen expressed on HUVECs in the presence (100 μg/mL) or absence of dilazep were determined. The TM activity and the total TM antigen levels in the cells were not affected by dilazep even after 24 hours of the treatment (data not shown).
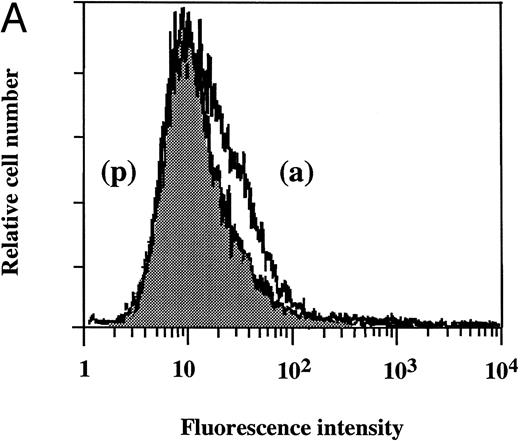
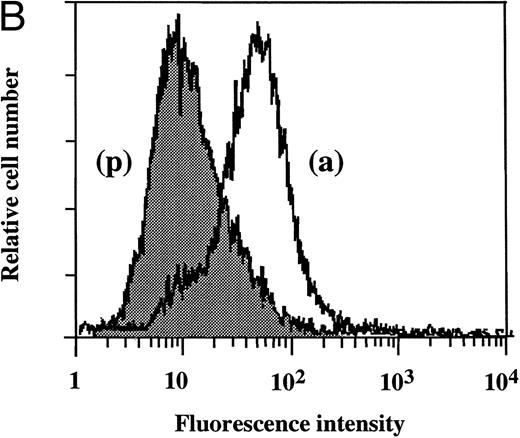
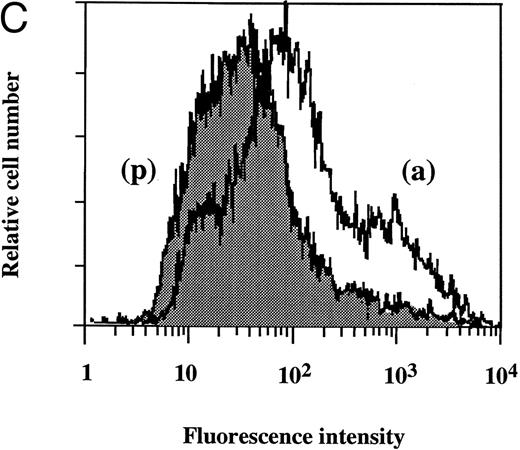
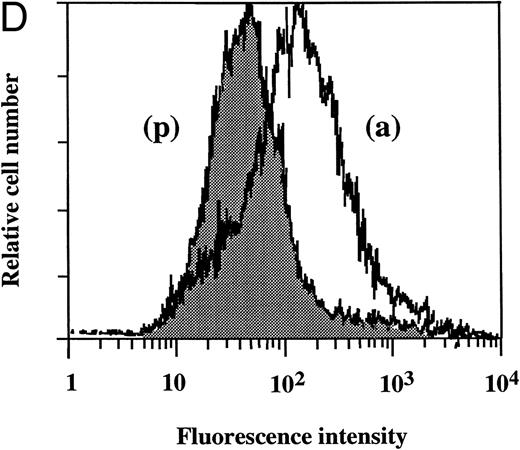
Flow cytometry of TF antigen expression.FACScan analysis was performed to determine whether dilazep reduces the amount of TF antigen expression on HUVEC surface induced by TNF, thrombin, or PMA. As shown in Fig 5, dilazep alone did not affect the fluorescence intensity on HUVECs, but markedly decreased the elevation of TF antigen levels on HUVECs induced by 1,000 U/mL TNF, 25 nmol/L thrombin, or 5 nmol/L PMA.
Flow cytometry of the effect of dilazep on TF antigen expression on the surface of HUVECs stimulated with TNF, thrombin, or PMA. HUVECs were stimulated for 5 hours with each stimulant. (A) Control, (B) 1,000 U/mL TNF, (C) 25 nmol/L thrombin, and (D) 5 nmol/L PMA, in the presence (p) or absence (a) of 100 μg/mL dilazep. The cells were then harvested, incubated with murine monoclonal antihuman TF IgG, and then incubated with FITC-labeled goat antimouse IgG-antibody. Fluorescence intensity was determined by FACScan as described in the Materials and Methods, and the data are displayed using a logarithmic scale.
Flow cytometry of the effect of dilazep on TF antigen expression on the surface of HUVECs stimulated with TNF, thrombin, or PMA. HUVECs were stimulated for 5 hours with each stimulant. (A) Control, (B) 1,000 U/mL TNF, (C) 25 nmol/L thrombin, and (D) 5 nmol/L PMA, in the presence (p) or absence (a) of 100 μg/mL dilazep. The cells were then harvested, incubated with murine monoclonal antihuman TF IgG, and then incubated with FITC-labeled goat antimouse IgG-antibody. Fluorescence intensity was determined by FACScan as described in the Materials and Methods, and the data are displayed using a logarithmic scale.
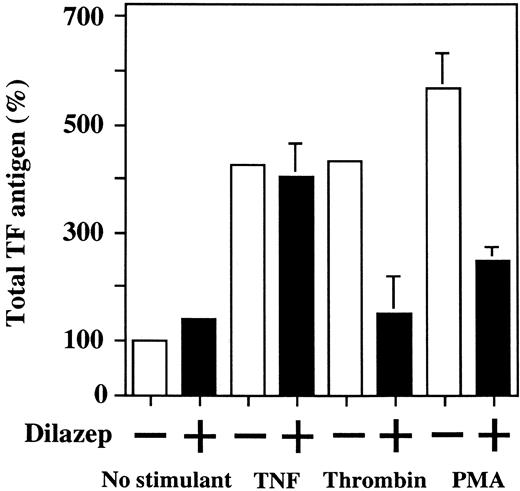
Effect of dilazep on total TF antigen level in HUVECs stimulated with TNF, thrombin, or PMA.To investigate whether the suppressive effect of dilazep on HUVEC surface TF activity depends on the decreased TF production by the cells, the total amount of TF antigen in HUVECs lysates was measured by ELISA. Upregulation of total TF antigen level was observed in HUVECs treated with 1,000 U/mL TNF, 25 nmol/L thrombin, and 5 nmol/L PMA. Dilazep (100 μg/mL) significantly inhibited the thrombin- and PMA-induced upregulation of total TF antigen levels (432% ± 31.4% v 158% ± 0.8% and 526% ± 12.9% v 210% ± 4.6%, respectively). However, dilazep did not inhibit the TNF-induced total TF antigen level (425% ± 1.1% v 405% ± 14.2%; Fig 6). Considering this latter result together with that shown in Fig 5B, dilazep appears to inhibit the transport of TNF-induced TF protein to the cell surface of HUVECs.
Effect of dilazep on total TF antigen level in HUVECs stimulated with TNF, thrombin, or PMA. HUVECs were treated for 5 hours with each stimulant: 1,000 U /mL TNF, 25 nmol/L thrombin, and 5 nmol/L PMA, in the presence of varying concentrations of dilazep (0 to 100 μg/mL). The total cell TF antigen levels in HUVECs were measured by ELISA (see the Materials and Methods). The data are expressed as the percentage of the control (TF antigen in HUVECs without stimulation in the absence of dilazep). The values represent the mean ± SD of four independent experiments.
Effect of dilazep on total TF antigen level in HUVECs stimulated with TNF, thrombin, or PMA. HUVECs were treated for 5 hours with each stimulant: 1,000 U /mL TNF, 25 nmol/L thrombin, and 5 nmol/L PMA, in the presence of varying concentrations of dilazep (0 to 100 μg/mL). The total cell TF antigen levels in HUVECs were measured by ELISA (see the Materials and Methods). The data are expressed as the percentage of the control (TF antigen in HUVECs without stimulation in the absence of dilazep). The values represent the mean ± SD of four independent experiments.
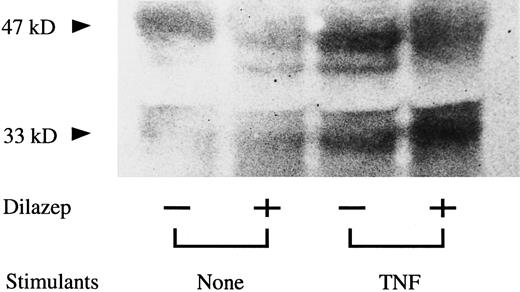
Effect of dilazep on the molecular mass of TNF-induced TF in HUVECs.To investigate the inhibitory effect of dilazep on TNF-induced TF expression on HUVECs, Western blot analysis was also performed. TNF-stimulated HUVECs exhibited a prominent band with approximate molecular mass of 47 kD, consistent with an increase in the mature form of TF. There was also an increase in a band at the 33-kD level. The addition of dilazep to TNF stimulation prevented the increase in the high molecular mass band (47 kD) but caused an increase in the band at the 33-kD level (Fig 7).
Western blot analysis of the effect of dilazep on the molecular mass of TF in HUVECs stimulated with TNF. HUVECs were treated for 5 hours with TNF (1,000 U /mL) in the presence of 100 μg/mL dilazep. The TF antigen in HUVECs were detected by Western blot analysis using polyclonal antihuman TF antibody (see the Materials and Methods).
Western blot analysis of the effect of dilazep on the molecular mass of TF in HUVECs stimulated with TNF. HUVECs were treated for 5 hours with TNF (1,000 U /mL) in the presence of 100 μg/mL dilazep. The TF antigen in HUVECs were detected by Western blot analysis using polyclonal antihuman TF antibody (see the Materials and Methods).
Effect of dilazep on TF mRNA expression induced by TNF, thrombin, or PMA in HUVECs.To analyze whether the effect of dilazep on TF antigen levels in HUVECs (stimulated with TNF, thrombin, or PMA) corresponds to the levels of TF mRNA in HUVECs, the effect of dilazep on the steady-state levels of TF mRNA was assessed in HUVECs using the RT-PCR method.27 Resting HUVECs and the cells treated with dilazep alone showed no detectable expression of TF mRNA (Fig 8A). As shown in Fig 8A and B, 1,000 U/mL TNF, 25 nmol/L thrombin, or 5 nmol/L PMA induced TF mRNA expression markedly after 3 hours of the treatment with each stimulant. Dilazep significantly inhibited the thrombin- or PMA-induced TF mRNA expression. However, the same amount of dilazep did not decrease the TF mRNA expression induced by TNF. These effects of dilazep on the TF mRNA expression appear to correspond well to that on the total TF antigen level in HUVECs shown in Fig 6.
The effect of dilazep on TF mRNA expression in HUVECs stimulated with TNF, thrombin, or PMA. (A) HUVECs were stimulated with each stimulant: 1,000 U/mL TNF, 25 nmol/L thrombin, or 5 nmol/L PMA for 3 hours in the presence (+) or absence (−) of 100 μg/mL dilazep. TF mRNA expression was analyzed using the RT-PCR method as described in the Materials and Methods. The amplified cDNA was blotted onto a nitrocellulose membrane and hybridized with the [32P]TF cDNA probe and then analyzed using an autoradiogram. (B) The data in (A) are depicted after normalization to equal amount of RNA loads based on the intensity of GAPDH mRNA. The data are expressed as the percentage of the control (each stimulant induced TF mRNA in the absence of dilazep). The values represent the mean ± SD of four independent experiments. Statistical analysis was performed using the Student's t-test (*P < .05).
The effect of dilazep on TF mRNA expression in HUVECs stimulated with TNF, thrombin, or PMA. (A) HUVECs were stimulated with each stimulant: 1,000 U/mL TNF, 25 nmol/L thrombin, or 5 nmol/L PMA for 3 hours in the presence (+) or absence (−) of 100 μg/mL dilazep. TF mRNA expression was analyzed using the RT-PCR method as described in the Materials and Methods. The amplified cDNA was blotted onto a nitrocellulose membrane and hybridized with the [32P]TF cDNA probe and then analyzed using an autoradiogram. (B) The data in (A) are depicted after normalization to equal amount of RNA loads based on the intensity of GAPDH mRNA. The data are expressed as the percentage of the control (each stimulant induced TF mRNA in the absence of dilazep). The values represent the mean ± SD of four independent experiments. Statistical analysis was performed using the Student's t-test (*P < .05).
Effect of adenosine receptor antagonist on the inhibitory effect of dilazep.Dilazep is believed to potentiate the effect of adenosine by blocking the uptake of adenosine.31 In an attempt to clarify whether the inhibitory effect of dilazep on TF expression is mediated by adenosine receptor, the effect of the adenosine receptor antagonist, 8-SPT, on the inhibitory effect of dilazep was investigated. As shown in Fig 9, 1 mmol/L 8-SPT alone did not affect the basal or stimulant-induced TF expression. Dilazep (100, 10, and 100 μg/mL) decreased the TF activity induced by 1,000 U/mL TNF, 25 nmol/L thrombin, and 5 nmol/L PMA, respectively. Downregulation of TF activity on HUVECs induced by dilazep was counteracted by 8-SPT, indicating that the inhibitory effect of dilazep on TF expression on HUVECs is mediated, at least in part, by adenosine receptor.
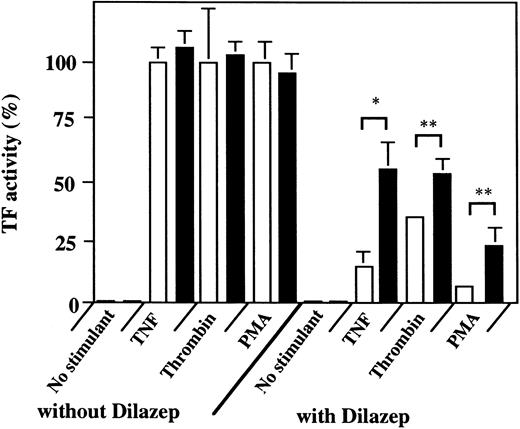
Effect of 8-SPT on the dilazep-induced downregulation of TF activity on the surface of HUVECs stimulated with TNF, thrombin, or PMA. HUVECs (2 × 105 cells/well) were incubated with (1 mmol/L, final concentration; ▪) or without (□) 8-SPT for 15 minutes and then with 100 μg/mL dilazep. After incubation of the cells with 1,000 U/mL TNF, 25 nmol/L thrombin, or 5 nmol/L PMA, in the presence or absence of dilazep, the cells were washed twice with 1 mL of HEPES-buffered saline containing 5 mmol/L CaCl2 . TF activity was determined as factor Xa generation on the surface of HUVECs as described in the Materials and Methods. The data are expressed as the percentage of the control (each stimulant induced TF activity in the absence of dilazep). Statistical analysis was performed using the Student's t-test (*P < .05; **P < .001).
Effect of 8-SPT on the dilazep-induced downregulation of TF activity on the surface of HUVECs stimulated with TNF, thrombin, or PMA. HUVECs (2 × 105 cells/well) were incubated with (1 mmol/L, final concentration; ▪) or without (□) 8-SPT for 15 minutes and then with 100 μg/mL dilazep. After incubation of the cells with 1,000 U/mL TNF, 25 nmol/L thrombin, or 5 nmol/L PMA, in the presence or absence of dilazep, the cells were washed twice with 1 mL of HEPES-buffered saline containing 5 mmol/L CaCl2 . TF activity was determined as factor Xa generation on the surface of HUVECs as described in the Materials and Methods. The data are expressed as the percentage of the control (each stimulant induced TF activity in the absence of dilazep). Statistical analysis was performed using the Student's t-test (*P < .05; **P < .001).
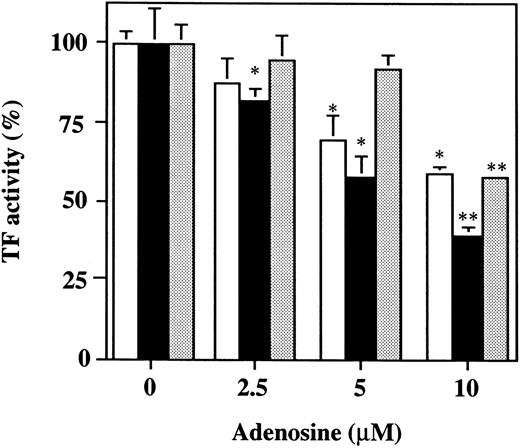
Effect of adenosine on the TF activity of HUVECs stimulated with TNF, thrombin, or PMA.Increasing concentrations of adenosine (0 to 10 μmol/L) decreased the TF activity expression on the surface of HUVECs stimulated with 1,000 U/mL TNF, 25 nmol/L thrombin, or 5 nmol/L PMA in a dose-dependent fashion (Fig 10). Coincubation with 10 μmol/L adenosine decreased approximately 50% the TF activity on HUVECs induced by each stimulant. These findings indicate that adenosine significantly blocks the TF expression in HUVECs stimulated with TNF, thrombin, or PMA. Viability of HUVECs as determined using WST-1 was not affected by adenosine at any concentration used in this experiment.
The effect of varying concentrations of adenosine on TF activity expression on HUVECs stimulated with TNF, thrombin, or PMA. HUVECs were treated for 5 hours with each stimulant: 1,000 U/mL TNF (□), 25 nmol/L thrombin (▪), or 5 nmol/L PMA () in the presence of varying concentrations of adenosine (0, 2.5, 5, and 10 μmol/L). TF activity on HUVEC surface was determined as factor Xa generation using S2222 (see the Materials and Methods). The data are expressed as the percentage of the control (each stimulant induced TF activity in the absence of adenosine). The values represent the mean ± SD of four independent experiments. Statistical analysis was performed using the Student's t-test (*P < .05; **P < .001).
The effect of varying concentrations of adenosine on TF activity expression on HUVECs stimulated with TNF, thrombin, or PMA. HUVECs were treated for 5 hours with each stimulant: 1,000 U/mL TNF (□), 25 nmol/L thrombin (▪), or 5 nmol/L PMA () in the presence of varying concentrations of adenosine (0, 2.5, 5, and 10 μmol/L). TF activity on HUVEC surface was determined as factor Xa generation using S2222 (see the Materials and Methods). The data are expressed as the percentage of the control (each stimulant induced TF activity in the absence of adenosine). The values represent the mean ± SD of four independent experiments. Statistical analysis was performed using the Student's t-test (*P < .05; **P < .001).
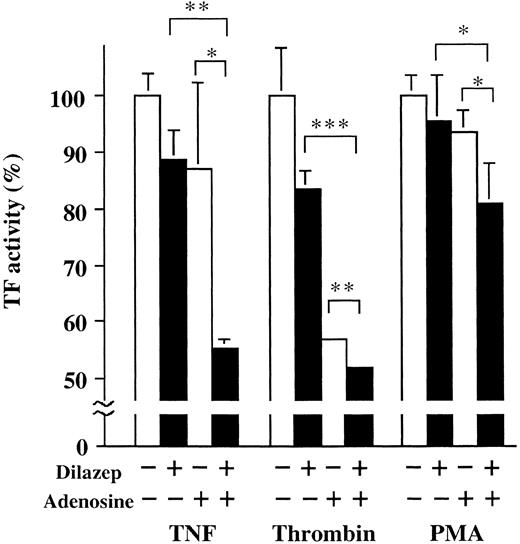
Additive effect of dilazep and adenosine on the TF activity of HUVECs stimulated with TNF, thrombin, or PMA.Isotope-technical studies using 3H-dilazep in humans showed that the therapeutic concentration of dilazep in the systemic circulation is around 1 μg/mL.32 The therapeutical concentration of dilazep is known to increase the concentration of adenosine in the extracellular space fluid up to micromolar levels.33 34 In our assays, we used a dose of dilazep equal to its therapeutic blood concentration in combination with adenosine. We found that dilazep, in combination with adenosine, inhibited the 1,000 U/mL TNF- and 25 nmol/L thrombin-induced TF expression in HUVECs more significantly (60%) than dilazep alone, as shown in Fig 11.
Additive effect of dilazep and adenosine on the TF activity of HUVECs stimulated with TNF, thrombin, or PMA. HUVECs (2 × 105 cells/well) were incubated with (1 μg /mL, final concentration) or without adenosine for 15 minutes and then with adenosine (2.5 μmol/L). After incubation of the cells with 1,000 U/mL TNF, 25 nmol/L thrombin, or 5 nmol/L PMA, in the presence or absence of dilazep or adenosine, the cells were washed twice with 1 mL of HEPES-buffered saline containing 5 mmol/L CaCl2 . TF activity was determined as factor Xa generation on the surface of HUVECs as described in the Materials and Methods. The data are expressed as the percentage of the control (each stimulant induced TF activity in the absence of both dilazep and adenosine). Statistical analysis was performed using the Student's t-test (*P < .05; **P < .005; ***P < .001).
Additive effect of dilazep and adenosine on the TF activity of HUVECs stimulated with TNF, thrombin, or PMA. HUVECs (2 × 105 cells/well) were incubated with (1 μg /mL, final concentration) or without adenosine for 15 minutes and then with adenosine (2.5 μmol/L). After incubation of the cells with 1,000 U/mL TNF, 25 nmol/L thrombin, or 5 nmol/L PMA, in the presence or absence of dilazep or adenosine, the cells were washed twice with 1 mL of HEPES-buffered saline containing 5 mmol/L CaCl2 . TF activity was determined as factor Xa generation on the surface of HUVECs as described in the Materials and Methods. The data are expressed as the percentage of the control (each stimulant induced TF activity in the absence of both dilazep and adenosine). Statistical analysis was performed using the Student's t-test (*P < .05; **P < .005; ***P < .001).
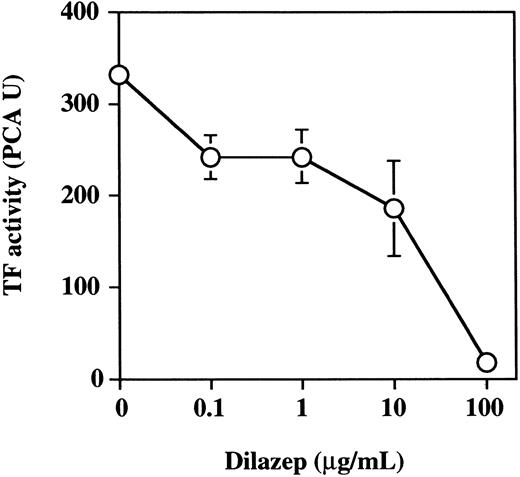
Effect of dilazep on TF activity of monocytes stimulated with TNF.Peripheral blood monocytes are known to participate in blood coagulation processes by expressing TF after being stimulated with inflammatory mediators.35 36 The effect of dilazep on TNF-induced TF expression on monocyte surface was evaluated. Peripheral blood monocytes were found to express 20.6 ± 3.2 PCA U per 7 × 105 mononuclear cells. TNF (1,000 U/mL) increased 17.5-fold the monocyte TF activity (352.5 ± 6.5 PCA U per 7 × 105 mononuclear cells). We then evaluated the effect of dilazep on TF expression by monocytes. Increasing the concentration of dilazep (0 to 100 μg/mL) inhibited the PCA of monocytes in a dose-dependent fashion (Fig 12). The concentration of dilazep required for inhibiting 50% of monocyte PCA was approximately 10 μg/mL. The minimal concentration of dilazep required for inhibiting the monocyte PCA was less than 0.1 μg/mL. Viability of mononuclear cells as determined by using WST-1 and 1-methoxy PMS was not affected by dilazep at any concentration used in this experiment.
The effect of varying concentrations of dilazep on TF activity expression on monocytes stimulated with TNF. Peripheral blood mononuclear cells (monocytes plus lymphocytes) were treated for 5 hours with 1,000 U /mL TNF in the presence of varying concentrations of dilazep (0 to 100 μg/mL). TF activity on monocyte surface was determined as PCA as described in the Materials and Methods. The values represent the mean ± SD of four independent experiments.
The effect of varying concentrations of dilazep on TF activity expression on monocytes stimulated with TNF. Peripheral blood mononuclear cells (monocytes plus lymphocytes) were treated for 5 hours with 1,000 U /mL TNF in the presence of varying concentrations of dilazep (0 to 100 μg/mL). TF activity on monocyte surface was determined as PCA as described in the Materials and Methods. The values represent the mean ± SD of four independent experiments.
DISCUSSION
Various cytokines, such as TNF, IL-1, and lymphotoxin, play relevant roles in several inflammatory processes and in the pathogenesis of atherosclerosis. Activation of endothelial cells by these mediators induces the expression of TF that converts the quiescent endothelium into a procoagulant surface. Increased thrombin generation at sites of vascular injury is believed to play a critical role in the pathogenesis of atherosclerosis. Antiplatelet therapy is commonly used in patients at risk of developing thrombotic disease. Dilazep is one of the antiplatelet drugs commonly used in European countries and Japan. Dilazep exerts antiplatelet activity by increasing the extracellular fluid level of adenosine in the systemic circulation by blocking adenosine uptake.13,14 Dilazep is also known to dilate coronary, cerebral, and renal vessels by blocking the calcium influx15,16 and to prevent the occurrence of thrombus formation in experimental animals.18 In preliminary clinical studies, we found that dilazep decreases the plasma level of various hemostatic markers (TAT, FDP-D-dimer, and fibrinogen) in patients with hypercoagulable state. Dilazep was also found to decrease the level of plasma TF antigen that is known to be a marker of endothelial cell or leukocyte activation.4 37 These findings prompted us to investigate the effect of dilazep on the procoagulant activity of HUVECs stimulated with TNF, thrombin, or PMA and on that of monocytes stimulated with TNF. The results of the present study showed that dilazep significantly inhibits the expression of TF in HUVECs and monocytes.
TF expression is known to be regulated at both transcriptional and posttranscriptional levels.6,25,38,39 In the current study, analysis of the TF mRNA expression showed that the thrombin- or PMA-induced TF expression in HUVECs is blocked by dilazep probably due to an effect on TF gene transcription and/or TF mRNA stability. PMA and thrombin are well-known activators of protein kinase C. This can be activated directly by PMA or indirectly by thrombin via the G protein-coupled receptor.40,41 Thus, dilazep probably inhibits the PMA- or thrombin-induced TF mRNA expression by blocking the protein kinase C-mediated signaling pathway. On the contrary, dilazep inhibited the HUVEC surface TF expression but it did not inhibit the expressions of total TF antigen and TF mRNA induced by TNF in HUVECs. This latter finding may be explained by the different signaling pathway of TNF-induced TF expression from that of thrombin- or PMA-induced TF expression. In this regard, TNF was previously found to induce TF mRNA expression in HUVECs by the sphingomyelinase/ceramide pathway.42 In addition, TNF-mediated activation of protein kinase C is in general not required for nuclear factor-κB activation or for protein expression in endothelial cells.43,44 On the other hand, the fact that dilazep inhibited HUVEC surface expression of TF (decrease in TF activity and in its surface antigen expression by factor Xa generation assay and FACScan, respectively) without blocking the expression of total TF antigen and TF mRNA in these cells, as measured by ELISA and RT-PCR, suggests that dilazep regulates TNF-induced TF surface expression at the posttranscriptional level. Alternation in the folding, processing, and translocation of TF may be the potential mechanisms by which dilazep exerts this effect. Another piece of evidence that suggests the posttranscriptional regulation of TNF-induced TF expression by HUVECs is the results of the Western blot analysis showing that dilazep reduced the accumulation of matured forms (47 kD) of TF but increased the formation of TF with lower molecular weight (33 kD) in TNF-treated HUVECs. Similar lower molecular weight TF formation has been previously reported in lipopolysaccharide-treated mononuclear cells by endogenous cellular antioxidant potentiation.25 Dilazep is also known to act as an antioxidant agent and to modulate cytoplasmic redox state on endothelial cells.17 The redox state regulates protein synthesis at posttranscriptional level by altering the stability and the translation rate of mRNA45,46 or by modifying protein folding or glycosylation.47-51 The antioxidant activity of dilazep might generate reduced conditions in cytoplasm resulting in unstable/immatured forms of TF. Thus, it is conceivable that dilazep inhibits the transport of TF to the cell surface by affecting the formation of matured forms of the protein in TNF-stimulated HUVECs.
Another point that needs clarification is the mechanism by which dilazep activates the signaling pathway of HUVECs for the transcriptional or posttranscriptional regulation of TF. Dilazep was previously reported to inhibit adenosine uptake by endothelial cells leading to increased plasma adenosine levels.31 In the current study, we evaluated whether this adenosine potentiating activity of dilazep downregulates TF expression in HUVECs. The inhibitory effect of dilazep on TF expression in HUVECs stimulated with TNF, thrombin, or PMA was counteracted by adenosine receptor antagonists, suggesting that the inhibitory effect of dilazep is mediated, at least in part, by adenosine receptors. Adenosine itself was also found to inhibit TF expression in HUVECs. All together, these findings suggest that adenosine and its receptor might play important roles in the regulation of TF expression on endothelial cells.
Our present study showed that dilazep (300 mg/d) dramatically decreases various hemostatic markers and that it significantly inhibits the TF expression induced by inflammatory agents in endothelial cells. These results suggest that dilazep may act as an anticoagulant agent in vivo besides its antiplatelet and vasodilating activities. The therapeutic blood concentration of dilazep was reported to be around 1 μg/mL.32 On the other hand, the concentration of adenosine in the extracellular space fluid is known to increase up to micromolar levels in patients treated with dilazep.33,34 This effect depends on dilazep-induced inhibition of adenosine uptake by several cells. In our present investigation, at this reported blood therapeutic concentration, dilazep showed a modest degree of inhibitory activity on TF expression by HUVECs. However, the dose of dilazep equal to the therapeutic blood concentration of the drug in combination with adenosine significantly inhibited the expression of TF by HUVECs in our assay conditions. For instance, we found that dilazep in combination with adenosine inhibited the TNF- and thrombin-induced endothelial cell TF expression more significantly (60%) than dilazep alone. Based on these findings, we believed that, in biologic conditions (in the presence of adenosine), dilazep would inhibit significantly the TF expression by activated endothelial cells. Moreover, in the present study, dilazep was also found to inhibit the TNF-induced monocyte TF expression, supporting further the potential clinical usefulness of dilazep. Overall, these findings suggest that dilazep does not act only as an antiplatelet agent but acts also as an anticoagulant agent when administered to patients with hypercoagulable states. The expression of TF induced by various inflammatory mediators on endothelial cell or monocyte surface is believed to play an essential role in the pathogenesis of atherosclerosis and veno-occlusive diseases.35,36,52 53 Dilazep may have the potential to reduce the procoagulant activity of endothelial cells and monocytes observed in these disorders.
In summary, this study showed that dilazep inhibits endothelial TF expression at both transcriptional and posttranscriptional levels and that its inhibitory effect is mediated, at least in part, by adenosine receptors. The results of this study also suggest that dilazep, besides its well-known antiplatelet effect, exerts a potent inhibitory activity on the procoagulant activity of endothelial cells and monocytes.
Supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (Grants No. 05454622, 06282225, and 06771216) and by Research Aid from Japan Foundation of Cardiovascular Research.
Address reprint requests to Koji Suzuki, PhD, Department of Molecular Pathobiology, Mie University School of Medicine, Edobashi 2-174, Tsu-city, Mie 514, Japan.

![Fig. 8. The effect of dilazep on TF mRNA expression in HUVECs stimulated with TNF, thrombin, or PMA. (A) HUVECs were stimulated with each stimulant: 1,000 U/mL TNF, 25 nmol/L thrombin, or 5 nmol/L PMA for 3 hours in the presence (+) or absence (−) of 100 μg/mL dilazep. TF mRNA expression was analyzed using the RT-PCR method as described in the Materials and Methods. The amplified cDNA was blotted onto a nitrocellulose membrane and hybridized with the [32P]TF cDNA probe and then analyzed using an autoradiogram. (B) The data in (A) are depicted after normalization to equal amount of RNA loads based on the intensity of GAPDH mRNA. The data are expressed as the percentage of the control (each stimulant induced TF mRNA in the absence of dilazep). The values represent the mean ± SD of four independent experiments. Statistical analysis was performed using the Student's t-test (*P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/6/10.1182_blood.v90.6.2345/3/m_bl_0015f8a.jpeg?Expires=1769103422&Signature=y16zvP8Z6yxoL3Z6rdR2XIuYkDTQbZd2su3LUFQhZgoeczh66cNMxKIl6jkE8RwpQYmE1VGxTvDMjTBhd~KU~j4onEmqRgIjIu-U9ztz2Ve~xLyQ4LThwiNbNmmGyiK1gsAL~-XLPukdGxaHX3DQOg6b-rDb~XmGs8ztNfZvGTpKAoOIAUxVfM4bP-AkccgBrWTH2PfON5ZZW5a071gg~gc6gZ7NGLJdL3qq9qMVD-F62gViw6uBELxPUdo3Ks03rics2f3ZOJpWyaBMIPx5exq2xKOnECaR6BPDbnQpeAS6zbCWfWb7ZOq6EaTH0vpFQAHB9MZv9FnDv-90IagzKw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. The effect of dilazep on TF mRNA expression in HUVECs stimulated with TNF, thrombin, or PMA. (A) HUVECs were stimulated with each stimulant: 1,000 U/mL TNF, 25 nmol/L thrombin, or 5 nmol/L PMA for 3 hours in the presence (+) or absence (−) of 100 μg/mL dilazep. TF mRNA expression was analyzed using the RT-PCR method as described in the Materials and Methods. The amplified cDNA was blotted onto a nitrocellulose membrane and hybridized with the [32P]TF cDNA probe and then analyzed using an autoradiogram. (B) The data in (A) are depicted after normalization to equal amount of RNA loads based on the intensity of GAPDH mRNA. The data are expressed as the percentage of the control (each stimulant induced TF mRNA in the absence of dilazep). The values represent the mean ± SD of four independent experiments. Statistical analysis was performed using the Student's t-test (*P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/6/10.1182_blood.v90.6.2345/3/m_bl_0015f8b.jpeg?Expires=1769103422&Signature=anMeNCwracRZvry9c2Aystuxv81fmHrIBzNnabh4e9VW61tGEnmF64uAw4sMVcWiGF~kfnAEKrw6x2z0eWXQgVtwRdnIOqV3nM2L4n0Ym3ZZ9RnETl2~7ILAuJPnWGnwOfV7qlOlQ9CYky-NcRAATmKOn1ZETTdy7HmFgG3lbNhlOWlVUYfbfjeWE35tgfP9hRJiddKUyEhUDOuofC30CZqG0EIoPlM9m2eP6hjk0joFT2oVZ~j1Lu17nrXQXZcNTTHcTE4ESsnNoAsKVeLDoRaEwDNo0SvcNihPL2a2IGSAmB-CTGMkdjMQexRAwnh-SQQzmuanNYZo0JGatQ06tg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal