Abstract
Efficient integration of transgenes at preselected chromosomal locations was achieved in mammalian cells by recombinase-mediated-cassette-exchange (RMCE), a novel procedure that makes use of the CRE recombinase together with Lox sites bearing different spacer regions. We have applied RMCE to the study of the human β-globin gene Locus Control Region by integrating at the same genetic locus in MEL cells, a LacZ gene driven by the human β-globin promoter linked to HS2 and HS3 alone or in combination with HS4. Expression studies at the cell population level and in individual cells before and after induction of differentiation with hemin or DMSO show that the presence of these enhancers is associated with variegated patterns of expression. We were able to show that the LCR fragments tested act by controlling both the probability of expression and the rate of transcription of the linked β-globin promoter. Both of these factors were also dependent on the state of differentiation of the MELc and on the presence of a second transcription unit located in cis. The ability to manipulate by RMCE constructs integrated into chromosomes should help in the creation of complex, rationally designed, artificial genetic loci.
IT IS WELL ESTABLISHED that the regulation of gene expression in higher eukaryotes is affected by chromatin structure. Therefore, it is important to study cis-acting elements that regulate gene expression in relation to the chromosomal context in which they are located.
Most techniques to permanently introduce a DNA fragment into cells result in integration of multiple copies of the transgenes at one, or a few, random chromosomal locations.1 In addition, because of the low frequency of chromosomal integrations after transfection, studies of nonselectable transgenes require cotransfection with a selectable gene. The cells produced by a typical transfection experiment therefore contain one to several hundred copies of transgenes, cointegrated with an unknown number of copies of selectable gene, at unknown chromosomal locations.
Comparisons between such transfections are difficult because the level of expression of the transgene depends on the number of integrated copies and the locus of integration, and because the multiple integrated copies of transgene and selectable marker interact with each other in complex and unpredictable ways. Although these problems can be mitigated by comparing pools of subclones, or by analyzing by Southern blots the array of copies in independent subclones, the lack of control over the transgene integration process has been a major hurdle for the study of the regulation of gene expression.
We have developed a new method, termed Recombinase Mediated Cassette Exchange (RMCE), that eliminates these problems. RMCE is based on the CRE site-specific recombinase combined with mutated Lox sites, and allows integration of a unique copy of a transgene, at a predetermined chromosomal locus.
We have used this new approach to study the enhancer function in mouse erythroleukemia cells (MELc) of the Dnase I hyper sensitive sites (HS) constituting the human β-globin Locus Control Region (LCR). The LCR is the major regulatory element of the five developmentally regulated β-globin genes. It is composed of five developmentally stable Dnase I hypersensitive sites (HS), termed HS 1 to 5, that are located upstream of the ε-gene.2-4 Studies of naturally occurring deletions have shown that the LCR is crucial for the regulation of the β-globin locus, because in its absence, the locus is late replicating, DnaseI resistant, and transcriptionally silent.5
Grosveld et al6 and others have performed experiments in transgenic mice suggesting that the LCR differ from other enhancers because it appears to have a dominant chromatin opening activity that suppresses position-effects. Analysis of artificially induced deletions of HS2 and HS3 in the endogenous mouse locus7,8 as well as in YACs and cosmids containing large fragments encompassing the entire human locus9-11 have shown that none of the sites are indispensable for LCR function, because all the deletions tested had relatively mild phenotypes. Nevertheless, HS2, 3, 4, and, to a lesser extent, HS1, have enhancer activity when tested individually in cell culture and in transgenic mice.12
Finally, it has also been shown that the β-globin LCR participates in the initiation of DNA replication. This was shown by the observation that the origin of replication located near the β-globin promoter and used to replicate the locus in both erythroid and nonerythroid cells is not used in the absence of the LCR.13 It is uncertain whether the LCR sequences involved in the regulation of transcription are the same as those responsible for replication control.
In this report, we have focused on the functional interactions between the human β-globin promoter and HS2 and HS3, alone or in combination with HS4, as a model for the study of the role of enhancers integrated into chromosomes.
Two models have been proposed to explain the increased levels of expression observed in the presence of enhancers. In the classical model, enhancers form complexes with promoters via looping of the intervening DNA and increase the rate of transcription. In situ hybridization studies have suggested that higher rate of transcription could be caused by an increase in the frequency of interaction between the LCR and the promoters of the globin genes rather than by an increase in the density of polymerase per promoter.14 The second model is based on the premise that enhancers act by establishing a permissive chromatin structure and affect the probability of expression within a given cell rather than the rate of transcription.15-18 According to this model, rates of transcription are set primarily by the promoter, the cellular environment, and by long-range chromatin effects, while enhancers act by suppressing or preventing silencing, and have little or no effect on the rate of transcription.
We report here that we have inserted by RMCE five different expression cassettes at a given chromosomal locus that was initially tagged by random integration of a single copy of a Hygromycin resistance gene flanked by appropriate Lox sites. Subsequent expression studies showed that various LCR derivatives affect both the rate of transcription and the probability of expression when they were linked to the human β-globin gene promoter.
MATERIALS AND METHODS
Plasmids
Construction of the plasmids used in this study was based on conventional techniques.19 Detailed sequence information on all the plasmids developed for this study is available upon request.
pL1PL2
The MC promoter was derived from an Xho I/Pst I fragment of pMCNeopA (Stratagene, La Jolla, CA). The L1 and L2 site were synthetic DNA fragments that were attached to the MC promoter by PCR.
pL1HYGL2
The Neo coding sequence was derived from pMCNeopA. The PGK-HYG gene was a generous gift of Dr R. Kucherlapathy (Albert Einstein College of Medicine, Bronx, NY). The FRT sequences present in this and several other plasmids were synthetic oligonucleotides.
Cassettes 0, 2, 3, and 234
HS 2, 3 and 4 were generated from the LAR-βs construct of Forrester et al.20 The HS2 fragment contains sequences from position 7764 to 9172 of genbank file HUMHBB; the HS3 fragment HUMHBB coordinates are 4273-5122; the HS234 fragment contained the two above fragments plus an HS4 fragment (HUMHBB coordinates: 951-2199). The β-globin promoter fragment included sequences −374 to +44 relative to the cap site. The LacZ fragment was derived from plasmid pCMVbeta (Clontech, Palo Alto, CA) and contains an SV40-derived intron, the Escherichia coli LacZ sequence (fused to the ATG of the drosophila ADH gene) and an SV40 derived poly-adenylation signal.
FLP Expression Vector
Plasmid POG 44 containing the FLP recombinase coding sequence driven by the CMV promoter was obtained from Stratagene (La Jolla, CA).
CRE Expression Vector
Three CRE expression plasmids (pSSR73, pBS185, and pCAG-CRE) were used interchangeably at various times during the study: The CRE coding sequence is driven by the RSV promoter in pSSR73 (described in ref 21), by the cytomegalovirus (CMV) promoter in pBS185 (Life Technologies, Gaithersburg, MD) and by the chicken acting promoter in pCAG-CRE (a generous gift of Dr P. Vassilli, Geneva, Switzerland).
GFP Expression Plasmid
Plasmid pEGFP-N1 was obtained from Clontech.
Cell Culture
MEL cells (clone 745) were grown in Dulbecco's modified Eagle's medium (DME) containing 10% fetal calf serum, penicillin, streptomycin, and fungizone. Hemin induction was performed by seeding 3 × 104 cells/mL in the above medium containing 75 μmol/L of hemin and culturing the cells for 4 days at 37°C. Dimethyl sulfoxide (DMSO) inductions were performed by seeding 3 × 104/mL cells in above medium plus 2% DMSO and culturing the cells for 8 days at 37°C. Induction of 99% to 100% of the cells was routinely obtained after DMSO induction (monitored by performing 2-7 diamino-fluorene staining22 ). The level of induction after culture in the presence of hemin could not be monitored the same way because hemin interferes with the assay. G418 and hygromycin were added at a concentration of 1 mg/mL when appropriate.
Electroporation
Electroporation was performed as follows: 3 to 4 × 106 cells resuspended in 400 μL of serum-containing medium, were mixed with DNA dissolved in PBS, incubated 5 minutes at room temperature, and electroporated at 230 V and 1,100 μF in a Cell-Porator (BRL, Bethesda, MD). Integrations at random loci were performed with 10 μg of DNA. RMCE were performed using 200 μg of the plasmid containing the cassette to be inserted and 50 μg of the CRE expression plasmid. Reducing the amount of DNA severely decreased the efficiency of RMCE.
Excision of MCNeo
Subclones used to performed the excision experiments were passaged twice in G418 48 hours before the transfection. Excision of MCNeo was performed by transfection of 3 × 106 cells with 200 μg of pOG 44 (an FLP recombinase expression plasmid) and 10 μg of pEGFP-N1 (a green fluorescent protein [GFP] expression plasmid). The 0.25% to 0.35% of cells expressing the most GFP (and presumably the most FLP) were then sorted into two 96-well plates at a density of 4 cells/well using a Becton Dickinson Flow cytometer (Becton Dickinson, San Jose, CA). To improve survival, the cells were sorted in wells containing 50% medium conditioned by MEL cells and 50% fresh medium. After incubation for a week at 37°C, 25 to 120 (average 55 ± 25) independent colonies derived from cells transiently transfected with high levels of FLP recombinase were recovered. Each of these colonies was then tested for loss of the MCNeo gene by culture in the presence of 1 mg/mL of G418: between 2 and 25 colonies (average 5.7 ± 7) from each sorting experiment had lost G418 resistance. FLP-mediated excision of the MCNeo gene was then confirmed on these subclones by Southern blotting using a probe derived from cassette 0 containing region −374 to +40 of the human β-globin promoter.
LacZ Detection
Chemiluminescence detections of β-GlobZ were performed using the Galacto-light plus kit (Tropix, Bedford, MA). The crude cell extracts were prepared at 4°C by rinsing 1.5 × 106 cells three times in phosphate-buffered saline (PBS), lysing them in 150 μL of lysis solution (100 mmol/L potassium phosphate, 0.2% Triton X-100, and 100 μg/mL of Pefabloc (Life Technologies) and eliminating the debris by centrifugation (5 minutes at 10,000g). The extracts were stored at −70°C. The assays were performed in triplicate as recommended by the manufacturer (using the extracts as is or diluted 8 times as required to stay in the linear range of the assay). For each clone analyzed in this study, at least two different extracts made from two different cultures were tested. To compensate for temperature and reaction timing variations on assays performed on different days the results were normalized to a control solution of purified β-galactosidase (final concentration of 1 pg/reaction). These corrected luminometer values were further normalized to the total amount of protein per extract as determined with the BioRad protein assay (performed in duplicate). During the differentiation process the amount of protein per cell varies; to take this into account the results were also normalized as follows: Multiple determinations of protein concentration of extract made from carefully counted cells showed that the average protein content/cell was 1.13-fold higher after hemin induction and 0.57-fold lower after DMSO induction. Results from hemin-induced and DMSO-induced were therefore multiplied by these numbers.
Fluorescence-Activated Cell Sorter (FACS)-gal Analysis
The assays were performed exactly as described by Fiering et al23 on a FACSCAN flow-cytometer (Becton Dickinson) and the Lysis II software package (Becton Dickinson). At least two negative controls were used in each experiment.
RESULTS
The experimental goal of these studies was to compare several expression cassettes containing various LCR derivatives without the complications that are typically associated with multicopy integrations and with site of integration-induced position effects. To achieve this goal we have devised a new method termed RMCE that allows the exchange of a chromosomal cassette flanked by mutated Lox sites with a cassette carried by a transfected plasmid. Using conventional transfection techniques and Southern blot to assess the number of integrated copy, we have introduced at a random chromosomal location, a single copy of a cassette containing mutated Lox sites, and we have used RMCE to integrate a unique copy of each cassette that we wanted to study at that particular chromosomal location.
Development of RMCE
Chromosomal integrations can be performed using the CRE enzyme, a site-specific recombinase that catalyzes recombinations between short DNA sequences termed Lox sites. The simplest way to integrate a plasmid into a chromosome using CRE is to transfect cells that contain a Lox site pre-integrated on a chromosome, with a plasmid containing the same Lox site. In the presence of CRE, the two Lox sites will recombine and the plasmid will integrate into the chromosome. However, because the recombination reactions are reversible, these integrations are unstable because the plasmid can re-excise itself. Since integrations are inter-molecular events while excisions are intra-molecular events, excisions are strongly favored. With this approach, site-specific chromosomal integrations are therefore feasible (Fukushige and Sauer et al24 ) but the efficiency is low because the plasmid tends to pop out of the integration site.
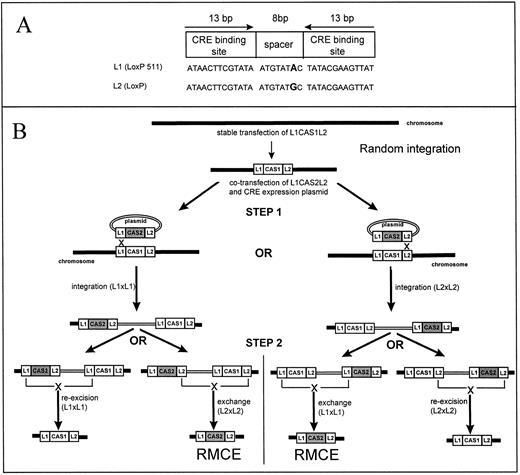
RMCE is a method that aims at eliminating the re-excision problem by taking advantage of Lox sites mutated in their spacer regions (Fig 1A). Such mutated Lox sites recombine readily with themselves but not with wild-type sites, or sites bearing different mutations.25 As described in Fig 1B, one can introduce a single copy of a cassette (cassette 1) flanked by two mutually incompatible Lox sites (L1 and L2) into a chromosome by random integration, and then exchange cassette 1 by another cassette (cassette 2). This exchange is a two-step process. In the first step of RMCE, a plasmid containing cassette 2 flanked by L1 and L2 is cotransfected with a CRE expression plasmid into the cells containing cassette 1. This results in the integration of the plasmid containing cassette 2 into cassette 1 because of an intermolecular recombination between either the two L1 or the two L2 sites. In the presence of CRE, however, these integrations are unstable and lead with a high frequency to secondary intra-molecular reactions between L1 or L2. These secondary reactions recreate either the starting locus, or, more interestingly, lead to the exchange of cassette 1 with cassette 2. Because L1 and L2 are mutually incompatible, these exchanges are stable even in the presence of recombinase.
RMCE. (A) Structure of a Lox Site: Lox sites are 34-bp double-stranded DNA fragments composed of two inverted binding sites for the CRE recombinase flanking an 8-bp spacer. The exact sequence of the spacer is not crucial for the recombination to occur, but the spacer sequence must be the same for two Lox sites to recombine.25 By introducing mutation in the spacer region, one can therefore create mutually incompatible Lox sites, that is, Lox sites that will recombine with themselves but not with each other. On the two last lines of the figure, the sequences of L1 and L2, the two mutually incompatible sites used in this study, are depicted. L1 and L2 differ by a G → A mutation at position +3 of their spacer regions. (B) Principle of RMCE: RMCE is a method that allows the exchange of a cassette located on a chromosome (cassette 1) by a cassette located on a plasmid (cassette 2). To perform RMCE a single-copy of cassette 1 flanked by L1 and L2 is placed on a chromosome by random integration (or by homologous recombination). A plasmid containing cassette 2 flanked by L1 and L2 is then cotransfected with a CRE expression plasmid in cells containing cassette 1. After the transfection, two reactions can occur (step 1): either the two L1 sites recombine or the two L2 sites recombine leading in either cases to integration of the plasmid carrying cassette 2 in the chromosome. If the L1 sites recombine leading in either cases to integration of the plasmid carrying cassette 2 in the chromosome. If the L1 sites recombine the structure on the left-hand side of the figure is obtained. If the L2 sites recombine the structure on the right-hand side of the figure is obtained. However, these structures are not the final outcome of the reaction because two L1 and L2 sites are now present in cis on the chromosome and can recombine at high efficiency (see text). These secondary recombinations (step 2) lead either to re-excision of the plasmid carrying cassette 2 or to the exchange of cassette 1 by cassette 2. When an insertion occur, it is stable because L1 and L2 cannot recombine with each other. Cassette 2 is therefore locked into the chromosome. The products of step 2 are also substrates for RMCE, the final proportion of exchanges versus re-excisions therefore depends on the number of copies of plasmid introduced in the cells. Theoretically, the equilibrium between exchange and re-excision can be tilted toward exchange by introducing large number of plasmid molecules in the cells. The thick lines represent chromosomal sequences. The double line plasmidic sequences. CAS1, cassette 1; CAS2, cassette 2. “X” symbolize CRE-mediated recombination.
RMCE. (A) Structure of a Lox Site: Lox sites are 34-bp double-stranded DNA fragments composed of two inverted binding sites for the CRE recombinase flanking an 8-bp spacer. The exact sequence of the spacer is not crucial for the recombination to occur, but the spacer sequence must be the same for two Lox sites to recombine.25 By introducing mutation in the spacer region, one can therefore create mutually incompatible Lox sites, that is, Lox sites that will recombine with themselves but not with each other. On the two last lines of the figure, the sequences of L1 and L2, the two mutually incompatible sites used in this study, are depicted. L1 and L2 differ by a G → A mutation at position +3 of their spacer regions. (B) Principle of RMCE: RMCE is a method that allows the exchange of a cassette located on a chromosome (cassette 1) by a cassette located on a plasmid (cassette 2). To perform RMCE a single-copy of cassette 1 flanked by L1 and L2 is placed on a chromosome by random integration (or by homologous recombination). A plasmid containing cassette 2 flanked by L1 and L2 is then cotransfected with a CRE expression plasmid in cells containing cassette 1. After the transfection, two reactions can occur (step 1): either the two L1 sites recombine or the two L2 sites recombine leading in either cases to integration of the plasmid carrying cassette 2 in the chromosome. If the L1 sites recombine leading in either cases to integration of the plasmid carrying cassette 2 in the chromosome. If the L1 sites recombine the structure on the left-hand side of the figure is obtained. If the L2 sites recombine the structure on the right-hand side of the figure is obtained. However, these structures are not the final outcome of the reaction because two L1 and L2 sites are now present in cis on the chromosome and can recombine at high efficiency (see text). These secondary recombinations (step 2) lead either to re-excision of the plasmid carrying cassette 2 or to the exchange of cassette 1 by cassette 2. When an insertion occur, it is stable because L1 and L2 cannot recombine with each other. Cassette 2 is therefore locked into the chromosome. The products of step 2 are also substrates for RMCE, the final proportion of exchanges versus re-excisions therefore depends on the number of copies of plasmid introduced in the cells. Theoretically, the equilibrium between exchange and re-excision can be tilted toward exchange by introducing large number of plasmid molecules in the cells. The thick lines represent chromosomal sequences. The double line plasmidic sequences. CAS1, cassette 1; CAS2, cassette 2. “X” symbolize CRE-mediated recombination.
We have shown the feasibility of inserting cassettes by RMCE into predetermined chromosome locations in MEL cells (MELc) using plasmids pL1HYGL2 and pL1PL2 (Fig 2A), two plasmids that form a “plug-and-socket” system that can trap RMCE reactions very efficiently. Plasmid pL1HYGL2 contains a “socket” made of the coding and poly-adenylation sequences of the MCNeo gene, and an RMCE cassette composed of the hygromycin resistance gene flanked by L1 and L2. Plasmid pL1PL2, the “plug,” contains the MC promoter flanked by L1 and L2. Neither pL1HYGL2 nor pL1PL2 alone confers resistance to G418, but if the plug is inserted into the socket by RMCE, the MCNeo gene that confers resistance to G418 is reconstituted.
Experimental strategy. (A) Step 1: To introduce targets for RMCE, we transfected the insert of plasmid pL1HYGL2 into MELc, and identified clones with single integrated copies by Southern blots. Two such clones were obtained; the insertion sites were termed Random Locus 1 and 2 (RL1 and RL2). These cells were hygromycin (Hyg) resistant but G418 sensitive because the Neo coding sequence (the socket, see text) lacks a promoter. Step 2: RMCE at RL1 and 2 were performed by cotransfecting a CRE expression plasmid, and plasmid pL1PL2 or its derivatives. Exchange of the PGK-HYG gene by the DNA fragment flanked by the L1 and L2 site in plasmid pL1PL2 and derivatives results in insertion of cassette X and in the reconstitution of the MCNeo gene. Therefore, MELc having undergone RMCE can be selected for in G418. The cells also loose Hyg resistance. Initial experiments to demonstrate the feasibility of RMCE were performed at the RL1 and RL2 loci using plasmid pL1PL2 (cassette X = nothing). Insertions with pL1PL2 derivatives (cassette X = cassettes 0, 2, 3 or 234 [see B]) were only performed at RL1. Step 3: After step 2, the reconstituted MCNeo gene is flanked by FLP Recognition Target (F) sites. It can therefore be excised by transient expression of an FLP expression plasmid. Because FLP-mediated excisions are rare events, a GFP plasmid was cotransfected and used to enrich in cells having been transfected. At the end of step 3 the only remaining plasmid sequences are therefore short FRT and L1 sequences flanking cassette X. (B) Cassettes 0, 2, 3, and 234 are represented by the “cassette X” box in (B) HS 2, 3, and 4 were generated from the LAR-βs construct of Forrester et al20 (see Materials and Methods). The β-globin promoter is represented by the “β-G” box in the diagram. The LacZ fragment contains an SV40-derived intron, the E coli LacZ sequence, and an SV40 derived poly-adenylation signal. All cassettes were inserted with the promoter of the β-globin gene facing away from the MC promoter (see Figs 4C and 5C).
Experimental strategy. (A) Step 1: To introduce targets for RMCE, we transfected the insert of plasmid pL1HYGL2 into MELc, and identified clones with single integrated copies by Southern blots. Two such clones were obtained; the insertion sites were termed Random Locus 1 and 2 (RL1 and RL2). These cells were hygromycin (Hyg) resistant but G418 sensitive because the Neo coding sequence (the socket, see text) lacks a promoter. Step 2: RMCE at RL1 and 2 were performed by cotransfecting a CRE expression plasmid, and plasmid pL1PL2 or its derivatives. Exchange of the PGK-HYG gene by the DNA fragment flanked by the L1 and L2 site in plasmid pL1PL2 and derivatives results in insertion of cassette X and in the reconstitution of the MCNeo gene. Therefore, MELc having undergone RMCE can be selected for in G418. The cells also loose Hyg resistance. Initial experiments to demonstrate the feasibility of RMCE were performed at the RL1 and RL2 loci using plasmid pL1PL2 (cassette X = nothing). Insertions with pL1PL2 derivatives (cassette X = cassettes 0, 2, 3 or 234 [see B]) were only performed at RL1. Step 3: After step 2, the reconstituted MCNeo gene is flanked by FLP Recognition Target (F) sites. It can therefore be excised by transient expression of an FLP expression plasmid. Because FLP-mediated excisions are rare events, a GFP plasmid was cotransfected and used to enrich in cells having been transfected. At the end of step 3 the only remaining plasmid sequences are therefore short FRT and L1 sequences flanking cassette X. (B) Cassettes 0, 2, 3, and 234 are represented by the “cassette X” box in (B) HS 2, 3, and 4 were generated from the LAR-βs construct of Forrester et al20 (see Materials and Methods). The β-globin promoter is represented by the “β-G” box in the diagram. The LacZ fragment contains an SV40-derived intron, the E coli LacZ sequence, and an SV40 derived poly-adenylation signal. All cassettes were inserted with the promoter of the β-globin gene facing away from the MC promoter (see Figs 4C and 5C).
Chromosomal loci containing the RMCE cassette and the socket were produced by random integration of a 3.8-kb fragment of pL1HYGL2 (Fig 2, step 1). The number of integrated copies of pL1HYGL2 was then determined by Southern blot (data not shown) and two single-copy clones were selected for use as recipient cells. The two random integration loci were termed RL 1 and RL 2. Culture of cells hemizygous for L1HYGL2 at RL 1 and 2 in G418-containing medium showed that the presence of the socket did not confer G418 resistance. Control transfections with either pL1PL2 or the CRE expression plasmid alone produced 0 or 1 G418-resistant colonies. This demonstrated that in this experimental system, very few G418-resistant colonies can be generated without all the necessary components for RMCE. We then transfected 3 × 106 cells hemizygous for L1HYGL2 at RL 1 and RL 2 with plasmid pL1PL2 and a CRE recombinase expression plasmid. Approximately 5,000 G418-resistant clones were obtained in each transfection. This suggested that RMCE had occurred, because the most likely explanation for the large number of G418-resistant clones obtained was that the MCNeo gene26 had been reconstituted by an exchange of the PGKHYG gene with the MC promoter (insertion of the plug into the socket, Fig 2A, step 2). Because 30% to 70% of the cells die during our electroporation procedure, about 1 in 200 to 1 in 500 surviving cells had been converted to G418 resistance by CRE-mediated insertion.
To verify that RMCE, and not some unforeseen mechanisms, had reactivated the Neo coding sequence, we tested the G418 resistant cells for loss of hygromycin resistance. All of 24 RL 1 and RL 2 independent G418-resistant clones tested had lost hygromycin resistance. Plating of pools of 1,000 G418-resistant cells showed that more than 99% of the cells had lost hygromycin resistance, suggesting that the overwhelming majority of the G418-resistant clones had acquired G418 resistance by RMCE.
To definitely establish that we had successfully performed RMCE, we extracted genomic DNA from the pools tested above and performed Southern blots. Digestions with Cla I/HindIII and Xho I showed that RMCE had taken place at RL1 and RL2 (data not shown). Taken together, these data show the feasibility and high efficiency of RMCE.
Application of RMCE to the Study of the LCR
Experimental strategy.The experimental strategy is depicted in Fig 2. Five cassettes were studied: cassette 0 contains the β-globZ reporter gene and no enhancer; cassettes 2, 3, and 234 contain the β-globZ gene linked, respectively, to HS2, HS3, and a fragment containing HS2, 3, and 4. Hypersensitive sites in cassettes 2, 3, and 234 are in their natural orientations, relative to the β-globin promoter. Cassette 234o2 contains the same LCR fragment than cassette 234 but inserted in the reverse orientation. These three enhancers were chosen because although the HSs of the LCR are thought to act in concert at their natural location, they are among the strongest enhancers active in erythroid cells even when acting alone. HS2 and 3 were tested individually, because it has been suggested that they might differ in their mechanisms of action since both the human and murine HS3 are active only when integrated into chromatin27-29 while HS2 is also active in transient transfection assays. Cassette 234 was chosen as an example of a DNA fragment that possesses most of the enhancer activity of the full LCR.20
Cassettes 0, 2, 3, 234, and 234o2, cloned 5′ of the MC promoter in plasmid pL1PL2, were integrated by RMCE at locus RL 1 using the plug-and-socket system described in the previous section (Fig 2, step 2). This selection scheme was chosen over simpler systems in which the selectable gene is also the reporter gene, because it allows the study of cassettes containing no enhancer or enhancers too weak to drive a selectable gene.
After the cassette exchange, the MCNeo gene was excised using FLP recombination target (FRT) sites that had been incorporated at appropriate locations in plasmid pL1HYGL2 and pL1PL2 (Fig 2, step 3). This step was included to eliminate potential interactions between MCNeo and the LCR-containing cassette. At the end of this procedure, each cassette was therefore integrated at RL1, in the absence of any vector sequences or selectable gene.
β-globZ expression was then determined at the individual cell level using the FACS-GAL procedure,23 and at the cell population level by chemiluminescence on lysates of whole cell populations. The average level of expression per active cell was then calculated by multiplying the proportion of expressing cells (derived from the FACS-GAL analysis) by the level of β-globZ expression in the whole cell population (derived from the chemiluminescence assay). This procedure was necessary because direct quantification of the level of β-gal expression per cells by FACS-GAL was not possible, because, in many samples, the fluorescein-di-galactopyranoside (FDG) substrate was in limiting quantity in the cells.
Like most permanent cell lines, MELc have a somewhat unstable karyotype. Since subcloning could result in chromosome loss that could affect gene expression, we attempted to minimized the extent of these clonal variations by studying pools: For each cassette, excisions of the MCNeo gene after RMCE (Fig 2, step 3) were performed on two independent subclones (subclones A and B), and all the subclones of subclones A or B that tested positive for excision of the MCNeo gene (ΔNeo subclones) were pooled. This yielded two independent pools of ΔNeo subclones (pool A and B) for each cassette. Expression studies were then performed on both pools.
The expression studies were performed in uninduced MELc, and in MELc in which hemin or DMSO were used to induce differentiation. DMSO induction is associated with accumulation of globin chains and a complex terminal differentiation process similar to normal in vivo erythroid differentiation.30 Hemin induction is also associated with globin accumulation, but by contrast with DMSO, hemin induction is reversible and is not associated with terminal differentiation.31 32 Whether MELc induced by hemin are blocked at an early stage of the normal erythroid pathway, undergo partial differentiation, or are following an alternate or abnormal differentiation pathway is not clear. In any case, cells produced after culture with DMSO and hemin differ widely in nuclear morphology and express different globin genes33; therefore, they provide two distinct environments in which to test enhancer function at the RL1 locus.
Finally, the expression studies were also performed in the presence of MCNeo at RL1 using the subclones A and B that were subsequently used for the excisions experiments.
Insertion of the Cassettes
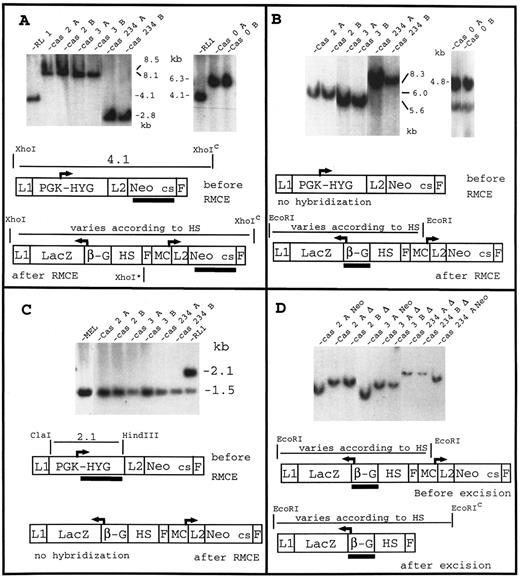
Each of the five cassettes was transfected in MELc hemizygous for PGKHYG at RL1, and 20 independent G418 colonies per cassette were analyzed by polymerase chain reaction (PCR) using RMCE-specific primers (data not shown). Ninety-eight of 100 clones analyzed appeared to have undergone RMCE. Three to 12 clones for each cassette were then analyzed by Southern blots using three different probes (Fig 3A through C). More than 90% of the clones studied had the predicted structure. The frequency of RMCE was between 0.2% and 1% of the cells that survived the transfection.
Insertion of cassettes 2, 3, 234 and 0 at the RL1 locus and excision of MCneo. Panels (A) through (C) demonstrate insertion of cassettes 0, 2, 3, and 234 by RMCE at the RL1 locus using three different probes. In all three panels, lanes labeled RL1 contains DNA with the PGK-Hyg gene inserted at RL1 while lanes A and B contains DNA isolated from two independent clones. The blots in panel (A) were probed with a DNA fragment containing the entire coding sequence of the Neomycin resistance gene. The band present when the PGK-Hyg gene is at RL1, disappear after RMCE, and is replaced by a band of a size characteristic of each cassette. In the case of cassette 234, presence of an internal Xho I site (XhoI*) leads to a band smaller than the band observed when the PGK-Hyg gene is at RL1. The restriction site marked by a superscript “c” is a chromosomal site located close to the integration site. In panel (B) the blots were probed with a segment of the β-globin promoter (region −374 to +40 relative to the cap site). The size of the fragment varies as expected, according to the size of the cassettes. As expected, the PGK-Hyg gene at the RL1 locus did not hybridize to the β-globin promoter probe (data not shown). In panel (C), the blots were probed with a segment of the PGK-Hyg gene containing the coding sequence of the Hyg gene and the mouse PGK polyA signal. The 1.5-kb band present in all lanes represents the endogenous PGK polyA gene and serves as a useful loading control; the upper band is a PGK-Hyg specific band (lane RL1): as expected it disappears when one of the cassettes is at RL1 (all other lanes). This shows that the PGK-Hyg gene is exchanged during RMCE rather than simply inactivated by the insertion. Panel (D) shows excision of the reconstituted MCNeo gene. For each cassette, one subclone containing the MCneo gene (for instance Cas 2 A Neo) and two subclones having lost the MCNeo gene (for instance Cas 2 A Δ, and Cas 2 B Δ) are shown. The blots were probed with a β-globin promoter fragment (see panel B). With all cassettes, excision is demonstrated by an increase in size of the fragment due to the loss of an EcoRI site present in the MC Neo promoter. Neo cs, neomycin resistance coding sequence (also includes polyA sequence); β-G, β-globin promoter; L1 and L2, Lox sites differing by a mutation in their spacer region; F, FLP recognition target (FRT); MC, MC promoter.
Insertion of cassettes 2, 3, 234 and 0 at the RL1 locus and excision of MCneo. Panels (A) through (C) demonstrate insertion of cassettes 0, 2, 3, and 234 by RMCE at the RL1 locus using three different probes. In all three panels, lanes labeled RL1 contains DNA with the PGK-Hyg gene inserted at RL1 while lanes A and B contains DNA isolated from two independent clones. The blots in panel (A) were probed with a DNA fragment containing the entire coding sequence of the Neomycin resistance gene. The band present when the PGK-Hyg gene is at RL1, disappear after RMCE, and is replaced by a band of a size characteristic of each cassette. In the case of cassette 234, presence of an internal Xho I site (XhoI*) leads to a band smaller than the band observed when the PGK-Hyg gene is at RL1. The restriction site marked by a superscript “c” is a chromosomal site located close to the integration site. In panel (B) the blots were probed with a segment of the β-globin promoter (region −374 to +40 relative to the cap site). The size of the fragment varies as expected, according to the size of the cassettes. As expected, the PGK-Hyg gene at the RL1 locus did not hybridize to the β-globin promoter probe (data not shown). In panel (C), the blots were probed with a segment of the PGK-Hyg gene containing the coding sequence of the Hyg gene and the mouse PGK polyA signal. The 1.5-kb band present in all lanes represents the endogenous PGK polyA gene and serves as a useful loading control; the upper band is a PGK-Hyg specific band (lane RL1): as expected it disappears when one of the cassettes is at RL1 (all other lanes). This shows that the PGK-Hyg gene is exchanged during RMCE rather than simply inactivated by the insertion. Panel (D) shows excision of the reconstituted MCNeo gene. For each cassette, one subclone containing the MCneo gene (for instance Cas 2 A Neo) and two subclones having lost the MCNeo gene (for instance Cas 2 A Δ, and Cas 2 B Δ) are shown. The blots were probed with a β-globin promoter fragment (see panel B). With all cassettes, excision is demonstrated by an increase in size of the fragment due to the loss of an EcoRI site present in the MC Neo promoter. Neo cs, neomycin resistance coding sequence (also includes polyA sequence); β-G, β-globin promoter; L1 and L2, Lox sites differing by a mutation in their spacer region; F, FLP recognition target (FRT); MC, MC promoter.
Excision of the MCNeo Gene
The excisions of MCNeo were performed by transient cotransfection of expression plasmids coding for the FLP recombinase and the GFP, followed by an enrichment in cells expressing a high level of FLP recombinase using GFP as a flow cytometry marker (see Materials and Methods). For cassette 2, two ΔNeo subclones were obtained from subclone A and four from subclone B. For cassette 3, three ΔNeo subclones were obtained from subclone A and seven from subclone B. For cassette 234, no ΔNeo subclones were obtained from subclone A and five from subclones B. ΔNeo clones obtained from the same subclones were pooled and the pools were termed pool A and B. In the case of cassette 0 and 234o2, the excisions were performed on pools of 10 independent clones. In each case, 10 ΔNeo subclones were obtained. These ΔNeo subclones were divided arbitrarily into 2 pools of 5 clones. The overall frequency of MCNeo excision was 0.017% ± 0.025% of the cells having survived the transfection and 10.15% ± 8.3% of the sorted GFP positive cells. Excisions were verified by Southern blots (Fig 3D).
Expression Studies
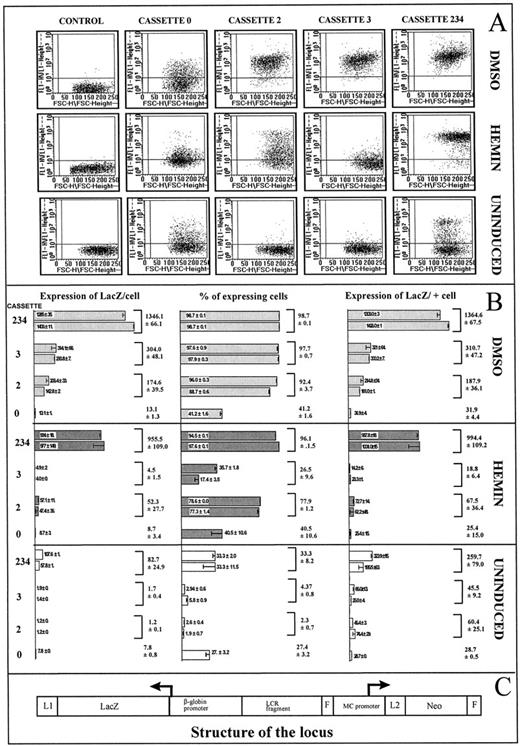
The results are illustrated and summarized in Figs 4 and 5. In Fig 4, the four cassettes are integrated at RL1 in the absence of MCNeo. In Fig 5, the four cassettes and the MCNeo gene are integrated at RL1. In Fig 4, two pools of several clones were studied for each cassette. In Fig 5, two independent clones were studied for each cassette.
Expression studies with cassettes 0, 2, 3, or 234 integrated at RL1 in the absence of MCNeo. (A) FACS-GAL analyses. All analyses were performed in the presence of 2 mmol/L propidium iodide. Debris and dead cells were excluded from the analyses by gating out cells with low SSC (Side-SCatter) and high (propidium iodide–induced) fluorescence in the red channel (FL2). Two thousand cells are shown in each graph, 10,000 cells were counted. The boxes in the first column are MEL cells with PGK-HYG at the RL1 locus and were used to defined the limit between the β-gal–negative and β-gal–positive populations. β-gal–positive cells are in the upper window (x axis = FSC [Forward Scatter]; y axis = green fluorescence channel [530-nm wavelength with a 30-nm bandwidth]). The lower limit of the window was set at the position at which between 0.3% to 0.4% of control cells appeared positive. The next four columns represent cells with cassettes 0, 2, 3, or 234 at RL1 before and after induction of differentiation by DMSO or hemin. Striking differences in the proportions of expressing cells can be observed. (B) Comparative analysis of cassettes 0, 2, 3, and 234 integrated at RL1. Levels of LacZ expression in cell population with cassettes 0, 2, 3, 234, and 234o2 integrated at RL1 were measured on cell lysates by chemiluminescence. The percentage of cells expressing β-GlobZ within each cell population was determined by FACS-Gal analyses (illustrated above). The level of expression of LacZ per positive cells was estimated by multiplying the level of LacZ expression in cell population by the proportion of positive cells after normalization (see text). For each cassette, the histograms represent the results obtained for two independent pools of several clones (pool A and B, see text). The means and standard deviation of the results obtained for pools A and B combined is also indicated on the right side of the histograms. The smallest level of β-gal expression represented in the histogram (0.8 × 10−15 g of β-gal/cell for cassette 0 in uninduced cells) was about 15 times higher than the background level observed with untransfected cells (data not shown) and was therefore well within the range of detection of our luminometer. This analysis clearly shows that both the probability of expression and the rate of transcription are enhancer and trans-acting factor dependent. (C) Structure of the locus. The structure of the RL1 locus after integration of the cassettes and excision of the MCNeo gene is depicted. LCR fragments were, respectively, HS2, HS3, and HS234 in cassettes 2, 3, and 234 (with HS234 in reverse orientation in HS234o2). In cassette 0, no LCR fragment was present.
Expression studies with cassettes 0, 2, 3, or 234 integrated at RL1 in the absence of MCNeo. (A) FACS-GAL analyses. All analyses were performed in the presence of 2 mmol/L propidium iodide. Debris and dead cells were excluded from the analyses by gating out cells with low SSC (Side-SCatter) and high (propidium iodide–induced) fluorescence in the red channel (FL2). Two thousand cells are shown in each graph, 10,000 cells were counted. The boxes in the first column are MEL cells with PGK-HYG at the RL1 locus and were used to defined the limit between the β-gal–negative and β-gal–positive populations. β-gal–positive cells are in the upper window (x axis = FSC [Forward Scatter]; y axis = green fluorescence channel [530-nm wavelength with a 30-nm bandwidth]). The lower limit of the window was set at the position at which between 0.3% to 0.4% of control cells appeared positive. The next four columns represent cells with cassettes 0, 2, 3, or 234 at RL1 before and after induction of differentiation by DMSO or hemin. Striking differences in the proportions of expressing cells can be observed. (B) Comparative analysis of cassettes 0, 2, 3, and 234 integrated at RL1. Levels of LacZ expression in cell population with cassettes 0, 2, 3, 234, and 234o2 integrated at RL1 were measured on cell lysates by chemiluminescence. The percentage of cells expressing β-GlobZ within each cell population was determined by FACS-Gal analyses (illustrated above). The level of expression of LacZ per positive cells was estimated by multiplying the level of LacZ expression in cell population by the proportion of positive cells after normalization (see text). For each cassette, the histograms represent the results obtained for two independent pools of several clones (pool A and B, see text). The means and standard deviation of the results obtained for pools A and B combined is also indicated on the right side of the histograms. The smallest level of β-gal expression represented in the histogram (0.8 × 10−15 g of β-gal/cell for cassette 0 in uninduced cells) was about 15 times higher than the background level observed with untransfected cells (data not shown) and was therefore well within the range of detection of our luminometer. This analysis clearly shows that both the probability of expression and the rate of transcription are enhancer and trans-acting factor dependent. (C) Structure of the locus. The structure of the RL1 locus after integration of the cassettes and excision of the MCNeo gene is depicted. LCR fragments were, respectively, HS2, HS3, and HS234 in cassettes 2, 3, and 234 (with HS234 in reverse orientation in HS234o2). In cassette 0, no LCR fragment was present.
Analysis of cells with cassettes 2, 3, or 234 integrated at RL1 in the presence of MCNeo. (A through C) All experiments were performed exactly as described in Fig 4. This analysis confirms the conclusion of Fig 3 and demonstrates that presence of a cis-linked transcription unit has significant effects on both probability of expression and rate of transcription.
Analysis of cells with cassettes 2, 3, or 234 integrated at RL1 in the presence of MCNeo. (A through C) All experiments were performed exactly as described in Fig 4. This analysis confirms the conclusion of Fig 3 and demonstrates that presence of a cis-linked transcription unit has significant effects on both probability of expression and rate of transcription.
Clonal variations.To determine if clonal variations would significantly affect the results of this study, we compared for each cassette the levels of β-globZ expression between pool A and B. As is evident in Fig 4, variation between pool with the same cassette integrated at RL1 are not significantly from variation because of experimental error. For each cassette, Student t-tests failed to show any statistically significant differences between pools A and B. In contrast, when different cassettes are inserted at RL1, Student t-tests show several statistically significant differences between β-globZ level of expression (see below). The same statistical analysis performed for the subclones A and B containing each cassette in the presence of the MCNeo gene (Fig 5) yielded similar results. We concluded that, whether pools of a few clones or individual subclones are studied, clonal variations due to random chromosome loss or some other mechanisms are relatively small and in the same order of magnitude as the experimental errors in this study.
Expression of β-globZ in the absence of MCNeo.The β-globZ gene inserted at RL1 without an enhancer (cassette 0) was expressed in 1.5% of uninduced MELc at an average level of expression of 60 × 10−15 g of β-gal per positive cells. As shown in Fig 4B, bottom panel, when HS2 and HS3 were driving the β-globZ gene (cassettes 2 and 3), the proportions of expressing cells were 28- and 4-fold higher while the average level of expression per positive cell was unchanged. When cassettes 234 and 234o2 were present the proportion of active cells was almost 100%, and the average levels of expression per positive cells was about 10-fold higher than in the presence of cassette 0 (t-test P value = .001). These results clearly suggest that enhancers control the proportion of expressing cells and the level of expression per positive cells.
Previous reports17 had clearly shown that transgenes integrated at ectopic loci could be subject to extinction. Therefore, we took care to passage the cells a similar number of times before measuring β-globZ activity. In addition, to assess the rate of extinction at the RL1 locus in the presence of cassettes 2, 3, and 234, the same measurements were repeated 4 weeks after the experiments described above. No major changes were observed in any of the cassettes (data not shown). Although long-term studies will be required to assess the stability of expression at RL1, this preliminary result indicates that expression at RL1 was not undergoing rapid extinction at the time of our initial measurements.
To extend this analysis, we then induced differentiation of the MELc. After hemin induction, the proportions of expressing cells and the average level of expression per positive cell were similar to uninduced levels for cassettes 0 and 3, suggesting that these two cassettes did not respond to hemin. In the case of cassette 2, the proportion of active cells more than doubled to 90% while the average level of expression per positive cells increased about sevenfold. In the case of cassette 234 and 234o2, the proportion of active cells was unchanged at 100% and the average level of expression per positive cell increased twofold to threefold. The average levels of expression per positive cell were, respectively, 7- and 23-fold higher in the presence of HS2 and HS234 than when there was no enhancer at RL1. The Student t-test showed that these differences were statistically significant: P = .0038 for the comparison of the mean LacZ expression level positive cells for cassette 0 and 2, and P = .00004 for the comparison between cassette 0 and cassette 234. Differences between cassette 2 and 234 were also statistically significant: P = .01.
These differences could be due to changes in the rate of transcription at the β-globin promoter when different enhancers are at RL1 or to different timing of induction. To discriminate between these two possibilities, the hemin induction period was prolonged from 4 to 8 days to ascertain that a new equilibrium had been reached. Expression levels and proportion of expressing cells were then determined as above. The results after 8 days of hemin induction were similar to the results obtained after 4 days of induction (data not shown). Therefore, we concluded that the differences observed were not caused by difference in the timing of induction. These results confirm the findings made in uninduced cells that both the probabilities of expression and the average levels of expression per positive cell vary widely when no or different enhancers are at the RL1 locus; they also show that HS2, an individual HS much less complex than HS234, can also modulate the level of expression per positive cell.
After DMSO induction, 39% of the cells were expressing β-globZ when cassette 0 was at RL1 and almost 100% of the cells were expressing when cassettes 2, 3, 234, or 234o2 were at the same location. The average level of expression per active cell was similar to the level observed in uninduced cells in the case of cassette 0, twofold higher in the case of cassette 234 and 234o2 and about 10-fold higher in the case of cassettes 2 or 3.
The average levels of expression per positive cell after DMSO induction were, respectively, 10-, 9-, and 20-fold higher when HS2, 3, 234(o2) were at RL1 than when no enhancer was present. The respective P values were .005, .00003, and .000001. Differences between the level of expression per positive cells when cassettes 2 or 3 were compared to cassette 234 were also statistically significant. These results clearly confirm the conclusions of the experiments in uninduced and hemin-induced cells and demonstrate that HS3 also can drive expression in all cells when the cellular environment is adequate.
As can be seen in Fig 4, the level of β-globZ expression per positive cells and the percentage of expressing cells appear to be positively correlated. To establish that this correlation was not a technical artifact, we ascertain that the sensitivity of the chemiluminescence β-gal assay did not decrease with increasing concentration of β-gal enzyme in the extract. Extracts from population of cells expressing β-globZ in all cells were mixed with extracts made from untransfected cells in various proportions and the levels of β-globZ expression were measured. As can be seen in Fig 6, this showed that the chemiluminescence assay is linear in the range tested.
The chemiluminescence assay is linear. Extracts from MELc populations in which all the cells expressed the LacZ gene (cells with cassette 234 at RL1) were sequentially diluted with extracts from nonexpressing cells and the levels of β-gal expression were measured using the chemiluminescence assay. Extracts from uninduced, hemin-, or DMSO-induced cells were used in three independent experiments. The means of the levels of β-gal expression (normalized to the 100% positive cells extract) obtained for all three types of extract is plotted against the percentage of positive cells per extracts. This shows that the chemiluminescence assay is almost perfectly linear over the range of interest. As shown by the small error bars, results for each type of extract plotted individually were similar.
The chemiluminescence assay is linear. Extracts from MELc populations in which all the cells expressed the LacZ gene (cells with cassette 234 at RL1) were sequentially diluted with extracts from nonexpressing cells and the levels of β-gal expression were measured using the chemiluminescence assay. Extracts from uninduced, hemin-, or DMSO-induced cells were used in three independent experiments. The means of the levels of β-gal expression (normalized to the 100% positive cells extract) obtained for all three types of extract is plotted against the percentage of positive cells per extracts. This shows that the chemiluminescence assay is almost perfectly linear over the range of interest. As shown by the small error bars, results for each type of extract plotted individually were similar.
Expression of β-globZ in the presence of MCNeo.To assess the influence of the immediate chromosomal environment on enhancer/promoter functional interactions, we repeated the experiments described above using the subclones in which MCNeo had not been removed (Fig 5).
When cassette 0 was at RL1, presence of MCNeo was associated with a marked increase in the proportion of expressing cells as compared with the same cassette tested in the absence of MCNeo, suggesting that the enhancer present in MCNeo also acts on the β-globin promoter. However, when cassettes 2, 3, or 234 were at RL1, the presence of MCNeo was associated with a decrease in the proportion of expressing cells, suggesting that the MC promoter/enhancer and the LCR fragments are interfering or competing with each other and/or with the β-globin promoter. To ascertain that the MCNeo gene was expressed in all cells, the FACS-GAL measurements were repeated after selection of the cells for 6 days in G418. For all cassettes, the proportions of expressing cells increased slightly but none of the increases reach statistical significance (data not shown). We concluded that forcing expression of MCNeo in all cells did not lead to expression of β-globZ in all cells or alternatively to complete extinction, but led to a variegated pattern of expression.
After induction of differentiation with DMSO or hemin, the influence of MCNeo on the interactions between the LCR fragment and the β-globin promoter was similar to that observed before induction. In the case of cassette 0, the probability of expression was higher in the presence of MCNeo while in the case of cassettes 2 and 234, the probability of expression and the level of expression per positive cell decreased in the presence of MCNeo. However, this decrease was less pronounced than in induced cultures. This suggests that when the MEL cells differentiate, the erythroid-specific interactions predominate. In the case of cassette 3, the negative interactions between MCNeo and HS3 are markedly decreased after hemin induction, suggesting that hemin induces changes in the trans-environment that silences HS3. This hypothesis is supported by the fact that when cassette 3 is at RL1 in the absence of MCNeo, the average level of expression per positive cell is lower after hemin induction than in undifferentiated cells.
As in the absence of MCNeo, the proportions of expressing cells and the average levels of expression per active cell depend greatly on the enhancer present. After DMSO induction the average level of expression per active cell was 40 times higher in the presence of cassette 234 than in the presence of cassette 0 at RL1.
DISCUSSION
RMCE is a new method for site specific chromosomal integrations that does not require any pre-existing mutations in the cell and should therefore be widely applicable. Because the technique allows insertion of different cassettes at the same locus, cis-acting regulatory sequences can be compared more rigorously than with classical transfection techniques. Therefore, RMCE could prove very useful for the study of gene regulation. By varying the integration locus, one should also be able to learn a great deal about position effects and the mechanism of creation of open chromatin domains. Combining RMCE with homologous recombination in ES cells should facilitate the creation of animals with ever more subtle mutations. Insertion based on FRT sites with spacer mutations had been previously reported in mammalian cells.34 However, the efficiency was very low probably because the FLP recombinase is 1,000 times less efficient than CRE in mammalian cells.21
In this report, we have focused on applying RMCE to the study of the mechanism of action of enhancers. Because all the comparisons were made between cells that differ only by the enhancer present at RL1, and because these enhancers are not believed to affect posttranscriptional processing, the variations in β-globZ levels of expression observed with different cassettes integrated at RL1 are very likely to reflect changes in the rate of transcription. In the rest of the discussion, differences in the level of expression of the β-globZ gene per active cell are therefore equated with changes in the rate of transcription.
Variegated Patterns of Expression
A striking result obtained in these studies is that, even in the presence of enhancers, expression of β-globZ at RL1 is variegated in uninduced MEL cells. The mechanisms of appearance of the variegation are probably multiple: one mechanism of creation of variegation is clearly linked to the presence of MCNeo. The capacity of MCNeo to induce variegation can be observed when cassettes 2 or 3 are at RL1 but is most obvious in the case of cassette 234: in the latter case, absence of MCNeo is associated with β-globZ expression in all cells, while presence of MCNeo is associated with β-globZ expression in fewer than 50% of the cells. The fact that selection with G418 to insure expression of MCNeo in all cells did not eliminate variegation of β-globZ suggests that at least some variegation is not caused by a global extinction of the whole locus due to proximity to hetero-chromatin. The nature of the variegation induced by MCNeo is therefore unclear but could be related to steric hindrance induced by transcription at the MC promoter (which is constituted of a tandem repeat of the enhancer region from the polyoma mutant PYF441 linked to the promoter of the HSV-TK gene26 ), to competition for HS234 between the TK and β-globin promoter, or to interferences between the polyoma enhancer and cassette 234.
Because variegated patterns of expression are also observed in the case of cassette 0, 2, and 3 in the absence of MCNeo, some other mechanism has to be implicated. One possibility would be that some other transcription units, located close to the RL1 locus acts as MCNeo, once MCNeo is excised. Alternatively, gradual silencing caused by differential hetero-chromatin spreading, a poorly understood phenomenon that has been invoked in numerous systems, could be involved: In the case of the β-globin genes, Walters et al17 have reported that at most loci, a β-Geo reporter gene was rapidly silenced when it was integrated as single-copy in the absence of an enhancer in K562 cells but did not observe silencing when HS2 was present at the same loci. These results differ from the observations presented here since we have observed variegation in the presence of HS2. However, because the cells, the selection strategy, and the integration locus were different in both studies, it is nevertheless possible that some of the variegation of expression observed in the study reported here could be the result of a gradual silencing process that we failed to detect in our 4-week study of stability of expression at RL1. This hypothesis implies that in MELc at RL1, HS2 and HS3 can slow gradual silencing but do not stop it. The fact that variegation of a LacZ reporter gene driven by a large LCR fragment was observed in transgenic mice supports the contention that LCR fragments do not necessarily stop position-effect variegation.35 Long-term studies of stability of expression at RL1 are in progress and should help answer this question.
Whether position-effects variegation observed in the presence and absence of MCNeo are related is unclear at this time. In any case, variegation of expression in MEL cells is not permanent because it can be rapidly overridden by inducing the MEL cells to differentiate along the erythroid lineage.
Enhancers Can Affect the Rate of Transcription
In uninduced MEL cells and in the absence of any enhancer at RL1, a small proportion of cells was expressing low but easily detectable level of β-globZ. The average level of expression per active cell when cassette 0 was at RL1 was defined as the basal level of transcription of the β-globin promoter at RL1. This basal rate of transcription was unchanged by hemin or DMSO induction.
In uninduced MEL cells, presence of cassette 234 or 234o2 was associated with a rate of transcription 10-fold higher than the basal rate of transcription. This clearly showed that the rate of transcription is not entirely set by long-range chromatin effects but that it can be affected by the presence of HS234, a large and complex enhancer. The fact that HS2 and HS3 did not increase the rate of transcription is in accordance with the observation of Walters et al16 that HS2 act primarily by increasing the proportion of expressing cells.
This result is confirmed and extended by the fact that after hemin induction the rate of transcription in the presence of HS2 and HS234 is, respectively, 8- and 23-fold higher than the basal rate of transcription and by the fact that after DMSO induction all three enhancers tested were capable of increasing the rate of transcription 10- to 20-fold over the basal rate of transcription.
An alternative interpretation of the induction studies would be that the differences in the level of expression of β-globZ are caused by differential timing of the silencing of the various cassettes by the hetero-chromatinization process that occurs during erythroid differentiation of MEL cells. Although it is technically difficult to eliminate this hypothesis in the case of DMSO induction, the fact that prolonging hemin induction from 4 to 8 days did not change the results shows that HS2 is not silenced at any point during hemin induction, and suggests that HS2 does modulate the rate of expression.
Taken globally these results show that the enhancers studied here can affect the level of expression of a linked gene by modulating the proportion of expressing cells and the rate of transcription.
Comparison of the rate of transcription and the proportion of expressing cells before and after induction of differentiation when the same cassettes are at RL1 reveals that, as expected, the rate of transcription is also greatly affected by the differentiation process. This also shows that there is a positive correlation between the proportion of expressing cells and the rate of transcription as the level of differentiation of the cells changes. This suggests that these two factors are closely intertwined and might be two facets of the same regulatory mechanism.
Interestingly, cassette 0 and cassette 3 respond very little or not at all to hemin induction, while cassette 2 and 234 respond strongly. This clearly suggests that at RL1 the response to hemin is entirely mediated by enhancers. Previous studies had shown that HS2 but not HS3 is hemin inducible in K562 cells36 and that the hemin response depended on the presence of the double NFE-2 site present in HS2. This suggests that the hemin response in MEL cells also might be mediated by the double NFE-2 binding sites.
How Do Enhancers and Promoters Interact?
Results of this study do not eliminate either the looping or the chromatin structure-based probabilistic models discussed in the introduction. However, we have made several observations that constrain any model. We have clearly shown that four enhancers affect the final level of expression in a cell population both by affecting the probability of expression and the rate of transcription. Therefore, any looping model must account for the fact that in uninduced MEL cells, enhancers can interact with the promoter in only some cells. The fact that the rate of transcription can be altered by enhancers rules out models in which the only role of enhancer is to open up the chromatin without changing the rate of transcription, but leaves open the possibility that long-range chromatin structure changes, and not direct interaction by looping, are critical in promoter/enhancer interactions. One of the goals of these studies was to determine if the enhancers tested differ by some qualitative properties that might help define distinct classes of enhancers. The facts that all the enhancers tested (HS2, HS3, and HS234 in two orientations) were subject to variegation and could increase the rate of transcription under some circumstances suggest, however, that they might all share the same mechanism of action at least when they are integrated at RL1.
In conclusion, we have shown that at the cell population level, expression of β-globZ integrated at one particular genetic locus varies almost a 1,000-fold depending on the enhancer present and the presence of another genetic unit. These variations were caused in part by the fact that the proportion of expressing cells can vary from less than 1% to 100%, and in part to the fact that the rate of transcription of the β-globin gene promoter varies up to 40-fold. Any model of interactions between promoters and enhancers must therefore account for this dual effect. Understanding these regulations is a prerequisite for the rational engineering of complex synthetic artificial genetic loci that could produce a regulated amount of protein useful to human endeavor. RMCE might prove to be a powerful tool to achieve this goal.
ACKNOWLEDGMENT
We are very grateful to Dr Ronald Nagel for his support throughout this project, and to Ilia Rochlin and Antonietta Marmorata for their excellent technical assistance. We also thank Dr A. Skoultchi and S. Fiering for critical review of the manuscript.
Supported by grants from the National Institute Of Health (Grants No. NIH HL38655, HL48374, and HL55435), by a Grant-in-Aid from the American Heart Association (Grant No. 95015110), and by a grant from Johnson & Johnson.
Address reprint requests to Eric E. Bouhassira, PhD, Division of Hematology, Department of Medicine, Albert Einstein College of Medicine, 1300 Morris Park Ave, Bronx, NY 10461.

![Fig. 2. Experimental strategy. (A) Step 1: To introduce targets for RMCE, we transfected the insert of plasmid pL1HYGL2 into MELc, and identified clones with single integrated copies by Southern blots. Two such clones were obtained; the insertion sites were termed Random Locus 1 and 2 (RL1 and RL2). These cells were hygromycin (Hyg) resistant but G418 sensitive because the Neo coding sequence (the socket, see text) lacks a promoter. Step 2: RMCE at RL1 and 2 were performed by cotransfecting a CRE expression plasmid, and plasmid pL1PL2 or its derivatives. Exchange of the PGK-HYG gene by the DNA fragment flanked by the L1 and L2 site in plasmid pL1PL2 and derivatives results in insertion of cassette X and in the reconstitution of the MCNeo gene. Therefore, MELc having undergone RMCE can be selected for in G418. The cells also loose Hyg resistance. Initial experiments to demonstrate the feasibility of RMCE were performed at the RL1 and RL2 loci using plasmid pL1PL2 (cassette X = nothing). Insertions with pL1PL2 derivatives (cassette X = cassettes 0, 2, 3 or 234 [see B]) were only performed at RL1. Step 3: After step 2, the reconstituted MCNeo gene is flanked by FLP Recognition Target (F) sites. It can therefore be excised by transient expression of an FLP expression plasmid. Because FLP-mediated excisions are rare events, a GFP plasmid was cotransfected and used to enrich in cells having been transfected. At the end of step 3 the only remaining plasmid sequences are therefore short FRT and L1 sequences flanking cassette X. (B) Cassettes 0, 2, 3, and 234 are represented by the “cassette X” box in (B) HS 2, 3, and 4 were generated from the LAR-βs construct of Forrester et al20 (see Materials and Methods). The β-globin promoter is represented by the “β-G” box in the diagram. The LacZ fragment contains an SV40-derived intron, the E coli LacZ sequence, and an SV40 derived poly-adenylation signal. All cassettes were inserted with the promoter of the β-globin gene facing away from the MC promoter (see Figs 4C and 5C).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3332/5/m_bl_0056f2.jpeg?Expires=1765218235&Signature=zcT7dP04t-CAAP~bFGMwzxDNE~qSvldzSnqsFHrmU1w6YdUCKjX~~J5epVXFIM7k-TXYqe1bG90VYyTt19JRENMvl5nth89bB4nGel9araQG1-MtiFnvPdRk2Fh66tlOkbUIVUHf6qhYRxhJv43Mp7sjoU-NSdJu6SCkFgjegZNqmZCnKH45AOa01q~6GdFMxBTwz3EN7AWntnb9LsI3bhClluQZ2qHT3a9zRgxK7QumHn04LLqFcblhgENAotpn41SqbQlRX0uEotrmB8tuUU3OIyqXAl1FXS2X7KSikZS3ImXY9z~XR1GsBQNqPqKUYT7vBMWXJ9ZqQ98tTVGo2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Expression studies with cassettes 0, 2, 3, or 234 integrated at RL1 in the absence of MCNeo. (A) FACS-GAL analyses. All analyses were performed in the presence of 2 mmol/L propidium iodide. Debris and dead cells were excluded from the analyses by gating out cells with low SSC (Side-SCatter) and high (propidium iodide–induced) fluorescence in the red channel (FL2). Two thousand cells are shown in each graph, 10,000 cells were counted. The boxes in the first column are MEL cells with PGK-HYG at the RL1 locus and were used to defined the limit between the β-gal–negative and β-gal–positive populations. β-gal–positive cells are in the upper window (x axis = FSC [Forward Scatter]; y axis = green fluorescence channel [530-nm wavelength with a 30-nm bandwidth]). The lower limit of the window was set at the position at which between 0.3% to 0.4% of control cells appeared positive. The next four columns represent cells with cassettes 0, 2, 3, or 234 at RL1 before and after induction of differentiation by DMSO or hemin. Striking differences in the proportions of expressing cells can be observed. (B) Comparative analysis of cassettes 0, 2, 3, and 234 integrated at RL1. Levels of LacZ expression in cell population with cassettes 0, 2, 3, 234, and 234o2 integrated at RL1 were measured on cell lysates by chemiluminescence. The percentage of cells expressing β-GlobZ within each cell population was determined by FACS-Gal analyses (illustrated above). The level of expression of LacZ per positive cells was estimated by multiplying the level of LacZ expression in cell population by the proportion of positive cells after normalization (see text). For each cassette, the histograms represent the results obtained for two independent pools of several clones (pool A and B, see text). The means and standard deviation of the results obtained for pools A and B combined is also indicated on the right side of the histograms. The smallest level of β-gal expression represented in the histogram (0.8 × 10−15 g of β-gal/cell for cassette 0 in uninduced cells) was about 15 times higher than the background level observed with untransfected cells (data not shown) and was therefore well within the range of detection of our luminometer. This analysis clearly shows that both the probability of expression and the rate of transcription are enhancer and trans-acting factor dependent. (C) Structure of the locus. The structure of the RL1 locus after integration of the cassettes and excision of the MCNeo gene is depicted. LCR fragments were, respectively, HS2, HS3, and HS234 in cassettes 2, 3, and 234 (with HS234 in reverse orientation in HS234o2). In cassette 0, no LCR fragment was present.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3332/5/m_bl_0056f4.jpeg?Expires=1765218235&Signature=OFh3C8hUFVJcmXKNw5Q52I~qp9U6gQimuYv3Owqc1IyewYOUnEaJwBD~lbvjVP1Zl2FNiZA0nyJyxDEweOrrA97e8hlY7vvDWjlEOHq9WhGG~q0v73HsKdE~FEMLSaY6vU92zmEb9r1v0d-EYW0MqThGMhgDCv3hti5uz8C92wjI7sVzJWwDpEkY6ELchy3fOd~xYW2zJT-4~yKgh6lTF4u8QVY4403PPwJYssJJdwlcKIZ4tK0bNtgsixxUKDLA6IlvpW~uWrKINrm7lxOpdFV~vMGYC84VUJ-UlOAGinI4OCOParmrd84~1eaL5JhnkOt6voXhLdj5xIFuu5jMUw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal