Abstract
The progenitors for cells of bone, cartilage, fat, and muscle are thought to be derived from mesenchymal stem cells but despite extensive study of stromal cell differentiation, neither mesenchymal stem cells or the more committed, tissue-specific progenitors have been well-characterized. In this study we used flow cytometry to isolate from fetal rat periosteum a population of small, slowly cycling cells with low cytoplasmic granularity (S cells) that display stem cell characteristics. On plating, S cells exhibited a 90% higher labeling index with [3H]-thymidine compared to unsorted cells and when grown in culture generated cartilage, adipocyte, and smooth muscle phenotypes, in addition to bone. Only the S-cell population showed extensive self-renewal of cells with osteogenic potential. Electron microscopy showed that S cells have high nuclear:cytoplasmic ratios with large condensed nuclei and a paucity of cytoplasmic organelles. Freshly sorted suspensions of immunocytochemically stained S cells did not express differentiation-associated markers such as type I, II, and III collagens, alkaline phosphatase, or osteopontin. However, after attachment, S cells became immunopositive for collagens I, II, III, osteopontin, and also for the cell surface receptor CD44, which mediates cell attachment to hyaluronan and osteopontin. These studies show that viable osteogenic precursor cells with the stem cell characteristics of self-renewal, high proliferative capacity, and multipotentiality can be enriched from heterogeneous stromal cell populations with simple flow cytometric methods. These cells may be useful for regeneration of stromal tissues.
THE ABILITY OF tissues and organs to develop, remodel, regenerate, and repair is dependent on the existence of stem cells that upon division form more differentiated progeny.1,2 The existence of stem cells has been well-documented in the epidermis,3 the intestinal epithelium,4 and the hematopoeitic system.5 In contrast, evidence of stem cells in mesenchymal tissues is largely indirect.6,7 In vivo and in vitro studies have provided evidence of osteogenic precursor cells in bone marrow (BM) and other stromal cell preparations,8-12 but the identity of cells in these tissues and their relationship to cells with classical stem cell characteristics1 2 has yet to be established.
Differentiation of mesenchymal cells has been extensively studied in osteogenesis.13-16 However, the lack of unique markers for osteoprogenitors, and the low estimated frequency of these precursor cells (0.0005% in BM17; 0.3% in the fetal rat calvariae18 ) has been an impediment in the search for osteogenic stem cells. Indeed single cell analysis of the phenotypic characteristics of osteogenic cells has indicated that the precursor cells may be heterogeneous.19 Nevertheless, attempts have been made to separate osteoprogenitor and osteogenic cells using density centrifugation17 and flow cytometry using cell surface markers.12,20,21 However, successful application of these approaches has been limited by the relatively poor viability of the sorted cells. Further, it is uncertain how closely these cells are related to putative osteogenic stem cells. Indeed, the use of positive selection to isolate progenitor cells would seem to select for relatively differentiated cells that have already begun to express phenotypic markers associated with osteoblastic differentiation (eg, alkaline phosphatase20 ).
In a recent study we used multiparametric flow cytometry analyses of osteopontin (OPN) expression, protein content, and cell cycle position to identify discrete subpopulations of osteogenic cells in fetal rat periosteum at different stages of culture. We identified at the time of peak proliferation a unique subpopulation of small, noncycling OPN− cells with low cytoplasmic granularity and low protein content.22 Since vital sorting based on cell size and cytoplasmic granularity have been used previously to separate primitive precursors from BM hematopoietic cells,23 and to enrich for stem cells for hematopoietic therapy,24 this approach was used to enrich for a viable subpopulation of cells with the characteristics of mesenchymal stem cells.
MATERIALS AND METHODS
Cell culture. Fetal rat calvarial cell (FRCC) populations were prepared by five sequential enzymatic digestion (I to V) of calvariae from 21-day-old fetuses of timed-pregnant Wistar rats as described previously.25 Cells from digestions II to V were plated in T-75 flasks and grown in α-minimal essential medium (α-MEM) containing 15% heat-inactivated fetal bovine serum (FBS) and antibiotics (100 μg/mL penicillin G, 50 μg/mL gentamicin sulfate, and 0.3 μg/mL fungizone). In some experiments we assessed the ability of FRCC and BM stromal cells to support hematopoeisis by culturing nonadherent cells from rat or mouse BM flushes in methylcellulose and Iscove's modified Dulbecco's medium (IMDM; Stem Cell Technologies, Vancouver, BC; 3 × 104 cells/mL).
Stromal cells were grown at 37°C in a humidified atmosphere of 95% air/5% CO2 . After 24-hours incubation, nonviable cells were washed away with phosphate-buffered saline (PBS). Cells from populations II to V were pooled to permit an analysis of the total osteogenic population as well as those fibroblastic cells that are derived from the fibrous periosteum in these fractions. Aliquots were electronically counted (ZM Coulter Counter; Hialeah, FL) and replated in T-75 flasks at a density of 2.25 × 105 cells per flask. Culture conditions were identical in all experiments except as outlined below. Before sorting, cells were plated for a period of 2 days.
Flow cytometry and cell sorting. Attached cells were procured using 5 mL of 0.01% trypsin in citrate buffer and cells from 5 T-75 flasks (∼4 × 106 cells) were resuspended in 2 mL α-MEM (phenol red-free, to eliminate artefactual fluorescence during flow cytometry) containing 15% filtered FBS and 10% antibiotics. Sorting was performed on a FACStar Plus flow cytometer (Becton Dickinson Immunocytochemistry Systems, Moutain View, CA) equipped with an argon ion laser (Coherent innova 70) operating at 250 mW beam power. Gating windows were established for forward light scatter (FSC) and side scatter (SSC). Particles with an average size <5 μm (determined by running standard size beads) were excluded. Two major subpopulations were sorted: cells with the lowest 15% FSC and lowest 15% SSC in the population (S cells) and the cells with the highest 15% FSC and highest 15% SSC (L cells). Three other populations were also collected: (1) FRCC cells were passed without sorting through the flow cytometer as a control group to evaluate the effect of passage through the flow cytometer on osteogenesis in vitro and other functional assays; (2) cells remaining after sorting the two main groups (S, L), were designated as S− and L−, respectively. All groups were collected in glass tubes containing 3 mL α-MEM with 15% FBS and 10% antibiotics.
Cell vitality. To assess cell vitality as a result of passage through the flow cytometer and sorting, sorted cells were plated overnight into 8-well chamber slides (n = 8 replicates; Nunc, Roskilde, Denmark) at a density of 1 × 103 cells per well. One slide was plated at the same density with unsorted FRCC. Slides were washed with PBS to remove nonviable cells and debris and fixed. Cells were washed, permeabilized with 0.01% Triton-X (BDH, Toronto, ON) to permit intracellular staining and incubated for 10 minutes at 4°C with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Boehringer, Mannheim, Germany; 1 μg/mL final concentration in 0.1% NP-40). Slides were analyzed by immunofluorescence microscopy. The mean number of cells and standard errors of the means per microscopic field area (25× objective) were computed. Statistical analysis of computation of P values for these data were determined using analysis of variance and Tukey's test.
OPN mRNA. Cells were analyzed for the presence of OPN mRNA by the reverse transcriptase polymerase chain reaction (RT-PCR) as described.22 RNA was extracted using the Mini-GT Protocol for preparation of total nucleic acids.26 The GeneAmp RNA PCR Kit (Perkin-Elmer Cetus, Norwalk, CT) was used for cDNA synthesis and PCR amplification following the supplier's protocol with minor modifications. Random hexamers were used to prime the cDNA synthesis (20 μL final volume) from the RNA of 200 cells as described.22 Seminested PCR was performed to confirm that the amplified product was derived from OPN cDNA. Amplification products were analyzed by electrophoresis in a 2% agarose gel in 0.5× Tris borate EDTA (TBE) and visualized with 0.5 μg/mL ethidium bromide.
Alkaline phosphatase activity and cell proliferation. Sorted cells were plated into 8-well chamber slides (n = 4 replicates; Nunc) at a plating density of 1 × 103 cells per well and were compared to unsorted FRCC plated at the same density. Cultures were analyzed on day 1, 2, 4, 6, 8, and 10. Due to the limited numbers of cells in the sorted subpopulations, only FRCC were analyzed on day 12. To assess the proportion of proliferating cells, wells were incubated with 3H-thymidine (1 μCi/mL) for 3 hours before the termination of the culture. Slides were fixed, stained for alkaline phosphatase (AP) activity, and prepared for radioautography with NTB-2 liquid emulsion (Kodak, Rochester, NY) as described.27 The following populations of cells were counted: 3H-thymidine labeled cells (>4 silver grains per nucleus), AP+ cells, and cells with both 3H-thymidine labeling and AP staining. All cells were counted in the same microscopic field area (40× objective; triplicate fields for each culture). The mean labeling indices of cells and standard errors of the means were computed.
Limiting dilution analysis. Cells were plated in 96-well plates at dilutions of 5, 25, 50, 100, 300, and 600 cells per well. Two replicate plates (192 wells) were obtained for each dilution. Cells were grown continuously for 24 days and the medium was changed every 2 to 3 days. Ascorbic acid (50 μg/mL) and 10 mmol/L sodium β-glycerophosphate (Sigma) were added at confluence. After 24 days, wells were fixed overnight in neutral buffered formalin and stained with von Kossa's reagent. The fraction of wells without bone nodules (F0 ; nonresponsive wells) was calculated for each plating density. This fraction was plotted against the number of cells plated per well for each one of the subpopulations. A linear regression was fitted with 95% confidence limits and correlation coefficients were calculated. Application of the Poisson distribution28 permitted estimation of the number of osteoprogenitor cells in each of the groups which upon division was capable of forming bone nodules.
Colony assays. For assessment of osteogenic capacity at relatively high cell densities, sorted cells were plated in 35-mm dishes at 3 × 104 cells per dish. Two sets of dishes were prepared, either untreated (plastic) or precoated with a thin film of collagen type I (Vitrogen; Celtrix, Santa Clara, Ca) following the supplier's protocol. Cultures were subsequently augmented with 50 μg/mL ascorbic acid and 10 mmol/L sodium β-glycerophosphate (Sigma) at confluence. The cultures were terminated on day 16, fixed overnight in neutral buffered formalin, and stained with von Kossa's reagent.29 The number of bone nodules was counted in each plastic dish. Triplicate fields (10× objective) for each culture were counted in triplicate dishes. The mean number of bone nodules and standard deviations were calculated for each group. The mean area of bone nodule was assessed by image analysis (R&M Biometrics, Nashville, TN) and expressed as μm2; 30 replicate nodules were measured for each group (10× objective). The mean nodule size and standard deviations were calculated and differences were evaluated using analysis of variance. Collagen-coated dishes exhibited diffuse patterns of mineralization that precluded enumeration of bone nodules.
Self-renewal capacity. To assess the self-renewal capacity of cells from the two main sorted subpopulations (S, L), and FRCC passed through the flow cytometer without sorting, cells were plated in T-25 flasks at a plating density of 7.5 × 104 cells per flasks and grown for 12 days (early mineralization stage22 ). Nonviable cells and debris were removed by washing twice with PBS and attached cells were procured with trypsin in citrate buffer. Visual inspection of cultures following trypsinization confirmed the complete removal of all cells. Aliquots were counted electronically and prepared for sorting as described above. Initially, cells were resorted into 3 subpopulations (parental FRCCs ; Ss ,Ls ) using the same criteria as the first sort. Subsequent sorts used expanded windows for the L subpopulation. Cells were seeded into 12-well chamber plates at a plating density of 1.5 × 104 cells per well and supplemented with ascorbic acid (50 μg/mL) and 10 mmol/L sodium β-glycerophosphate at confluence. Cultures were terminated after 16 days of culture, stained with von Kossa's reagent and analyzed for bone nodule formation. The mean and standard deviation of the number of bone nodules were determined from duplicate fields (10× objective) counted for each culture in 6 replicate dishes.
Electron microscopy. Transmission electron microscopy was performed to assess the morphological characteristics of the subpopulations sorted by flow cytometry. Sorted subpopulations were either pelleted or plated overnight, and then fixed with 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer, pH 7.3, postfixed with osmium tetroxide, dehydrated in an ethanol series, and embedded in spurr epoxy resin. Thin sections (7 nm) were stained with uranyl acetate and lead citrate and examined under an electron microscope.
Immunostaining. The two sorted subpopulations (S and L) were characterized by antibody staining and the staining patterns were compared with unsorted FRCC. Two sets of staining procedures were used. In the first procedure, 1.5 × 104 cells were sorted by flow cytometry, collected in tubes, fixed immediately, stained in suspension, and cytospin preparations prepared as described.10 In the second procedure, sorted cells were plated in 8-well chamber slides (Nunc) at a plating density of 2 × 103 cells per well. Cells were grown overnight and washed twice before fixation. Replicate wells (n = 4) were obtained for each subculture. Staining procedures were identical in the two procedures and were done simultaneously.
Antibodies to collagen I, II, and III were used as described previously.30,31 Mouse antirat OPN monoclonal antibody (MPIIIB1O1) was obtained from the Developmental Studies Hybridoma Bank (Johns Hopkins University, Baltimore, MD under contract from NICHD). Intracellular staining of OPN by this antibody was characterized previously.22 Biotin-conjugated mouse antirat CD44 (Pgp-1, H-CAM; Clone OX-49; Pharmingen, San Diego, CA) was used to stain cells, as OPN and hyaluronan, which is expressed early in development, are important ligands for CD44.32 Cells immunostained for collagens type I and II were fixed with methanol at −20°C for 15 minutes. Samples immunostained for collagen III, OPN, CD44 were fixed with 2% paraformaldehyde in Ca2+ and Mg2+-free PBS for 30 minutes at 4°C. Washing and dilution of all antibodies was in 0.25% bovine serum albumin (BSA) in PBS (Ca2+ and Mg2+-free) except as outlined below. After two washes cells were incubated with the following diluted antibodies: an affinity purified sheep antirat collagen I (1:20) was incubated for 1 hour at 4°C; an affinity purified rabbit antirat collagen II (1:50) was incubated for 1 hour at 4°C; an affinity purified sheep antipig collagen III (1:20) was incubated for 1 hour at 4°C; OPN (1:800) and CD44 (1:200) were both incubated for 45 minutes at 4°C followed by a 10-minute incubation at 22°C. All samples were washed twice again with the BSA solution. Collagen type I and III stained samples were incubated with fluorescein isothyiocyanate (FITC)-conjugated rabbit antisheep antibody diluted 1:50 in the BSA solution and incubated for 1 hour at 4°C. Collagen II stained samples were incubated with FITC-conjugated goat antirabbit F(ab)2 fragments diluted 1:20 in the same BSA solution and incubated for 1 hour at 4°C; OPN samples were incubated with FITC-conjugated sheep antimouse antibody diluted 1:100 in the BSA solution and incubated for 30 minutes at 4°C; CD44 stained samples were incubated with streptavidin FITC-conjugated antibody diluted 1:50 in the BSA solution and incubated for 1 hour at 4°C.
Stained samples were washed with PBS (Ca2+ and Mg2+-free), and counterstained with DAPI for 10 minutes at 4°C. Slides were washed twice again with PBS (Ca2+ and Mg2+-free) and cytospins of the cell suspension were prepared. Slides were coverslipped with Permount (Fisher, Toronto, Ontario, Canada) and analyzed by immunofluorescence microscopy. DAPI fluorescence was used to ensure the presence of cells and then cells were examined for specific proteins with the FITC-labeled reagents. FITC fluorescence was graded as: none, low, medium, and high. The relative percentage of stained cells was estimated from counts of the relative number of FITC+ cells divided by the number of DAPI nuclei.
RESULTS
We assessed whether FRCC could support hematopoiesis, thereby indicating their potential as stromal cells. Nonadherent hematopoietic cells from either rat or mouse femoral BM flushes did not form colonies when plated in methylcellulose, IMDM, and 2% FBS. However, when incubated with growth factors (3 U/mL erythropoietin, 10 ng/mL mrIL-3, 10 ng/mL hrIL-6, and 50 ng/mL mr Stem Cell Factor; all from Stem Cell Technologies), colony-forming units (CFC-C) were observed (CFC-C −49 ± 3.9/35-mm dish). Similarly, when cells were incubated with IMDM and 2% FBS and plated on feeder cell layers of either high density rat BM stromal cells (CFC-C −57 ± 4.6/35-mm dish) or on high density FRCC (CFC-C −54 ± 3.45/35-mm dish), there was colony development and with no statistically different colony counts compared to the number of colonies in cultures supplemented with hematopoietic growth factors. We conclude that FRCC are competent stromal cells.
Cell sorting. Cells isolated from fetal calvariae were sorted following culture for 2 days, a time of maximal proliferative activity in the FRCC cultures and when conditions for enriching for S cells was optimal. As determined from previous studies,22 S cells were characterized on the basis of low forward angle light scatter, low cytoplasmic granularity, low protein content and enrichment in G1 and S phases of the cell cycle. A typical profile of FRCCs sorted on the basis of light scatter is shown in Fig 1A and is representative of 7 separate sorting experiments that were conducted. Cell counts showed that both the S and L cell populations comprised 6% to 11% of the total number of fractionated cells.
Separation of FRCC by flow cytometry. (A) A representative flow cytometry plot of FRCC separated according to light scatter characteristics reflecting cytoplasmic granularity and cell size. Seven independent replicates of the sorting procedure were used to isolate the S (lowest 15% FSC and lowest 25% SSC) and L (highest 15% FSC and highest 25% SSC) subpopulations. This cytogram shows debris close to the origin but particles smaller than 5 μm were excluded from the sort. (B) Histogram showing the number of sorted cells (▪; mean ± SEM) in each population that attached to glass after overnight plating compared to replated FRCC from the same cultures (▨). Counts were done on DAPI-stained cells and expressed as number per 100 cells plated. (C) Analysis of OPN mRNA expression in sorted cells measured by RT-PCR. Lanes 1 through 5, S cells; FRCC; L cells; S− cells; L− cells. First, amplification of an 871-bp fragment, encompassing most of the OPN sequence, was performed using total RNA extracted from the cells (left side). This was followed by amplification of a 486-bp fragment using the 871 fragment as template (right side), which confirmed OPN expression in all populations except the S population.
Separation of FRCC by flow cytometry. (A) A representative flow cytometry plot of FRCC separated according to light scatter characteristics reflecting cytoplasmic granularity and cell size. Seven independent replicates of the sorting procedure were used to isolate the S (lowest 15% FSC and lowest 25% SSC) and L (highest 15% FSC and highest 25% SSC) subpopulations. This cytogram shows debris close to the origin but particles smaller than 5 μm were excluded from the sort. (B) Histogram showing the number of sorted cells (▪; mean ± SEM) in each population that attached to glass after overnight plating compared to replated FRCC from the same cultures (▨). Counts were done on DAPI-stained cells and expressed as number per 100 cells plated. (C) Analysis of OPN mRNA expression in sorted cells measured by RT-PCR. Lanes 1 through 5, S cells; FRCC; L cells; S− cells; L− cells. First, amplification of an 871-bp fragment, encompassing most of the OPN sequence, was performed using total RNA extracted from the cells (left side). This was followed by amplification of a 486-bp fragment using the 871 fragment as template (right side), which confirmed OPN expression in all populations except the S population.
Analysis of cells that attached following overnight plating (Fig 1B) showed that, in comparison to unsorted FRCC, passage of cells through the flow cytometer reduced FRCC vitality only slightly (P > .2). However, the L and L− subpopulations showed ∼20% to 25% loss of vitality (P < .05) compared to unsorted FRCC, whereas the S subpopulation exhibited a 40% loss of vitality (P < .05), which was a significantly higher loss than observed for all the other populations (P < .05). The lower viability of S cells may in part reflect the inclusion of small, apoptotic cells which were included in the sorting window although we note that these cells can be readily deleted from the sort by the use of pulse processing software (Becton Dickinson).
To verify that the S subpopulation was OPN−, RNA prepared from the sorted subpopulations (200 propidium iodide− cells were used for each preparation) was subjected to RT-PCR using primers based on the rat OPN sequence as described by Zohar et al.22 Initial amplification of the 871 base pair (bp) sequence of the translated OPN mRNA generated a positive response in all the sorted subpopulations except the S cells, which were negative in four replicates and weakly positive in one replicate. When the primary PCR product was re-amplified using nested primers corresponding to the carboxy-terminal half of OPN, the resulting 486-bp product confirmed the presence of OPN mRNA in all the subpopulations except S cells, which were negative in all replicates (Fig 1C).
Alkaline phosphatase activity and proliferative capacity. The [3H]thymidine labeling index (Fig 2A) for the S subpopulation was the highest of all the subpopulations in the first 48 hours of culture. In the first 24 hours of culture the S subpopulation exhibited a 90% higher labeling index than the unsorted FRCC and >100% more than the L subpopulation. S-cell nuclei were heavily labeled with silver grains (>20 grains/nucleus) whereas the other subpopulations exhibited only 5 to 12 grains for labeled cells. The two sorted subpopulations (S, L) showed decreased proliferation during the matrix formation phase of the culture (days 4 through 6) while FRCC maintained a relatively constant % of labeled cells. As cultures multilayered and approached mineralization (days 8 through 12), there was another increase in the percentage of labeled cells for all groups. Although at day 8 there was no difference between the subpopulations, the S subpopulation at day 10 exhibited threefold more labeled cells than the other two groups.
Analysis of cell proliferation and alkaline phosphatase expression. (A) The proportion of 3H-thymidine–labeled cells at different stages of cellular differentiation was determined (mean labeling index ± SEM) after a 3-hour pulse of 3H-thymidine to detect proliferating cells. (B) The proportion of cells expressing alkaline phosphatase (AP index) at the same time points analyzed for cellular proliferation was determined by staining cells with Naphthol AS Phosphate substrate containing Fast Blue BB salts and the proportion of blue stained cells was counted (mean index ± SEM). (C) Proportion of cells exhibiting both labeling for [3H]-thymidine and AP staining (mean ± SEM).
Analysis of cell proliferation and alkaline phosphatase expression. (A) The proportion of 3H-thymidine–labeled cells at different stages of cellular differentiation was determined (mean labeling index ± SEM) after a 3-hour pulse of 3H-thymidine to detect proliferating cells. (B) The proportion of cells expressing alkaline phosphatase (AP index) at the same time points analyzed for cellular proliferation was determined by staining cells with Naphthol AS Phosphate substrate containing Fast Blue BB salts and the proportion of blue stained cells was counted (mean index ± SEM). (C) Proportion of cells exhibiting both labeling for [3H]-thymidine and AP staining (mean ± SEM).
AP activity (Fig 2B), used as a marker of osteoblastic differentiation, was detected in ∼35% of cells in the FRCC and L groups during the first 24 hours of culture whereas S cells did not show any detectable AP activity over this time period. All groups showed increased proportions of AP+ cells during the matrix formation phase in which the highest proportion were in the S and L groups (70% of the cells) and 60% of the cells in the FRCC. The L cells exhibited high proportions of AP+ cells until day 8 when there was a decrease to only 30% of the cells. A similar decline was observed in the FRCC on day 10 and these two groups showed increased proportions of AP cells by 5% to 15% as they approached mineralization. The S population did not lose the high proportion of AP+ cells acquired during culture: at least 60% of the cells on day 6 were AP+.
The same fields were analyzed for cells exhibiting dual labeling for [3H]-thymidine and AP, a measure of the proportion of transit amplifying cells. In the first 4 days only cultures of the parental FRCC and L cells showed dual-labeled cells (Fig 2C). The peak proportion for the L population was on day 2 when 40% of the cells in the culture were double labeled whereas only 23% were double labeled for the FRCC. The S group did not show dual-labeled cells until day 6 when all groups exhibited similar proportions of dual-labeled cells (20% to 30% of the cells). All groups showed an increase in the proportion of dual-labeled cells as they approached mineralization: 60% of the cells in the S group compared to 15% to 20% in the FRCC and L groups were double labeled.
Analysis of osteoprogenitor cells. Limiting dilution analysis was used to assess cell cooperativity in bone nodule formation and the relative proportions of transit amplifying cells that could divide in culture and produce bone nodules.18 The analyses showed a linear relationship between the number of cells plated per well and the fraction of nonresponsive wells. The lowest R2 value for all groups was 0.83 in the FRCC (Fig 3A), showing a strong linear correlation and single hit kinetics, as evaluated by counting of von Kossa stained bone nodules. Thus, the progenitor cells that divided to form nodules did not require assistance from other cell types.
Limiting dilution analyses of bone nodule-forming capacity. Linear regression analysis was performed to show the relationship between the fraction of the nonresponsive wells against plated cell number for the different sorted cell populations and the parent FRCC population. Results are expressed as mean ± 95% confidence limits. R is the correlation coefficient. F0.37 is an estimate of the mean number of cells that would include one progenitor cell, based on application of the Poisson distribution.
Limiting dilution analyses of bone nodule-forming capacity. Linear regression analysis was performed to show the relationship between the fraction of the nonresponsive wells against plated cell number for the different sorted cell populations and the parent FRCC population. Results are expressed as mean ± 95% confidence limits. R is the correlation coefficient. F0.37 is an estimate of the mean number of cells that would include one progenitor cell, based on application of the Poisson distribution.
Based on the single event theory28 the proportion of amplifying osteoprogenitors was estimated for each of the subpopulations. By calculating the probability of no response at F0 = 0.37, we estimated that the proportion of these progenitors in the FRCC was 1:620 cells, threefold less than the S subpopulation that we estimated as 1:202 (Fig 3B) or ∼1:120 when adjusted for cell viability (Fig 1B). The L group showed threefold lower numbers in relation to FRCC (Fig 3C; 1:1750) that was almost ninefold less than S cells. The S− subpopulation contained more progenitors than L cells (Fig 3D; 1:968), but this was still fourfold to fivefold less than the S subpopulation. Thus, over the time period of culture, all populations were capable of generating transit amplifying cells for the osteogenic lineage, but the relative frequency of these cells was much higher in the S population.
Osteogenic capacity of sorted subpopulations. We plated cells on either tissue culture plastic or on collagen-coated plastic as earlier work has shown that collagen influences the development and maintenance of the osteoblastic phenotype in primary and passaged rat calvarial osteoblasts.33 Within 24 hours after flow cytometry, all sorted subpopulations attached to plastic or collagen-coated dishes. On the basis of phase contrast microscopy and staining with oil red O, alcian blue, and toluidine blue, there was the morphological appearance of fat cells, chondrocytes, and smooth muscle cells (see below) in addition to the presence of osteogenic cells that are the focus of this report. On both collagen and plastic substrata, cell proliferation was most rapid in the S group. Indeed, only the S and L− populations had reached confluence on collagen gels by day 13, at which time a diffuse pattern of mineralization was evident. In two of four replicate experiments the FRCC, S−, and L subpopulations showed only limited mineralization after 20 days on collagen gels and never to the same degree as observed in the S and L− populations. S cells grown on plastic formed rapidly mineralizing bone nodules by day 9, as observed by phase-contrast microscopy. In contrast, mineralized bone nodules were not evident in the L− and other subpopulations before day 12. However, on day 13 all groups (data not shown) had produced bone nodules except the the L population which formed small nodules 3 to 4 days later. Notably, bone nodules formed by the S cells were significantly larger than those formed by the other populations (see below).
Staining of day 16 cultures with Von Kossa's reagent (Fig 4A) confirmed the observations by phase contrast microscopy. Analysis of bone nodule-forming capacity of different subpopulations showed that in collagen-coated dishes, most of the dish in S and L− subpopulations was covered with mineralized tissue. There were small foci of mineralization in the S− group, but no bone nodules in the L group or FRCC. Quantification of bone nodules on plastic dishes (Fig 4B) showed fourfold higher numbers of nodules in the S group than the FRCC population and 10-fold larger size of individual bone nodules (P < .001). In contrast, the number of nodules in the L group was threefold lower than the FRCC population which was similar to the S− subpopulation, and twofold lower than the L− subpopulation. There were no significant differences in the size of nodules produced by the parental FRCC and L cells while the nodules produced by S− cells were fourfold larger than FRCC and bone nodules produced by L− cells were sixfold larger than FRCC (P < .001).
Analysis of bone nodule formation. (A) Photograph of day 16 cultures stained by Von Kossa to show mineralization. All subpopulations exhibit mineralization when grown on plastic dishes (P) but S and L− cells exhibit larger numbers of bone nodules. Mineralization is observed only for S and L− cells when grown on collagen-coated (C) dishes. (B) Bone nodule number and size (μm) determined after Von Kossa staining of the day 16 cultures grown on plastic dishes (mean ± SEM).
Analysis of bone nodule formation. (A) Photograph of day 16 cultures stained by Von Kossa to show mineralization. All subpopulations exhibit mineralization when grown on plastic dishes (P) but S and L− cells exhibit larger numbers of bone nodules. Mineralization is observed only for S and L− cells when grown on collagen-coated (C) dishes. (B) Bone nodule number and size (μm) determined after Von Kossa staining of the day 16 cultures grown on plastic dishes (mean ± SEM).
Self-renewal capacity. As counts of bone nodules showed that all cultures contained measureable numbers of amplifying osteoprogenitor cells albeit at different proportions, we conducted a more rigourous assay to estimate the self-renewal capacity of more primitive, undifferentiated cells. After 12 days of growth, S and L cells and FRCC were individually subjected to a second round of flow cytometry to produce Ss , Ls and parental (unsorted) FRCCs populations of resorted cells. Notably, on passage through the flow cytometer, 43% of S− cells retained the same forward scatter and side scatter characteristics of the initially sorted population. In contrast, 0% of the L− cells exhibited the cytological characteristics of S− cells. Consequently we expanded the sorting windows for the Ls population to include those cells with the highest 50% side scatter and highest 50% forward scatter. After plating, the S−-cell populations from all groups were confluent by day 7 to 8. Phase contrast microscopy of Ss cells showed a progressive increase in cell numbers and foci of bone nodule formation for each sorted population. However, Ls and FRCCs groups exhibited decreased cell density from day 8 onwards. Ls cells showed cell degeneration, as indicated by enlargement of cell bodies, an increased number of cytoplasmic vacuoles and detachment of cell processes. By day 14 there were few cells remaining in the Ls group with somewhat higher cell numbers in the FRCCs . Von Kossa staining of day 16 cultures showed abundant bone nodule formation in cells from the Ss group, but only barely detectable numbers of bone nodules were observed in the FRCCs and even lower numbers in the other subpopulations. The mean bone nodules counts per field were: FRCC−s 1.1 ± 1.07; L−s 0.0; S−s 54.5 ± 5.02.
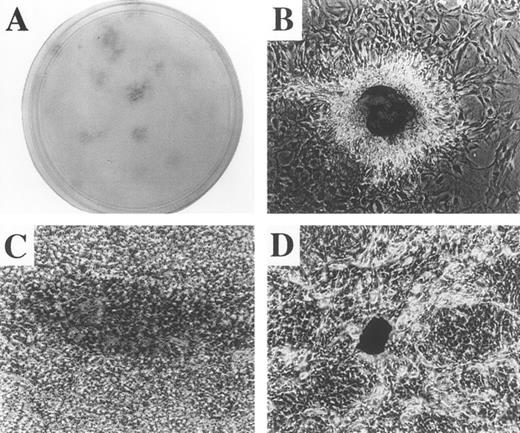
Pluripotentiality. In S−-cell cultures plated at low density (1,000 cells per 100-mm diameter dish; 45 days growth), isolated colonies were marked and monitored over time. From serial morphological observation we determined that the colonies arose from single cells (Fig 5A). Many of the colonies stained positively for oil red (a marker of adipocytes), for alcian blue (a marker of cartilage), and for von Kossa's reagent (a marker for osteogenesis; Fig 5B through D). In some colonies derived from S−-cell cultures, stained cells of a single phenotype were colocalized with cells in the same cluster that were of other phenotypes (ie, fat/cartilage or bone/fat). In contrast, stained colonies were either absent or were very rare in L−-cell cultures.
Evidence for pluripotentiality by formation of multiple cell types at clonal cell densities. Sorted S−cells were plated at very low density (1,000 cells/100-mm diameter plate) and cultured for 45 days (A) × 4 magnification. Note the discrete, isolated colonies distributed througout the dish. Colonies were marked, observed over time, and found to arise from single cells. Colonies were stained for cartilage by alcian blue (B), for adipocytes by oil red (C) and for bone by von Kossa's reagent (D). (B) through (D) × 400 magnification.
Evidence for pluripotentiality by formation of multiple cell types at clonal cell densities. Sorted S−cells were plated at very low density (1,000 cells/100-mm diameter plate) and cultured for 45 days (A) × 4 magnification. Note the discrete, isolated colonies distributed througout the dish. Colonies were marked, observed over time, and found to arise from single cells. Colonies were stained for cartilage by alcian blue (B), for adipocytes by oil red (C) and for bone by von Kossa's reagent (D). (B) through (D) × 400 magnification.
Ultrastructural characterization. Electron microscopy of the sorted cells verified the flow cytometric measurement of size and cytoplasmic granularity which was used for sorting in this study (data not shown). L cells exhibited the largest size and most well-developed endoplasmic reticulum and vacuolar apparatus while S cells were the smallest and least developed in terms of cytoplasmic structure. Higher magnification showed that S cells had a high nuclear/cytoplasmic ratio, condensed nucleus, and low amount of cytoplasmic organelles. L cells showed relatively small nuclei and a cytoplasm rich in organelles.
Immunofluorescence of cytospins and spread cells. Immunocytochemical analysis of cytospin preparations showed large numbers of DAPI-stained nuclei for all preparations. S cells did not stain for OPN (Table 1). FRCC exhibited intracellular OPN staining in ∼80% of the cells and staining intensity ranged from low to high; 60% of the L cells were stained for OPN at medium intensity. High-intensity collagen I staining was detected in 70% of the FRCC while low to medium staining was seen in <50% of the L cells. Medium intensity collagen II staining was detected in more than 30% of the FRCC and 70% of the L cells showed medium-high–intensity staining for type II collagen. None of the S cells were immunopositive for collagens I or II. Collagen III staining was seen in only a few S and FRCC cells (<5%) with low intensity while 20% of the L cells exhibited low to medium collagen-III staining. Immunoreactivity for CD44 was not observed in any of the groups.
Phenotype of S and L Cells
| Protein Detected . | Sorted-Cytospin . | Plated Overnight . | ||
|---|---|---|---|---|
| . | S . | L . | S . | L . |
| Osteopontin | − | +++ (60%) | +++ (90%) | +++ (70%) |
| Collagen-I | − | ++ (50%) | +++ (40%) | +++ (60%) |
| Collagen-II | − | ++ (70%) | +++ (60%) | +++ (80%) |
| Collagen-III | ± (3%) | + (20%) | ++ (25%) | + (40%) |
| CD-44 | − | − | +++ (40%) | ++ (40%) |
| Protein Detected . | Sorted-Cytospin . | Plated Overnight . | ||
|---|---|---|---|---|
| . | S . | L . | S . | L . |
| Osteopontin | − | +++ (60%) | +++ (90%) | +++ (70%) |
| Collagen-I | − | ++ (50%) | +++ (40%) | +++ (60%) |
| Collagen-II | − | ++ (70%) | +++ (60%) | +++ (80%) |
| Collagen-III | ± (3%) | + (20%) | ++ (25%) | + (40%) |
| CD-44 | − | − | +++ (40%) | ++ (40%) |
Immunostaining of cells that were sorted and analyzed (sorted-cytospin) or sorted and plated overnight from S- and L-cell subpopulations. Fluorescence intensity given estimate of level of expression: − none, + low, ++ medium, +++ high, ++++ very high; () indicates percentage of cells expressing the specific phenotypic marker. DAPI staining was used to show present of viable cells in each population; irrelevant antibody and 2nd Ab alone was used to show the absence of nonspecific staining.
Analysis of sorted cells plated overnight showed large numbers of attached cells stained with DAPI. High intensity intracellular OPN staining was observed in most of the plated S cells and bright staining was also observed in 70% of the FRCC and the L cells (Table 1). All groups exhibited a perimembranous, focal adhesion type of OPN staining.22 Larger intracellular clusters of OPN staining were observed in the L cells. High intensity but diffuse intracellular staining for collagen I was observed in 40% of S cells and >60% of the FRCC and L cells. High-intensity diffuse intracellular staining for collagen II was observed in 40% of S cells and FRCC and 80% of the L cells. Bright staining for collagen III was observed in 25% of the S cells while 80% of the FRCC cells showed medium intensity staining and 40% of the L cells showed low-medium intensity. High intensity surface-staining for CD44 was observed in 40% of S cells and medium staining for CD44 was observed in ∼40% of FRCC and L cells.
DISCUSSION
Stem cell enrichment. Stromal cells derived from bone and other mesenchymal tissues comprise a heterogeneous population that include cells with high proliferative capacity and multipotentiality, indicative of the presence of stem cells.7 However, before our study, cells with the classical features of stem cells have not been clearly identified in cell populations derived from stromal tissues and only limited progress has been made in isolating these cells.12 We have shown here that sorting cells on the basis of size and cytoplasmic granularity enriches for a population of slowly cycling cells that did not express differentiation associated markers and which upon plating develops high-proliferative capacity, multipotentiality, and capacity for self-renewal. In contrast, the remaining S− and L populations were depleted of stem cells as shown by their lack of self-renewal capacity, their limited proliferative capacity, and their reduced ability to form bone nodules. As these populations contained abundant proportions of cells that were stained for alkaline phosphatase and were labeled with 3H-thymidine, they likely comprise transit amplifying cells.2 Some of these putative amplifying cells entered the osteogenic lineage and were therefore capable of producing bone nodules; but they exhibited almost no self-renewal capacity.
Since recovery of viable cells following selection and isolation is problematic,12,20 we used FRCC primary cultures as a model since this system contains a relatively high proportion of osteoprogenitor cells18 and stem cells are likely to be present in the periosteal tissue surrounding bone.6 Second, we sorted for a slow cycling, OPN−, small cell population with low granularity and protein content, identified previously in FRCC22 after 2 days of culture, a time at which enrichment with osteoprogenitor cells was anticipated.16,34 Although flow cytometry based on selection of cells for bone lineage markers is potentially more discriminating (eg, Long et al21 ) and can isolate cells capable of producing bone nodules in vitro,20 such cells are already committed to osteoblastic differentiation and lose their osteogenic capacity following flow sorting.20 Nevertheless, despite using relatively atraumatic procedures for cell separation, cell viability was lowest in the S subpopulation (60%), which may reflect the paucity of cell attachment receptors such as CD44 and/or the inclusion of small apoptotic cells.
Cell characterization. Although multiple cell lineages, including adipocytes, chondrocytes, and smooth muscle cells were generated when S cells were grown in culture, we focused our studies on the more prominent, osteogenic potential of these cells. When grown on either plastic or collagen substrata, S cells exhibited the sequential expression of phenotypic markers associated with the progressive phases of osteogenesis that have been characterized in unfractionated calvarial cell populations.14,15 Proliferation was the dominant process in the first 48 hours of all cultured subpopulations. As shown in double labeling experiments, >80% of S cells proliferated, but did not exhibit AP activity, which is an early marker of osteogenic differentiation. The major shifts in AP activity and proliferation of S cells over time in culture indicate that some of these cells, which initially did not express OPN or collagen type I, have the ability to rapidly mature and differentiate, as would be expected for osteoprogenitor cells. Notably, there appeared to be an apparently reciprocal relationship between the relative proportions of proliferating L and S subpopulations of FRCC cultures. On day 2 the L subpopulation exhibited the highest proportions of proliferating, AP+ cells whereas the S population contained the lowest proportion of these cells. As cultures approached the mineralization stage, the L population, as well as the parental FRCC population, showed large reductions in the proportions of dual-labeled cells while the S population exhibited very high proportions of dual-labeled cells (60%). However, at the time of bone nodule mineralization, all subpopulations exhibited increased proliferation, indicating that bone nodule-forming cells might undergo clonal expansion during bone nodule formation. In support of this concept a quiescent cell population has been shown to proliferate extensively at this phase of culture,22 while clonal expansion has been observed at a comparable stage of adipocyte development.35
Whereas OPN, type I, II, and III collagens and AP activity were initially absent in suspensions of sorted S cells, these proteins were rapidly induced on plating. Indeed within 24 hours, there was strong staining for types I and II collagen and OPN. Thus, attachment and spreading at low plating density promoted the differentiation of S−-cell subpopulation progenitors into various lineages of which osteogenesis appears prominent. In this context the use of monoclonal antibodies to detect differentiation stage-specific markers has been shown to be a useful approach for isolating the progeny of stem cells in hematopoietic and epithelial tissues. However, in bone cell lineages, restriction points have been determined only for cells that are already committed.21,36 Thus, the use of known early expression markers by osteoblast lineage cells20 enables isolation of osteoprogenitors but not of stem cells.2 Identification of novel early proteins in this lineage might be related to the unique properties of the matrix-dependent differentiation process in osteogenesis.
As shown in this study, only S cells (S and L− subpopulations) were able to populate type I collagen-coated dishes and form a significant number of bone nodules. Evidently type I collagen substrata33 drive osteoblast lineage cells to rapid maturation and terminal differentiation which in turn lead to either a lack of bone nodule formation in highly differentiated cells (ie, the L subpopulation) or a limited number of bone nodules in more heterogenous cells (ie, the S− subpopulation).
Diversity of osteoprogenitors. It has been suggested that at low plating densities bone nodules are produced from cells, which arise from a single osteoprogenitor.18 To estimate the relative proportions of osteoprogenitors in our sorted populations, we used a limiting dilution analysis. The estimated proportion of progenitors in the S cultures (1:202; or 1:120 if corrected for cell viability) was threefold higher than the parental, FRCC population. However, as differentiation of multiple lineages evidently occurs in the S− cell subpopulation, the number of stem cells in the S subpopulation is likely to be much higher than that indicated simply by the proportion of osteogenic progenitors.
Previous studies have indicated that osteoprogenitor cells in fetal rat calvarial cultures are alkaline phosphatase positive and have limited self-renewal capacity,20 characteristics that are consistent with the L− cells described in our report. However the S− cells also generate bone nodules albeit at a much higher frequency and unlike L cells, are capable of extensive self-renewal. Further, the nodules produced by the S cells are 10-fold larger than the L cells, indicating that the osteogenic, transit amplifying cells produced by the S cells undergo at least 3 more cell divisions than the progeny of L cells. Thus, when nodule formation, nodule size, and self-renewal capacity are used as the criteria for osteoprogenitor cells, it is evident that there are different classes of progenitor cells which can ultimately produce bone-forming cells. Further, the wide phenotypic differences between the sorted L and S− cell populations suggest the existence of very different types of subpopulations. Notably, stem cells alter their behavior markedly when their environment or compartment size is altered.2 Consistent with this prediction and as discussed above, we observed dramatic phenotypic changes of S− cells within hours after plating. These alterations were not observed in L− cells, indicating that at least in culture, the progenitors in the L and S− cell populations behave very differently.
Stromal progenitor cells. Collectively, our studies have characterized a relatively undifferentiated cell population derived from fetal rat periosteum. The features of small size, low granularity, low cytoplasmic to nuclear ratio and undetectable expression of osteopontin, collagens, and alkaline phosphatase activity are consistent with the expectations of a mesenchymal stem cell (Table 2). Further, on plating, these cells proliferated rapidly and generated new self-renewing cells as well as transit amplifying cells (lineage directed cells) that expressed early lineage markers, such as collagen I, AP, and OPN for the osteogenic lineage. Further differentiation of these cells was promoted by the formation of a collagen matrix beneath the cells, consistent with the observation that tissue nodules are formed following the production of an extracellular matrix by fully differentiated cells.13 At this stage it is conceivable that the size of the tissue nodule is also increased through the clonal expansion of osteoblast precursors. During the differentiation process, a large number of cells at different stages of differentiation are produced: the more highly differentiated cells (ie, the L population) having a more limited proliferative capacity. If this paradigm of cellular differentiation in vitro parallels the normal processes occurring during the maintenance and repair of stromal tissues in vivo, then it might be expected that the heterogeneity of cell populations isolated from stromal tissues19 is a consequence of progressive differentiation and one in which the number of stem cells is retained at low levels.
Comparisons of S and L Populations Using Stem Cell Criteria
| . | Sorted S . | Sorted L . | Plated S . | Plated L . |
|---|---|---|---|---|
| Cytology | ||||
| Protein cell content | + | ++++ | ||
| Cell size | + | ++++ | ||
| Cytoplasmic granularity | + | ++++ | ||
| Nuclear/cytoplasm ratio | High | Low | ||
| Proliferation | ||||
| S-phase cells | 80% | 37% | ||
| Cell cycle | G1/G0 | All stages | All stages | All stages |
| Double labeled 3H/AP | 3% | 25% | ||
| Substrate: | ||||
| Plastic dishes | ++++ | ++ | ||
| Collagen-I coated dishes | ++++ | − | ||
| Differentiation assays | ||||
| No. bone nodules/field | 20 | 1.5 | ||
| Area of bone nodules/mm2 | 11.5 | 1.61 | ||
| Substrate: | ||||
| Bone nodules on collagen dishes | ++++ | − | ||
| Bone nodules on plastic | ++++ | + | ||
| Osteoprogenitor frequency | 1:202 | 1:1750 | ||
| Alkaline phosphatase | − | + (8%) | ++ (40%) | |
| Self-renewal | ||||
| 2nd passage sorting purity | 43% | 0% | ||
| No. bone nodules/field | 5.5 | 0.00 | ||
| Pluripotentiality | ||||
| Bone | ++++ | + | ||
| Cartilage | +++ | +/− | ||
| Adipocytes | ++ | + |
| . | Sorted S . | Sorted L . | Plated S . | Plated L . |
|---|---|---|---|---|
| Cytology | ||||
| Protein cell content | + | ++++ | ||
| Cell size | + | ++++ | ||
| Cytoplasmic granularity | + | ++++ | ||
| Nuclear/cytoplasm ratio | High | Low | ||
| Proliferation | ||||
| S-phase cells | 80% | 37% | ||
| Cell cycle | G1/G0 | All stages | All stages | All stages |
| Double labeled 3H/AP | 3% | 25% | ||
| Substrate: | ||||
| Plastic dishes | ++++ | ++ | ||
| Collagen-I coated dishes | ++++ | − | ||
| Differentiation assays | ||||
| No. bone nodules/field | 20 | 1.5 | ||
| Area of bone nodules/mm2 | 11.5 | 1.61 | ||
| Substrate: | ||||
| Bone nodules on collagen dishes | ++++ | − | ||
| Bone nodules on plastic | ++++ | + | ||
| Osteoprogenitor frequency | 1:202 | 1:1750 | ||
| Alkaline phosphatase | − | + (8%) | ++ (40%) | |
| Self-renewal | ||||
| 2nd passage sorting purity | 43% | 0% | ||
| No. bone nodules/field | 5.5 | 0.00 | ||
| Pluripotentiality | ||||
| Bone | ++++ | + | ||
| Cartilage | +++ | +/− | ||
| Adipocytes | ++ | + |
Characterization of S- and L-cell subpopulations including previous data22 and in this study. For each parameter, intensity or level of expression is described: − none, + low, ++ medium, +++ high, ++++ very high; () indicates percentage of cells expressing the specific phenotypic marker. For measures of proliferation, proportion of S-phase and double-labeled cells are expressed as percentages. Numbers and area of bone nodules reported per microscopic field. Osteoprogenitor frequencies were computed from limiting dilution analyses and are expressed as prevalence of progenitors in whole cell population that are capable of forming bone nodules. In criteria for self-renewal, sorting purity was calculated from the proportion of S− cells that exhibited the same scatter characteristics as the initial sort. Collectively, the data show that S− cells are enriched for stem cell markers as described in reference 2.
The application of the flow cytometry sorting method described here for the enrichment of stem cells provides a simple, reproducible approach to help delineate the stages of bone cell differentiation. Further, the ability of flow cytometry to enrich for stem cells has significant potential for enhancing clinical procedures aimed at regenerating stromal tissues, particularly in the elderly where the numbers of stem cells are believed to be low.
ACKNOWLEDGMENT
The expert assistance of Dr Harry H.K. Moe and Wilson Lee and the advice of Dr S. Cheifetz (University of Toronto, Toronto, Canada) in these studies is gratefully appreciated. We appreciate the suggestions of Dr Russell Taichman (University of Michigan, Ann Arbor, MI) for his help with the hematopoietic cell growth experiments.
Supported by a Harron Fellowship (to R.Z.), and the studies were funded by an MRC of Canada Group Grant (to C.M. and J.S.)
Address reprint requests to Ron Zohar, DMD, 4384 Medical Sciences Bldg, University of Toronto, 8 Taddle Creek Rd, Toronto, Ontario, Canada, M5S 1A8.


![Fig. 2. Analysis of cell proliferation and alkaline phosphatase expression. (A) The proportion of 3H-thymidine–labeled cells at different stages of cellular differentiation was determined (mean labeling index ± SEM) after a 3-hour pulse of 3H-thymidine to detect proliferating cells. (B) The proportion of cells expressing alkaline phosphatase (AP index) at the same time points analyzed for cellular proliferation was determined by staining cells with Naphthol AS Phosphate substrate containing Fast Blue BB salts and the proportion of blue stained cells was counted (mean index ± SEM). (C) Proportion of cells exhibiting both labeling for [3H]-thymidine and AP staining (mean ± SEM).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/9/10.1182_blood.v90.9.3471/4/m_bl_0048f2.jpeg?Expires=1764957341&Signature=RYkWb~FzhuVRKvYBKdx0dJBsKgxKagmqXrSqubPQG6UM8FdNLlfD6tF198tIslHDWW4QVq35zUbqkRR1Dd05d0m~LtInzZkFEVSZYXMVOJiZwr26jjIP60IGJvSQWEp4GoVng7cKQzbK4xq-qdhMSeAB~ebXxDlqa4wVNVTadR40N1HhT0FjlaCZIYd6Jr8d~dPvu8YSS7gLc7jqIDqW1W~C0tFHvNXCRtjYVLL8jT6I-mKTXjnr3~SS54EFIB1w06VdS4ig~eXYbKAp8UGGmvoB8Hzyi-GqcDxhRliFyR9UvO8W-REFMAUjPS~8S6XnAj1NcY8w0BhZyiJNv0WdNg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal