Abstract
Defects in immune response are often reported in patients with multiple myeloma (MM). Because dendritic cells (DCs) are key effectors in promoting cellular immunity and are potential vectors for immunotherapy, we have evaluated the ability of MM patients' apheresis cells to generate DCs in short-term cultures. We report here the obtaining of a virtually pure population of DCs (89.7% ± 6%, n = 18) after culturing adherent apheresis cells for 7 days with granulocyte-macrophage colony-stimulating factor (GM-CSF ) and interleukin-4 (IL-4). These cells exhibited all the phenotypic characteristics (CD1a+, HLA-DR+, CD80+, CD40+, CD14−) and the MLR stimulating capacity of mature DCs. The number of DCs reached 12.1% of the initial apheresis cell number put into culture. As DC precursors involved in this model were CD34− cells, the unabsorbed cells resulting from clinical-grade CD34 purification were a reliable source of DCs, even after freezing. The proliferation of DC precursors could be increased 10-fold by adding IL-3 and tumor necrosis factor-α together with GM-CSF and IL-4. Thus, CD34− apheresis cells from patients with MM offer an interesting source for generating pure, functional, and potentially proliferating DCs.
DENDRITIC CELLS (DCs) are able, in vivo, to capture, process, and present foreign antigenic peptides to T lymphocytes.1 They are the most potent professional antigen presenting cells (APCs) to initiate immune response owing to their widespread localization in all sites of antigen entry, their high expression of immunomodulatory molecules necessary for T-cell activation, and their production of cytokines, especially interleukin-12 (IL-12), which induces the development of Th1 cellular response.2-4 Several studies have shown that DCs are powerful vectors for antitumoral immunotherapy strategies in animal and human models.5-8 Strikingly, the first clinical trial using DCs in human B-cell lymphoma shows the efficiency and the safety of this approach.8 DCs could therefore constitute an attractive alternative in all malignancies that remain incurable by conventional therapies.9 Multiple myeloma (MM), a B-cell neoplasia with very poor prognosis, might be a good model because of some already identified tumoral antigens, such as monoclonal Ig or Mucin-1 (MUC-1) glycopeptide.10
Several steps in the maturation and function of DCs have been identified. Immature DCs, such as epidermal Langerhans cells (LC), pick up antigens with great efficiency.11-13 These cells mature and migrate to the secondary lymphoid organs where they express high levels of major histocompatability complex (MHC) class II, prime naive T lymphocytes14-17 and stimulate memory cells. In vitro, several cytokines and activation molecules modulate DC maturation. In particular, tumor necrosis factor-α (TNF-α) induces an irreversible downregulation of DC antigen-presentation capacity.11 In contrast, DCs cultured for a long time with granulocyte-macrophage colony-stimulating factor (GM-CSF ) and interleukin-4 (IL-4) retain their ability to internalize soluble particles, to cleave them in peptides, and to present peptide-HLA complexes.11
One of the problems for a clinical use of DCs is their very low frequency in accessible body samples, especially in blood. The medullary origin of DCs has been strengthened by the recent obtention of dendritic cell colony-forming units (CFU-DC) from CD34+ bone marrow (BM) cells in vitro.18 Therefore, several groups have developed methodological approaches to generate DCs by using CD34+ hematopoietic progenitor cells purified from BM,19,20 peripheral blood (PB),21-23 or neonatal cord blood.24 25 Stimulation of these cells with GM-CSF and TNF-α allows the generation of a heterogeneous cell population including a variable percentage of well-defined DCs (CD1a+, CD14−, MHC class II++) that are potent T-lymphocyte activators. Addition of IL-4 increases the percentage of CD1a+ cells while stem cell factor (SCF ) and flt3/flk2 ligand synergize with GM-CSF and IL-4 to enhance the overall cell yield by expanding CD34+ cell myeloid progeny.
However, it is well known that DCs can be obtained from adherent cells already committed to the monocytic lineage.26 27 Therefore, we have established a simple, efficient, and rapid method to generate, in the presence of GM-CSF and IL-4 and without any additional purification step, pure and functional dendritic cells, able to endocyte antigens via their mannose receptors, to activate allogenic T lymphocytes and to stimulate autologous T cells in response to recall antigens. IL-3, in combination with TNF-α, can enhance DC proliferation and count. We show that the dendritic precursors involved in this model are CD34−. In particular, they are still present in nonabsorbed cells resulting from clinical-grade procedures of CD34+ cell purification, making these nonabsorbed cells a suitable source for DC generation.
MATERIALS AND METHODS
Patients and leukapheresis collection protocol. Seventeen patients with MM (median age, 59 years) and 1 patient with primary plasma cell leukemia (PCL, patient 9) were evaluated in this study. According to the Durie-Salmon classification, 3 patients were of stage I, 2 of stage II, 7 of stage IIIA, and 5 of stage IIIB. Seven patients had IgGκ MM, 5 IgGλ MM, 2 IgAκ MM, 1 IgAλ MM, 1 Bence-Jones κ MM, and 2 Bence-Jones λ MM.
Reproducible Generation of CD1a+ DCs From Apheresis Cells From Patients With MM Mobilized by High-Dose Cyclophosphamide and G-CSF
| Patient . | % of CD1a+ Cells . | DC Yield (% of input cells) . |
|---|---|---|
| 1 | 91 | 16 |
| 2 | 87 | 9 |
| 3 | 96 | 10 |
| 4 | 98 | 12 |
| 5 | 86 | 10 |
| 6 | 97 | 10 |
| 7 | 81 | 20 |
| 8 | 88 | 13 |
| 9 | 85 | 14 |
| 10 | 93 | 11 |
| 11 | 80 | 14 |
| 12 | 87 | 10 |
| 13 | 83 | 12 |
| 14 | 98 | 12 |
| 15 | 97 | 10 |
| 16 | 85 | 13 |
| 17 | 95 | 11 |
| 18 | 88 | 11 |
| Mean ± SD | 89.7 ± 6 | 12.1 ± 2.7 |
| Range | 80-98 | 9-20 |
| Patient . | % of CD1a+ Cells . | DC Yield (% of input cells) . |
|---|---|---|
| 1 | 91 | 16 |
| 2 | 87 | 9 |
| 3 | 96 | 10 |
| 4 | 98 | 12 |
| 5 | 86 | 10 |
| 6 | 97 | 10 |
| 7 | 81 | 20 |
| 8 | 88 | 13 |
| 9 | 85 | 14 |
| 10 | 93 | 11 |
| 11 | 80 | 14 |
| 12 | 87 | 10 |
| 13 | 83 | 12 |
| 14 | 98 | 12 |
| 15 | 97 | 10 |
| 16 | 85 | 13 |
| 17 | 95 | 11 |
| 18 | 88 | 11 |
| Mean ± SD | 89.7 ± 6 | 12.1 ± 2.7 |
| Range | 80-98 | 9-20 |
Apheresis cells were cultured with GM-CSF and IL-4 as described in Materials and Methods. After 7 days, cells were obtained, counted, and CD1a expression was determined by flow cytometry. The cell viability was always superior to 90%. The DC yield is the ratio of the number of CD1a+DCs recovered compared with the number of apheresis cells put into culture.
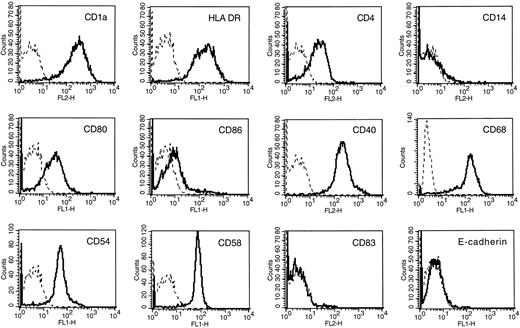
Dendritic cell phenotype of cultured adherent AC. Flow cytometric analysis was performed after 7 days of culture in the presence of GM-CSF and IL-4. Isotype-matched control murine MoAbs were used as negative controls (dotted lines). Fluorescence profiles were obtained on a FACScan apparatus.
Dendritic cell phenotype of cultured adherent AC. Flow cytometric analysis was performed after 7 days of culture in the presence of GM-CSF and IL-4. Isotype-matched control murine MoAbs were used as negative controls (dotted lines). Fluorescence profiles were obtained on a FACScan apparatus.
After written informed consent, patients were treated with high-dose cyclophosphamide (3 g/m2) and human recombinant granulocyte colony-stimulating-factor (rhuG-CSF, filgrastim; NEUPOGEN, Amgen-Roche, Neuilly sur Seine, France; daily injection of 5 μg/kg) to mobilize CD34+ cells in the peripheral blood. PB mononuclear cells (PBMC) were collected during a 5-hour apheresis.
Cell purification procedures. The ability to generate DCs was assayed in four cell subpopulations: apheresis cells (AC), CD34+ cells (CD34+ AC), ACs nonabsorbed on CD34 columns (NA-AC), and ACs completely devoid of CD34 cells (CD34− AC). ACs were obtained from all patients by standard centrifugation of ACs on Ficoll-Hypaque (Biowittaker, Walkersville, MD). They were used immediately or frozen at −80°C in 4% of human serum albumin and 10% of dimethyl sulfoxide (DMSO). CD34+ ACs were purified by the clinical-grade methodology from Baxter (Isolex 300; Baxter International Inc, Deerfield, IL) according to the manufacturer's instructions. In this methodology, CD34+ cells were labeled with an anti-CD34 monoclonal antibody (MoAb) and then rosetted with Dynal magnetic beads coated with sheep-antimouse Ig (Dynal, Oslo, Norway). Rosetted cells were retained on a magnet and the magnetic beads and anti-CD34 MoAb were further released by incubation with a peptide recognizing the same CD34 epitope as the anti-CD34 MoAb. This methodology yielded a highly pure population of CD34+ cells (95.2% ± 2.3%, see Table 2). Generally, few tumoral plasmocytes were present in ACs (<1%) except in patients with PCL. This contamination can be monitored by staining ACs with anti syndecan-1 MoAb which specifically recognizes myeloma cells in tumoral samples.28 29 CD34 purification allowed a depletion of these tumoral cells which represented then less than 0.001%. Cells (NA-AC) that were not retained on the magnet still contained some CD34+ cells. To assay the ability of ACs completely devoid of CD34+ cells (CD34− AC) to generate DCs, we used a small-scale purification system with Dynal magnetic beads. Magnetic beads were used at a ratio of 10 beads to 1 target cell and the cells were exposed twice to the magnet to remove all CD34+ cells (see Table 2). For each population, the cell viability was greater than 95% as determined by trypan blue exclusion and the percentage of CD34+ cells was analyzed by direct flow cytometry using phycoerythrin (PE)-conjugated CD34 MoAb (Immunotech, Marseille, France) on a FACScan apparatus (Becton Dickinson, San Jose, CA).
CD34+ Percentages in Primary Cells and CD1a+ Percentages in Cultured Cells for the Four Cell Subpopulations Used as DC Precursors
| Patient . | Starting Cell Population . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Apheresis Cells . | CD34 Depleted Cells . | Nonabsorbed Cells . | CD34 Purified Cells . | . | . | . | . | . | ||||||||
| . | CD34+ Cells in Primary Cells (%) . | CD1a+ Cells in Cultured Cells (%) . | CD1a+ DC Yield (% of input cells) . | CD34+ Cells in Primary Cells (%) . | CD1a+ Cells in Cultured Cells (%) . | CD1a+ DC Yield (% of input cells) . | CD34+ Cells in Primary Cells (%) . | CD1a+ Cells in Cultured Cells (%) . | CD1a+ DC Yield (% of input cells) . | CD34+ Cells in Primary Cells (%) . | CD1a+ Cells in Cultured Cells (%) . | CD1a+ DC Yield (% of input cells) . | . | . | . | . | . |
| 8 | 2.0 | 87.8 | 13 | 0 | 90.1 | 11 | 1.3 | 86.8 | 14 | 96.4 | 47.9 | 24.4 | |||||
| 9 | 3.3 | 84.8 | 14 | 0.07 | 84.7 | 10 | 0.9 | 81.4 | 14 | 96.8 | 28.1 | 16.3 | |||||
| 11 | 5.0 | 79.7 | 14 | 0.08 | 80.3 | 12 | 1.2 | 80.6 | 13 | 96.8 | 53.3 | 28.8 | |||||
| 12 | 1.9 | 87 | 10 | 0.05 | 86.5 | 11 | 1 | 84.2 | 10 | 94.4 | 36.2 | 12.3 | |||||
| 13 | 3.8 | 82.6 | 12 | 0 | 79.5 | 11 | 3.3 | 79.9 | 12 | 91.5 | 43.6 | 29.7 | |||||
| Mean ± SD | 3.2 ± 1.3 | 84.4 ± 3.3 | 12.6 ± 1.7 | 0.04 ± 0.04 | 84.2 ± 4.4 | 11.0 ± 0.7 | 1.5 ± 1.0 | 82.6 ± 2.9 | 12.6 ± 1.7 | 95.2 ± 2.3 | 41.8 ± 9.9 | 22.3 ± 7.7 | |||||
| Patient . | Starting Cell Population . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Apheresis Cells . | CD34 Depleted Cells . | Nonabsorbed Cells . | CD34 Purified Cells . | . | . | . | . | . | ||||||||
| . | CD34+ Cells in Primary Cells (%) . | CD1a+ Cells in Cultured Cells (%) . | CD1a+ DC Yield (% of input cells) . | CD34+ Cells in Primary Cells (%) . | CD1a+ Cells in Cultured Cells (%) . | CD1a+ DC Yield (% of input cells) . | CD34+ Cells in Primary Cells (%) . | CD1a+ Cells in Cultured Cells (%) . | CD1a+ DC Yield (% of input cells) . | CD34+ Cells in Primary Cells (%) . | CD1a+ Cells in Cultured Cells (%) . | CD1a+ DC Yield (% of input cells) . | . | . | . | . | . |
| 8 | 2.0 | 87.8 | 13 | 0 | 90.1 | 11 | 1.3 | 86.8 | 14 | 96.4 | 47.9 | 24.4 | |||||
| 9 | 3.3 | 84.8 | 14 | 0.07 | 84.7 | 10 | 0.9 | 81.4 | 14 | 96.8 | 28.1 | 16.3 | |||||
| 11 | 5.0 | 79.7 | 14 | 0.08 | 80.3 | 12 | 1.2 | 80.6 | 13 | 96.8 | 53.3 | 28.8 | |||||
| 12 | 1.9 | 87 | 10 | 0.05 | 86.5 | 11 | 1 | 84.2 | 10 | 94.4 | 36.2 | 12.3 | |||||
| 13 | 3.8 | 82.6 | 12 | 0 | 79.5 | 11 | 3.3 | 79.9 | 12 | 91.5 | 43.6 | 29.7 | |||||
| Mean ± SD | 3.2 ± 1.3 | 84.4 ± 3.3 | 12.6 ± 1.7 | 0.04 ± 0.04 | 84.2 ± 4.4 | 11.0 ± 0.7 | 1.5 ± 1.0 | 82.6 ± 2.9 | 12.6 ± 1.7 | 95.2 ± 2.3 | 41.8 ± 9.9 | 22.3 ± 7.7 | |||||
For AC, CD34 depleted AC and NA-AC, cells were cultured for 7 days in the presence of GM-CSF and IL-4. CD34+ cells were culutred for 14 days with GM-CSF, TNF-α, SCF, and IL-4. Cultured cells were stained with PE-conjugated anti-CD1a MoAb or PE-conjugated control IgG1 MoAb. The percentage of CD1a+ cells and the DC yield in the different culture groups were compared with a t-test for pairs.
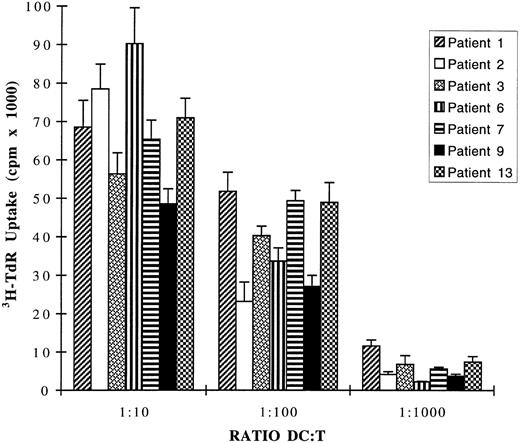
CD1a+ DCs obtained from leukapheresis of seven patients strongly stimulate allogenic T-lymphocyte proliferation. DCs were generated by 7 days of culture of ACs from 7 MM patients in the presence of GM-CSF and IL-4. These DCs were washed three times, irradiated at 30 Gy, and used in MLR reaction as stimulator cells for 1.5 × 105 allogenic T cells from healthy volunteers. Cell proliferation was evaluated by a 12-hour pulse with 3H-TdR after 5-day cocultures. The MLR obtained with DCs from the patient with PCL (patient 9) is shown by the black histograms. Data are expressed as mean ± SD of sextuplet independent cultures. 3H-TdR incorporation rates of irradiated DCs or purified T cells were less than 600 cpm.
CD1a+ DCs obtained from leukapheresis of seven patients strongly stimulate allogenic T-lymphocyte proliferation. DCs were generated by 7 days of culture of ACs from 7 MM patients in the presence of GM-CSF and IL-4. These DCs were washed three times, irradiated at 30 Gy, and used in MLR reaction as stimulator cells for 1.5 × 105 allogenic T cells from healthy volunteers. Cell proliferation was evaluated by a 12-hour pulse with 3H-TdR after 5-day cocultures. The MLR obtained with DCs from the patient with PCL (patient 9) is shown by the black histograms. Data are expressed as mean ± SD of sextuplet independent cultures. 3H-TdR incorporation rates of irradiated DCs or purified T cells were less than 600 cpm.
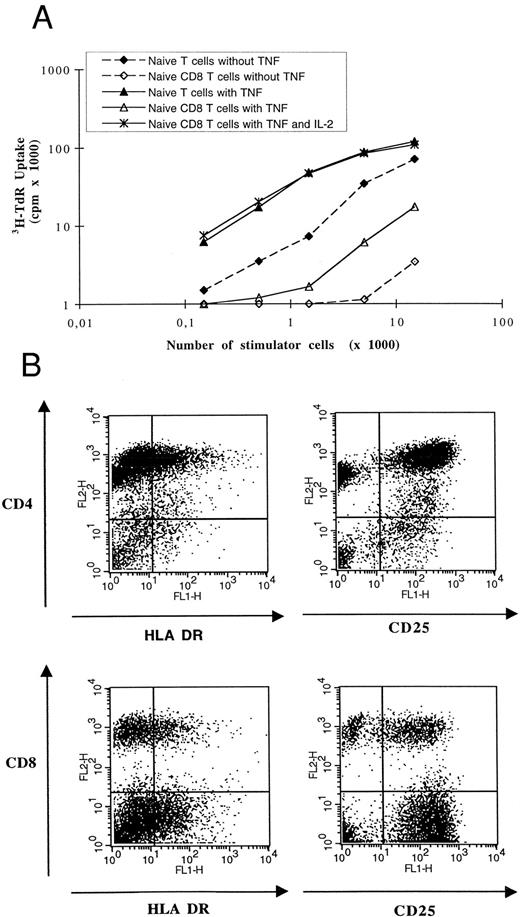
AC-derived DCs induced activation and proliferation of naive allogenic T cells. (A) Graded numbers of irradiated DCs cultured with or without TNF-α were used as stimulator cells for 105 allogenic CD45RA+CD3+ T cells or 105 allogenic CD45RA+CD8+CD3+ T cells. IL-2 was added at 50 U/mL when indicated. Cell proliferation was evaluated by a 12-hour pulse with 3H-TdR. 3H-TdR incorporation of purified naive T cells ± IL-2 were substracted. Results are representative of four experiments using DCs and T cells from two distinct donors. (B) After 5 days of coculture of 104 TNF-α–treated DCs and 105 allogenic CD45RA+ CD3+ naive T lymphocytes, cells were obtained and stained with PE-CD4 or PE-CD8 MoAbs and FITC–HLA-DR or FITC-CD25 MoAbs. Negative controls were obtained with irrelevant isotype-matched murine MoAbs. No viable CD1a+ DC was detectable at this time. At the beginning of coculture, less than 1% of T cells expressed HLA-DR and less than 3% expressed CD25.
AC-derived DCs induced activation and proliferation of naive allogenic T cells. (A) Graded numbers of irradiated DCs cultured with or without TNF-α were used as stimulator cells for 105 allogenic CD45RA+CD3+ T cells or 105 allogenic CD45RA+CD8+CD3+ T cells. IL-2 was added at 50 U/mL when indicated. Cell proliferation was evaluated by a 12-hour pulse with 3H-TdR. 3H-TdR incorporation of purified naive T cells ± IL-2 were substracted. Results are representative of four experiments using DCs and T cells from two distinct donors. (B) After 5 days of coculture of 104 TNF-α–treated DCs and 105 allogenic CD45RA+ CD3+ naive T lymphocytes, cells were obtained and stained with PE-CD4 or PE-CD8 MoAbs and FITC–HLA-DR or FITC-CD25 MoAbs. Negative controls were obtained with irrelevant isotype-matched murine MoAbs. No viable CD1a+ DC was detectable at this time. At the beginning of coculture, less than 1% of T cells expressed HLA-DR and less than 3% expressed CD25.
Presentation of TT to autologous T cells. DCs generated in the presence of GM-CSF and IL-4 were cultured at 104 cells/well with 105 autologous highly purified T cells. The cultures were set up in the presence of increasing amounts of TT and T-cell proliferation was monitored by 3H-TdR uptake on day 6. Backgrounds caused by autologous MLR were substracted. The data are representative of two experiments performed with DCs and T cells from two patients with MM.
Presentation of TT to autologous T cells. DCs generated in the presence of GM-CSF and IL-4 were cultured at 104 cells/well with 105 autologous highly purified T cells. The cultures were set up in the presence of increasing amounts of TT and T-cell proliferation was monitored by 3H-TdR uptake on day 6. Backgrounds caused by autologous MLR were substracted. The data are representative of two experiments performed with DCs and T cells from two patients with MM.
FITC-dextran endocytosis by AC-derived and CD34+AC-derived DCs. DCs obtained from ACs in the presence of GM-CSF and IL-4 without (A) or with (B) TNF-α or from CD34+ progenitors in the presence of GM-CSF, IL-4, TNF-α and SCF (C) were incubated for various lengths of time in medium containing 1 mg/mL of FITC-dextran and analyzed on a FACScan apparatus after extensive washings. Solid lines, background uptake at 4°C; dotted lines, 7 minutes at 37°C, broken lines, 15 minutes at 37°C; bold lines, 30 minutes at 37°C. The data are representative of four experiments.
FITC-dextran endocytosis by AC-derived and CD34+AC-derived DCs. DCs obtained from ACs in the presence of GM-CSF and IL-4 without (A) or with (B) TNF-α or from CD34+ progenitors in the presence of GM-CSF, IL-4, TNF-α and SCF (C) were incubated for various lengths of time in medium containing 1 mg/mL of FITC-dextran and analyzed on a FACScan apparatus after extensive washings. Solid lines, background uptake at 4°C; dotted lines, 7 minutes at 37°C, broken lines, 15 minutes at 37°C; bold lines, 30 minutes at 37°C. The data are representative of four experiments.
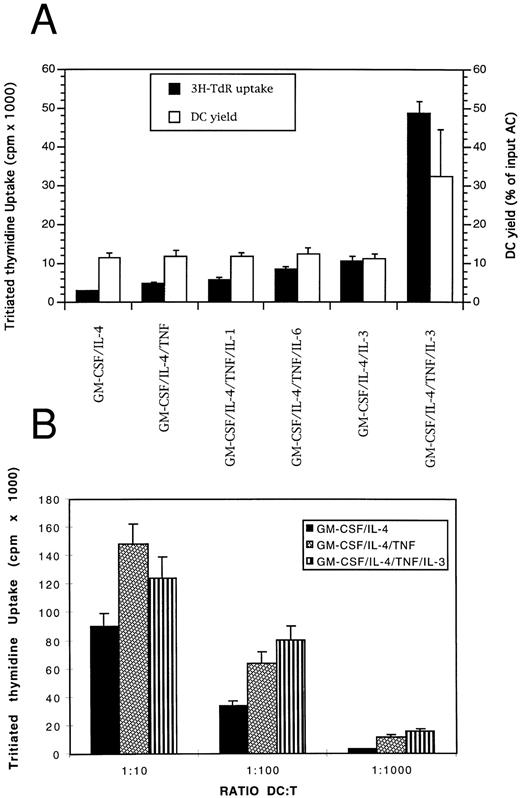
IL-3 and TNF-α enhance DC precursor proliferation and growth without altering their phenotype or their functionality. (A) Adherent ACs were cultured for 4 days in the presence of different cytokine combinations as described in Materials and Methods. Cell proliferation was evaluated by a 12-hour pulse with 3H-TdR and results are expressed as mean ± SD of sextuplet cultures. One representative experiment out of four performed with ACs from four MM patients is shown. The number of DCs generated in the different culture groups was counted and expressed as the percentage of the initial cell number put into culture. Results are the mean ± SD of the DC yields obtained in the four experiments using ACs from the four patients. (B) Graded numbers of DCs were used as stimulator cells for 1.5 × 105 thawed allogenic T cells. 3H-TdR incorporation of irradiated (30 Gy) DCs or purified T cells were less than 800 cpm.
IL-3 and TNF-α enhance DC precursor proliferation and growth without altering their phenotype or their functionality. (A) Adherent ACs were cultured for 4 days in the presence of different cytokine combinations as described in Materials and Methods. Cell proliferation was evaluated by a 12-hour pulse with 3H-TdR and results are expressed as mean ± SD of sextuplet cultures. One representative experiment out of four performed with ACs from four MM patients is shown. The number of DCs generated in the different culture groups was counted and expressed as the percentage of the initial cell number put into culture. Results are the mean ± SD of the DC yields obtained in the four experiments using ACs from the four patients. (B) Graded numbers of DCs were used as stimulator cells for 1.5 × 105 thawed allogenic T cells. 3H-TdR incorporation of irradiated (30 Gy) DCs or purified T cells were less than 800 cpm.
Generation of DCs from adherent cells. Six million AC, NA-AC, or CD34− ACs were plated in 2 mL of RPMI 1640 supplemented with 2 mmol/L of L glutamine and 10% heat-inactivated fetal calf serum (FCS) in each well of 6-well flat-bottomed plates (Nunc, Roskilde, Denmark). Nonadherent cells were discarded by gentle rinsing after 2 hours incubation at 37°C in 5% CO2 . Adherent cells were cultured in RPMI 1640-10% FCS with 1,000 U/mL of rhGM-CSF (specific activity: 107 U/mg; LEUCOMAX, Sandoz, Basel, Switzerland) and 500 U/mL of rhIL-4 (specific activity, 2 × 107 U/mg; Genzyme, Cambridge, MA). When indicated, rhTNF-α (specific activity, 2 × 107 U/mg; R & D Systems, Minneapolis, MN) was used at 10 ng/mL. Cultures were maintained at 37°C in 5% CO2 by replacing culture medium and cytokines every 4 days for a maximum of 5 weeks.
For cell proliferation assays, adherent ACs were cultured in the presence of rhGM-CSF, rhIL-4, and various combinations of rhTNF-α and/or rhIL-6 (Ares Serono, Geneva, Switzerland) at 3 ng/mL, rhIL-1α (specific activity 108 U/mL; R & D Systems), rhIL-3 (specific activity, 106 U/mL; R & D Systems) at 10 U/mL, rhSCF (specific activity, 2 × 105 U/mg; R & D Systems) at 50 ng/mL. At day 4, cells were obtained, seeded in 96-well round-bottomed microplates (Nunc), and pulsed for 12 hours with 1 μCi of tritiated thymidine (specific activity, 25 Ci/mmol; Amersham, Buckingham, UK). Results were expressed as mean cpm ± SD determined in sextuplet culture wells.
The development of DCs was monitored by the detection of large, stellate cells with typical motile veils in phase contrast microscopy. After 7 days, no more cell proliferation occurred and the cell viability determined by trypan blue exclusion was always higher than 90%. The percentage of DCs was determined by a phenotypic analysis made on day 7. After saturation of the nonspecific binding sites with 30% human pooled AB serum (SAB), cells were labeled with the following MoAbs: CD1a, CD14, CD4, CD83, and CD40 antibodies conjugated with PE; CD54, CD58, CD80 (B7-1) and HLA-DR antibodies conjugated with fluorescein (FITC) and noncoupled anti–E-cadherin (Immunotech). FITC-anti CD86 (B7-2) was purchased from Pharmingen (San Diego, CA) and intracellular staining for CD68 was performed using EBM11 MoAb (Dako, Glostrup, Denmark) and Fix and Perm kit (Caltag Laboratories, Burlingame, CA). To confirm the absence of contaminant tumoral cells in the final DC population, we used the FITC-conjugated anti-syndecan-1 MoAb (MI15 MoAb). Negative controls were done with corresponding irrelevant-matched murine MoAbs.
Cell-cycle analysis. Cell-cycle analysis was performed on AC-derived DCs and on CD34− AC-derived DCs cultured either in the presence of GM-CSF and IL-4 or with GM-CSF, IL-4, TNF-α and IL-3 by propidium iodide (PI) staining. Briefly, cells were washed in phosphate-buffered saline (PBS), resuspended in 70% ethanol in PBS, and incubated at 4°C overnight. They were then resuspended in PBS supplemented with 100 μg/mL of RNase A (Sigma Chemical Co, St Louis, MO) and 50 μg/mL of PI (Sigma) and incubated at room temperature for 1 hour before flow cytometric analysis on a FACScan apparatus. The percentages of cells in G0/G1, S, and G2/M phases were evaluated by analyzing the data with ModFitLT software (Becton Dickinson).
Generation of DCs from CD34+ AC. Cultures were initiated at 2 × 104 CD34+ AC/mL in 6-well flat-bottomed plates in 5 mL of RPMI-10% FCS supplemented with rhGM-CSF (500 U/mL), TNF-α (20 ng/mL), and SCF (50 ng/mL). IL-4 (500 U/mL) was added when indicated on the first day of culture or only after 1 week. Every 5 days, cultures were split at 105 cells/mL with medium containing fresh cytokines. The cell yield and the membrane marker expression were determined on day 14. To compare the respective potential of ACs and CD34+ ACs to generate DCs in relation to their respective concentration in an apheresis sample, we evaluated the total number of DCs obtained from these two cell populations present in the same count of AC. The yield of DCs generated from ACs could then be calculated as follows:
The yield of DCs generated from CD34+ ACs was determined at the same time as:
Since CD14+ cells were still present on day 14 of culture of CD34+ AC, a double staining was performed using either PE-anti CD1a (Pharmingen) and FITC-anti CD86, FITC-anti CD80, FITC-anti HLA-DR, FITC-anti CD40 or FITC-anti CD1a and PE-anti CD83, PE-anti CD14 MoAbs (Immunotech).
Exposure to human myeloma cell line (HMCL) supernatants. Four HMCL were used: XG-1, XG-4, XG-6, and RPMI 8226. Growth of three of them (XG-1, XG-4, and XG-6) was completely dependent on exogenous IL-6 (3 ng/mL). Their detailed characteristics have been reported elsewere.30 RPMI 8226 (obtained from American Type Culture Collection [ATCC], Rockville, MD) was an IL-6–independent HMCL. Cells were cultured at 106 cells/mL for 3 days and supernatants were then collected, filtered through a 0.2-μm filter, and stored at −80°C.
We performed cultures of adherent ACs and CD34+ cells in the presence of 20% of supernatants added on day 0. The medium was replaced every 4 days with fresh medium containing cytokines and 20% of supernatants. Phenotypic and functional analysis were performed on day 7 or on day 14 of culture as described above.
Allogenic mixed lymphocyte reaction (MLR). PB from two healthy volunteers was collected under EDTA after written informed consent. T lymphocytes were purified either by rosetting with sheep red blood cells (SRBC) or by depletion of non-T cells with MoAbs and magnetic beads. T cells were first purified by two cycles of 1 hour rosetting with 2-aminoethyl-isothiouronium bromide (AET; Sigma Chemical Co)-treated SRBC as previously described.31 After isotonic SRBC lysis, the percentage of CD3+ cells was always greater than 92%. To minimize an activation of T lymphocytes by the SRBC-rosetting, studies on autologous T cells and on allogenic naive T cells were performed on lymphocytes purified by immunomagnetic depletion. Monocytes and B cells were depleted using CD14- and CD19-coated microbeads (Dynal). Then, non-T cells were removed by incubating with a cocktail of MoAbs CD16, CD56, and HLA-DR (Immunotech) and goat-antimouse Ig microbeads. After 2 rounds of purification, the purity of CD3+ cells was always higher than 97%. CD45RA+ CD3+ naive T cells were further purified by removing CD45RO+ memory T cells and CD8+ CD45RA+ CD3+ naive CD8 T cells were purified by removing CD45RO+ and CD4+ cells using anti CD45RO and anti CD4 MoAbs and magnetic beads. The purity of the T-cell subpopulations was greater than 97%. Cells were frozen in 50% FCS-10% DMSO at 20 × 106 cells per vial.
DCs, generated in cultures of 8 to 14 days, were collected, irradiated at 30 Gy, and washed three times to remove exogeneous cytokines. Graded numbers of irradiated DC cells were added to 1.5 × 105 allogenic T cells or to 105 naive (CD45RO depleted) allogenic T cells in 200 μL of RPMI-5% SAB culture medium in 96-well round-bottomed microplates. After 5 days of culture, cells were pulsed with 1 μCi of 3H-thymidine for 12 hours, procured, and counted. Tests were performed in sextuplet and results were expressed as mean cpm ± SD.
To determine which T-cell subpopulation responded to DC stimulation, a double staining was performed at day 5 of coculture of DCs and naive T cells using PE-anti CD4, PE-anti CD8, FITC-anti CD25, and FITC-anti HLA DR MoAbs (Immunotech).
Recall antigen presentation assay. To measure the ability of presentation of a soluble antigen, 104 AC-derived DCs were cultured with 105 highly purified autologous T cells in the presence of increasing amounts of tetanus toxin (TT; List Biological Laboratories Inc, Campbell, CA). Tritiated thymidine uptake was measured on day 6. Background proliferation caused by autologous MLR without TT was substracted.
Study of endocytosis at the cellular level. Capacity of endocytosis through mannose receptor was assessed using lysine-fixable FITC-dextran, molecular weight (MW) = 40,000, purchased from Molecular Probes Inc (Eugene, OR), as described.32 Briefly, AC-derived DCs were collected at day 5 and CD34+AC-derived DCs at day 12 of culture and were incubated at 37°C with 1 mg/mL of FITC-dextran for different times in RPMI 1640-10% FCS-25 mmol/L HEPES. Cells were then washed four times with cold PBS containing 1% FCS and 0.02% NaN3 and were analyzed on a FACScan apparatus. The background uptake was measured by incubating cells at 4°C.
IL-12 measurement. AC were cultured for 7 days with GM-CSF and IL-4 and resulting DCs were plated at 3 × 105/mL in RPMI-10% FCS in the presence of CD40 stimulation. This stimulation was performed using MoAb 89 anti-CD40 antibody and irradiated (40 Gy) murine L cells stably transfected by RFcγII (CD32) (kindly provided by Dr J. Banchereau, Schering-Plough, Dardilly, France). L cells were added at 3 × 104 cells/mL to DCs with 0.5 μg/mL of MoAb 89. Supernatants were collected after 72 hours of coculture and assayed, in serial dilutions in duplicate, for IL-12 concentration using an ELISA kit that detected only the biologically active p75 heterodimer (Genzyme).
Generation of DCs from adherent cells in serum-free medium. AC were treated as described above except that serum-free medium X-VIVO 15 (Biowhittaker, Gagny, France) was used instead of RPMI 1640-10% FCS for adherence and culture. Endocytosis capacity of cultured cells was studied at day 5 and phenotypic analysis and MLR were done at day 7 using the same methodologies as for AC-derived DCs generated in RPMI-10% FCS.
RESULTS
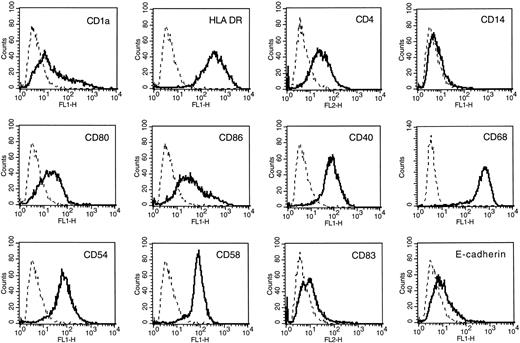
Reproducible obtaining of CD1a+ DCs from MM patients. When adherent ACs were cultured for 3 or 4 days in the presence of GM-CSF and IL-4, cells with typical morphological features of DCs were detected under phase-contrast microscopy. At day 7, the culture contained loosely adherent large cell clusters and isolated cells with cytoplasmic mobile projections. No adherent macrophage-like cells were detectable. Flow cytometric analysis indicated that 89.7% (range, 80% to 98%) of recovered cells were CD1a+ (Table 1) and expressed all surface markers of mature DCs: HLA-DR, CD4, CD80, CD40, and few CD86 (Fig 1). No CD83 or E-cadherin expression were detectable. Treatment of these AC-derived DCs by TNF-α at day 5 increased significantly the level of CD86, HLA-DR, CD80, CD54, and CD58 at day 7 and induced CD83 expression (data not shown). The number of DCs generated on day 7 was 12.1 ± 2.7% of the number of ACs put into culture (Table 1). The cell yield and the purity were significantly lower when starting from apheresis collected without previous mobilization by cyclophosphamide and G-CSF (respectively, 7% ± 1.3% and 69% ± 5%; n = 3). Viability and phenotype of AC-derived DCs could be maintained for 5 weeks by changing culture medium and cytokines twice a week. However, no further increase in cell number was found. We found that addition of culture supernatants (up to 20% of final culture medium) from 4 HMCL (XG-1, XG-4, XG-6, and RPMI 8226) did not modify the phenotype or the MLR capacity of AC-derived DCs even if the DC cell yield was decreased (20% to 30%, data not shown).
These AC-derived DCs were tested for their ability to stimulate T lymphocytes in primary MLR. As shown in Fig 2, CD1a+ DCs obtained from ACs from seven patients in the presence of GM-CSF and IL-4 elicited a vigorous proliferation of allogenic T cells. Several studies have shown that DCs are the only cells able to prime naive T CD4+ cells. Therefore, we evaluated AC-derived DCs from MM patients for their ability to stimulate allogenic naive T cells. In primary MLR, DCs induced proliferation of alloreactive CD45RA+CD3+ cells and this stimulation was enhanced by addition of TNF-α for the last 48 hours of DC cultures (Fig 3A). Because DCs induced only a low proliferation of highly purified alloreactive naive CD8 T cells (CD45RA+CD8+ CD3+), we determined, by double-staining, whether both CD4 and CD8 naive T cells could be stimulated by our DCs (Fig 3B). After 5 days of coculture of CD45RA+CD3+ cells and allogenic AC-derived DCs treated by TNF-α, about 34% naive T CD4+ were HLA-DR+ (22% to 45%, n = 4) and 72% expressed CD25 (62% to 77%, n = 4). In the same cultures, 36% naive T CD8+ cells were HLA-DR+ (21% to 48%, n = 4) and 71% CD25+ (55% to 83%, n = 4). Thus, even if CD8+ naive T cells alone could not proliferate in response to DC stimulation, AC-derived DCs were able to activate naive allogenic T CD4+ and T CD8+ lymphocytes with the same efficiency. One major difference between activated CD4+ and CD8+ T cells is their capacity to produce.33 Therefore, we performed an MLR with AC-derived DCs and CD45RA+CD8+CD3+ T cells in the presence of 50 U/mL of IL-2 and found a CD8 T-cell proliferation similar to that obtained with a mixture of CD4 and CD8 T cells (Fig 3A). We also found that AC-derived DCs could present soluble TT as a recall antigen to autologous purified T cells (Fig 4). Another important feature of DCs is their potential for endocytosis through mannose receptor. Accordingly, AC-derived DCs were very effective in endocyting FITC-dextran (Fig 5A). Endocytosis was dramatically decreased (60% to 80% of inhibition) when TNF-α was added to the culture medium (Fig 5B). Finally, we triggered AC-derived DCs with anti-CD40 MoAb anchored on CD32-transfected cells to evaluate their ability to produce IL-12. Using a specific ELISA assay that detects only the biologically active IL-12 p75 heterodimer, we found that AC-derived DCs, triggered by anti-CD40, produced large amounts of p75 (1.5 to 5 ng/mL, n = 3), compared with basal production of resting DCs (<50 pg/mL).
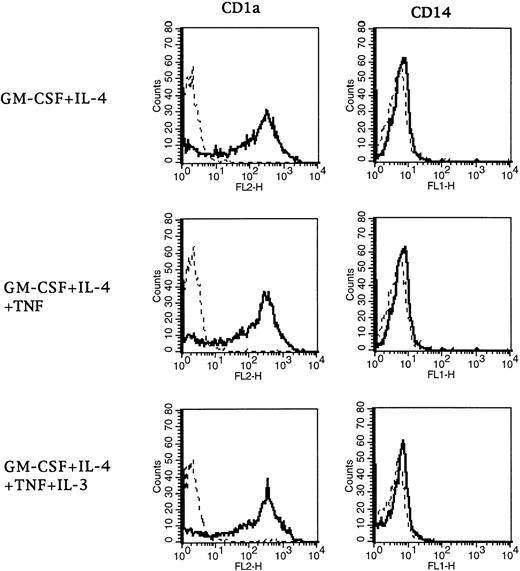
Phenotype of DCs generated with the four-cytokine combination. After 7 days of culture, DCs were stained with CD1a and CD14 MoAbs. Controls (dotted lines) were isotype-matched irrelevant MoAbs.
Phenotype of DCs generated with the four-cytokine combination. After 7 days of culture, DCs were stained with CD1a and CD14 MoAbs. Controls (dotted lines) were isotype-matched irrelevant MoAbs.
Addition of IL-3 and TNF-α Increased the Percentages of Cells in the S and G2/M Phases of Cell Cycle
| . | Analysis of Cell Cycle . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | DCs Obtained With GM-CSF/IL-4 . | DCs Obtained With GM-CSF/IL-4/IL-3/TNF-α . | . | . | . | . | ||||
| . | G0/G1 (%) . | S (%) . | G2/M (%) . | G0/G1 (%) . | S (%) . | G2/M (%) . | . | . | . | . |
| Patient 1 | ||||||||||
| Day 2 | 99.1 | 0.8 | 0.1 | 98.3 | 1 | 0.7 | ||||
| Day 3 | 97.8 | 1.2 | 0.8 | 95.5 | 4 | 0.5 | ||||
| Day 5 | 97.6 | 1.4 | 1 | 94.1 | 4.5 | 1.4 | ||||
| Day 7 | 98.4 | 0.9 | 0.7 | 96.4 | 1.8 | 1.8 | ||||
| Patient 2 | ||||||||||
| Day 2 | 99.4 | 0.6 | 0 | 98.5 | 1.2 | 0.3 | ||||
| Day 3 | 98.2 | 1.1 | 0.7 | 95.4 | 2 | 2.6 | ||||
| Day 5 | 98.5 | 0.8 | 0.7 | 95.5 | 2.9 | 1.6 | ||||
| Day 7 | 99 | 0.6 | 0.4 | 97.9 | 1.1 | 1 | ||||
| Patient 3 | ||||||||||
| Day 2 | 99 | 1 | 0 | 97.1 | 1 | 1.9 | ||||
| Day 3 | 99.5 | 0.2 | 0.3 | 96.5 | 2.2 | 1.3 | ||||
| Day 5 | 99.5 | 0.2 | 0.3 | 93.3 | 5 | 1.7 | ||||
| Day 7 | 99.5 | 0.3 | 0.2 | 98 | 1.5 | 0.5 | ||||
| . | Analysis of Cell Cycle . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | DCs Obtained With GM-CSF/IL-4 . | DCs Obtained With GM-CSF/IL-4/IL-3/TNF-α . | . | . | . | . | ||||
| . | G0/G1 (%) . | S (%) . | G2/M (%) . | G0/G1 (%) . | S (%) . | G2/M (%) . | . | . | . | . |
| Patient 1 | ||||||||||
| Day 2 | 99.1 | 0.8 | 0.1 | 98.3 | 1 | 0.7 | ||||
| Day 3 | 97.8 | 1.2 | 0.8 | 95.5 | 4 | 0.5 | ||||
| Day 5 | 97.6 | 1.4 | 1 | 94.1 | 4.5 | 1.4 | ||||
| Day 7 | 98.4 | 0.9 | 0.7 | 96.4 | 1.8 | 1.8 | ||||
| Patient 2 | ||||||||||
| Day 2 | 99.4 | 0.6 | 0 | 98.5 | 1.2 | 0.3 | ||||
| Day 3 | 98.2 | 1.1 | 0.7 | 95.4 | 2 | 2.6 | ||||
| Day 5 | 98.5 | 0.8 | 0.7 | 95.5 | 2.9 | 1.6 | ||||
| Day 7 | 99 | 0.6 | 0.4 | 97.9 | 1.1 | 1 | ||||
| Patient 3 | ||||||||||
| Day 2 | 99 | 1 | 0 | 97.1 | 1 | 1.9 | ||||
| Day 3 | 99.5 | 0.2 | 0.3 | 96.5 | 2.2 | 1.3 | ||||
| Day 5 | 99.5 | 0.2 | 0.3 | 93.3 | 5 | 1.7 | ||||
| Day 7 | 99.5 | 0.3 | 0.2 | 98 | 1.5 | 0.5 | ||||
CD34 were extensively depleted from AC and CD34− AC were cultured with either GM-CSF and IL-4 or GM-CSF, IL-4, IL-3, and TNF-α. The distribution of the cells in the different phases of the cell cycle was evaluated by PI staining and analysis with ModFitLT software on days 2, 3, 5, and 7 of culture.
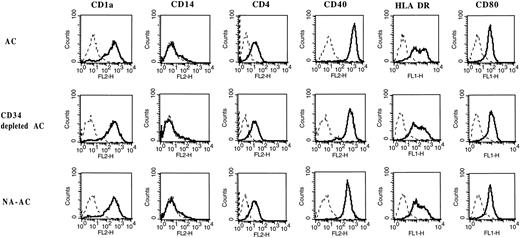
Cell-surface phenotype of cells stained after 7 days of culture with GM-CSF and IL-4. This representative analysis out of five shows no difference between AC, CD34− AC, and NA-AC derived DCs either in lineage (CD4, CD14, CD1a) or in costimulatory antigen (CD40, MHC class II, CD80) expression. Controls (dotted lines) were isotype-matched irrelevant antibodies.
Cell-surface phenotype of cells stained after 7 days of culture with GM-CSF and IL-4. This representative analysis out of five shows no difference between AC, CD34− AC, and NA-AC derived DCs either in lineage (CD4, CD14, CD1a) or in costimulatory antigen (CD40, MHC class II, CD80) expression. Controls (dotted lines) were isotype-matched irrelevant antibodies.
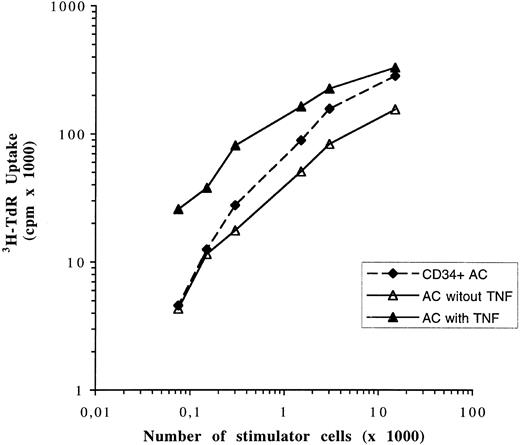
DCs generated from CD34 depleted and unabsorbed cells are as potent as AC-derived DCs in stimulating allogenic T-cell proliferation. Graded numbers of DCs obtained after 7 days of culture in the presence of GM-CSF and IL-4 were used as stimulator cells for 1.5 × 105 allogenic purified T cells in 5-day cultures. Cell proliferation was assayed after a 12-hour pulse with 3H-TdR. Data are expressed as mean ± SD of sextuplet cultures. 3H-TdR incorporation of irradiated (30 Gy) DCs or purified T cells were less than 800 cpm. Results are representative of three experiments using cells from three different patients.
DCs generated from CD34 depleted and unabsorbed cells are as potent as AC-derived DCs in stimulating allogenic T-cell proliferation. Graded numbers of DCs obtained after 7 days of culture in the presence of GM-CSF and IL-4 were used as stimulator cells for 1.5 × 105 allogenic purified T cells in 5-day cultures. Cell proliferation was assayed after a 12-hour pulse with 3H-TdR. Data are expressed as mean ± SD of sextuplet cultures. 3H-TdR incorporation of irradiated (30 Gy) DCs or purified T cells were less than 800 cpm. Results are representative of three experiments using cells from three different patients.
Expression of CD1a, CD14, and accessory molecules on 14-day cultured CD34+ cells. After 14 days of CD34+ cell culture with GM-CSF, TNF-α, IL-4, and SCF, cells were obtained and double-stained with FITC- or PE- anti-CD1a and HLA-DR, CD80, CD86, CD40, and CD83 MoAbs. Irrelevant mouse Igs were used as a negative control.
Expression of CD1a, CD14, and accessory molecules on 14-day cultured CD34+ cells. After 14 days of CD34+ cell culture with GM-CSF, TNF-α, IL-4, and SCF, cells were obtained and double-stained with FITC- or PE- anti-CD1a and HLA-DR, CD80, CD86, CD40, and CD83 MoAbs. Irrelevant mouse Igs were used as a negative control.
In conclusion, we have obtained from MM patients' ACs DCs that are able to stimulate naive allogenic T cells, capture antigen by mannose receptor-mediated endocytosis, present processed recall antigen to autologous T cells, and produce active IL-12. Interestingly, it was still possible to obtain a large amount of functional DCs from a patient with plasma cell leukemia (Table 1 and Fig 2, patient 9). No syndecan-1+ tumoral plasma cells were present in the final culture (data not shown) although ACs initially contained 36% of syndecan-1+ myeloma cells.
Stimulation of DC precursor proliferation by IL-3. To increase the proliferation and the number of DCs, we assayed the effect of IL-1, IL-3, IL-6, TNF-α, and SCF either alone or used in combination (Fig 6A). IL-3 enhanced by 10-fold tritiated thymidine uptake when associated with TNF-α. Similarly, cell-cycle analysis with PI showed that the peak of proliferation was reached between days 3 and 5 of culture and that the percentages of cells in S and G2/M phases were 3- to 10-fold higher in the presence of the four cytokines than with GM-CSF and IL-4 alone (data not shown). In addition, the association of IL-3 and TNF-α increased the yield of DC generation 3-fold (range, 2- to 3.7-fold, n = 4; Fig 6A). These last two sets of data indicated that the number of DCs in the S phase was increased by almost 10-fold in agreement with the 10-fold increase in tritiated thymidine incorporation. With IL-3/TNF-α/GM-CSF/IL-4, the mean percentage of CD1a+CD14− DCs was 83.7% compared to 83.5% with only GM-CSF and IL-4 in the same experiments (Fig 7, n = 7). The mean final CD1a+ DC yield then reached 32.5% of the ACs put in culture (range, 20% to 47%, n = 4) with the four cytokines compared to 11.5% (range, 10% to 13%, n = 4) for the same patients with only GM-CSF and IL-4 (Fig 6A). Moreover, DCs obtained after 7 days of culture in the presence of GM-CSF/IL-4/IL-3/TNF-α stimulated allogenic T lymphocytes with the same efficiency as DCs obtained without IL-3 (Fig 6B). These results showed that IL-3, in the presence of GM-CSF, IL-4, and TNF-α, enhanced DC precursor proliferation and growth without modifying the DC functionality. In contrast, neither IL-1, IL-6, nor SCF clearly improved DC proliferation on day 4 nor DC yield on day 7, even in the presence of TNF-α.
CD34+ cells were not involved in DC obtaining from AC. For five patients we evaluated the ability of four cell populations to generate DCs: AC, CD34+ cells, nonabsorbed cells (NA-AC) obtained after large scale and clinical-grade CD34 selection, and ACs completely devoid of CD34+ cells (Table 2). The large-scale purification of CD34+ cells yielded a virtually pure population of CD34+ cells (95.2%, Table 2). However, cells that were not captured by the magnet still contained a significant percentage of CD34+ cells (ie, NA-AC: 1.5%, Table 2). A cell population completely devoid of CD34+ cells was obtained by increasing the ratio of magnetic beads to target cells and repeating the depletion twice (CD34− cells, 0.04% CD34+ cells, Table 2).
After 7 days of culture, no difference was found between the percentage of CD1a+ cells in culture of AC, CD34− AC, and NA-AC (Table 2). The yields of DC generation compared with the number of input cells were similar, respectively: 12.6% ± 1.7%; 11% ± 0.7%; and 12.6% ± 1.7% (Table 2). Analysis of the cell cycle showed the same results for CD34− ACs as for AC, ie, a higher percentage of cells in the S and G2/M phases (mean, fivefold) in the presence of TNF-α and IL-3 added to GM-CSF and IL-4 than with only the two cytokines (Table 3). In addition, cells generated from these three cell subpopulations had the same surface-marker characteristics of DCs: CD1a, HLA-DR, CD80, CD40, and lack of CD14 (Fig 8). Finally, DCs obtained from CD34− ACs and NA-AC were as potent as AC-derived DCs in stimulating allogenic T cells (Fig 9).
Comparison of DCs generated from adherent ACs and from CD34+ cells. To date, no study has compared the ability to generate DCs from adherent DC progenitors and from CD34+ cells or the phenotype and the function of these DCs. For five patients with MM, DCs were generated either from adherent ACs as indicated above or from purified CD34+ cells (Table 3). As stated in previous reports,20-22 when these cells were cultured with GM-CSF, SCF, and TNF-α only, several cell populations were found on day 14 of the culture: typical DC clusters, adherent cells resembling macrophages, and isolated, round small cells. At this time, double staining showed that only 27.2% ± 8.1% of cultured cells were CD1a+ cells, of which about 30% displayed reactivity with anti-CD14 MoAb (n = 5) (data not shown). In addition, cultures contained 17.3% ± 1.8% of typical CD14+CD1a− macrophages. With this cytokine combination, the yield of DCs generated from CD34+ AC, calculated as described in Materials and Methods, reached 96.4% ± 25.9% of ACs (n = 5). However, if the CD1a+ cells only were considered, the yield of DCs generated from CD34+AC was 28.6% ± 10.2% of ACs (n = 5).
After a 2-week culture in the presence of GM-CSF/SCF/TNF-α and IL-4, CD34+ cells generated a higher percentage of CD1a+ DCs (41.8% ± 9.9%, n = 5) (Table 2 and Fig 10). Morphologically, no adherent macrophage-like cell was present and less than 0.5% of cultured cells stained with anti-CD14 antibody. Addition of IL-4 reduced the total CD34+AC-derived cell yield, which was only 53% ± 12.4% of AC, but an absolute number of DCs was retained with a CD34+AC-derived DC yield of 22.3% ± 7.7% of ACs (Table 2). Similar results were obtained when IL-4 was added only after 1 week of culture. Thereafter, since it provided the most homogeneous cell population with the best CD1a+ percentage, we studied only CD34+ AC-derived cells resulting from a culture with GM-CSF/SCF/TNF-α and IL-4. Flow-cytometric analysis showed that CD40 and CD80 costimulatory molecules were expressed on CD1a+ cells but not on CD1a− cells and that even CD1a+ cells displayed a weaker reactivity to HLA-DR MoAb than AC-derived DCs (Fig 10). In addition, we showed that the allogenic T-lymphocyte stimulation capacity, evaluated by MLR (Fig 11), and the receptor-mediated endocytic capacity, evaluated by FITC-dextran endocytosis (Fig 5C), were lower for CD34+AC-derived cells than for AC-derived DCs.
AC-derived DCs display a higher T-cell activation capacity than CD34+AC-derived DCs when growing in the same cytokine conditions. Graded numbers of AC-derived DCs generated after 7 days of culture in the presence of GM-CSF and IL-4 with (▴) or without (▵) TNF-α and CD34+AC-derived DCs generated after 14 days of culture in the presence of GM-CSF/IL-4/TNF-α (▪) were used in MLR as stimulator cells for 1.5 × 105 thawed allogenic T cells. Cells were pulsed with 3H-TdR for 12 hours after 5 days of coculture. Data are expressed as means of sextuplet cultures (one of two experiments). 3H-TdR incorporation rates of irradiated DCs or purified T cells were always less than 900 cpm.
AC-derived DCs display a higher T-cell activation capacity than CD34+AC-derived DCs when growing in the same cytokine conditions. Graded numbers of AC-derived DCs generated after 7 days of culture in the presence of GM-CSF and IL-4 with (▴) or without (▵) TNF-α and CD34+AC-derived DCs generated after 14 days of culture in the presence of GM-CSF/IL-4/TNF-α (▪) were used in MLR as stimulator cells for 1.5 × 105 thawed allogenic T cells. Cells were pulsed with 3H-TdR for 12 hours after 5 days of coculture. Data are expressed as means of sextuplet cultures (one of two experiments). 3H-TdR incorporation rates of irradiated DCs or purified T cells were always less than 900 cpm.
When 20% of HMCL supernatant was added on day 0 of CD34+ culture, no difference was detected in DC phenotype or in DC ability to stimulate allogenic T cells. However, we have shown a slight increase (20% to 50%) of the total CD34+AC-derived cell yield (data not shown).
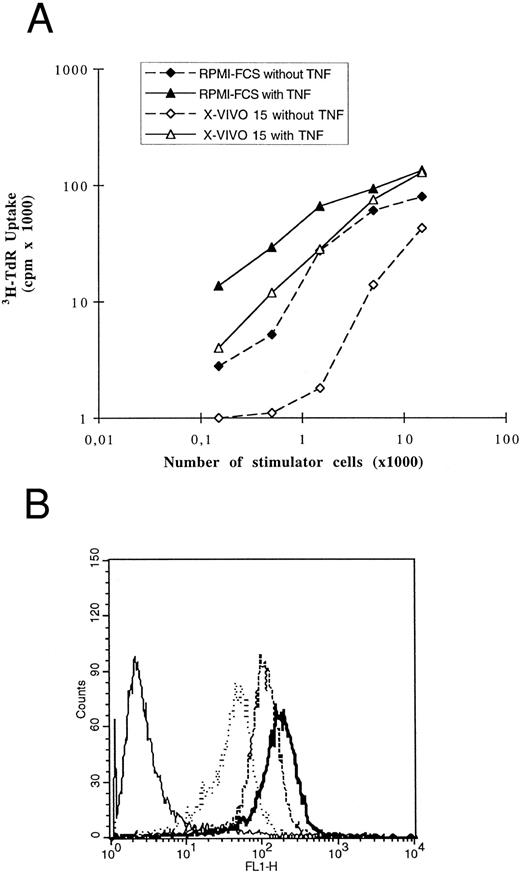
Generation of AC-derived DCs in serum-free medium. We have evaluated the ability to generate DCs in a serum-free culture medium to avoid the use of FCS or autologous serum that contained uncontrolled concentrations of autologous and tumoral antigens, especially the monoclonal Ig. The X-VIVO 15 medium could be used to generate DCs from mobilized ACs from MM patients. After 7 days of culture, the yield of DC generation was 9% (n = 5) and their phenotype was close to that of AC-derived DCs obtained with FCS: CD14− and HLA-DR+, CD4+, CD80+, and CD40+ (Fig 12). Interestingly, CD86 expression was higher in the X-VIVO 15 medium. However, these cells expressed little or no CD1a antigen. Because CD1a was differently present according to DC differentiation or localization, we tested the functionality of cells obtained in X-VIVO 15. Their FITC-dextran endocytosis was similar to that of DCs obtained in RPMI-10% FCS (Fig 13B). Furthermore, even if cells generated in serum-free medium with GM-CSF and IL-4 were poor allogenic T-cell stimulators, addition of TNF-α enhanced this capacity which reached that of DCs generated with FCS (Fig 13A). At the same time, TNF-α increased the expression of some accessory molecules such as CD80, CD86, CD54, and CD58 and induced CD83 exactly as described for DCs obtained in RPMI-10% FCS (data not shown). Therefore, we conclude that cells obtained from adherent ACs cultured in X-VIVO 15 medium supplemented with GM-CSF and IL-4 and with a further addition of TNF-α belong to the DC lineage and could be used to present exogenous antigens to T cells.
Cells obtained in X-VIVO 15 medium exhibited a partial DC phenotype. Flow cytometric analysis was performed exactly as described in the legend to Fig 1.
Cells obtained in X-VIVO 15 medium exhibited a partial DC phenotype. Flow cytometric analysis was performed exactly as described in the legend to Fig 1.
Cells obtained in X-VIVO 15 medium were functional DCs. (A) DCs obtained from the same ACs cultured either in RPMI-10% FCS (solid symbols) or in X-VIVO 15 medium (open symbols) were used in MLR with 1.5 × 105 allogenic highly purified CD3+ T cells obtained by immunomagnetic depletion. TNF-α was added (triangle) or not (square) at day 5 of DC culture. This comparison was performed with ACs from two patients and gave the same results. (B) DCs obtained after 5 days of culture in X-VIVO 15 supplemented with GM-CSF and IL-4 were incubated with FITC-dextran and analyzed as described in the legend to Fig 5. These data are representative of three experiments.
Cells obtained in X-VIVO 15 medium were functional DCs. (A) DCs obtained from the same ACs cultured either in RPMI-10% FCS (solid symbols) or in X-VIVO 15 medium (open symbols) were used in MLR with 1.5 × 105 allogenic highly purified CD3+ T cells obtained by immunomagnetic depletion. TNF-α was added (triangle) or not (square) at day 5 of DC culture. This comparison was performed with ACs from two patients and gave the same results. (B) DCs obtained after 5 days of culture in X-VIVO 15 supplemented with GM-CSF and IL-4 were incubated with FITC-dextran and analyzed as described in the legend to Fig 5. These data are representative of three experiments.
DISCUSSION
This study was performed to evaluate the possibility of generating large amounts of pure, functional DCs from patients with MM. We have shown that AC, obtained after high-dose cyclophosphamide and G-CSF mobilization, are of particular interest. We show that, without any purification procedure, we can reproducibly obtain a homogeneous population of about 90% of CD1a+ cells that express high levels of accessory molecules (CD40, CD80, HLA-DR), lack the CD14 monocyte marker, are very powerful for stimulating allogenic T lymphocytes and autologous T cells in response to recall antigens, and produce active p75 IL-12 in response to CD40 ligation. We have demonstrated that they can activate naive T cells CD4+ and CD8+ and induce them to proliferate even if IL-2 addition is necessary for optimal proliferation of naive CD8 T cells, as described in other models.33 Thus, we may conclude that these cells are mature DCs. The method we describe is very simple because it requires only two cytokines (GM-CSF and IL-4), 1 week of ex vivo culture, and no need for cell purification, therefore limiting the cost and the risks associated with long-term cultures. In addition, the mean yield of DC generation was 12.1% of ACs put in culture and was homogeneous among 18 different patients (range, 80% to 98%). Because fresh AC are not always regularly available for the same patient, it is noteworthy that pure DCs can be reproducibly obtained with this methodology from thawed AC. In addition, we have proven that CD34+ cells are not involved in the DC generation from adherent AC. In particular, we have shown that the nonabsorbed cells, resulting from clinical-grade CD34 purification, are able to generate DCs with the same purity and the same yield as whole AC. Thus, the same ACs can be used to purify CD34+ cells for autologous stem cell transplantation and to generate DCs on separate occasions from frozen unabsorbed cells. Recent in vivo data indicate that only a few million DCs might be sufficient to generate an antitumor response when injected on repeated occasions.8 Because several billion DCs might be generated from the residual ACs after CD34 purification, the number of DCs should not be a limiting component to start clinical trials.
Compared with the obtaining of DCs from AC, four cytokines and a 2-week culture are needed to obtain DCs from CD34+ cells.21,22 In addition, the cultured cells comprised 42% of CD1a+ DCs only, which are the cells able to efficiently activate T lymphocytes. Moreover, comparative MLR show clearly that bulk CD34+AC-derived DCs are less efficient in stimulating T lymphocytes than AC-derived DCs in the same culture conditions. One can hypothesize that the difference in CD1a+ percentage between the two cell populations (about 50%) is responsible for this discrepancy.22 Finally, CD34+AC-derived DCs, probably in part because of the need for TNF-α addition, are not very efficient for active endocytosis mediated by mannose receptor. Concerning the cell yield, it is obvious that CD34+ cell proliferation, which is stimulated by SCF, made it possible to obtain a higher number of cells (4 to 8 times more depending on culture conditions), but if CD1a+ DCs are considered this cell yield is only about 2.5 times better than that obtained with adherent ACs cultured in the presence of GM-CSF and IL-4.
In vivo, MM cells produce several cytokines that can modulate DC obtention and function in vitro as recently described for IL-1034,35 or VEGF.36 However, when ACs or CD34+ cells are cultured in the presence of 20% of supernatants obtained from HMCL, the only detectable effect is a modification of DC yield without any alteration of DC phenotype nor functionality (data not shown). The cell yield is lowered when starting from ACs and increased when using CD34+ cells as previously described with other tumoral cells.36 This suggests that, even if some tumoral cells interfere with DC maturation and function, it is not the case for MM cells.
Recently, Rettig et al37 have reported that medullar stromal cells with some phenotypic characteristics of DCs could be infected by Kaposi's sarcoma-associated herpesvirus (KSHV) in MM patients. KSHV has been previously detected in Kaposi's sarcoma lesions, in multicentric Castelman's disease, and in body-cavity–based or pleural effusion lymphomas. Its genome contains several open reading frames with striking homology to known cellular genes.38 It especially encodes a viral IL-6,39 which could contribute to MM pathogenesis in promoting tumor cell growth. It would thus be very important to determine if AC-derived or CD34+AC-derived DCs are infected by KSHV and produce viral IL-6.
When using AC, addition of IL-3 and TNF-α makes it possible to enhance CD34− DC precursor proliferation with an increase of cells in the S phase and a threefold increase of the number of DCs generated in culture. The MLR capacity was not modified. The role of TNF-α in inducing DC generation from early precursors24 might be due in part to its ability to upregulate the β-chain of the IL-3/GM-CSF/IL-5 receptor complex.40-42 On the other hand, it has been involved in monocytic differentiation of myeloid cell lines.43 Caux et al44 have already shown that IL-3 can replace GM-CSF and cooperate with TNF-α for DCs development from cord blood CD34+ progenitors. Because GM-CSF and IL-3 exert overlapping activities by using the common β-receptor chain, it is a wonder that they could have additional effects on DC precursors in the presence of TNF-α. We used ACs obtained following a recovery from aplasia that contained a heterogeneous blending of precursors at all maturation stages of the monocytic and dendritic pathways. A differential expression of GM-CSF and IL-3 receptor α-chains by different cell populations might explain this synergy of IL-3 and GM-CSF. This increase in DC precursor proliferation would be of great interest to generate antitumoral or anti-infectious T lymphocytes because a lot of gene transfer strategies are dependent on cell proliferation. Indeed, several studies suggest that DCs generated from CD34+ cells can be engineered to express exogeneous antigens and are therefore able to induce specific T-cell activation.45 We have to determine now whether proliferating AC-derived DCs could be efficient for genetic manipulations.46
TNF-α, which downregulates the endocytic activity of DCs but enhances costimulatory antigen expression at their surface,11 is not required to obtain DCs from AC. Thus, it would be interesting to generate DCs ex vivo without TNF-α in the presence of identified tumoral antigens to favor protein uptake and to add TNF-α at the end of the culture to improve T-lymphocyte activation.7,47,48 Another advantage of the use of adherent ACs would be the possibility of modulating DC maturation during the culture. In patients with MM, two tumoral antigens are already potentially interesting. One of them is the monoclonal Ig of which VH and VL idiotypes have mutations specific to the tumoral clone and identical throughout the disease course.49,50 It is noteworthy that anti-idiotypic cytotoxic T cells have recently been obtained from a patient with MM by using tumoral immunoglobulin pulsed DCs as an immunogen.51 Another potentially interesting antigen is MUC-1 that is expressed on myeloma cell lines and is immunogenic.10 MUC-1 pulsed DCs have recently shown to be interesting immunogens.45 Generation of functional DCs in serum-free medium, devoid of xenogenic antigens and of circulating tumoral antigens, raises the possibility of obtaining clinical-grade DCs able to present selected peptides, provided in appropriate concentration, to autologous T lymphocytes in MM patients to generate efficient antitumoral immunity.
Supported by grants from ARC (Paris, France), LFNC (Paris, France), and AFS (Paris, France).
Address reprint requests to Bernard Klein, PhD, Institute for Molecular Genetics, CNRS, 1919 Route de Mende, 34033 Montpellier, France.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal