Abstract
Although much is known about the intracellular phospholipase C (PLC) specific for inositol phospholipids, few data are available about the presence of a less common PLC at the external side of the membrane bilayer of some cell types. This ectoenzyme seems to play particular roles in cellular function by hydrolyzing inositol lipids located on the outer leaflet of the plasma membrane. Here, we provide the first evidence that peripheral T lymphocytes express a discrete level of a PLCγ1 at the outer leaflet of the plasma membrane. Flow cytometry showed that the PLCγ1-positive (PLCγ1+) cells (∼37%) were CD8+ and CD45RA+. Biochemical evidence indicated that (1) this ectoenzyme displays a mass similar to the cytoplasmic form, (2) it is phosphorylated on tyrosine residues, and (3) its activity is Ca2+-dependent. In addition, this enzyme appeared to be correlated with the proliferative state of the cell, since stimulation with phytohemagglutinin (PHA) downregulated both its expression and activity, which were restored by treatment with an antiproliferative agent like natural interferon beta. Moreover, the different kinetics of formation of its hydrolytic products, inositol 1 phosphate and inositol 1:2 cyclic phosphate (Ins(1)P and Ins(1:2 cycl)P), formed upon incubation of the lymphocytes with [3H]-lyso-phosphatidylinositol (PI), allow the hypothesis of a selective involvement of the two inositol phosphates in the mechanisms regulating the metabolism of particular T-lymphocyte subsets.
IT IS WELL ESTABLISHED that phosphatidylinositol (PI)-specific phospholipase C (PLC) plays a pivotal role in signal transduction in a wide range of cellular systems.1-5 PLC-dependent phosphoinositide hydrolysis occurs both at the plasma membrane and in the nuclear compartment,6 and most of the relevant research has focused on the role of intracellular PLC isoform activity. Only limited data are available about the less common expression of PLC at the external surface of some cell types.7-11 This surface activity may be involved in the regulation of cell growth.12-14 Here, we report the first evidence of substantial levels in human T lymphocytes of cell surface PLC (ecto-PLC) isoform γ1. We also report that its expression varies during the modulation of cell proliferation. This analysis was accomplished by means of confocal microscopy and flow cytometry. In a parallel study, we tested the activity of this external enzyme using inositol 2 3H-monoacyl-PI ([3H]-lyso-PI) as a substrate and analyzing the formation of the specific hydrolytic products.
MATERIALS AND METHODS
Cell culture and interferon treatment.
Lymphocytes were obtained from normal blood donors by Ficoll-Hypaque separation (Sigma Chemical Co, St Louis, MO). To test normal resting T lymphocytes, samples were previously depleted from adherent cells by incubating the cell suspension in tissue culture dishes overnight at 37°C in a controlled atmosphere. For polyclonal activation, cells were cultured in HEPES-buffered RPMI 1640, 10% fetal calf serum, glutamine, and antibiotics in the presence of 20 μL/mL phytohemagglutinin (PHA) at 37°C in 5% CO2 and high humidity. For the experiments, human interferon beta (Serono Co, Geneva, Switzerland; 1,000 IU/mL) was administered for different times after 24 hours of PHA stimulation. Viability of the cells was determined by the Trypan blue exclusion test, which indicated that 95% of the cells were still viable after 4 days of culture.
Immunocytochemical and confocal analysis.
Lymphocytes were washed in phosphate-buffered saline (PBS) and incubated with anti-PLCγ1, δ1, or β1 MoAb (Upstate Biotechnology Inc, Lake Placid, NY; 1:300) and then with fluorescein-conjugated goat Fab anti-mouse IgG (Sigma; 1:64) for 60 minutes. The samples were always kept in an ice bath during the incubation period to avoid the capping and/or internalization of Ag-Ab complex. All antibody solutions were diluted in PBS and 4% goat and 4% human Ig to avoid cross-reactivity or unspecific binding. The reactions performed using either anti-CD2 MoAb (anti-leu-5b, 1:300 as a cell surface marker; Becton Dickinson, Meylan, France) or anti-STAT 91/84 protein (Affinity, Mamhead, Exeter, UK; 5 μg/mL as intracellular marker to check integrity of the membrane) instead of the primary antibody or only with the secondary antibody represented the controls for the experimental procedures. In addition, to better characterize the identity of the ectoenzyme, a different anti-PLCγ1 (Santa Cruz, Santa Cruz, CA; 1:100) was tested. After washing in PBS, a drop of each sample was layered on a slide, mounted, and observed with a Leica TCS 4D equipped with an argon ion laser (Leica, Heidelberg, Germany), attached to Leitz DMRB fitted with a 100×/1.3 NA oil immersion objective (Leitz, Heidelberg, Germany). For the image acquisition, fluorescein isothiocyanate (FITC) was excited with the blue (488 nm) line of the argon ion laser. Images were stored on a computer with a scanning mode format of 512 × 512 pixels.
Multicolor immunofluorescence staining and flow cytometric analysis.
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood by Ficoll-Hypaque density gradient centrifugation. Two-color immunofluorescence was used to examine PLCγ1 expression by CD4+, CD8+, CD45RA+, and CD45RO+ cells. PBMCs were first treated with anti-PLCγ1 (UBI) or, as a control, with mouse IgG (Coulter, Miami, FL) and counterstained with F(ab′) fragments of FITC-conjugated anti-mouse IgG (Sigma). Next, the cells were stained for determination of T-cell subpopulations with phycoerythrin (PE)-conjugated anti-CD4, -CD8, -CD45RA, and -CD45RO MoAbs (Sigma) or isotype-matched mouse IgG. Cells were analyzed on a fluorescent-activated cell scan (FACScan) using Lysis II software (Becton Dickinson). The region for the lymphoid population was then selected. Ten thousand events were accumulated and analyzed for fluorescence.
Preparation of cell extracts.
Resting T lymphocytes were washed in PBS, homogenized in a homogenization buffer (20 mmol/L Tris, pH 7.4, 100 mmol/L NaCl, 5 mmol/L MgCl2, 0.2 mmol/L Na3VO4, 10 μg/mL aprotinin, and 10 μg/mL leupeptin), and kept for 30 minutes on ice. The lysates were centrifuged at 8,000 g for 15 minutes at 4°C. The supernatants were recovered as the cytosolic fraction. Homogenization buffer containing 50 mmol/L Triton X-100 was added to the particulate fraction to solubilize membrane proteins. After incubation at 22°C for 20 minutes, samples were centrifuged at 400,000 g for 20 minutes at 4°C, and the supernatants were collected as the solubilized membrane fraction.
Immunoprecipitation of PLCγ1.
Lysates from membrane and cytosolic fractions normalized at 400 μg protein were incubated at 4°C for 60 minutes with 10 μg anti-PLCγ1 MoAb (UBI) previously coupled to goat anti-mouse IgG magnetic beads. Immunocomplexes were collected by a magnet and washed several times with ristocetin-induced platelet agglutination buffer (PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate [SDS]) in the presence of protease inhibitors. Equal amounts of immunocomplexes were subjected to immunoblot analysis.
Immunoblot analysis.
Cytosolic and membrane extracts or equal amounts of PLCγ1 immunocomplexes from the two fractions were diluted in SDS sample buffer, electrophoresed in 8% SDS–polyacrylamide gel electrophoresis (PAGE), transferred onto nitrocellulose membrane, and incubated for 1 hour at room temperature with anti-PLCγ1 antibody (UBI or Santa Cruz; 1 μg/mL) or anti-phosphotyrosine (PY-20) MoAb (Affinity; 1:1,000) in wash buffer (10 mmol/L Tris pH 7.5, 100 mmol/L NaCl, 0.1% Tween 20), and 5% nonfat milk. Immunoreactive bands were detected by a chemiluminescence system (ECL; Amersham Corp, Buckinghamshire, UK) using peroxidase-conjugated secondary antibodies diluted in wash buffer with 5% nonfat milk.
When required, blots were stripped of bound antibodies by incubating the membranes in wash buffer containing 2% SDS at 80°C for 40 minutes. After washing, membranes were reprobed with the secondary antibody to confirm removal of the primary antibody. Blots were then incubated with anti-PLCγ1, and the antibody binding was detected by the ECL system as previously described.
Internal controls obtained by incubating the membranes only with secondary antibodies always yielded negative results.
Preparation of radiolabeled lyso-PI.
Tritium-labeled lyso-PI was prepared as described by Volwerk et al.13 PI conversion to lyso-PI and complete removal of fatty acids were verified by thin-layer chromatography (chloroform:methanol:water 65:25:4). [3H]-lyso-PI was dried, dissolved in water at 1.125 mmol/L, and stored frozen at −20°C until use. The specific activity (5.50 Ci/mol) was verified by liquid scintillation counting.
Kinetic analysis of [3H]-lyso-PI uptake and ecto-PLC activity.
Ecto-PLC activity was determined as described by Volwerk et al.13 An equal number of lymphocytes (1 × 106) in the different experimental conditions (ie, unstimulated, PHA-stimulated, and PHA-stimulated plus 30 minutes, 90 minutes, 6 hours, and 24 hours of interferon beta, respectively) were incubated at 4°C up to 30 minutes with 11.25 μmol/L [3H]-lyso-PI. At the indicated times, aliquots from the samples were centrifuged and the amount of water-soluble radiolabeled products formed was measured by extracting a portion of the incubation medium with chloroform:methanol:concentrated HCl (66:33:1). Since lyso-PI is highly water-soluble, the aqueous phase was extracted again with chloroform so that removal of this lipid was more complete. The aqueous phase was then used for liquid scintillation counting. As controls, samples containing EGTA (0.5 mmol/L) were always tested in parallel. Direct scintillation counting of the incubation medium of these EGTA-containing samples yielded the measure of spontaneous uptake of lyso-PI by the cells.
Identification of inositol phosphate products.
Identification of inositol phosphates produced from hydrolysis of [3H]-lyso-PI by the ecto-PLC of T lymphocytes was performed by loading the aqueous phase onto a small (1 mL) Dowex AG1-X8 anion-exchange resin (formate form) column (Bio-Rad, Hercules, CA) as described by Berridge et al.15The columns were previously calibrated with standard samples consisting of [3H]-lyso-PI, sn-glycero-3-phospho-d-1myo-inositol (Sigma), dl-myo-inositol-1 monophosphate (Sigma), and dl-myo-inositol 1,2 cyclic monophosphate (Sigma). Eluted fractions were collected and processed for scintillation counting.
RESULTS AND DISCUSSION
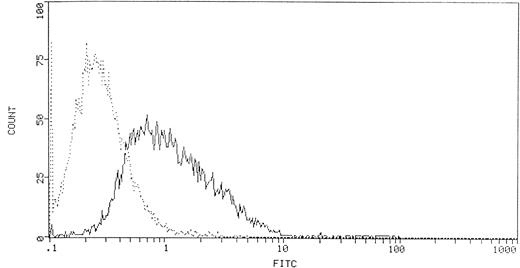
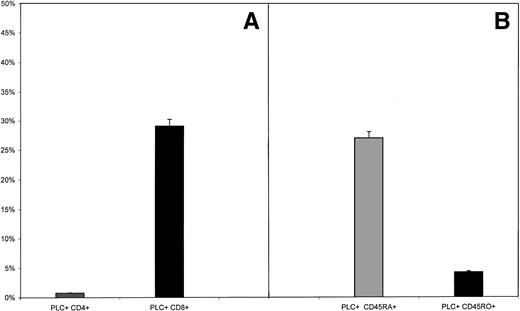
The presence of PLCγ1 at the external side of T lymphocytes was revealed by confocal microscopy on unfixed cells as a bright ring-shaped fluorescence (Fig 1A). The reaction accomplished with the anti-PLCβ1 and -PLCδ1 MoAbs always yielded negative results. Flow cytometric analysis showed that PLCγ1+ cells represented 36.4% ± 4.8% of peripheral lymphocytes (Fig 2). To assess whether the enzyme expression correlated with given functional phenotypes, we then analyzed PLCγ1 expression in specific subtypes of peripheral T lymphocytes (CD4+, CD8+, CD45RA+, and CD45RO+ cells). Flow cytometry was performed as two-color analysis, and the results are expressed as the percentage of total lymphocytes positive for a given phenotype. PLCγ1+ cells were almost exclusively CD8+cells, whereas the percentage of circulating PLCγ1+CD4+ cells was found to be low (Fig3A). To investigate whether expression of the enzyme was correlated with “naive” or “memory” functional phenotypes, we extended the analysis to CD45RA+ and CD45RO+populations. The results indicated that PBMCs showed a significant percentage of PLCγ1+CD45RA+ cells, whereas the ectoenzyme was expressed only on a small percentage of circulating CD45RO+ cells (Fig 3B).
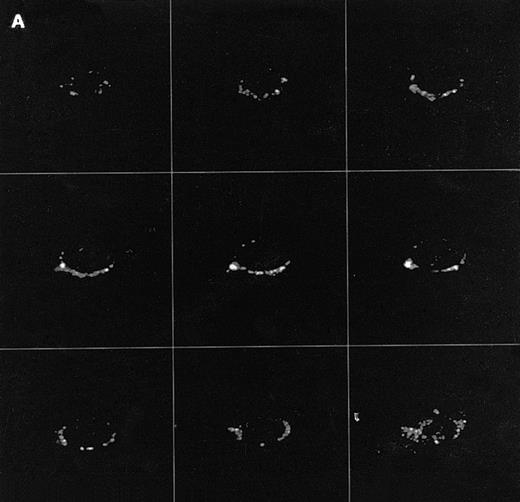
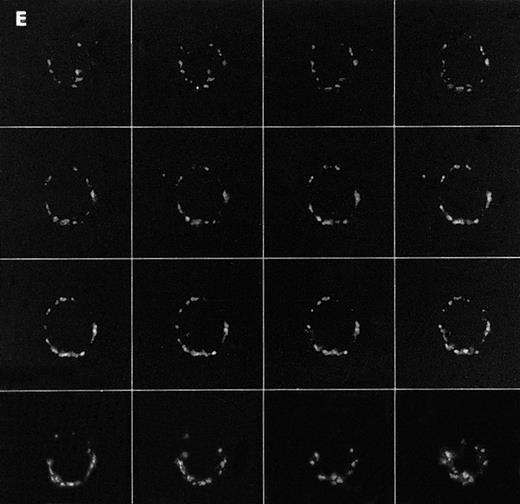
Confocal analysis of PLCγ1 expression at the external leaflet of the plasma membrane in human T lymphocytes. (A) Unstimulated cell (increments of 0.85 μm in z-axis; cell size, 8 μm); (B) PHA-stimulated cell (increments of 0.9 μm in z-axis; cell size, 17 μm); (C) PHA-stimulated cell incubated for 90 minutes with interferon beta (increments of 0.75 μm in z-axis; cell size, 14 μm). (D) PHA-stimulated cell incubated for 24 hours with interferon beta (increments of 0.54 μm in z-axis; cell size, 10 μm); (E) cell labeled with anti-Leu-5b MoAb (CD 2), used as an internal control (increments of 0.56 μm in z-axis; cell size, 11 μm). Note that the membrane labeling evident in resting lymphocytes became weaker in PHA-stimulated cells, and again was strongly detectable after addition of interferon. As internal controls, cells were incubated with anti-Leu-5b MoAb, a T-lymphocyte surface marker, which strongly labeled the membranes (Fig 2E), while the reactions performed in the absence of the primary antibody yielded consistently negative results. Results obtained by incubating lymphocytes with anti-STAT 91/84 protein as an intracellular marker (to check membrane integrity) and with anti-PLC β and δ isoforms did not disclose any FITC membrane labeling.
Confocal analysis of PLCγ1 expression at the external leaflet of the plasma membrane in human T lymphocytes. (A) Unstimulated cell (increments of 0.85 μm in z-axis; cell size, 8 μm); (B) PHA-stimulated cell (increments of 0.9 μm in z-axis; cell size, 17 μm); (C) PHA-stimulated cell incubated for 90 minutes with interferon beta (increments of 0.75 μm in z-axis; cell size, 14 μm). (D) PHA-stimulated cell incubated for 24 hours with interferon beta (increments of 0.54 μm in z-axis; cell size, 10 μm); (E) cell labeled with anti-Leu-5b MoAb (CD 2), used as an internal control (increments of 0.56 μm in z-axis; cell size, 11 μm). Note that the membrane labeling evident in resting lymphocytes became weaker in PHA-stimulated cells, and again was strongly detectable after addition of interferon. As internal controls, cells were incubated with anti-Leu-5b MoAb, a T-lymphocyte surface marker, which strongly labeled the membranes (Fig 2E), while the reactions performed in the absence of the primary antibody yielded consistently negative results. Results obtained by incubating lymphocytes with anti-STAT 91/84 protein as an intracellular marker (to check membrane integrity) and with anti-PLC β and δ isoforms did not disclose any FITC membrane labeling.
FACScan histogram of ecto-PLCγ1+lymphocytes. The percentage of ecto-PLCγ1+ lymphocytes was obtained by calculating the mean ± SD of 10 experiments.
FACScan histogram of ecto-PLCγ1+lymphocytes. The percentage of ecto-PLCγ1+ lymphocytes was obtained by calculating the mean ± SD of 10 experiments.
Expression of PLCγ1 at the external leaflet of the plasma membrane in T-cell subsets. PBMCs were double-stained with anti-PLCγ1, evidenced by a FITC-conjugated secondary antibody, and with either PE-labeled anti-CD4, anti-CD8, anti-CD45RA, or anti-CD45RO antibody. (A) Results showed that the enzyme is expressed on the membrane of CD8+ cells (29.2% ± 5.1%), while the percentage of circulating PLCγ1+CD4+lymphocytes is very low (1.1% ± 0.2%, P < .05). (B) When expression of the enzyme on naive and memory cells was considered, PBMCs displayed a significant percentage of PLCγ1+CD45RA+ (27.2% ± 1.7%), whereas the phenotype PLCγ1+CD45RO+was slightly detectable (4.2% ± 0.6%, P < .005). Significance of the results was determined by Student's ttest. n = 10 samples.
Expression of PLCγ1 at the external leaflet of the plasma membrane in T-cell subsets. PBMCs were double-stained with anti-PLCγ1, evidenced by a FITC-conjugated secondary antibody, and with either PE-labeled anti-CD4, anti-CD8, anti-CD45RA, or anti-CD45RO antibody. (A) Results showed that the enzyme is expressed on the membrane of CD8+ cells (29.2% ± 5.1%), while the percentage of circulating PLCγ1+CD4+lymphocytes is very low (1.1% ± 0.2%, P < .05). (B) When expression of the enzyme on naive and memory cells was considered, PBMCs displayed a significant percentage of PLCγ1+CD45RA+ (27.2% ± 1.7%), whereas the phenotype PLCγ1+CD45RO+was slightly detectable (4.2% ± 0.6%, P < .005). Significance of the results was determined by Student's ttest. n = 10 samples.
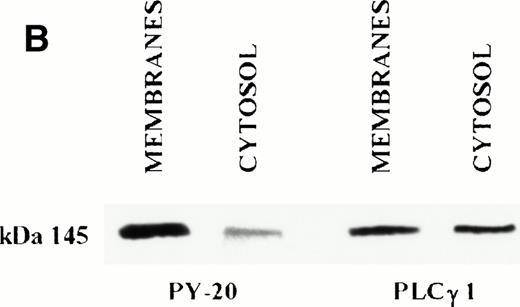
These results might suggest a regulation of the ectoenzyme along the differentiation pathway from “naive” to “memory” CD8+ cells, but this hypothesis requires accurate investigation. To better characterize the apparently novel PLCγ1, we performed an anti-PLCγ1 immunoblot analysis of the membrane and cytosolic fractions from peripheral T lymphocytes. The anti-PLCγ1 MoAb showed a single band with a molecular weight that was apparently the same in both extracts, thus indicating that the external PLCγ1 shares the same mass as the cytosolic one (Fig4A). Since the immunocytochemical and immunoblot detections were acquired using a mixed monoclonal preparation (anti-PLCγ1; UBI), to confirm the identity of the enzyme, we repeated the reactions using a different anti-PLCγ1 antibody (Santa Cruz). The latter, according to the manufacturer, is directed against the epitope corresponding to amino acids 1249 to 1261 within the carboxyl-terminal domain of PLCγ1. The results showed no difference with respect to previous experiments (not shown), further confirming the enzyme identity. In addition, these experiments indicated that the enzyme exposes the above-reported epitope from the external leaflet of the membrane, since it was clearly recognized by the immunocytochemical reaction.
(A) Immunoblot analysis of PLCγ1 in membranes and cytosolic fractions from resting T lymphocytes. Proteins from membrane and cytosolic fractions were separated by SDS-PAGE, blotted onto nitrocellulose, and reacted with anti-PLCγ1 antibody. The ECL system revealed for each fraction one band apparently at the same molecular weight. Control is represented by a cell lysate from a PLCγ1+ line (Jurkat); 145 corresponds to molecular weight (in kilodaltons) assessed on the basis of standard comigration. (B) Tyrosine phosphorylation level of PLCγ1 immunoprecipitated from membranes and cytosolic fractions. PLCγ1 immunoprecipitated from membrane and cytosolic fractions was probed with anti-phosphotyrosine antibody (PY-20) and revealed by the ECL system. Membrane PLCγ1 was consistently phosphorylated, while no or weak reactions were detected in the cytosolic enzyme. After stripping anti-PY-20, the same blot was reprobed with anti-PLCγ1 antibody to show that the levels of the protein loaded on the gel were comparable in both samples. Result is representative of 3 different experiments.
(A) Immunoblot analysis of PLCγ1 in membranes and cytosolic fractions from resting T lymphocytes. Proteins from membrane and cytosolic fractions were separated by SDS-PAGE, blotted onto nitrocellulose, and reacted with anti-PLCγ1 antibody. The ECL system revealed for each fraction one band apparently at the same molecular weight. Control is represented by a cell lysate from a PLCγ1+ line (Jurkat); 145 corresponds to molecular weight (in kilodaltons) assessed on the basis of standard comigration. (B) Tyrosine phosphorylation level of PLCγ1 immunoprecipitated from membranes and cytosolic fractions. PLCγ1 immunoprecipitated from membrane and cytosolic fractions was probed with anti-phosphotyrosine antibody (PY-20) and revealed by the ECL system. Membrane PLCγ1 was consistently phosphorylated, while no or weak reactions were detected in the cytosolic enzyme. After stripping anti-PY-20, the same blot was reprobed with anti-PLCγ1 antibody to show that the levels of the protein loaded on the gel were comparable in both samples. Result is representative of 3 different experiments.
Since tyrosine phosphorylation is believed to be required for activation of the intracellular PLCγ1, we determined the phosphorylation levels of this cell surface enzyme. PLCγ1 was immunoprecipitated from the membranes and, as an internal control, from the cytosolic extracts of peripheral T cells. Equal amounts of the immunoprecipitates were subjected to Western blot analysis using an anti-phosphotyrosine MoAb (PY-20). The ECL system showed that PLCγ1 from the membranes was clearly phosphorylated on tyrosine residues, whereas the cytosolic enzyme displayed weak reactivity due to the absence of any activation trigger (ie, TCR/CD3 engagement, etc.; Fig4B). Since it has been convincingly demonstrated that PLCγ1 resides in the cytoplasm of resting T cells and is recruited to the membrane only upon activation,16 it appears unlikely that the phosphorylated enzyme in the membrane fraction is the cytoplasmic one recruited to the internal leaflet of the plasma membrane.
We determined next whether the expression of ecto-PLCγ1 was correlated with the proliferative state of the cells by extending the investigations to lymphocytes treated first with an activating agent like PHA and then with an antiproliferative drug such as natural interferon beta, whose antireplicative effect on T lymphocytes has already been reported.17 Treatment of PHA-stimulated lymphocytes with interferon beta indeed slowed the cell proliferation within 24 hours of treatment, leading to a progressive reduction of the growth rate over the following 2 days (not shown). Flow cytometric analysis indicated that treatment of the cells with PHA and with PHA plus interferon did not change either the percentage of PLCγ1+ lymphocytes or the distribution between T-cell subsets (not shown). On the other hand, the confocal microscopic images showed that PHA alone induced a downregulation of ecto-PLCγ1 expression (Fig 1B), which was promptly restored by addition of interferon to the culture medium. This effect was clearly detectable after 90 minutes of interferon treatment and was still evident after 24 hours (Fig 1C and D). Such results therefore strongly suggest a relationship between the proliferative state of the cells and ecto-PLCγ1 expression.
To investigate whether the functional properties of the enzyme were also modulated by PHA alone and PHA plus interferon treatment, we next investigated its activity by incubating the cells with [3H]-lyso-PI and measuring the production of inositol phosphates released in the medium. [3H]-lyso-PI, due to its high water solubility, forms micelles that rapidly transfer to the cell membrane. Indeed, the kinetics of the spontaneous uptake of this lipid by lymphocytes showed that lyso-PI in the medium plateaued at about 25% within 5 minutes in all samples, in this way becoming accessible to the ectoenzyme; beyond 5 minutes, the rate of PI transfer was evidently reduced (Fig5). In all samples, the formation of radiolabeled inositol phosphates (measured as the aqueous extractable cpm), which paralleled the disappearance of PI from the medium, was approximately linear over a 5-minute period. Beyond that time, such production plateaued, weakly increasing between 5 and 30 minutes (Fig6a). The rapid kinetics of formation of radiolabeled inositol phosphates in the external medium upon addition of [3H]-lyso-PI reasonably excludes the possibility that such production could derive from a sequence of events including (1) lipid internalization into the cell, (2) its cleavage by an intracellular phospholipase, and (3) diffusion through the membrane of the hydrolytic products to the external medium. A parallel incubation of [3H]-lyso-PI in the medium without cells produced low levels of radioactivity, further indicating that the radiolabeled production was due to a cellular phospholipase rather than to unspecific enzymatic activities in the medium.
Kinetics of [3H]-lyso-PI uptake. Transfer of [3H]-lyso-PI from donor vesicles to the cell membrane of lymphocytes was measured by scintillation counting of the incubation medium from EGTA-containing samples. The 100% value was obtained by measuring an aliquot of solution of 11.25 μmol/L [3H]-lyso-PI (concentration of substrate added to the cells). The kinetics of spontaneous uptake of the lipid by the cells shows that its concentrations in the medium plateaued at about 25% within 5 minutes. Beyond this time, the rate of lyso-PI transfer was evidently reduced. Data are the mean ± SD of at least 3 separate experiments.
Kinetics of [3H]-lyso-PI uptake. Transfer of [3H]-lyso-PI from donor vesicles to the cell membrane of lymphocytes was measured by scintillation counting of the incubation medium from EGTA-containing samples. The 100% value was obtained by measuring an aliquot of solution of 11.25 μmol/L [3H]-lyso-PI (concentration of substrate added to the cells). The kinetics of spontaneous uptake of the lipid by the cells shows that its concentrations in the medium plateaued at about 25% within 5 minutes. Beyond this time, the rate of lyso-PI transfer was evidently reduced. Data are the mean ± SD of at least 3 separate experiments.
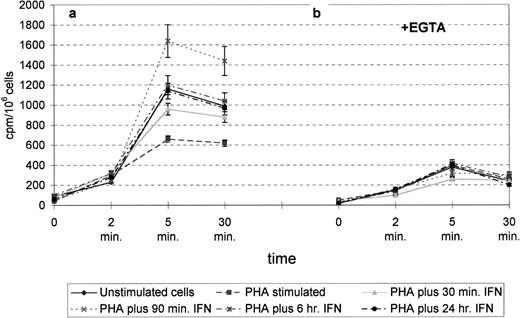
Formation of water-soluble radiolabeled products in the absence (a) or presence (b) of EGTA. Assays were performed for the indicated times in the absence or presence of 0.5 mmol/L EGTA. In the absence of EGTA, production increased linearly at least fivefold over a 5-minute period, plateauing between 5 and 30 minutes. When EGTA was added to the reactions, recovery of radioactivity dramatically decreased, indicating its Ca2+ dependency. Data are the mean ± SD of at least 3 different experiments.
Formation of water-soluble radiolabeled products in the absence (a) or presence (b) of EGTA. Assays were performed for the indicated times in the absence or presence of 0.5 mmol/L EGTA. In the absence of EGTA, production increased linearly at least fivefold over a 5-minute period, plateauing between 5 and 30 minutes. When EGTA was added to the reactions, recovery of radioactivity dramatically decreased, indicating its Ca2+ dependency. Data are the mean ± SD of at least 3 different experiments.
Scintillation analysis showed that compared with PHA-stimulated cells, larger amounts of radiolabeled inositol phosphates were produced in resting and interferon-treated cells, with a main peak in the 90-minute interferon-treated samples (Fig 6a). These results fit well with the PLCγ1 overexpression observed by confocal microscopy in the corresponding samples (Fig 1).
The reaction appeared to be Ca2+-dependent, since the presence of EGTA in the incubation medium strongly impaired the recovery of radioactivity of water-soluble material in all samples (Fig6b). Such dependency sets this PLC apart from the bacterial andTrypanosoma brucei forms, which do not require Ca2+for their activity.18 19 Moreover, the almost complete inactivation of the enzyme observed upon impairment of Ca2+in the medium indicates that the enzyme also exposes the Ca2+-binding site(s) outside the cell membrane.
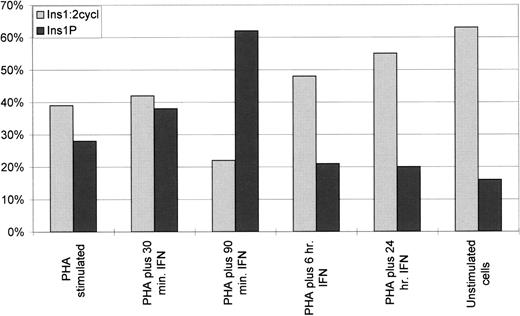
To verify that lyso-PI cleavage occurred through the action of PLC rather than other enzymes (ie, lysophospholipase or phospholipase A1 or D), we tested for the specific radiolabeled products of PLC activity, inositol 1 phosphate (Ins(1)P) and inositol 1:2 cyclic phosphate (Ins(1:2 cycl)P), by loading the water-soluble material on a small (1 mL) chromatographic Dowex AG1-X8 column.15 By this method, we were able to identify two main fractions corresponding to Ins(1)P and Ins(1:2 cycl)P (Fig 7), along with small amounts of glycero-phospho-myo-inositol, and traces of radioactivity related to possible contaminants (not shown). Scintillation counting of the chromatographic products showed that, together, Ins(1)P and Ins(1:2 cycl)P appeared particularly high in the resting cells and in interferon-treated cells (Fig 7). These results strongly suggest a relationship of ecto-PLC expression and activity to cell proliferation, and are in accordance with previous data indicating increased activity of an ecto-PLC in fibroblastic lines during inhibition of cell growth.12-14
Percentage of radiolabeled inositol phosphates in the water-soluble material (100%). Lymphocytes in the different experimental conditions were incubated in a reaction mixture containing 11.25 μmol/L lyso-PI up to 30 minutes. Once the reaction was stopped, the water-soluble products extracted from the incubation medium were chromatographed over a 1-mL column of Dowex AG 1-X8 resin, and the fractions were analyzed by scintillation counting. The peak is evident for Ins(1)P in 90-min interferon-treated samples, while Ins(1:2 cycl)P constantly increases along the time of treatment and accumulates to significant concentrations in 24-hour treated samples and in unstimulated cells. Data are the mean of 3 experiments differing ≤5% SD.
Percentage of radiolabeled inositol phosphates in the water-soluble material (100%). Lymphocytes in the different experimental conditions were incubated in a reaction mixture containing 11.25 μmol/L lyso-PI up to 30 minutes. Once the reaction was stopped, the water-soluble products extracted from the incubation medium were chromatographed over a 1-mL column of Dowex AG 1-X8 resin, and the fractions were analyzed by scintillation counting. The peak is evident for Ins(1)P in 90-min interferon-treated samples, while Ins(1:2 cycl)P constantly increases along the time of treatment and accumulates to significant concentrations in 24-hour treated samples and in unstimulated cells. Data are the mean of 3 experiments differing ≤5% SD.
It is known that PLC catalytic activity results in the formation of diacylglycerol (DAG) other than inositol phosphates, and both of these second messengers could contribute to the regulation of the proliferative state of the cells. Since DAG is able to activate protein kinase C (PKC), we checked for changes in PKC activity in the different experimental conditions, but the results did not indicate any substantial differences among the samples (not shown). This finding thus suggests the inositol phosphates generated by the action of ecto-PLC as possible regulatory molecules in the signaling route controlling lymphocyte metabolism. It is well known indeed that inside the cell, these second messengers, even at low concentration, can modulate a wide range of cellular activities.20 In addition, the hypothesis of selective involvement of Ins(1)P and Ins(1:2 cycl)P in the mechanisms regulating T-lymphocyte behavior might be taken into account. Indeed, based on the finding of a peak of radiolabeled Ins(1)P in the 90-minute interferon-treated cells and the constant increase of Ins(1:2 cycl)P along the time of interferon treatment, a different involvement of the two molecules could be supposed: Ins(1)P might be a primary signal for entry in the metabolic shutdown of proliferation, while Ins(1:2 cycl)P might be required for establishment and maintenance of the quiescence. The finding that Ins(1:2 cycl)P accumulates to a significant concentration in 24-hour interferon-treated cells and in resting cells (ie, unstimulated) is in line with the suggestion of a distinct regulatory role for these two compounds. This is also in accordance with previous studies in which PLC (albeit the intracellular enzyme) is reportedly able to catalyze the formation of Ins(1)P or Ins(1:2 cycl)P by totally independent pathways.21 Moreover, it has been previously described that noncyclic and cyclic inositol phosphates show completely different kinetics, the former being rapidly metabolized and the latter displaying a slow turnover.22 These findings allow us to hypothesize that distinct inositol phosphates, generated by hydrolysis of the aliquot of PI present on the outer leaflet of the membrane,23,24 might contribute to regulation of the lymphocyte metabolic machinery. PLCγ1 hydrolytic products might thus be capable of transbilayer diffusion and/or receptor-mediated endocytosis, with subsequent autocrine or paracrine action on cell proliferation.
Taken together, the data presented here provide the first evidence of the presence of a PLCγ1 on the external surface of some T-cell subsets, which is apparently involved in the regulation of the replicative machinery. PLCγ1+ lymphocytes were essentially CD8+ and CD45RA+. The structural and kinetic characterization of the enzyme indicate that (1) it has an apparent mass of 145 kD and reacts with antibodies directed against PLCγ1, (2) it is phosphorylated on tyrosine residues, and (3) its activity is calcium-dependent.
In terms of cell surface disposition, significant regions of the enzyme, such as the catalytic active sites, the carboxyl-terminal portion recognized by the anti-PLCγ1 antibody, and the calcium-binding site(s), appear to be extracellular domains of the enzyme and therefore are not directly involved in the mechanisms of membrane attachment.
This peculiar localization strongly sets this protein apart from the other known mammalian PLCs, although some biochemical properties are shared with the intracellular form. Although the possibility of a synthesis from modified mRNA cannot be completely ruled out, the relatively rapid increase of the enzyme expression and activity after interferon treatment would suggest a translocation to the cell surface from the intracellular stores. If this is the case, phosphorylation might play an important role in the transfer, since the enzyme at the external surface appears to be phosphorylated and active. It is conceivable that conformational changes induced by phosphorylation may be responsible for the exposure of hydrophobic residues able, in turn, to anchor the protein to the membrane.
ACKNOWLEDGMENT
This work is dedicated to the memory of Valerio.
Supported by a MURST 60% grant (1995-1996).
Address reprint requests to Sebastiano Miscia, MD, Istituto di Morfologia Umana Normale, Università G. D'Annunzio, Via dei Vestini 6, 66100 Chieti, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 5. Kinetics of [3H]-lyso-PI uptake. Transfer of [3H]-lyso-PI from donor vesicles to the cell membrane of lymphocytes was measured by scintillation counting of the incubation medium from EGTA-containing samples. The 100% value was obtained by measuring an aliquot of solution of 11.25 μmol/L [3H]-lyso-PI (concentration of substrate added to the cells). The kinetics of spontaneous uptake of the lipid by the cells shows that its concentrations in the medium plateaued at about 25% within 5 minutes. Beyond this time, the rate of lyso-PI transfer was evidently reduced. Data are the mean ± SD of at least 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/10/10.1182_blood.v91.10.3833/3/m_blod41041005y.jpeg?Expires=1767708311&Signature=e6Mk~thNFin4l~RgW6JeSYHjABT-bIx0aewthkYbGYHinq9-eQTyTXQlrkL3K7Iy7zgFtRTAgTed3lZBA5jv5GUrDxafbUxJYfbSJkXWralsiYDVgGwhUUPpMaQfyjta3lTQYJc0NUTwtIioIRI3xxHAHe05bXn03ey-xWzHwuGIf65deoweBOqgV5jI5d9w4LAKnGc14TXtLQgTRdzeU9Bm9Tx2~ugs~fzGSQhkBQNYzf52eTxPPyVmu-9iHMq5Yjf9Ymqsr7Z8ruJK0SNj0Fd35nyOUszh0A5xepvf3hgOtBauZ8KRsufMjUU2TlkLz4-TuLbGDoKUMW8ou4CNlQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal