Abstract
The “zinc-finger” transcription factor GATA-1 was first shown in cells of erythroid lineage. It is also expressed in cells of other hematopoietic lineages including megakaryocytes, mast cells, and eosinophils. GATA-1 is now considered to be one of the central regulators in hematopoietic cell differentiation. To further analyze the role of GATA-1 in controlling differentiation from hematopoietic stem cells, we investigated the phenotypic changes induced by the overexpression of murine GATA-1 in the murine myeloid leukemic cell line, M1. Forced expression of GATA-1 induced the appearance of erythroid cells and megakaryocytes as assessed by cellular morphology, acetylcholinesterase activity, and expression of platelet factor 4 and β-globin mRNA synthesis. Because the c-mpl ligand, thrombopoietin, plays an important role in megakaryopoiesis, the expression of c-mpl and c-mpl ligand (thrombopoietin) mRNA was analyzed by Northern blot and reverse transcription-polymerase chain reaction (RT-PCR) in M1 cells overexpressing GATA-1. The c-mpl ligand mRNA was equally expressed both in parental M1 cells and in those transfected with the GATA-1 expression vector. In contrast, the mRNA expression of c-mpl was increased only in GATA-1 expressing M1 cells differentiated towards erythroid and megakaryocyte lineages. The increased expression of c-mpl mRNA induced by GATA-1 raised the question as to whether or not GATA-1 transactivated the c-mpl promoter. The activity of the c-mpl promoter in the presence of cotransfected GATA-1 was significantly increased compared with that of the control. A plasmid with the mutated GATA-binding site did not show transactivation ability in the cotransfection with a GATA expression vector. These findings suggest that the upregulation of c-mpl induced by GATA-1 expression in M1 cells is closely associated with erythroid and megakaryocytic differentiation.
THE DIFFERENTIATION of hematopoietic stem cells is regulated in part by the binding of hematopoietic growth factors to their receptors.1 However, the molecular basis for the lineage commitment of hematopoietic progenitors is still unclear. In the erythroid lineage, GATA-1, Tal-1/SCL, or Rbtn2 transcription factors are involved in the differentiation of erythroid cells.2,3 In the myeloid lineage, PU.1, first identified as the Spi-1 oncogene activated in viral induced erythroleukemias,4,5 may be one of the principal regulators in myeloid differentiation because it activates numerous myeloid target genes.6-13 It has been shown that p45 of the erythroid transcription factor NF-E2 acts as an essential factor for megakaryocyte maturation.14 Thus, studies of the role of transcription factors in the commitment of hematopoietic stem cells have suggested that various nuclear proteins are associated with lineage-specific gene expression.
GATA-1 was originally isolated for its ability to bind a core consensus (A/T)GATA(A/G) site present in the promoters or enhancers of many erythroid genes. Studies of GATA-1 gene disruption have shown that GATA-1 plays a pivotal role in the differentiation of the erythroid lineage.15 However, GATA-1 is also expressed in cells of other lineages, including mast cells, eosinophils, and megakaryocytes.16-18 Forced GATA-1 expression in avian Myb-Ets–transformed myeloblasts results in reprogramming of myeloblasts towards the eosinophil and megakaryocyte lineages.19 On the other hand, targeted disruption of the mouse GATA-1 gene prevents primitive and definitive erythroid development.15 To date, it is generally accepted that GATA-1 plays an important role in hematopoietic stem cell differentiation towards erythroid and myeloid lineages.
To further analyze the roles of GATA-1 in regulating the differentiation of hematopoietic stem cells towards the myeloid lineage, we investigated phenotypic changes in the murine myeloid leukemia M1 cell line, which is a well-established in vitro model for studying the differentiation pathway from immature blast cells to mature macrophage, stably transfected with and expressing the murine GATA-1 gene.
MATERIALS AND METHODS
Cell culture.
The murine myeloid leukemia cell, M1, originally established by Ichikawa,20 was maintained at 37°C under 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated newborn calf serum (ICN Biochemicals Japan Co, Osaka, Japan), 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were passaged twice a week at 2 × 105 cells/mL.
Plasmids and electroporation.
We used a murine GATA-1 expression plasmid containing the SV40 enhancer and adenovirus major late promoter (pMG-mGATA1), which provided constitutive expression of GATA-1 mRNA in neomycin-resistant, stably transfected cells. For electroporation, 1.5 × 107cells were pelleted and resuspended in 0.5 mL of electroporation buffer, 21 mmol/L HEPES (pH 7.05), 137 mmol/L NaCl, 5 mmol/L KCl, 0.7 mmol/L Na2HPO4, and 6 mmol/L glucose containing 20 μg Nde I-linearized plasmid DNA and left at room temperature for 5 minutes before electroporation at 320 V, 960 μF, using a Bio-Rad Gene Pulser (Bio-Rad Laboratories, Richmond, VA). The cells were kept on ice for 15 minutes, then resuspended in 10 mL of complete DMEM. After 48 hours, the cells were seeded in Methocel containing 1 mg/mL of G418 (Geneticin; Sigma, St Louis, MO). Two to 3 weeks later, G418-resistant colonies were transferred into liquid medium containing 0.4 mg/mL of G418.
Cytochemical staining.
Cells on cytocentrifuge slides were visualized by means of Wright-Giemsa and acetylcholine esterase staining.
Fluorescence-activated cell sorting (FACS) analysis.
M1 clonal lines transfected with the GATA-1 expression vector, M1GATA-Y1, M1GATA-Y22, and M1GATA-Y25 clone, were stained with biotinylated TER-119 monoclonal antibody (MoAb; PharMingen, San Diego, CA), followed by fluorescein isothiocyanate (FITC)-conjugated streptavidin (Becton Dickinson Immunocytometry System, San Jose, CA). Stained cells were analyzed by FACSvantage (Becton Dickinson Immunocytometry System).
Northern blot analysis.
Total RNA was extracted from M1 cells and their subclones with guanidine isothiocyanate.21 Poly(A)+ RNA was separated by oligotex-dT30 (Takara Shuzo, Japan). The total RNA (20 μg/lane) was denatured in formamide-formaldehyde followed by electrophoresis in a 1% agarose-formaldehyde gel. The RNA was then transferred onto Hybond-N nylon membranes (Amersham International Plc, Buckinghamshire, UK) and hybridized with radiolabeled cDNA probes encoding murine (m) GATA-1,22 mGATA-2,23mβ-globin,24 human (h) platelet factor 4 (PF4),25 mCD34,26 MafK,27 NF-E2 (p45),28 and 18S ribosomal RNA. The membranes were washed twice in 2X SSC containing 0.2% sodium dodecyl sulfate (SDS) at 53°C for 30 minutes and then washed twice in 0.2X SSC containing 0.2% SDS at 55°C for 30 minutes.
Reverse transcription-polymerase chain reaction (RT-PCR).
For murine c-mpl ligand (thrombopoietin), the 5′ primer sequence used was 5′-TGATGGCAGCACGAGGACAGTTGGAA-3′ and the 3′ primer sequence used was 5′-GTGAGGTTCCAGCAAAGAGCCCATG-3′, corresponding to exon 4 and exon 5, respectively.29 30 For murine β-actin, the 5′ primer sequence used was 5′-CTAGACTTCGAGCAGGAGAT-3′ and the 3′ primer sequence used was 5′-GCTCAGTAACAGTCCGCCTAGA-3′. The isolated total RNA was reverse-transcribed in a 20 μL total buffer containing 50 mmol/L Tris-HCl (pH 8.3), 75 mmol/L KCl, 3 mmol/L MgCl2, 10 mmol/L dithiothreitol, a 10 mmol/L dNTP mixture, 100 pmol/L random hexamer oligonucleotides (Takara Shuzo, Kyoto, Japan), and 200 U Moloney murine leukemia virus reverse transcriptase (BRL, Gaithersburg, MD). Double-stranded DNA was then synthesized from the single-stranded DNA with 1 U Thermus aquaticus (Taq) polymerase (Takara Shuzo) and two pairs of oligonucleotides (c-mpl ligand and β-actin), using 25 PCR cycles on a thermocycler (Perkin-Elmer Cetus PCR 1000, Norwalk, CT). Each cycle included denaturation at 94°C for 1 minute, reannealing of the primer, fragmentation at 55°C for 2 minutes, and polymerization at 72°C for 2 minutes.
Isolation of the murine c-mpl promoter.
The 5′ sequence containing exon 1 of the murine c-mpl gene was amplified and cloned from Sal I/BamHI-digested murine genomic DNA by PCR based on the published sequence (31) with primers A (5′-ACGCGTCGACCTGTTCTGACAGCCATATGC-3′) and B (5′-CGCGGATCCCTTCTCCGGCACTGTGTGCCT-3′). The synthesized fragment was 324 bp long, extending from bp −312 to +12 with the transcription start site designated +1.31 This PCR fragment was digested with Sal I and BamHI and cloned upstream of the herpes simplex virus thymidine kinase (TK) promoter followed by firefly luciferase cDNA, pTKLuc/m c-mpl.32 To generate the construct containing murine c-mpl promoter with a mutation in the GATA-binding site (bp −85 to −80), pTKLuc/m c-mpl was used as the template. A 6-bp linker containing a Xho I site (sequence 5′-CTCGAG-3′) was substituted for the wild-type sequence of the −312 bp murine c-mpl promoter construct between bp −85 and −80 using oligonucleotide-directed PCR mutagenesis.33 The PCR fragment was cloned into the luciferase vector pTKLuc, and the resulting construct was sequenced to confirm the correct placement of the linker oligonucleotide.
Transient transfection.
Jurkat cells were electroporated at 250 V, 960 μF, using a Bio-Rad Gene Pulser. The cells were harvested 24 hours posttransfection into 0.5 mL of lysis buffer, and 0.1 mL of this lysate was added to 0.3 mL of assay buffer for luciferase assays. Details of the transfection and luciferase assay procedures were as described.34 The transfection efficiency was normalized to the levels of growth hormone produced by 0.5 μg of cotransfected plasmid containing the cytomegalovirus (CMV) promoter directing human growth hormone gene expression (CMV-hGH). Growth hormone concentrations were measured by radioimmunoassay (Nichols Institute, San Juan Capistrano, CA). Data are expressed as the fold induction over that with the pTKLuc/m c-mpl.
RESULTS
GATA-1 induces the differentiation of the erythroid and megakaryocytic lineages from a murine myeloid leukemic cell line, M1.
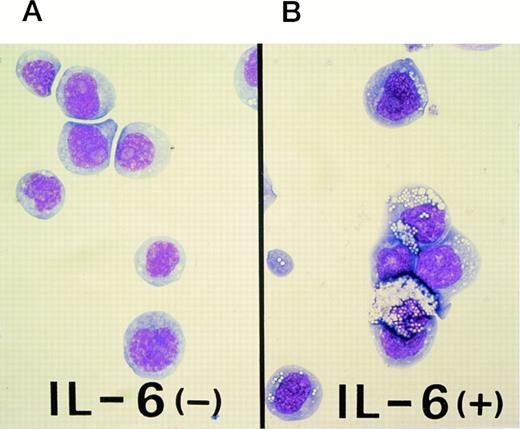
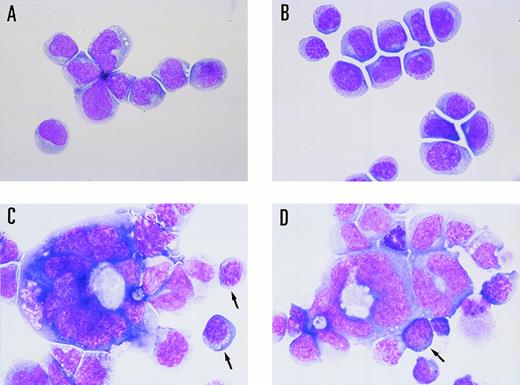
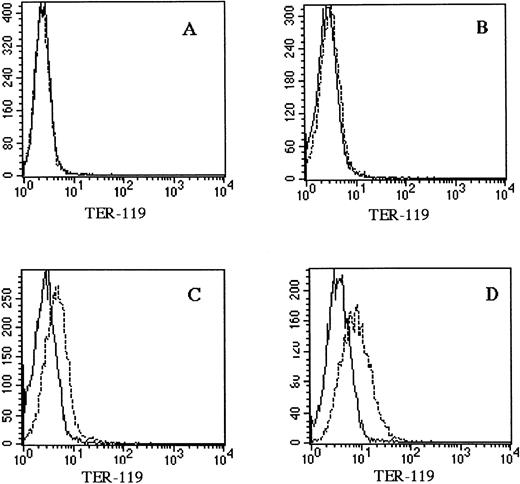
The M1 cell line was originally established in vitro from a spontaneous myeloid leukemia of SL strain mice.20 This cell line expresses abundant CD34, a marker of hematopoietic stem cells, and can differentiate towards the macrophage lineage in response to interleukin-6 (IL-6; Fig 1). As shown in Fig 1B, M1 cells were capable of differentiating towards macrophages that could phagocytize latex beads after stimulation with IL-6. However, M1 cells have not been shown to differentiate towards erythroid and/or megakaryocytic lineages. A linearized murine GATA-1 expression vector containing a neomycin-resistance cassette was electroporated into M1 cells. G418-resistant transfectants were selected, and clonal lines were generated by in vitro colony formation. Three G418-resistant colonies, M1GATA-Y1, M1GATA-Y22, and M1GATA-Y25, were selected for further analysis. Wright-Giemsa staining showed that the M1 clone transfected with a control vector (Fig 2A) and the M1GATA-Y1 clone (Fig 2B) were similar to the parental M1 cells. In contrast, the M1GATA-Y22 and -Y25 clones were distinctly different from the parental M1 cells, consisting of blast cells, erythroblasts, and megakaryocytes (Fig 2C and D). To confirm the presence of erythroblasts in M1 clonal lines transfected with the GATA-1 expression vector, FACS analysis was performed using TER-119 MoAb, which is specific for the erythroid lineage.35 As shown in Fig 3, TER-119 reacted with M1GATA-Y22 and M1GATA-Y25 clones, but not with M1GATA-Y1. This result indicated the erythroid characteristics of M1GATA-Y22 and M1GATA-Y25 clones. The megakaryocytic characteristics of these GATA-1–expressing clones were confirmed by Wright-Giemsa and acetylcholinesterase (ACh E)-specific (data not shown) staining.
Differentiation of M1 cells to phagocytic cells induced by IL-6. (A) Uninduced parental M1 cells, which are blast-like cells. (B) M1 cells were induced to differentiate to phagocytic cells (macrophages) by culturing the cells at an initial concentration of 2 × 105 cells/mL for up to 2 days in the presence of 50 ng/mL IL-6. These cells represent phagocytized latex beads.
Differentiation of M1 cells to phagocytic cells induced by IL-6. (A) Uninduced parental M1 cells, which are blast-like cells. (B) M1 cells were induced to differentiate to phagocytic cells (macrophages) by culturing the cells at an initial concentration of 2 × 105 cells/mL for up to 2 days in the presence of 50 ng/mL IL-6. These cells represent phagocytized latex beads.
Morphological changes induced by electroporation of the GATA-1 expression vector. (A) M1 clone transfected with the control vector (M1/pMG2), (B) M1GATA-Y1 clone, (C) M1GATA-Y22 clone, and (D) M1GATA-Y25 clone. Arrows indicate the erythroblasts.
Morphological changes induced by electroporation of the GATA-1 expression vector. (A) M1 clone transfected with the control vector (M1/pMG2), (B) M1GATA-Y1 clone, (C) M1GATA-Y22 clone, and (D) M1GATA-Y25 clone. Arrows indicate the erythroblasts.
Expression of the erythroid lineage in M1 clonal lines transfected with the GATA-1 expression vector. Cells were incubated with biotinylated TER-119 and FITC-conjugated streptavidin. (A) M1 clone transfected with the control vector (M1/pMG2), (B) M1GATA-Y1 clone, (C) M1GATA-Y22 clone, (D) M1GATA-Y25 clone. (_____), unstained cells; (----), stained cells.
Expression of the erythroid lineage in M1 clonal lines transfected with the GATA-1 expression vector. Cells were incubated with biotinylated TER-119 and FITC-conjugated streptavidin. (A) M1 clone transfected with the control vector (M1/pMG2), (B) M1GATA-Y1 clone, (C) M1GATA-Y22 clone, (D) M1GATA-Y25 clone. (_____), unstained cells; (----), stained cells.
Northern blot analysis of erythroid and megakaryocytic lineage markers.
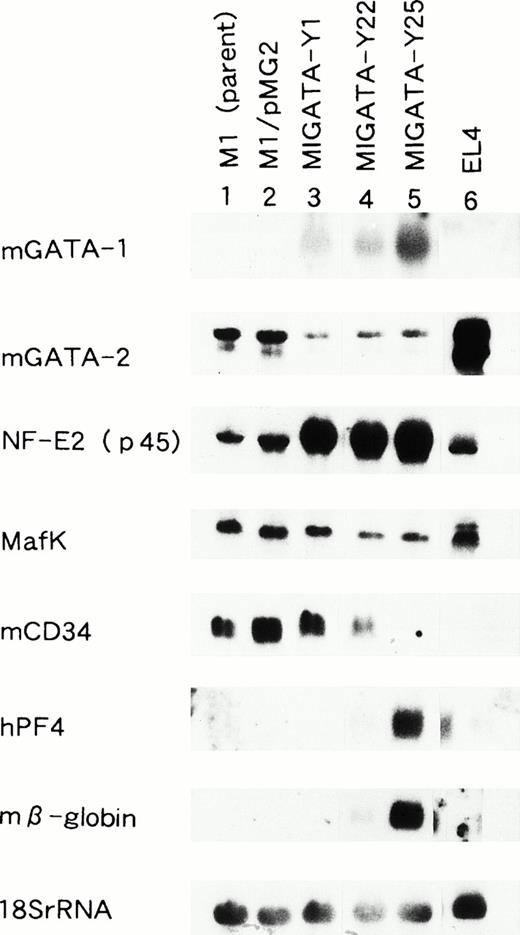
Morphological studies indicated that the selected clones of M1 cells transfected with mGATA-1, M1GATA-Y22, and M1GATA-Y25 possessed the characteristics of erythroblasts and megakaryocytes. To determine whether these clones expressed cellular markers of the erythroid or megakaryocytic lineages, and whether or not the expression level of other transcription factors were altered, we performed Northern blot analyses for the expression of mRNAs for mGATA-1, mGATA-2, NF-E2 (p45), Maf K, mCD34, human platelet factor 4 (hPF4), and mβ-globin (Fig 4). In all of these clones, M1GATA-Y1, -Y22, and -Y25, mGATA-1 mRNA was expressed. In contrast, the parental M1 cells (M1) and M1 clone transfected with a control vector (M1/pMG2) did not express GATA-1. The level of GATA-2 mRNA expression was lower in the M1 clones expressing GATA-1 compared with parental M1 and M1/pMG2 cells. The mRNAs encoding β-globin, an erythroid marker, and PF4, a megakaryocytic marker, were abundantly expressed in the M1GATA-Y25 clone. In the M1GATA-Y22 clone, less β-globin mRNA was expressed and PF4 mRNA expression was minimal. However, neither β-globin nor PF4 were expressed in the M1GATA-Y1 clone. The level of the mRNA encoding mCD34 was decreased in the M1 clones differentiated towards the erythroid and megakaryocytic lineages. NF-E2 p45 and Maf K form heterodimers with the NF-E2 site (TGCTGAGTCAT/C). Whereas the Maf K mRNA was expressed equally in all these clones, M1GATA-Y1, M1GATA-Y22, and M1GATA-Y25, as well as in the parental M1 cells and M1/pMG2 clone, the mRNA encoding NF-E2 p45 was increased only in the M1 clones expressing GATA-1. These findings indicated that M1 clones expressing GATA-1 possess the characteristics of both erythroid and megakaryocytic cells.
Northern blot analysis of M1 clonal lines transfected with the GATA-1 expression vector. Lane 1, parental M1 cells; lane 2, M1 cells transfected with the control vector; lane 3, M1GATA-Y1 clone; lane 4, M1GATA-Y22 clone; lane 5, M1GATA-Y25 clone; lane 6, EL-4 murine thymoma cell line. The membrane containing 15 μg total RNA from each cell line was sequentially hybridized with murine GATA-1, murine GATA-2, NF-E2 (p45), Maf K, murine CD34, human platelet factor 4 (hPF 4), murine β-globin, and 18S rRNA probes.
Northern blot analysis of M1 clonal lines transfected with the GATA-1 expression vector. Lane 1, parental M1 cells; lane 2, M1 cells transfected with the control vector; lane 3, M1GATA-Y1 clone; lane 4, M1GATA-Y22 clone; lane 5, M1GATA-Y25 clone; lane 6, EL-4 murine thymoma cell line. The membrane containing 15 μg total RNA from each cell line was sequentially hybridized with murine GATA-1, murine GATA-2, NF-E2 (p45), Maf K, murine CD34, human platelet factor 4 (hPF 4), murine β-globin, and 18S rRNA probes.
c-mpl and c-mpl ligand (thrombopoietin) mRNA expression.
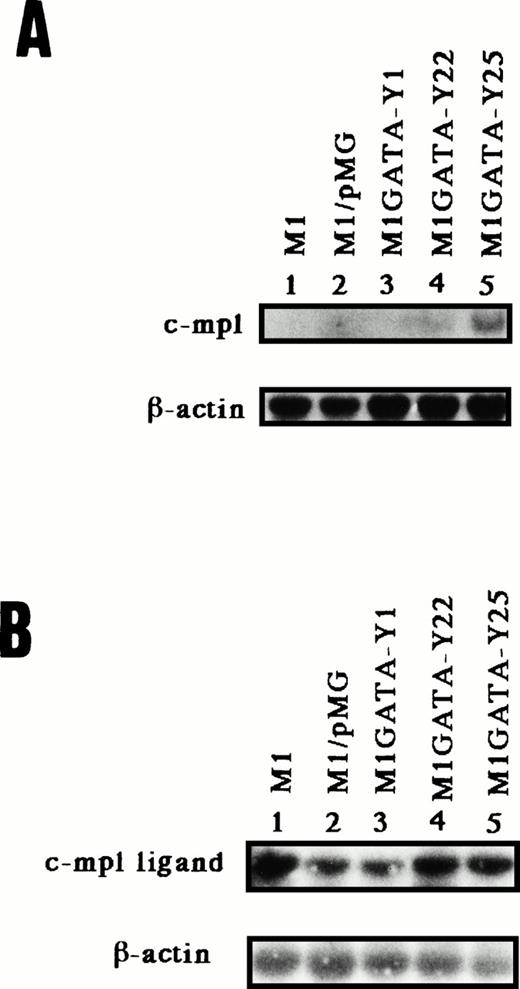
It has been reported that c-mpl and thrombopoietin play pivotal roles in megakaryocyte development.36 Accordingly, to determine whether the c-mpl or c-mpl ligand are expressed in M1 clones that differentiate into megakaryocytes, we examined the expression of the mRNA for the c-mpl and c-mpl ligand by Northern blot or RT-PCR followed by Southern blot, respectively. Then each primer used for RT-PCR of c-mpl ligand was derived from the different exons. Northern blot analysis showed that the expression of mRNA for c-mpl was detected in the M1GATA-Y22 and -25 clones but not M1, M1 cells transfected with a control vector (M1/pMG), and M1GATA-Y1 clone (Fig 5A). In contrast, the mRNA for thrombopoietin was expressed in all clones of cells transfected with the GATA-1 expression vector, as well as in M1 and M1/pMG cells (Fig5B). These data suggested that c-mpl expression in stable GATA-1–expressing M1 clones differentiated towards megakaryocytes (M1GATA-Y22 and -Y25) is closely associated with megakaryocytic differentiation.
mRNA expression of murine c-mpl by Northern blot (A) and that of murine c-mpl ligand (thrombopoietin) by RT-PCR followed by Southern blot (B) in the M1 clonal lines stably transfected with the GATA-1 expression vector. The membrane containing 5 μg poly(A)+ RNA from each cell was hybridized with murine c-mpl and β-actin cDNA (A). Murine β-actin was the internal control for RT-PCR (B). Lane 1, parental M1 cells; lane 2, M1 cels transfected with control vector; lane 3, M1GATA-Y1 clone; lane 4, M1GATA-Y22 clone; lane 5, M1GATA-Y25 clone.
mRNA expression of murine c-mpl by Northern blot (A) and that of murine c-mpl ligand (thrombopoietin) by RT-PCR followed by Southern blot (B) in the M1 clonal lines stably transfected with the GATA-1 expression vector. The membrane containing 5 μg poly(A)+ RNA from each cell was hybridized with murine c-mpl and β-actin cDNA (A). Murine β-actin was the internal control for RT-PCR (B). Lane 1, parental M1 cells; lane 2, M1 cels transfected with control vector; lane 3, M1GATA-Y1 clone; lane 4, M1GATA-Y22 clone; lane 5, M1GATA-Y25 clone.
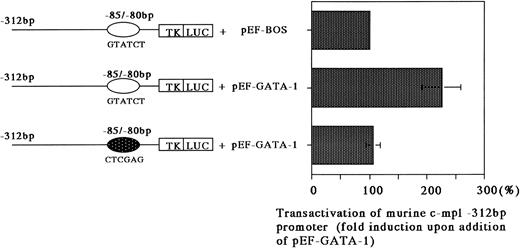
GATA-1 transactivates the c-mpl promoter.
The previously stated results suggested that c-mpl expression, as regulated through GATA-1, may be important for megakaryocytic differentiation. Therefore, we addressed whether or not GATA-1 can transactivate the c-mpl promoter in Jurkat cells that do not express c-mpl or GATA-1. The promoter region and the first exon of murine c-mpl gene (bp −312 to +12) were cloned upstream of the TK promoter followed by firefly luciferase cDNA.32 Constructs containing the intact murine c-mpl promoter with the GATA motif (bp −85 to −80) and the mutated promoter with a 6-bp linker substitution of the GATA-binding site (bp −85 to −80) were cotransfected into Jurkat cells with the human GATA-1 expression vector pEF-hGATA-1. GATA-1 was able to enhance the activity of a construct that contained a GATA-binding site upstream of the minimal TK promoter by an average of 2.3-fold (Fig 6). A plasmid with the mutated GATA-binding site did not show transactivation greater than that of the minimal TK promoter (Fig 6). These findings support the functional importance of GATA-1 in the regulation of c-mpl expression via binding to its recognition site.
Transactivation of a minimal TK promoter containing the murine c-mpl GATA-binding site. Jurkat cells were transfected with either pTKLuc/m c-mpl containing a wild-type GATA-binding site, or pTKLuc/m c-mpl containing a mutated GATA-binding site, bp −85 to −80, with and without 10 μg of pEF-hGATA-1. The results are expressed as the average fold increase in ability on addition of pEF-hGATA-1. The error bars represent the standard error of the mean of at least four experiments. Luciferase assay was normalized to β-galactosidase produced by a cotransfected CMV-β Gal plasmid.
Transactivation of a minimal TK promoter containing the murine c-mpl GATA-binding site. Jurkat cells were transfected with either pTKLuc/m c-mpl containing a wild-type GATA-binding site, or pTKLuc/m c-mpl containing a mutated GATA-binding site, bp −85 to −80, with and without 10 μg of pEF-hGATA-1. The results are expressed as the average fold increase in ability on addition of pEF-hGATA-1. The error bars represent the standard error of the mean of at least four experiments. Luciferase assay was normalized to β-galactosidase produced by a cotransfected CMV-β Gal plasmid.
DISCUSSION
We found that the overexpression of GATA-1 in the murine myeloid leukemic cell line, M1, leads to erythroid and megakaryocytic differentiation. Erythroid cells were confirmed by the morphology, expression of TER-119, and β-globin mRNA. Megakaryocytes were verified by the morphology, acetylcholinesterase staining and the expression of PF4 mRNA. M1 clones differentiated by forced GATA-1 expression were heterogenous in nature and formed cells of both the erythroid and megakaryocytic lineages. This finding suggests the presence of bilineage precursor cells capable of differentiating towards erythroid and megakaryocytic lineages in response to GATA-1 expression. Visvader et al37 have reported that introducing a GATA-1 expression vector into the early myeloid cell line 416B, which originally differentiated along the megakaryocytic and granulocytic pathways, resulted in the most restricted differentiation to the megakaryocytic lineage. Whereas 416B cells showed megakaryocytic characteristics before introducing a GATA-1 expression vector, the M1 cell line has been known for its potential for granulocyte-macrophage differentiation on stimulation with leukemia inhibitory factor (LIF) or IL-6, but not for megakaryocytic differentiation. This indicates that GATA-1 regulates the switching of the differentiation from bilineage precursors to cells of either the myeloid or erythroid/megakaryocyte lineage.
M1GATA-Y22 and -Y25 clones which expressed abundant GATA-1 mRNA differentiated towards erythroid and megakaryocyte lineages, but M1GATA-Y1, which expressed a low level of GATA-1 mRNA, did not. This result suggested that the high levels of GATA-1 expression are required for the erythroblastic or megakaryocytic differentiation of the M1 cell line by the GATA-1 transgene. Visvader et al37 have also reported that clones expressing a low level of GATA-1 mRNA did not show any megakaryocytic differentiation. In addition, it has been suggested that high levels of GATA-1 correlate with the differentiation of thromboblasts and erythroblasts, whereas intermediate levels induce eosinophil development.19
The c-mpl ligand, thrombopoietin, has been cloned and shown to induce progenitor cells to differentiate into platelet-producing megakaryocytes.29 In this study, mRNAs encoding c-mpl ligand were expressed in parental M1 cells and in all the stably transfected clones of M1 cells expressing GATA-1. In contrast, c-mpl receptor mRNA was expressed only in the clones of M1 cells that differentiated towards erythroblasts and megakaryocytes. In addition, we showed that GATA-1 could significantly transactivate the c-mpl promoter activity via the GATA-binding consensus site. In our study, although the magnitude of transactivation of the c-mpl promoter by GATA-1 was slight, the mutation at the GATA motif (bp −85 to −80) in the c-mpl promoter abolished the transactivation activity by GATA-1 (Fig 6). This indicates that GATA-1 is one of the transcription factors associated with the expression of the gene encoding c-mpl. Alexander and Dunn31 have speculated that Ets and GATA factors play an important role in expressing the c-mpl gene. In the analysis of human c-mpl gene promoter, it has been shown that GATA-1 binds with low affinity to a unique GATA motif at −70 bp in the human c-mpl promoter, and destruction of this site resulted in a 15% decrease of the promoter activity.38,39 From our data, although GATA-1 transactivated the murine mpl promoter activity, it did not indicate the full promoter activity. Transcription factors other than GATA-1, such as Ets factor, which bound immediately downstream of the GATA motif in the murine c-mpl promoter, may act synergistically with GATA-1 in the mpl gene expression. Deveaux et al39 have shown that the c-mpl promoter can be bound by GATA-1 and ETS proteins, including ETS-1, Fli-1, and Elf-1, and that the ETS motif adjacent to the GATA site is crucial for its expression. Also, it has been shown that the interaction between Sp1 and Ets factors play an important role in transcription of the megakaryocyte-specific αIIb gene.40 It is important to analyze which ets transcription factors are expressed during megakaryocyte differentiation. Besides, GATA-1 might induce other transcription factors that enhance c-mpl promoter activity. Further studies are needed on the c-mpl promoter, including an examination of the binding of GATA-1 in the murine c-mpl promoter by means of an electrophoretic mobility shift assay (EMSA) or the detailed functional promoter activity by linker-scanning mutants. Our findings suggested that the stably transfected GATA-1–expressing M1 clones differentiate towards megakaryocytes via an autocrine mechanism involving the expression of both c-mpl ligand and c-mpl receptor. However, at present, it is difficult to justify the autocrine mechanism via the expression of c-mpl ligand and c-mpl receptor by GATA-1. It has been reported that cells of the 32D promyelocytic line transfected with murine c-mpl are morphologically similar to early megakaryocytes but lack expression of the megakaryocyte markers, acetylcholinesterase and PF4.41 These findings suggest that in 32D cells, signaling through c-mpl and thrombopoietin may not be sufficient for megakaryocytic differentiation. Recently, it has been shown that in vivo overexpression via a retroviral vector of c-mpl leads to a fatal myeloproliferative syndrome characterized by hyperproliferation of the erythroblastic compartment.42 In addition, it has been reported that mpl ligand enhances proliferation of erythroid progenitors.43 44 This evidence may suggest that the c-mpl induced by GATA-1 is involved in the proliferation and differentiation of cells of not only the megakaryocytic but also the erythroid lineages.
In all clones of M1 cells overexpressing GATA-1, expression of the mRNA for mGATA-2 was downregulated (Fig 3). In mammalian hematopoietic cells, GATA-2 mRNA is expressed at high levels in megakaryocytes and mast cells but at very low or undetectable levels in murine erythroleukemia cell lines, in which mGATA-1 mRNA expression is increased (data not shown). In addition, the level of GATA-2 mRNA, which is expressed in quiescent hematopoietic progenitor cells, declines with erythroid and granulocytic differentiation, whereas GATA-1 mRNA expression is induced during both erythroid and megakaryocytic differentiation.45 46 These findings suggest that GATA-1 suppresses GATA-2 gene expression.
CD34 is expressed on pluripotential hematopoietic stem cells, and its expression declines with differentiation.47 Expression of mCD34 mRNA was also decreased in M1 cells overexpressing mGATA-1, which were differentiated towards erythroid or megakaryocytic lineages (Fig4). This evidence suggested that GATA-1 exerts a negative effect on CD34 gene expression or perhaps induces transcription factors that bind to a negatively acting cis element of the mCD34 promoter.
In conclusion, forced expression of GATA-1 in the myeloid leukemic cell line, M1, induces the differentiation towards erythroid and megakaryocytic lineage associated with the induction of c-mpl gene.
ACKNOWLEDGMENT
We are grateful to Dr M. Poncz for providing the human platelet factor 4 cDNA probe and Dr M. Hashiyama for the FACS analysis.
Supported by Grants-in-Aid from the Ministry of Education, Science and Culture of Japan.
Address reprint requests to Yuji Yamaguchi, MD, Department of Cell Differentiation, Institute of Molecular Embryology and Genetics, Kumamoto University School of Medicine, Honjo 2-2-1, Kumamoto, 860 Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal