The purpose was to verify the 5-year results of the MOPPEBVCAD chemotherapy regimen with limited radiotherapy in relation to the promising preliminary data. Mechlorethamine, vincristine, procarbazine, prednisone, epidoxorubicin, bleomycin, vinblastine, lomustine, melphalan, and vindesine were delivered according to a schedule derived through hybridization, intensification, and shortening of the corresponding alternating CAD/MOPP/ABV regimen. Radiotherapy was restricted to sites of bulky involvement or to areas that responded incompletely to chemotherapy. This multicenter, controlled, nonrandomized trial involved 145 eligible patients. Radiotherapy was administered to 47 patients, 46 of whom were in complete remission after chemotherapy. Remissions were complete in 137 patients (94%), partial in 4 (3%), and null in the remaining 4. Tumor-specific, overall, relapse-free, and failure-free survival at 5 years were 0.89, 0.86, 0.82, and 0.78, respectively. Hematologic toxicity was considerable, whereas nonhematologic side effects were fully acceptable. Most of the unfavorable prognostic factors lost their clinical weight. Only age and lymphocyte depletion histologic type were statistically correlated with major follow-up endpoints; performance status and bone marrow involvement were subordinate to age. Seven patients developed a second cancer (including 3 myelodysplasias). MOPPEBVCAD with selected radiotherapy is a highly effective regimen in advanced Hodgkin's disease. Early and late toxicity are no more severe than what would be expected with other alternating or hybrid regimens. A comparison with ABVD, which is currently considered the standard regimen for advanced Hodgkin's disease, is needed.
IN THE LAST DECADE, increasing evidence made it clear that the treatment of advanced Hodgkin's disease (HD) should be based on two distinct chemotherapy (CT) regimens, possibly combined with variable radiotherapy (RT), ie, MOPP (mechlorethamine, vincristine, procarbazine, and prednisone) and ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine). Both regimens have been tested on large patient series, both achieve equivalent results,1,2 both are non–cross-resistant,3 and both have no better four- or five-drug variants.4-8
However, in the 1980s, clinical application of the mathematical model of Goldie and Coldman9 to the drug sensitivity of tumor cells according to their spontaneous mutation rate led many investigators to design CT schedules that alternated some of the most effective regimens, chiefly MOPP and ABVD. Therefore, together with the alternating MOPP/ABVD schedule,10 many other similar combinations were designed, such as MOPP/CABS,11MOP-BAP,12 MOPP-CAVmP,13BCVPP/ABD,14 LOPP/EVAP,15ChlVPP/PABIOE,16 but none of them seemed to be superior to MOPP. In the Cancer and Leukemia Group B randomized trial, MOPP/ABVD was as effective as ABVD alone and more effective than MOPP with regard to complete remission rate and failure-free survival, but it did not show statistically significant better survival.17
The Goldie and Coldman model reached its extreme exploitation in CT regimens that delivered, within each cycle, all the drugs previously scheduled in alternating courses. This strategy allowed tumor cells more exposure to drugs having different mechanisms of action, which should also contribute to reducing the chance that resistant cell clones develop and grow. To this aim, the so-called hybrid regimens were designed, such as MOPP/ABV,18 MA/MA,19ChlVPP/EVA,20 and BEACOPP.21 So far, such hybrid protocols have not shown results clearly superior to those obtained with their alternating counterparts or even with ABVD alone, which, moreover, proved to be less toxic during a short follow-up.22
In agreement with the ongoing evolution of CT for advanced HD, in 1987 the Italian Lymphoma Study Group (GISL) designed a shortened, hybridized, and intensified version of Straus' alternating regimen CAD/MOPP/ABV23 and combined it with optional use of RT on very selected and limited areas. The preliminary data on feasibility, early results, and toxicity of this modified CT program were very encouraging.24
We report here the final results of this multicenter trial using hybrid MOPP/EBV/CAD (or, simply, MOPPEBVCAD) CT, with or without limited RT.
MATERIALS AND METHODS
Patient population.
The criteria for enrollment in the study were (1) unequivocal histologic diagnosis of HD; (2) age of 15 to 75 years; (3) no previous treatment; and (4) advanced or unfavorably presenting disease, defined as stage II B, III B, IV or subdiaphragmatic stage III A with lymphocyte-depleted nodular sclerosis or lymphocyte-depletion histologic types, or all histologic types of stage III2A. One hundred and fifty-five patients were entered into the study between January 1, 1988 and September 30, 1993, the date on which the trial was closed. Ten patients were subsequently excluded from the series: 5 on account of histologic reevaluation, which changed the diagnosis to anaplastic large-cell (CD30+) lymphoma (3 cases), T-cell–rich B-cell lymphoma (1 patient), and undifferentiated anaplastic carcinoma of the thyroid (1 case), respectively, and 5 because of staging inaccuracy (2 cases with actual stage IIA disease) or protocol violations (3 patients who received 1 or 2 cycles of MOPPEBVCAD then continued with either ABVD [2 cases] or MOPP [1 case] for reasons other than clinical, less frequent administration, distance from the hospital, etc).
Therefore, a total of 145 patients were eligible for the study and their clinical characteristics are listed in Table 1. The annual enrollment rate also reflects the increasing number of institutions that joined the GISL during the years of this investigation; the enrollment rate was as follows: 8% (1988), 16% (1989), 19% (1990), 23% (1991), 20% (1992), and 14% (first 9 months of 1993).
Clinical Characteristics of the Patients Studied
| Characteristics . | No. . | % . | Mean ± 1 SD . |
|---|---|---|---|
| Total | 145 | 100 | |
| Gender | |||
| Male | 85 | 59 | |
| Female | 60 | 41 | |
| Age (median, 35 yr; range, 16-75 yr) | |||
| 15-19 | 17 | 12 | |
| 20-29 | 43 | 29 | |
| 30-39 | 27 | 19 | |
| 40-49 | 27 | 19 | |
| 50-59 | 10 | 7 | |
| 60-69 | 17 | 11 | |
| 70-75 | 4 | 3 | |
| Histology | |||
| Lymphocyte predominance | 2 | 1 | |
| Nodular sclerosis | 68 | 46 | |
| Lymphocyte depleted nodular sclerosis | 20 | 14 | |
| Mixed cellularity | 42 | 30 | |
| Lymphocyte depletion | 13 | 9 | |
| Stage | |||
| II B | 45 | 31 | |
| III2 A | 22 | 15 | |
| III B | 39 | 27 | |
| IV A | 11 | 8 | |
| IV B | 28 | 19 | |
| Mediastinal bulk | 47 | 32 | |
| Spleen involvement | 29 | 20 | |
| Extranodal involvement | |||
| Bone marrow | 22 | 15 | |
| Lung | 9 | 6 | |
| Liver | 6 | 4 | |
| Other visceral organs | 3 | 2 | |
| Hb <100 g/L | 16 | 11 | 119 ± 20 |
| ESR >40 mm 1st hour | 95 | 66 | 61.6 ± 37.6 |
| Serum LDH >450 U/L | 33 | 23 | 400 ± 225 |
| Serum albumin <30 g/dL | 26 | 18 | 3.64 ± 0.59 |
| SNLG prognostic index >0.5 | 52 | 36 | 0.39 ± 0.2 |
| IDHD probability of surviving at 5 yr | 0.71 ± 0.18 |
| Characteristics . | No. . | % . | Mean ± 1 SD . |
|---|---|---|---|
| Total | 145 | 100 | |
| Gender | |||
| Male | 85 | 59 | |
| Female | 60 | 41 | |
| Age (median, 35 yr; range, 16-75 yr) | |||
| 15-19 | 17 | 12 | |
| 20-29 | 43 | 29 | |
| 30-39 | 27 | 19 | |
| 40-49 | 27 | 19 | |
| 50-59 | 10 | 7 | |
| 60-69 | 17 | 11 | |
| 70-75 | 4 | 3 | |
| Histology | |||
| Lymphocyte predominance | 2 | 1 | |
| Nodular sclerosis | 68 | 46 | |
| Lymphocyte depleted nodular sclerosis | 20 | 14 | |
| Mixed cellularity | 42 | 30 | |
| Lymphocyte depletion | 13 | 9 | |
| Stage | |||
| II B | 45 | 31 | |
| III2 A | 22 | 15 | |
| III B | 39 | 27 | |
| IV A | 11 | 8 | |
| IV B | 28 | 19 | |
| Mediastinal bulk | 47 | 32 | |
| Spleen involvement | 29 | 20 | |
| Extranodal involvement | |||
| Bone marrow | 22 | 15 | |
| Lung | 9 | 6 | |
| Liver | 6 | 4 | |
| Other visceral organs | 3 | 2 | |
| Hb <100 g/L | 16 | 11 | 119 ± 20 |
| ESR >40 mm 1st hour | 95 | 66 | 61.6 ± 37.6 |
| Serum LDH >450 U/L | 33 | 23 | 400 ± 225 |
| Serum albumin <30 g/dL | 26 | 18 | 3.64 ± 0.59 |
| SNLG prognostic index >0.5 | 52 | 36 | 0.39 ± 0.2 |
| IDHD probability of surviving at 5 yr | 0.71 ± 0.18 |
An exact estimate of the expected sample size of the study was not made, because this protocol was part of a general plan that divided treatment strategy into three different risk groups.25 A preliminary report was published on the first 80 patients, who were evaluable at the end of 1991, regarding the feasibility, tolerability, and early clinical results of the study.24 At that time, the advisability of stopping the trial and starting a randomized study to compare MOPPEBVCAD with one of the best current regimens was clear. However, the GISL rate of patient accrual was not sufficient to sustain a randomized study until other groups began to cooperate.
Disease stage was investigated according to the requirements of the Cotswolds Meeting.26 In particular, staging procedures routinely involved chest roentgenogram, computed tomography of the thorax and abdomen, ultrasonography of the abdomen, and bone marrow biopsy. None of the patients was staged with exploratory laparotomy and splenectomy. Every clinical, radiologic, or laboratory abnormality found at pretreatment staging was retested at the end of treatment to evaluate response.
In 1995, pathologists were called on to revise the histologic assessment of all cases of their own center and to submit equivocal specimens to two external pathologists; 27 cases underwent such intercenter reevaluation.
Chemotherapy.
The criteria for hybridization, dose intensification, and schedule shortening through which MOPPEBVCAD was derived from the alternating three-drug combination CAD/MOPP/ABV have already been detailed elsewhere.24 Six cycles of chemotherapy were planned. Drug doses and administration schedules are listed in Table 2, which also reports drug-dose modifications according to blood counts. Growth factors were not available until the end of 1991. However, starting in 1992, their use was very restricted and they were used only in cases of severe febrile neutropenia. Dose intensity was calculated according to the criteria reported by Hryniuk27 and the examples and suggestions offered by DeVita et al.28
MOPPEBVCAD Hybrid Regimen: Drug Doses and Time Schedule, With Dose Reduction According to Blood Cell Counts
| Drugs . | Dose (mg/m2) . | Route . | Days . |
|---|---|---|---|
| Mechlorethamine (NH2) | 6 | IV | 1 cycles 1, 3, and 5, only |
| Lomustine (CCNU) | 100 | Oral | 1 cycles 2, 4, and 6, only |
| Vindesine (VDZ) | 3 | IV | 1 |
| Melphalan (Alk) | 6 | Oral | 1-3 |
| Prednisone (Pred) | 40 | Oral | 1-14 |
| Epidoxorubicin (Epi) | 40 | IV | 8 |
| Vincristine (VCR) | 1.4 | IV | 8 |
| Procarbazine (PCZ) | 100 | Oral | 8-14 |
| Vinblastine (VBL) | 6 | IV | 15 |
| Bleomycin (BLM) | 10 | IV | 15 |
| Drugs . | Dose (mg/m2) . | Route . | Days . |
|---|---|---|---|
| Mechlorethamine (NH2) | 6 | IV | 1 cycles 1, 3, and 5, only |
| Lomustine (CCNU) | 100 | Oral | 1 cycles 2, 4, and 6, only |
| Vindesine (VDZ) | 3 | IV | 1 |
| Melphalan (Alk) | 6 | Oral | 1-3 |
| Prednisone (Pred) | 40 | Oral | 1-14 |
| Epidoxorubicin (Epi) | 40 | IV | 8 |
| Vincristine (VCR) | 1.4 | IV | 8 |
| Procarbazine (PCZ) | 100 | Oral | 8-14 |
| Vinblastine (VBL) | 6 | IV | 15 |
| Bleomycin (BLM) | 10 | IV | 15 |
| Leukocyte Count (109/L) . | Platelet Count (109/L) . | Doses and Drugs . |
|---|---|---|
| >4 | >150 | 100% All drugs |
| 3.000-3.999 | 100-149 | 100% VCR, BLM, Pred, |
| 50% VBL, VDZ, Alk, HN2, Epi, CCNU, PCZ | ||
| 2.000-2.999 | 50-99 | 100% VCR, BLM, Pred |
| 25% VDZ, VBL, Alk, NH2, Epi, PCZ | ||
| 0% CCNU | ||
| 1.500-1.999 | 50-99 | 100% BLM, Pred |
| 50% VCR | ||
| 25% VDZ, VBL, Alk, NH2, Epi, PCZ | ||
| 0% CCNU | ||
| <1.500 | <50 | No drugs: reevaluation after 1 wk |
| Leukocyte Count (109/L) . | Platelet Count (109/L) . | Doses and Drugs . |
|---|---|---|
| >4 | >150 | 100% All drugs |
| 3.000-3.999 | 100-149 | 100% VCR, BLM, Pred, |
| 50% VBL, VDZ, Alk, HN2, Epi, CCNU, PCZ | ||
| 2.000-2.999 | 50-99 | 100% VCR, BLM, Pred |
| 25% VDZ, VBL, Alk, NH2, Epi, PCZ | ||
| 0% CCNU | ||
| 1.500-1.999 | 50-99 | 100% BLM, Pred |
| 50% VCR | ||
| 25% VDZ, VBL, Alk, NH2, Epi, PCZ | ||
| 0% CCNU | ||
| <1.500 | <50 | No drugs: reevaluation after 1 wk |
When leukocytes are less than 3.0 and/or platelets are less than 100 × 109/L before the start of a new cycle, a 1 week delay is preferred to dose reductions.
Abbreviation: IV, intravenous.
Radiotherapy.
RT was not routinely associated with CT but was administered to a limited number of patients and only to 1 or 2 selected areas corresponding to previous bulky involvement or to masses that were only slowly or partially reduced during CT. RT had to be administered after CT and total doses could not exceed 35 Gy. The decision to treat, which sites to treat, and which dose to deliver were left to the clinicians and radiotherapists of each institution. In 4 patients, the extensive initial involvement of supradiaphragmatic areas required truly extended-field mantle RT.
Assessment of response and statistical analysis.
Complete remission (CR) was defined as complete regression of measured lesions and disappearance of all other objective evidence of lymphoma for at least 3 months. Partial remission (PR) consisted of a decrease of more than 50% in the sum of the products of the diameters of the measurable lesions. No response (NR) was anything less than a 50% decrease in measurable lesions. According to the Cotswolds Meeting recommendation,26 the category of unconfirmed or uncertain complete remission (CR[u]) was accepted to denote patients in normal health with no clinical evidence of HD but with some radiological abnormality in a site of previous disease. However, uncertainty about the completeness of such a remission had to be resolved within 6 months from the end of therapy. The definition of bulky masses met the criteria coded in the Cotswolds Meeting, ie, for a mediastinal mass, when its maximum width exceeded one third of the internal transverse diameter of the thorax at the level of the disc between T5 and T6 vertebrae and, for any extramediastinal mass, when its largest diameter was greater than 10 cm. A mass was judged to respond slowly when less than a PR was achieved after the third cycle in patients responding at least partially at the end of chemotherapy.
The Scotland and Newcastle Lymphoma Group (SNLG) prognostic index29 and the International Database on Hodgkin's Disease (IDHD)30 estimate of the probability of surviving year by year were retrospectively calculated for each patient according to the investigators' guidelines. These parameters were used as synthetic risk predictors derived from some of the most recent systematic investigations directed at selecting the best prognostic factors in HD.
Toxicity was measured according to standard ECOG criteria.31
Overall survival (OS) was determined from the date of diagnosis to the date of last observation or death (from any cause). The same time frame was used to calculate tumor mortality (TM), with the exception of censoring deaths occurring in complete remission of HD and not directly due to therapy. Relapse-free survival (RFS) for complete responders was measured from the date of therapy completion to the date of last observation or relapse. Failure-free survival (FFS) was computed from the start of treatment to one of the following events: death from any cause, disease progression during treatment, no CR at the end of treatment, or relapse.
Survival curves were calculated using the method of Kaplan and Meier.32 Deaths due to causes other than HD or treatment were not censored, except for RFS.33
Many clinical features were scrutinized singly at diagnosis to evaluate their individual role in discriminating OS, RFS, and FFS. In this univariate study, the Kaplan and Meier estimate was used for qualitative variables, whereas a simple linear regression analysis applied to the proportional hazard model34 was used for quantitative parameters. A multiple regression analysis was then performed, within the same proportional hazard model, to select the best clinical features related to OS, RFS, and FFS, respectively. In these analyses, the SNLG index and the IDHD estimate of the survival probability at 5 years were used as single, distinct covariates.
RESULTS
Of the 145 eligible patients, 137 (94%) achieved CR, 136 with chemotherapy alone. Four obtained only a PR (3%) and the other 4 did not respond at all (3%).
A total of 844 cycles were administered and evaluated; the mean number of cycles per patient was 5.8 (range, 3 to 8). Six patients received more than 6 cycles due to slowly responding symptoms or adenomegalies. All but 1 of them reached CR.
In 18 patients, treatment was stopped before the sixth cycle because of severe hematological toxicity (14 cases), infection (1 case ofPneumocystis carinii pneumonia and 1 of B-virus hepatitis), or patient refusal of further therapy (2 cases). Four of these 18 subjects died with persisting evidence of disease, whereas another relapsed after 3 months of CR and died of the disease during salvage treatment. The remaining 13 achieved stable CR.
CT was administered on an outpatient basis in the great majority of cases and only 18 subjects had to be hospitalized for complications.
Dose intensity was 0.76 ± 0.12 for the first 3 cycles and 0.72 ± 0.11 over all 6 cycles. According to the criteria for dose adjustment on the basis of white blood cell and platelet counts, delaying therapy was preferred to decreasing drug doses if severe myelosuppression occurred near the beginning of a new cycle, whereas the opposite strategy was followed when myelosuppression appeared before completion of the cycle. In 1992, growth factors became available in Italy, but the possibility of further dose intensification was not pursued. Granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) were used strictly on clinical demand, ie, when the neutrophil count decreased to less than 0.5 × 109/L and fever or other signs of infection were present. Twenty-four patients in all (16.5%) received at least a few days of therapy with G-CSF or GM-CSF.
Table 3 shows the toxicity associated with administration of the drug regimen.
Maximal Grades of Acute Hematological and Nonhematological Toxicity Experienced by Patients (Percentages of 145 Cases)
| . | ECOG Grade . | ||||
|---|---|---|---|---|---|
| 0 . | I . | II . | III . | IV . | |
| Hemoglobin | 16 | 51 | 19 | 10 | 4 |
| White blood cell count | 0 | 25 | 32 | 26 | 17 |
| Neutrophil count | 0 | 20 | 29 | 30 | 21 |
| Platelet count | 9 | 14 | 21 | 29 | 27 |
| Nausea and vomiting | 21 | 28 | 37 | 14 | 0 |
| Alopecia | 12 | 29 | 32 | 27 | 0 |
| Neurologic toxicity | 26 | 47 | 20 | 7 | 0 |
| Mucositis | 45 | 48 | 7 | 0 | 0 |
| . | ECOG Grade . | ||||
|---|---|---|---|---|---|
| 0 . | I . | II . | III . | IV . | |
| Hemoglobin | 16 | 51 | 19 | 10 | 4 |
| White blood cell count | 0 | 25 | 32 | 26 | 17 |
| Neutrophil count | 0 | 20 | 29 | 30 | 21 |
| Platelet count | 9 | 14 | 21 | 29 | 27 |
| Nausea and vomiting | 21 | 28 | 37 | 14 | 0 |
| Alopecia | 12 | 29 | 32 | 27 | 0 |
| Neurologic toxicity | 26 | 47 | 20 | 7 | 0 |
| Mucositis | 45 | 48 | 7 | 0 | 0 |
Hematologic toxicity was more severe (and more frequently dose-limiting) than nonhematological side effects. Eleven patients (8%) required transfusion of at least 1 U of concentrated erythrocytes. Thirty-seven febrile episodes were recorded throughout the treatment of the 30 patients (21%; Table 3) who registered at least one neutrophil count less than 0.5 × 109/L. In this regard, the criteria adopted in 1992 for the use of growth factors helped to limit the length and severity of serious infections in the second part of the trial, but did not affect their number throughout the study. One P carinii pneumonia, 1 cytomegalovirus infection, 1 acute B-virus hepatitis (likely posttransfusional), and 2 cases of thoracic herpes zoster were recorded; interruption of chemotherapy due to these illnesses had no effect on clinical response. Seven subjects developed prolonged asymptomatic pancytopenia, presenting a hemoglobin level of less than 10.0 g/dL, a white blood cell count of less than 2 × 109/L, and a platelet count of less than 100 × 109/L for 3 months or longer after the end of therapy.
Nonhematological toxicity was acceptable. Most of the cases of grade 2-3 nausea and/or vomiting were recorded before the antiserotoninergic receptor drugs became available, so that this side effect can be considered well controlled. Neurotoxicity required dose reduction of vincristine and vinblastine in 7 subjects. Mucositis was always mild. Two cases of pulmonary fibrosis were observed: the first (of grade 2 intensity) occurred during chemotherapy and was related to the administration of bleomycin (which was promptly stopped); the second (of grade 3 intensity) became evident 2 months after radiotherapy. One case of probable cardiotoxicity due to anthracycline consisting of a reversible episode of flutter-fibrillation occurred in a 33-year-old patient during the second cycle. Epidoxorubicin was omitted from this subject's subsequent cycles.
RT was administered in 47 patients (32%), 46 of whom were evaluated to be in CR or in CRu after completion of chemotherapy. Patients received RT with different supervoltage equipment in 13 distinct radiotherapy units. Dose fractions varied from 0.16 to 0.22 Gy and total doses ranged from 26 to 42 Gy (median, 35 Gy). The number of irradiated anatomic sites was 1 in 21 patients, 2 in 12, and 3 in 4. The areas to be irradiated were selected according to their bulky involvement at diagnosis or slow or partial response to chemotherapy. Extended-field radiotherapy was delivered in 10 patients (above the diaphragm in 9 and below the diaphragm in 1) either because of the extensive and massive involvement of other lymph node sites besides the one evaluated as bulky or because chemotherapy interruption (for complications, patient refusal to continue, etc) was anticipated.
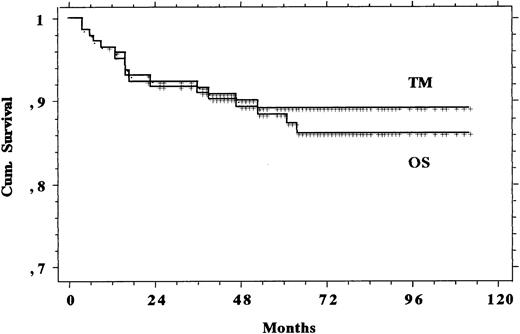
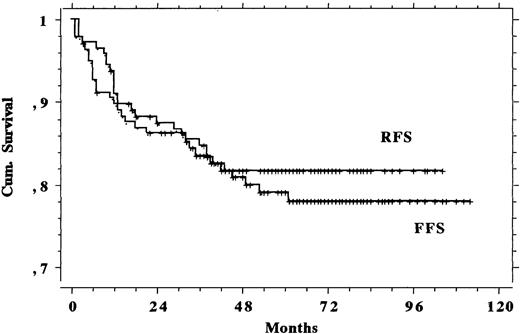
The median follow-up was 66 months (range, 25 to 114 months). Figure 1 shows the TM and OS and Fig 2 shows the RFS and FFS recorded in the eligible study population. Table 4offers a summary check of CR, PR, and death rates and OS, TM, RFS, and FFS both in the 145 eligible patients and in the total population of 155 subjects that were recruited and whom we intended to treat. Differences shown in Table 4 were negligible, and therefore only the eligible patients were analyzed. Of the 4 PR patients, 2 underwent high-dose chemotherapy with autologous bone marrow transplantation (1 was rescued and is still alive in CR and 1 did not respond and subsequently died) and 2 responded poorly to additional therapies as well and still survive with evidence of disease. The other 4 subjects with nonresponding or progressive disease during chemotherapy died within 5 months of diagnosis: 1 of complications to therapy and 3 of the disease.
Curves of TM and OS recorded in the 145 patients with advanced HD treated with MOPPEBVCAD.
Curves of TM and OS recorded in the 145 patients with advanced HD treated with MOPPEBVCAD.
Comparison of Results Obtained in the 145 Patients Eligible for the Study and in the Total Population of Subjects That Were Recruited and Treated
| Patients . | Response . | Deaths . | Survival . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TM . | OS . | RFS . | FFS . | ||||||||
| CR . | PR . | 3 yr . | 5 yr . | 3 yr . | 5 yr . | 3 yr . | 5 yr . | 3 yr . | 5 yr . | ||
| Eligible (145) | 0.944 | 0.028 | 0.124 | 0.915 | 0.889 | 0.908 | 0.881 | 0.833 | 0.814 | 0.845 | 0.786 |
| All treated (155) | 0.935 | 0.032 | 0.135 | 0.902 | 0.878 | 0.896 | 0.872 | 0.838 | 0.820 | 0.838 | 0.785 |
| Patients . | Response . | Deaths . | Survival . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TM . | OS . | RFS . | FFS . | ||||||||
| CR . | PR . | 3 yr . | 5 yr . | 3 yr . | 5 yr . | 3 yr . | 5 yr . | 3 yr . | 5 yr . | ||
| Eligible (145) | 0.944 | 0.028 | 0.124 | 0.915 | 0.889 | 0.908 | 0.881 | 0.833 | 0.814 | 0.845 | 0.786 |
| All treated (155) | 0.935 | 0.032 | 0.135 | 0.902 | 0.878 | 0.896 | 0.872 | 0.838 | 0.820 | 0.838 | 0.785 |
Among the 137 CR, 24 relapses were recorded. Five of these 24 patients underwent high-dose chemotherapy with autologous bone marrow transplantation: 3 of them died of unresponsive disease, 1 responded partially and is alive with evidence of disease, and 1 achieved a new CR. The relapses of the other 19 were treated with different conventional regimens containing three or four drugs or with alternating or hybrid ones delivering six drugs or more; 8 patients obtained a new CR and 6 are still alive (1 died of myelodysplastic syndrome and 1 died of infections [the subject was human immunodeficiency virus (HIV)-positive]), 1 responded partially to several subsequent therapies and is alive with evidence of disease, and 10 died of the disease after little or no response.
A synchronous tumor was diagnosed in 1 patient and metachronous ones developed in 7 other subjects. The clinical characteristics of these patients and the types of tumors recorded are listed in Table 5. These patients shared no definite clinical features, in particular not high drug dose intensity, frequent association of RT, or elevated incidence of relapses requiring further treatment. Both patients who developed small-cell lung carcinoma were smokers.
Characteristics of Those Patients Who Developed a Second Cancer After HD
| Patient . | Sex/Age (yr) . | Histology . | Stage . | Bulk . | RT . | DI (6 cycles) . | Clinical Response . | Relapse . | Time4-150 (mo) . | Present Status . | Second Cancer . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M.C. | M/62 | MC | III B | + | − | 0.76 | CR | − | 0 | Deceased | SCLC |
| M.G. | M/71 | LD | IV B | − | − | 0.68 | CR | − | 25 | Alive | Sigmoid carcinoma |
| F.A. | M/44 | MC | IIIS A | − | − | 0.69 | CR | + | 50 | Deceased | RAEB |
| S.A. | M/61 | LD | II B | + | + | 0.67 | CR | − | 55 | Deceased | SCLC |
| G.P. | M/43 | NS | IV A | − | − | 0.68 | CR | − | 55 | Alive | RAEB |
| F.P. | M/58 | NS | IV A | − | − | 0.77 | CR | − | 60 | Alive | Colon carcinoma |
| G.M. | F/29 | MC | III A | + | + | 0.86 | CR | + | 62 | Alive | Frontal bone eosinophilic granuloma |
| S.F. | F/27 | NS | III A | − | − | 0.90 | CR | − | 77 | Alive | RAEB |
| Patient . | Sex/Age (yr) . | Histology . | Stage . | Bulk . | RT . | DI (6 cycles) . | Clinical Response . | Relapse . | Time4-150 (mo) . | Present Status . | Second Cancer . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M.C. | M/62 | MC | III B | + | − | 0.76 | CR | − | 0 | Deceased | SCLC |
| M.G. | M/71 | LD | IV B | − | − | 0.68 | CR | − | 25 | Alive | Sigmoid carcinoma |
| F.A. | M/44 | MC | IIIS A | − | − | 0.69 | CR | + | 50 | Deceased | RAEB |
| S.A. | M/61 | LD | II B | + | + | 0.67 | CR | − | 55 | Deceased | SCLC |
| G.P. | M/43 | NS | IV A | − | − | 0.68 | CR | − | 55 | Alive | RAEB |
| F.P. | M/58 | NS | IV A | − | − | 0.77 | CR | − | 60 | Alive | Colon carcinoma |
| G.M. | F/29 | MC | III A | + | + | 0.86 | CR | + | 62 | Alive | Frontal bone eosinophilic granuloma |
| S.F. | F/27 | NS | III A | − | − | 0.90 | CR | − | 77 | Alive | RAEB |
The table also reports the patient whose second tumor, diagnosed at the end of therapy for HD, should properly be considered a synchronous cancer (first line).
Abbreviations: DI, dose intensity; SCLC, small-cell lung cancer; RAEB, refractory anemia with excess of blasts.
Time from the end of therapy to the diagnosis of second cancer.
In all, 18 patients died: 13 of HD progression after either first-line CT failure or one or more relapses, 1 of a pulmonary infection fromKlebsiella pneumoniae in second CR (the HIV-positive patient), 1 of severe gastric hemorrhage during CT (to which the disease did not respond), and 3 of second tumors.
A large list of factors reputed to be important for HD prognosis were scrutinized in a univariate analysis in relation to OS, RFS, and FFS and are reported in Table 6. Surprisingly, some of the best-known and most important prognostic factors in HD, such as stage, presence of bulky disease, extranodal involvement, erythrocyte sedimentation rate, hemoglobin concentration, serum lactate dehydrogenase, serum albumin level, and treatment with combined radiotherapy, did not influence any of the survival time parameters. Those that were found to exert a statistically significant role with respect to at least one follow-up variable (age, histologic type, performance status, bone marrow involvement, dose intensity of the first 3 CT cycles, SNLG prognostic index, and IDHD 5-year OS estimate) underwent multivariate analysis in the final Cox model, and only age and histologic type retained good statistical importance for OS and FFS, with histology being the only decisive factor for RFS (Table 7). When age was removed from the model, its prognostic role was replaced by bone marrow involvement and performance status for OS and by performance status alone for FFS. Practically speaking, patients more than 45 to 50 years of age (or, alternatively, with involved bone marrow and Karnofsky index ≤70) presenting lymphocyte depletion histologic type have a high probability of poor or short-lasting response.
List of the Clinical Variables Analyzed With a Univariate Technique in Relation to OS, RFS, and FFS
| Clinical Variables . | OS (P) . | RFS (P) . | FFS (P) . |
|---|---|---|---|
| Sex5-150 | .1744 | .5736 | .5230 |
| Age5-151 | 4 × 10−5 | .1118 | .0034 |
| Histology ([LP + NS] v MC v LD) | .0065 | .0019 | .0013 |
| Stage (II v III v IV) | .1566 | .2503 | .2607 |
| Systemic symptoms5-150 | .7488 | .5736 | .7723 |
| Karnofsky index5-151 | .0003 | .2020 | .0085 |
| Bulky disease5-150 | .6047 | .6805 | .4041 |
| Bone marrow involvement5-150 | .0005 | .0472 | .0077 |
| Visceral involvement5-150 | .8196 | .7952 | .8052 |
| ESR5-151 | .7235 | .5916 | .5435 |
| Hb5-151 | .1795 | .7675 | .6559 |
| LDH5-151 | .5742 | .6926 | .5763 |
| Serum albumin5-151 | .6649 | .3793 | .8086 |
| DI of CT cycles 1 to 35-151 | .0346 | .6931 | .3905 |
| DI of whole CT5-151 | .6236 | .6900 | .3691 |
| Radiotherapy5-150 | .6242 | .5227 | .4193 |
| SLNG prognostic index5-151 | .0006 | .1972 | .0396 |
| IDHD 5-yr OS estimate5-151 | 7 × 10−5 | .0371 | .0007 |
| Clinical Variables . | OS (P) . | RFS (P) . | FFS (P) . |
|---|---|---|---|
| Sex5-150 | .1744 | .5736 | .5230 |
| Age5-151 | 4 × 10−5 | .1118 | .0034 |
| Histology ([LP + NS] v MC v LD) | .0065 | .0019 | .0013 |
| Stage (II v III v IV) | .1566 | .2503 | .2607 |
| Systemic symptoms5-150 | .7488 | .5736 | .7723 |
| Karnofsky index5-151 | .0003 | .2020 | .0085 |
| Bulky disease5-150 | .6047 | .6805 | .4041 |
| Bone marrow involvement5-150 | .0005 | .0472 | .0077 |
| Visceral involvement5-150 | .8196 | .7952 | .8052 |
| ESR5-151 | .7235 | .5916 | .5435 |
| Hb5-151 | .1795 | .7675 | .6559 |
| LDH5-151 | .5742 | .6926 | .5763 |
| Serum albumin5-151 | .6649 | .3793 | .8086 |
| DI of CT cycles 1 to 35-151 | .0346 | .6931 | .3905 |
| DI of whole CT5-151 | .6236 | .6900 | .3691 |
| Radiotherapy5-150 | .6242 | .5227 | .4193 |
| SLNG prognostic index5-151 | .0006 | .1972 | .0396 |
| IDHD 5-yr OS estimate5-151 | 7 × 10−5 | .0371 | .0007 |
The reported levels of statistical significance derive from a log-rank test for qualitative variables and from a likelihood ratio test in a proportional hazards regression model for quantitative variables.
Binary data, ie, present (male, given) or absent (female, not given).
Data used in a continuous distribution.
Clinical Characteristics Most Strictly Related to OS, RFS, and FFS After MOPPEBVCAD (+RT)
| Clinical Covariates . | OS (P) . | RFS (P) . | FFS (P) . | ||
|---|---|---|---|---|---|
| Age | .0005 | — | — | .0104 | — |
| Histology | .0671 | .0862 | .0012 | .0016 | .0019 |
| Karnofsky index | >.1 | .0633 | — | >.1 | .0257 |
| Bone marrow involvement | >.1 | .0021 | >.1 | >.1 | >.1 |
| DI of CT cycles 1-3 | >.1 | >.1 | — | — | — |
| SLNG prognostic index | >.1 | >.1 | — | >.1 | >.1 |
| IDHD 5-yr OS estimate | >.1 | >.1 | >.1 | >.1 | >.1 |
| Clinical Covariates . | OS (P) . | RFS (P) . | FFS (P) . | ||
|---|---|---|---|---|---|
| Age | .0005 | — | — | .0104 | — |
| Histology | .0671 | .0862 | .0012 | .0016 | .0019 |
| Karnofsky index | >.1 | .0633 | — | >.1 | .0257 |
| Bone marrow involvement | >.1 | .0021 | >.1 | >.1 | >.1 |
| DI of CT cycles 1-3 | >.1 | >.1 | — | — | — |
| SLNG prognostic index | >.1 | >.1 | — | >.1 | >.1 |
| IDHD 5-yr OS estimate | >.1 | >.1 | >.1 | >.1 | >.1 |
Results of multivariate analysis with a proportional hazards regression model. Covariates entering the study were the best ones emerging from univariate analysis (bold and italic fonts indicate statistical probability of the null hypothesis <.05 or ranging from .10 to .05, respectively).
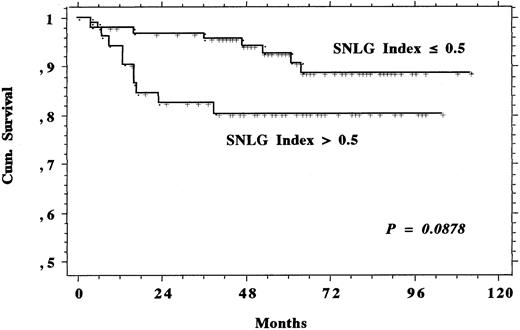
Systematic calculation of the SNLG index at diagnosis for each patient led to the identification of 52 subjects who had to be considered at high risk (ie, with an index >0.5, corresponding to a 60% probability of dying within 4 years, according to the work of Proctor et al29). Survival of such high-risk subjects (Fig 3) tended to be shorter than that of the other patients in the series, but the difference was not statistically significant and the survival plateaux, rather near each other, reached by both the curves do not lead us to think that prolonging the observation time would improve the statistical difference.
Survival comparison between the 52 patients having a SNLG index greater than 0.5, ie, absolutely the best prognosis according to Proctor et al,29 and the remaining 93 members of the study population.
Survival comparison between the 52 patients having a SNLG index greater than 0.5, ie, absolutely the best prognosis according to Proctor et al,29 and the remaining 93 members of the study population.
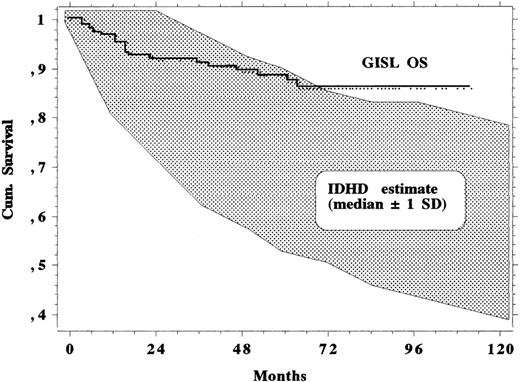
Moreover, the IDHD estimate of the probability of surviving at any given time was computed for each subject and made it possible to calculate the expected median survival of the whole population for any given year with a ±1 standard deviation confidence interval. As shown in Fig 4, it is clear that the MOPPEBVCAD protocol allows patients to survive longer than expected, all clinical patient characteristics of the patients being equal, with respect to the experience of the 1970s and 1980s.
Survival curve of the 145 study patients together with a band of ±1 SD around the expected median survival, which was estimated in the same patient series according to IDHD clinical experience and computation techniques.30
Survival curve of the 145 study patients together with a band of ±1 SD around the expected median survival, which was estimated in the same patient series according to IDHD clinical experience and computation techniques.30
DISCUSSION
The basic idea that underlay the formulation of the MOPPEBVCAD CT regimen, optionally combined with limited RT, for advanced HD was to try to improve an already existing treatment program, which had to be well known as widely tested and highly effective, by introducing selected changes. In 1987, the GISL chose Straus' alternating regimen23 as the original reference model, because, in the middle 1980s, it showed some of the relatively best figures for CR and RFS, associated with less frequent nausea and vomiting (which were serious clinical problems in those years). Importantly, these results seemed to agree fully with the theoretical models of drug sensitivity related to cell kinetics. Thus, hybridization of the drugs of the alternating CAD/MOPP/ABV into the MOPPEBVCAD schedule offered closer compliance with the Goldie and Coldman theory.9
Contemporary intensification of doses was obtained by administering in 6 cycles approximately the same cumulative drug dosage delivered with 9 cycles of the alternating CAD/MOPP/ABV and by reducing the cycle length from 35 to 28 days. The resulting planned intensification, according to the criteria reported by Hryniuk,27 was 1.54. Therefore, the average relative dose intensity actually delivered in our series with the hybrid regimen, which was 0.72, corresponded to 1.10 of the projected doses in the first 6 cycles of the reference regimen. Because the average observed-to-expected total drug delivery ratio during the initial 6 months with the alternating regimen proved to be 0.81,23 a true dose intensification of about 36% was actually reached with the hybridized CT regimen.
Conversely, to minimize early and late toxicity without compromising results, CT intensification was coupled with a marked reduction in the RT program. Whereas irradiation of all nodal regions initially involved was provided between the sixth and seventh cycle of the alternating CAD/MOPP/ABV regimen, only lesions evaluated as bulky at diagnosis or slowly or partially responding during CT (which are the sites with the highest probability of clinical failure) were irradiated after hybrid MOPPEBVCAD. The consequence of this decision was that about two thirds of our patients did not undergo RT and, where it was used, only one or two anatomic sites (rarely more) were irradiated.
Clinical results regarding the unfavorable subset of HD patients are interesting, because consistently excellent figures for CR rate, TM, OS, RFS, and FFS were achieved. Table 8lists the results of some of the major and recently reported series on advanced HD. Even though true and exact comparisons cannot be made among series with differences in patient composition, length of follow-up, therapy duration (mainly, number of cycles), and supportive care, our data seem to be similar to or better than those from the best protocols with alternating or hybrid chemotherapy. Only 2 of the 24 relapses in our study were recorded after 36 months of follow-up and none after 43 months; thus, our initial reserve about long-term results can be removed.
Results of Some of the Main Alternating or Hybrid Multiple-Drug CT Regimens in the Treatment of Advanced HD
| Study . | Treatment . | No. of Patients . | CR (%) . | OS . | RFS . | FFS . | Follow-Up (median, mo) . |
|---|---|---|---|---|---|---|---|
| Canellos et al17 | MOPP (no RT) | 123 | 67 | 66 | — | 50 | 60 |
| ABVD (no RT) | 115 | 82 | 73 | — | 61 | 60 | |
| MOPP/ABVD alt. (no RT) | 123 | 83 | 75 | — | 65 | 60 | |
| Hancock et al15 | LOPP + IF RT | 295 | 57 | 66 | 52 | — | 60 |
| LOPP/EVAP alt. + IF RT | 299 | 64 | 75 | 72 | — | 60 | |
| Cullen et al16 | ChlVPP/PABIOE + IF RT | 216 | 85 | 78 | — | 68 | 60 |
| Radford et al20 * | MVPP + IF RT | 208 | 58 | 71 | — | (PFS: 66) | 54 |
| ChlVPP/EVA hybr. + IF RT | 211 | 72 | 80 | — | (PFS: 80) | 54 | |
| Bartlett et al41 | MDVPVBE (Stanford V) (+IF RT) | 65 | — | 96 | — | 87 | 24 |
| Viviani et al19 | MOPP/ABVD alt. + IF RT | 211 | 91 | 74 | 74 | 67 | 108 |
| MOPP/ABVD hybr. + IF RT | 204 | 89 | 72 | 76 | 69 | 108 | |
| Diehl et al21 | BEACOPP + IF RT | 30 | 93 | 96 | 96 | 89 | 40 |
| Martinelli et al40 | ChlVPP/ABVVp16 | 28 | 95 | 75 | — | 70 | 48 |
| This series | MOPPEBVCAD (+IF RT) | 145 | 94 | 86 | 82 | 78 | 66 |
| Study . | Treatment . | No. of Patients . | CR (%) . | OS . | RFS . | FFS . | Follow-Up (median, mo) . |
|---|---|---|---|---|---|---|---|
| Canellos et al17 | MOPP (no RT) | 123 | 67 | 66 | — | 50 | 60 |
| ABVD (no RT) | 115 | 82 | 73 | — | 61 | 60 | |
| MOPP/ABVD alt. (no RT) | 123 | 83 | 75 | — | 65 | 60 | |
| Hancock et al15 | LOPP + IF RT | 295 | 57 | 66 | 52 | — | 60 |
| LOPP/EVAP alt. + IF RT | 299 | 64 | 75 | 72 | — | 60 | |
| Cullen et al16 | ChlVPP/PABIOE + IF RT | 216 | 85 | 78 | — | 68 | 60 |
| Radford et al20 * | MVPP + IF RT | 208 | 58 | 71 | — | (PFS: 66) | 54 |
| ChlVPP/EVA hybr. + IF RT | 211 | 72 | 80 | — | (PFS: 80) | 54 | |
| Bartlett et al41 | MDVPVBE (Stanford V) (+IF RT) | 65 | — | 96 | — | 87 | 24 |
| Viviani et al19 | MOPP/ABVD alt. + IF RT | 211 | 91 | 74 | 74 | 67 | 108 |
| MOPP/ABVD hybr. + IF RT | 204 | 89 | 72 | 76 | 69 | 108 | |
| Diehl et al21 | BEACOPP + IF RT | 30 | 93 | 96 | 96 | 89 | 40 |
| Martinelli et al40 | ChlVPP/ABVVp16 | 28 | 95 | 75 | — | 70 | 48 |
| This series | MOPPEBVCAD (+IF RT) | 145 | 94 | 86 | 82 | 78 | 66 |
*Fifteen percent of the patients with stage IB-IIA.
Furthermore, some of the most important and strongest prognostic factors seemed to be mitigated or even eliminated by this therapy: stage, bulky disease, involvement of the spleen or extranodal sites, B systemic symptoms, high levels of LDH and/or ESR, and low hemoglobin and/or serum albumin concentration proved to have no more influence on response to therapy, survival, or probability of relapse in patients treated with MOPPEBVCAD. This was particularly clear when outcome data were tested while considering two powerful composite prognostic systems, the IDHD survival estimate and the SNLG prognostic index. The survival of patients treated with the MOPPEBVCAD protocol exceeded the IDHD estimation of an expected survival band of 1 standard deviation around the median. Among patients treated with MOPPEBVCAD, there were no longer clear differences in survival (and RFS or FFS) between those with a SNLG prognostic index greater than 0.5 (whose probability of dying within 48 months should be 60%29) and those with a lower index value.
With MOPPEBVCAD therapy, only two clinical factors retain a crucial prognostic role: age and lymphocyte depletion histologic type. However, in practice, it is clinically rather difficult to use age to select those patients who are probably destined to fail to respond to MOPPEBVCAD therapy for a program of alternative experimental treatment. As a matter of fact, nearly the same age limits that would identify higher-risk patients should contraindicate such treatment options. Excluding age from the prognostic evaluation and accepting that it should simply discriminate otherwise selected high-risk patients only on the grounds of the technical feasibility of probably investigational therapy, the second and third most unfavorable prognostic factors for patients treated with MOPPEBVCAD become performance status and bone marrow involvement at diagnosis. Performance status can be accepted as a sufficiently useful factor because, first, it is not necessarily in conflict with other treatment options, even transplantation (being subject to pre-therapy improvement, if necessary) and, second, its highest discriminant effect is placed between 70 and 80 of the Karnofsky scale (ECOG, 1 to 2). Therefore, apart from age greater than 45 to 50 years, patients with lymphocyte depletion histologic type, bone marrow involvement, and/or Karnofsky index ≤70 (or ECOG grade ≤2) should enter clinical trials to compare one of the best chemotherapy regimens developed so far with one of the most promising experimental treatments available. Caution is needed when considering these prognostic results, despite the highly significant statistical levels reached, because they actually derive from a low number of observed failures. These results require further confirmation.
It is likely that the development of treatment protocols with increasing effectiveness in HD will require a search for particular prognostic factors related to a given CT regimen. Consideration of these features, according to the specific treatment one intends to use, would be important for early selection of patients with a definite risk for specifically designed clinical trials.
A few comments are needed on results and toxicity.
Acute nonhematological toxicity has to be considered fully acceptable and comparable with that of many alternating or hybrid regimens containing seven to eight drugs or more. Hematological toxicity was remarkable but controllable. First of all, only 1 toxic death was recorded, from gastrointestinal hemorrhage, in a man who did not show a response and presented severe thrombocytopenia after the second cycle of therapy. Second, growth factors, which became available during the last 2 years of the trial, were used only on demand in a strict minority of patients (16%). It is likely that moderate and intelligent use of them can contribute to reducing the consequences of myelotoxicity, particularly those related to heavy neutropenia.
Finally, as for intermediate and late toxicity, 1 case of nonsevere pulmonary fibrosis, 1 of mild cardiotoxicity (recurrent accesses of atrial fibrillation), and 7 second cancers (3 of which were fatal) were diagnosed during the follow-up. Although this series might not be considered completely mature in this regard, the absolute incidence per type of cancer appears to be lower than what is generally expected when dealing with MOPP and MOPP derivatives and probably higher than that recorded with ABVD. Staging without splenectomy, shortening the time of CT administration, reducing cumulative individual drug doses scheduled in the original alternating regimen from which MOPPEBVCAD derives, and eliminating RT in the majority of patients (with a reduction of the irradiated areas in the remaining ones) are all factors that potentially lower the relative risk of second cancer. Actually, the planned dose intensity for each drug in MOPPEBVCAD CT is not absolutely high when compared with what is commonly delivered in other popular CT regimens for HD. For example, the 6-cycle cumulative dose of mechlorethamine and vincristine is 25% and that of procarbazine 50% of what is provided in 6 cycles of the MOPP regimen1; the total dose of vinblastine and epidoxorubicin (assumed to be equivalent to a 40% lower dose of doxorubicin35) is 50% and that of bleomycin is 75% of what is planned in 6-cycle ABVD therapy2; CCNU doses are 50% of those delivered in the CVPP regimen36 or 66% of those in CEP37 CT. Finally, the cumulative dose of melphalan represents 70% of what is administered to patients with multiple myeloma over the same period at a current dosage of 0.25 mg/kg/d for 4 days every 6 weeks. On the other hand, a relatively high proportion of elderly patients (21% >50 years of age), advanced stage disease, increased dose intensity, association of several, albeit different, and potentially leukemogeneic drugs can be factors favoring the onset of a second cancer.
Unfortunately, the majority of the available studies on second cancer incidence among HD patients either involve too long a period of recruitment to reach a sufficient number of participants and unavoidably collect heterogeneously treated patients or are focused on relative risk with respect to a control population. The information we need for each trial (and for each arm of randomized studies) is represented by the number of second cancers actually recorded (or their cumulative incidence) and by the actual median follow-up length, but the availability of both in the literature is not common.
Indeed, our incidences of second myelodysplasia (2.1%), truly secondary lung cancer (0.7%), and colorectal cancer (1.4%) are similar to those of Viviani et al19 after alternating or hybrid MOPP/ABVD with or without RT, ie, 2.6%, 0.9%, and 0.5%, respectively. The experience of the Royal Marsden Hospital investigators38 after MOPP or MOPP-derived regimens also seems to be comparable, because the cumulative incidence at 5 and 10 years is 0.6 and 1.9 for second leukemia and related disorders, 0.2 and 1.3 for lung cancer, and 1.7 and 2.3 for other solid tumors, excluding skin cancers, respectively. The data on actuarial risk for second leukemia, lung cancer, and solid tumors that can be extrapolated from the study by Abrahamsen et al,39 on a population of patients with all disease stages, and from that by Martinelli et al,40 on advanced-stage patients only, are fully comparable, too. Thus, MOPPEBVCAD does not carry a higher risk of secondary tumors than what is observed in other series of patients treated with other alternating or hybrid drug combinations.
Only 2 patients in our series had relapsed before the onset of second cancer, so a role for extended administration of potentially oncogenic drugs as salvage therapy can be claimed in a minority of patients. Similarly, only the patient who developed lung cancer and the one who suffered a unifocal eosinophilic granuloma were treated with both CT and RT; therefore, this combined therapy cannot be considered a major causative factor.
Fertility was not specifically studied in these high-risk patients. Nevertheless, it is known to have been preserved in some of them. A 26-year-old woman and a 35-year-old man generated normal offspring 29 and 38 months, respectively, after treatment.
In conclusion, MOPPEBVCAD CT with optional limited RT can be considered a highly effective protocol for advanced HD. Its early hematologic toxicity is equivalent to that of other alternating or hybrid seven- or eight-drug regimens and might be better controlled with a limited use of growth factors. Its late toxicity is at least no greater than that of other alternating or hybrid regimens, particularly those including MOPP drugs. Randomized studies are needed and two are currently under way to compare other popular regimens with MOPPEBVCAD and to explore a less toxic version of it.
Supported in part by grants from the Associazione Italiana Ricerche sul Cancro, Milano, and the Fondazione Adolfo Ferrata and Edoardo Storti, Pavia, Italy.
Address reprint requests to Paolo G. Gobbi, MD, Medicina Interna e Oncologia Medica, Università di Pavia, IRCCS Policlinico S. Matteo, 27100 Pavia, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal