The mechanism by which interleukin-6 (IL-6) protects multiple myeloma (MM) plasma cells from apoptosis induced by anti-fas antibodies and dexamethasone was studied. Anti-apoptotic concentrations of IL-6 had no effect on cell-cycle distribution or activation of RAF-1 or ERK in dexamethasone- or anti–fas-treated 8226 and UCLA #1 MM cell lines. However, IL-6–dependent protection of viability correlated with an inhibition of dexamethasone- and anti–fas-induced activation ofjun kinase (JNK) and AP-1 transactivation. To test the hypothesis that cytokine-induced protection was mediated through inhibition of JNK/c-jun, we also inhibited c-junfunction in 8226 cells via introduction of a mutant dominant negative c-jun construct. Mutant c-jun–containing MM cells were also resistant to anti–fas-induced apoptosis but were significantly more sensitive to dexamethasone-induced apoptosis. These results support the notion that IL-6 protects MM cells against anti-fas through its inhibitory effects on JNK/c-junbut indicate protection against dexamethasone occurs through separate, yet unknown pathways.
INTERLEUKIN-6 (IL-6) is a well-known growth factor for multiple myeloma (MM) plasma cells. This cytokine is capable of inhibiting plasma cell apoptosis1,2 as well as stimulating some MM cell types to proliferate.3,4 Some experimental evidence indicates these two cytokine-dependent effects are dissociable and, thus, likely mediated by distinct mechanisms.2 Because the predominant effect of IL-6 in nontransformed B-lineage cells is that of inducing differentiation,5 a process which normally results in apoptosis of the terminally differentiated plasma cell, there must be inherent differences in IL-6 signaling in malignant plasma cells which result in continued cell growth and viability. Elucidation of these signaling pathways and differences may, thus, provide clues for future therapeutic manipulations that could be relatively selective for the malignant plasma cell clone while sparing normal B cells.
We have been actively studying the potential signal transduction pathways used by IL-6 to inhibit apoptosis in MM plasma cells. After receptor binding to IL-6 and homodimerization of gp 130, the signal-transducing molecule of this cytokine, there are two major pathways activated6: (1) Activation of JAK and TYK kinases followed by tyrosine phosphorylation of STAT 3 and, to a lesser extent, STAT 1 transcription factors; and (2) activation of ras by exchange factors such as SOS with subsequent activation of kinase cascades involving mitogen-activated protein kinases (MAPKs). Of these two major pathways, the latter MAPK cascades have been implicated as important regulatory pathways in several other in vitro models of apoptosis.7-11
One of these MAPK pathways is mediated by sequential activation of RAS/RAF/MEK/ and ERK.12 The ERKS (1 and 2) are then capable of activating transcription factors such as ELK-1/SAP-1.13More recently, a second MAPK pathway has been detected that involves JUN N-terminal kinase (JNK) or stress-activated protein kinase (SAPK).14,15 Activation of JNK/SAPK in this pathway is also dependent on prior ras activation14,16 and the signaling proceeds through a kinase cascade involving MEKK and SEK1 in an analagous fashion to the RAF/MEK/ERK cascade.17 Through phosphorylation, JNK activates the transcription factors c-junand ATF-2.18,19 Dimerization of c-jun with otherjun family members or with c-fos leads to formation of the AP-1 transcriptional activating complex. Thus, subsequent to receptor triggering, activated ras appears to direct signals into one or both kinase pathways, either RAF/MEK/ERK or MEKK/SEK1/JNK. The relative activation of each pathway versus the other may ultimately influence the outcome of receptor triggering. For example, the balance between ERK and JNK/SAPK pathway signaling is critical for determining whether neuronal cells survive or undergo apoptosis.7
Recent work by Chauhan et al20 showed an activation of SAPK by anti-fas during apoptosis of MM plasma cells. The ability of IL-6 to protect against anti–fas-induced apoptosis correlated with its ability to inhibit anti–fas-induced SAPK activation. Because experiments with neuronal cells7 indicate anti-apoptotic influences could be exerted by activation of the ERK pathway and/or inhibition of the JNK pathway, these latter studies with MM cells suggested that differential activation/inhibition signals through the two parallel MAPK cascades might regulate IL-6–induced protection of MM plasma cells against apoptosis. However, while showing a correlation, the study of Chauhan et al20did not prove a causal relationship between IL-6–dependent protection and inhibition of the JNK pathway. Thus, to further investigate this issue, we also studied the effects of IL-6 on both MAPK pathways in MM cells that were protected against apoptosis induced by dexamethasone and anti-fas antibody. In similar fashion to the work of Chauhan et al,20 we also found that JNK activity and subsequent c-jun transactivation induced during MM cell apoptosis was inhibited by protective concentrations of IL-6. To test whether these specific inhibitory effects of IL-6 were crucial to protection of MM cell viability, we introduced a dominant negative c-jun into MM cells, which resulted in inhibition ofjun activity induced by anti-fas or dexamethasone which was comparable to the inhibitory effects of IL-6. These dominant negative c-jun containing MM cells were resistant to apoptosis induced by anti-fas but not by dexamethasone. These data support the hypothesis that IL-6 protects MM cells from anti-fas through its inhibition of c-jun activity but also indicate that protection against dexamethasone proceeds along separate pathways.
MATERIALS AND METHODS
Cell lines.
The myeloma cell line 8226 was a kind gift from J. Epstein (Little Rock, AR). AF-10 MM cells were a kind gift of James Berenson (UCLA, Los Angeles, CA). UCLA #1 MM cells is a cell line started from the peripheral blood of a patient with plasma cell leukemia. It has the morphology of plasma cells, and expresses high amounts of CD38 as well as the identical Ig isotype of the patient's M protein. Both lines were maintained in RPMI media, supplemented with 10% fetal bovine serum, L-glutamine, nonessential amino acids, sodium pyruvate, and antibiotics.
Reagents.
Human recombinant IL-6 was from R&D Labs (Minneapolis, MN). Anti-MAPK and anti–RAF-1 antibodies were purchased from Santa Cruz Biotech Inc (Santa Cruz, CA). Antiphosphotyrosine antibody was obtained from UBI (Lake Placid, NY). Dexamethasone and myeline basic protein (MBP) was purchased from Sigma (St Louis, MO). γ-32P-ATP was obtained from Amersham Labs (Arlington Heights, IL). Anti-fasantibody was obtained from Kamiya Inc (Thousand Oaks, CA). All other chemicals were obtained from Sigma Labs.
Induction of apoptosis.
Cells were treated with anti-fas antibody or a control antibody of the same isotype. When dexamethasone was used to induce apoptosis, controls contained identical concentrations of alcohol (always <0.1%).
Immunoblotting.
MM cells were stimulated with or without IL-6 or with PMA for 5 to 15 minutes. Cells were then lysed in lysis buffer (50 mmol/L Tris pH 7.4, 150 mmol/L NaCl, 1% NP40, 1 mmol/L Na3VO4, 10 mmol/L NaF, 2 mmol/L phenylmethylsulfonyl fluoride [PMSF], 0.5 mmol/L EDTA, 10 μg/mL leupeptin, and 10 μg/mL aprotinin). One milligram of protein of each sample was then precipitated with 5 μg of antiphosphotyrosine antibody at 4°C with constant shaking for 1 hour, followed by addition of 35 μL of protein A-Sepharose and protein G-Sepharose. After another 1-hour reaction, the immunocomplexes were washed once with lysis buffer, twice with washing buffer (same as lysis buffer except NP40 was decreased to 0.1% and EDTA was removed), and the samples were boiled with 30 μL of sodium dodecyl sulfate (SDS)-sample buffer and resolved on 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The separated samples were transferred onto membranes and blotted with anti-MAPK or antiphosphotyrosine antibodies. The bands were detected by an enhanced chemiluminescence (ECL) system.
In vitro kinase assay for MAP kinase activity.
The assay was performed as previously described.21 Briefly, cells were treated for 5 to 15 minutes, were washed twice, and lysed in lysis buffer (20 mmol/L Tris, pH 7.4, 2 mmol/L MgCl2, 10 mmol/L β-glycerophosphate, 10 mmol/L p-nitrophenylphosphate, 1 mmol/L EGTA, 0.1 mmol/L Na3VO4, 10 mmol/L NaF, 0.5% Triton X-100, and 10 μg/mL aprotinin). Five micrograms of each lysate was incubated with 5 micrograms of MBP in kinase buffer (20 mmol/L HEPES, pH 7.6, 20 mmol/L MgCl2, 10 mmol/L β-glycerophosphate, 20 mmol/L p-nitrophosphate, 0.5 mmol/L Na3VO4, 2 mmol/L dithiothreitol [DTT], 50 μmol/L ATP0 plus 20 μCi of γ-32P-ATP) for 30 minutes at room temperature. SDS-sample buffer was then added and the samples boiled for 3 minutes. Samples were separated on SDS-PAGE. After separation, the gel was dried and exposed on film at −70°C overnight.
In vitro kinase for jun kinase activity.
The GST-jun vector was a kind gift of M. Karin (San Diego, CA). The GST-jun substrate was expressed in bacteria and then purified with glutathione (GSH)-beads. The purity and semi-quantitation of the substrate was monitored by SDS-PAGE. Cells were pretreated with IL-6 for increasing durations and then stimulated with anti-fas or dexamethasone. They were then lysed in JEB buffer (25 mmol/L HEPES, pH 7.7, 300 mmol/L NaCl, 1.5 mmol/L MgCl2, 0.1 mmol/L EDTA, 0.1% Triton X-100, 20 mmol/L β-glycerophosphate, 0.1 mmol/L Na3VO4, 0.5 mmol/L PMSF, 10 μg/mL aprotinin, and 10 μg/mL leupeptin), the lysates were centrifuged at 14,000 rpm for 10 minutes, and the supernatants saved. Twenty-five microliters of GST-jun-agarose was then added, followed by rocking at 4°C for 2 hours. The mixtures were washed twice in HBIB buffer (20 mmol/L HEPES, pH 7.7, 50 mmol/L NaCl, 0.1 mmol/L EDTA, 2.5 mmol/L MgCl2, and 0.05% Triton X-100). Kinase buffer was then added (25 μL of 0.5 μCi γ32P-ATP, 20 μmol/L ATP, 20 mmol/L MgCl2, 20 mmol/L HEPES, pH 7.6, 20 mmol/L β-glycerophosphate, 20 mmol/L PNPP, 0.1 mmol/L Na3VO4, and 2 mmol/L DTT) and the mixtures were incubated at 30°C for 30 minutes. The mixtures were then resolved on an 8% SDS-PAGE, and the gel was dried and exposed on film.
In some experiments, lysates were first incubated with specific antibody to SAPK/JNK (Santa Cruz Biotechnology) for 2 hours at 4°C before adding protein A-Sepharose for 1 hour. Immune complexes were then washed with JEB buffer, followed by kinase buffer and then resuspended in kinase buffer containing GST-jun and γ32P-ATP.
Immune complex kinase assay for RAF-1 activity.
The assay was performed as previously described.22 Briefly, cells were lysed in 25 mmol/L Tris (pH 8.0), 150 mmol/L NaCl, 0.1% SDS, 0.5% Na-deoxycholate, 1% NP40, 10% glycerol, 2 mmol/L EDTA, 1 mmol/L Na3VO4, 1 mmol/L PMSF, 20 μmol/L leupeptin, and 5 μg/mL aprotinin. Raf-1 was then immunoprecipitated using protein A-Sepharose preadsorbed with anti–RAF-1 antibody and the complex was washed twice in 20 mmol/L Tris (pH 7.4), 150 mmol/L NaCl, 1% Triton-X-100, 10% glycerol, 2 mmol/L EDTA, 1 mmol/L Na3VO4, and protease inhibitors. A final wash in kinase buffer (25 mmol/L HEPES (pH 7.4), 150 mmol/L NaCl, 25 mmol/L glycerol phosphate, 1 mmol/L DTT, 5 mmol/L MgCl2) was performed before incubation in 30 μL of kinase buffer containing purified recombinant MEK protein (generated from GST-MEK vector), 10 μmol/L ATP, and 20 μCi γ32P-ATP for 30 minutes at room temperature. Samples were centrifuged at 16,000g for 1 minute and the supernatant containing MEK and the pellet containing RAF-1 immune complexes were separated on SDS-PAGE. Phosphorylated MEK was detected by autoradiography in the dried gel and RAF-1 was transferred to nitrocellulose and detected by immunoblotting.
Detection of apoptosis.
DNA electrophoresis was performed as previously described.2After extraction in PCI, 5 to 10 μg of DNA per lane was electrophoresed in a 1% agarose gel for 2 hours at 45 V and the gels were visualized with ethidium bromide. Viability was determined by dye exclusion assays using trypan blue. Briefly, cells were seeded at a concentration of 2 to 4 × 105/mL in six-well tissue culture plates and drugs were added at the designated time points. At specified incubation times, cell viability was determined by trypan blue staining and percent viability was determined in at least 300 cells. Triplicate wells were run for each group and the standard deviation of the groups was always less than 5% of the mean viability. The percent apoptotic nuclei was determined by 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) staining. Cells were first fixed with 3.7% formaldehyde in phosphate-buffered saline (PBS) at room temperature for 10 minutes and then washed with PBS. Fixed cells were then stained with 1 μg/mL DAPI in PBS at room temperature for 15 minutes. After washing three times, cells were resuspended in glycerol:PBS (10:1) and were mounted onto glass slides and covered with a coverslip. The slide was examined under 400× magnification using a fluorescent microscope with a 340/380 nm excitation filter and LP 430-nm barrier filter. At least 300 nuclei were examined per group. The diphenylamine DNA fragmentation assay was performed by first lysing cells in 0.5 mL of buffer containing 0.5% Triton X-100, 25 mmol/L Tris (pH 8.0), 10 mmol/L EGTA, and 10 mmol/L EDTA for 15 minutes on ice. Samples were then centrifuged for 20 minutes at 13,000g to separate fragmented (supernatant) from intact chromatin (pellet). DNA content of each fraction was determined using the diphenylamine reagent and results are expressed as percentages of DNA in each sample that resisted sedimentation at 13,000g.
AP-1–dependent transcription assay.
As previously described,23 cells were cotransfected with equal amounts of cytomegalovirus (CMV) β gal and the reporter containing three copies of the tetradecanoyl phorbol acetate response element (TRE) present in the collagenase promoter fused to the chloramphenicol acetyltransferase gene (TRE-CAT). Transfection was by the DEAE-dextran method. Forty-eight hours posttransfection, cells were treated with the appropriate drugs for 1 hour. Cells were then washed with Tris HCl (pH 7.5) and incubated in fresh media for 24 hours. Cells were then obtained and the CAT was extracted. The colorimetric β-galactosidase assay provided a rough indication of transfection efficiency, from which we determined how much cell extract to use in the CAT assay. CAT activity was measured with (14C)chloramphenicol as substrate. The percentage converted to acetylated forms was quantified with a PhosphorImager (Molecular Dynamics, Chicago, IL).
Transduction of 8226 cells with mutant c-jun.
The cDNA encoding mutant jun was originally obtained from I. Verma (Salk Institute, La Jolla, CA). It was subcloned into the retroviral vector pSRα MSVtkNeo. Retrovirus stocks were prepared by transient transfection of 293 T cells with the ecotropic ψ-packaging plasmid. Indicator lines were generated by infection with the appropriate retrovirus stock and by selection for 2 to 3 weeks in G418. Naı̈ve 293 T cells were then newly infected with viral supernatant from the indicator lines containing the empty retroviral vector (neo control) or vector containing the mutant c-jun. Forty-eight hours later, high-titer viral supernatant was collected and used to transduce 8226 MM cells. 8226 cells were plated in complete media containing 5% fetal bovine serum (FBS) at 5 × 106 cells/plate and incubated at 37°C overnight. Media was aspirated and 1 mL of viral supernatant containing 1 μg/μL polybrene was added to each plate. Cells were incubated with the viral supernatant for 1 hour at 37°C, after which 10 mL of RPMI media was added and culture was continued. Forty-eight hours later, selection in 0.5 mg/mL G418 was initiated.
Cell-cycle analysis.
Cells were stained with hypotonic propidium iodide (50 μg/mL in 0.1% sodium citrate and 0.1% Triton X-100) for 1 hour at 4°C. They were kept in the dark at 4°C before analysis. Cell-cycle distribution was then determined by analyzing 10,000 events on a FACScan flow cytometer (Becton Dickinson, San Jose, CA). The DNA data were fitted to a cell-cycle distribution analysis by use of the MODFIT program for MAC V2.0.
Statistics.
The t-test was used to determine significance of differences between groups.
RESULTS
IL-6 protects MM cells from dexamethasone and anti–fas-induced apoptosis.
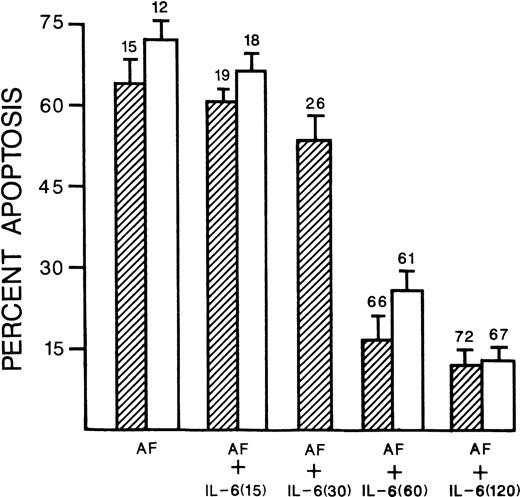
We have used the 8226 and UCLA #1 MM cell lines for the following mechanistic studies because, though expressing competent IL-6 receptors, exogenous IL-6 only protects against apoptosis but does not stimulate proliferation. Thus, we can isolate cytokine-induced effects on apoptosis without potential obfuscating effects on proliferation. These two MM cell lines undergo apoptotic death within 72 hours of incubation with 10−6 mol/L dexamethasone and within 20 hours of incubation in 0.5 μg/mL of anti-fas. Co-incubation with IL-6, at 1,000 U/mL, significantly inhibited both dexamethasone- and anti–fas-induced apoptosis. Figure 1 shows the ability of IL-6 to block endonucleosomal fragmentation induced by anti-fas or dexamethasone in both 8226 and UCLA #1 target cells. Figure 2 shows that IL-6–dependent protection against anti–fas-induced apoptosis is also demonstrated by scoring apoptotic nuclei on DAPI-stained cytospins and viability by dye exclusion assays (mean percent viability shown above each bar). Pretreatment with IL-6 for 60 or 120 minutes is more effective than shorter treatments for subsequent survival of anti–fas-challenged MM targets (Fig 2). Dye exclusion and DAPI staining of 8226 cells challenged with dexamethasone also showed significant protection afforded by IL-6 (63% viability and 35% apoptosis induced by dexamethasone [10−6 mol/L]v 81% and 9% when IL-6 is present [1,000 U/mL]). Similar results were seen with UCLA #1 cells treated with 10−6 mol/L dexamethasone (59% viability and 32% apoptosis v 79% viability and 12% apoptosis when IL-6 is present).
IL-6 protects against anti-fas and dexamethasone. 8226 (lanes A through F) and UCLA #1 cells (lanes G through K) were cultured in media alone for 48 hours (lanes A and G), IL-6 alone for 48 hours (1,000 U/mL, lane B), anti-fas for 20 hours (0.5 μg/mL, lanes C and H), dexamethasone for 48 hours (10−6 mol/L, lanes E and J) or the combination of anti-fas + IL-6 (lanes D and I) or dexamethasone + IL-6 (lanes F and K). In the anti-fas + IL-6 combination, IL-6 was present for 1 hour before addition of anti-fas. DNA was then extracted and electrophoresed. Culture of both cell lines with a control antibody of identical isotype to the anti-fas antibody resulted in normal, intact, high-molecular-weight DNA (identical to that shown in lanes A and G [not shown]).
IL-6 protects against anti-fas and dexamethasone. 8226 (lanes A through F) and UCLA #1 cells (lanes G through K) were cultured in media alone for 48 hours (lanes A and G), IL-6 alone for 48 hours (1,000 U/mL, lane B), anti-fas for 20 hours (0.5 μg/mL, lanes C and H), dexamethasone for 48 hours (10−6 mol/L, lanes E and J) or the combination of anti-fas + IL-6 (lanes D and I) or dexamethasone + IL-6 (lanes F and K). In the anti-fas + IL-6 combination, IL-6 was present for 1 hour before addition of anti-fas. DNA was then extracted and electrophoresed. Culture of both cell lines with a control antibody of identical isotype to the anti-fas antibody resulted in normal, intact, high-molecular-weight DNA (identical to that shown in lanes A and G [not shown]).
IL-6 protects against anti-fas. 8226 cells (▨) or UCLA #1 cells (□) cultured in anti-fas (AF, 0.5 μg/mL) alone for 20 hours, or anti-fas + IL-6 (1,000 U/mL). As shown in the figure, the IL-6 was present for either 15, 30, 60, or 120 minutes before addition of anti-fas. Results are the percent apoptosis from DAPI-stained cytospins, mean ± SD of three separate experiments. 8226 cells cultured in media alone for 20 hours showed only 3.5% apoptosis in this assay. Above each bar is shown the mean percent viability (from dye exclusion assays of the three experiments). 8226 cells cultured in media alone for 20 hours showed a viability of 88%. Only the presence of IL-6 for 60 or 120 minutes significantly (P < .05) decreased the % apoptosis and increased the percent viability relative to the cells cultured in anti-fasalone.
IL-6 protects against anti-fas. 8226 cells (▨) or UCLA #1 cells (□) cultured in anti-fas (AF, 0.5 μg/mL) alone for 20 hours, or anti-fas + IL-6 (1,000 U/mL). As shown in the figure, the IL-6 was present for either 15, 30, 60, or 120 minutes before addition of anti-fas. Results are the percent apoptosis from DAPI-stained cytospins, mean ± SD of three separate experiments. 8226 cells cultured in media alone for 20 hours showed only 3.5% apoptosis in this assay. Above each bar is shown the mean percent viability (from dye exclusion assays of the three experiments). 8226 cells cultured in media alone for 20 hours showed a viability of 88%. Only the presence of IL-6 for 60 or 120 minutes significantly (P < .05) decreased the % apoptosis and increased the percent viability relative to the cells cultured in anti-fasalone.
IL-6–induced protection is not caused by alteration of cell-cycle distribution.
In M1 myeloid leukemia blasts, IL-6 protects against p53-induced apoptosis and complements the antiproliferative effect of p53 resulting in cell-cycle exit with as high as 95% of cells in a quiescent G0 state.24 It is possible that this extreme blockage of target cells in a dormant state may actually protect them from p53-induced apoptotic death. In similar fashion, the ability of IL-6 to protect cells against tumor necrosis factor (TNF) cytotoxicity correlates with the cytokine's ability to arrest cells in G1.25 Although IL-6 stimulation of unchallenged 8226 and UCLA #1 cells neither stimulates nor inhibits in vitro growth, we considered the possibility that IL-6 could be interacting with dexamethasone or anti-fas for an enhanced antiproliferative effect similar to the combination of p53 and IL-6 or TNF and IL-6 with resulting protection against apoptosis. However, cell-cycle analysis (Table 1) showed that, in contrast to M1 leukemia cells, IL-6 did not complement dexamethasone-induced cell cycle exit in these cells. As expected, at both 48 and 72 hours, dexamethasone alone decreased the percentage of cells in S/G2M by approximately 50% with a concurrent increase in distribution of cells in G0/G1. This inhibition of cell-cycle transit through S and G2M induced by dexamethasone was not enhanced by concurrent IL-6 treatment. Thus, a synergistic increase in cell-cycle exit is not present and cannot, therefore, explain the ability of IL-6 to protect against apoptosis. The results in Table 1 also show that the inhibition of cell-cycle transit through S and G2M induced by dexamethasone was not prevented by concurrent IL-6 exposure. Thus, the palliative effect of IL-6 in these cultures is specific for apoptosis as the cytokine could not protect against dexamethasone-induced cytostasis. This lack of protection against cytostasis is similar to results we previously obtained when viable cell recoveries were determined.2Exposure of these cells to anti-fas for 20 hours did not significantly affect cell-cycle distribution (not shown).
Effects of IL-6 and Dexamethasone on Cell-Cycle Distribution
| Time . | Stimulation . | 8226 . | UCLA #1 . | ||
|---|---|---|---|---|---|
| G0/G1 . | S/G2 + M . | G0/G1 . | S/G2 + M . | ||
| 48 h | Control | 54 | 46 | 65 | 35 |
| IL-6 | 62 | 38 | 60 | 40 | |
| Dex | 76-150 | 24-150 | 85-150 | 15-150 | |
| Dex + IL-6 | 71-150 | 29-150 | 81-150 | 19-150 | |
| 72 h | Control | 53 | 47 | 69 | 31 |
| IL-6 | 47 | 53 | 64 | 36 | |
| Dex | 79-150 | 21-150 | 89-150 | 11-150 | |
| Dex + IL-6 | 76-150 | 24-150 | 82-150 | 18-150 | |
| Time . | Stimulation . | 8226 . | UCLA #1 . | ||
|---|---|---|---|---|---|
| G0/G1 . | S/G2 + M . | G0/G1 . | S/G2 + M . | ||
| 48 h | Control | 54 | 46 | 65 | 35 |
| IL-6 | 62 | 38 | 60 | 40 | |
| Dex | 76-150 | 24-150 | 85-150 | 15-150 | |
| Dex + IL-6 | 71-150 | 29-150 | 81-150 | 19-150 | |
| 72 h | Control | 53 | 47 | 69 | 31 |
| IL-6 | 47 | 53 | 64 | 36 | |
| Dex | 79-150 | 21-150 | 89-150 | 11-150 | |
| Dex + IL-6 | 76-150 | 24-150 | 82-150 | 18-150 | |
Cell-cycle analysis performed by hypotonic PI staining of 8226 or UCLA #1 MM cells 48 or 72 hours after culture in media (control), IL-6 (1,000 U/mL), dexamethasone (Dex; 10−6 mol/L) or Dex + IL-6. Results are percentage of cells in G0/G1versus S/G2 + M, mean of three separate experiments. The standard deviations were all <15% of the mean values.
Significantly different from corresponding control (media), P< .05.
IL-6 protects against apoptosis in the absence of activating effects on RAF-1 and ERKs.
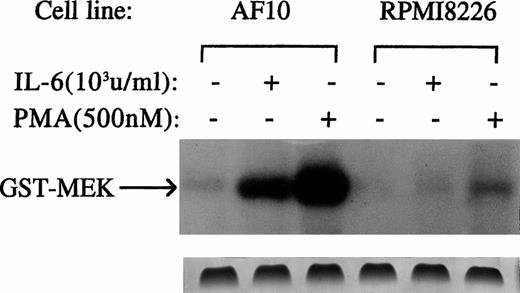
We first tested effects of anti-apoptotic concentrations of IL-6 on theras-dependent RAF-1/MEK/ERK pathway. RAF-1 activation was investigated by use of an in vitro kinase assay using MEK as a substrate. We used AF-10 MM cells as a positive control because prior studies26,27 confirmed the ability of IL-6 to activate the RAS/RAF/MEK/ERK pathway in these cells. Activation of the ERK pathway may mediate IL-6–dependent stimulation of proliferation in AF-10 cells.26 27 AF-10 and 8226 target cells were treated with or without IL-6 (1,000 U/mL) for 5 minutes. RAF-1 was then immunoprecipitated from cell lysates and tested for its enzymatic activity against GST-MEK. As shown in Fig3, IL-6 was capable of activating RAF-1 in AF-10 cells but could not activate RAF-1 activity in 8226 target cells. Coomassie blue staining confirmed equal loading of protein in each individual lane (Fig 3, lower band). Furthermore, in Western blot analyses not shown, reblotting immunoprecipitated RAF-1 with an anti–RAF-1 antibody confirmed that equal amounts of RAF-1 protein were immunoprecipitated from cell extracts. In addition, when cells were treated with phorbol myristate acetate (PMA) (500 nmol/L for 5 minutes), RAF-1 activation was clearly detected in 8226 cells (Fig 3). These latter control experiments show that 8226 cells contain RAF-1 that is capable of being activated by appropriate stimuli but is not susceptible to activation by IL-6 when used in concentrations that protect these same MM cells from apoptosis. Repeated in vitro kinase assays testing longer exposures of cells to IL-6 (10 or 15 minutes, not shown) consistently showed no IL-6–dependent RAF-1 activation in 8226 cells, although positive activation was seen in AF-10 cells.
Protective concentrations of IL-6 do not activate RAF-1. 8226 and AF-10 MM cells were cultured with or without IL-6 (1,000 U/mL) or PMA (500 nmol/L) for 5 minutes. RAF-1 was then immunoprecipitated and tested for enzymatic activity against the substrate GST-MEK in an in vitro kinase assay. Similar results, ie, a lack of IL-6–dependent activation of RAF-1 in UCLA #1 cells was also shown (not shown). Bottom panel shows Coomassie blue staining of the gel.
Protective concentrations of IL-6 do not activate RAF-1. 8226 and AF-10 MM cells were cultured with or without IL-6 (1,000 U/mL) or PMA (500 nmol/L) for 5 minutes. RAF-1 was then immunoprecipitated and tested for enzymatic activity against the substrate GST-MEK in an in vitro kinase assay. Similar results, ie, a lack of IL-6–dependent activation of RAF-1 in UCLA #1 cells was also shown (not shown). Bottom panel shows Coomassie blue staining of the gel.
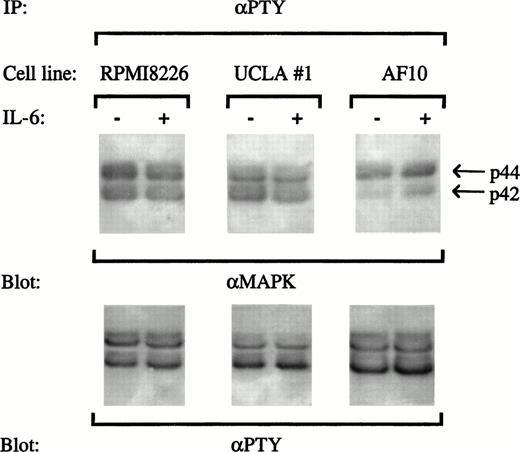
Activation of ERKs was tested in two ways. First, the three MM cell lines (8226, UCLA #1, or AF-10) were treated with or without IL-6 (1,000 U/mL for 15 minutes), and tyrosine phosphorylated proteins were immunoprecipitated from extracts with an antiphosphotyrosine antibody. We then immunoblotted with anti-MAPK1 (ERK 1; p44) and anti-MAPK2 (ERK 2; p42) antibodies. As shown in Fig 4, both ERK 1 and ERK 2 were constitutively tyrosine phosphorylated in 8226, UCLA #1, and AF-10 target cells. However, no further stimulation of ERK activation was demonstrated upon stimulation of 8226 or UCLA #1 cells with IL-6. In contrast, IL-6 efficiently activated ERK 2 (p42) in AF-10 target cells. Immunoblotting with antiphosphotyrosine antibodies (Fig4) confirmed equal amounts of phosphotyrosine proteins were applied to the lanes. This assay was repeated twice with similar results.
Protective concentrations of IL-6 do not induce tyrosine phosphorylation of ERK-1 or ERK-2. 8226, UCLA #1, and AF-10 cells were treated with or without IL-6 (1,000 U/mL) for 15 minutes and extracts were immunoprecipitated with an antiphosphotyrosine antibody (IP:αPTY). Immunoprecipitates were then blotted with anti-ERK antibodies (anti-MAPK, upper blot) and with an antiphosphotyrosine antibody (αPTY, lower blot).
Protective concentrations of IL-6 do not induce tyrosine phosphorylation of ERK-1 or ERK-2. 8226, UCLA #1, and AF-10 cells were treated with or without IL-6 (1,000 U/mL) for 15 minutes and extracts were immunoprecipitated with an antiphosphotyrosine antibody (IP:αPTY). Immunoprecipitates were then blotted with anti-ERK antibodies (anti-MAPK, upper blot) and with an antiphosphotyrosine antibody (αPTY, lower blot).
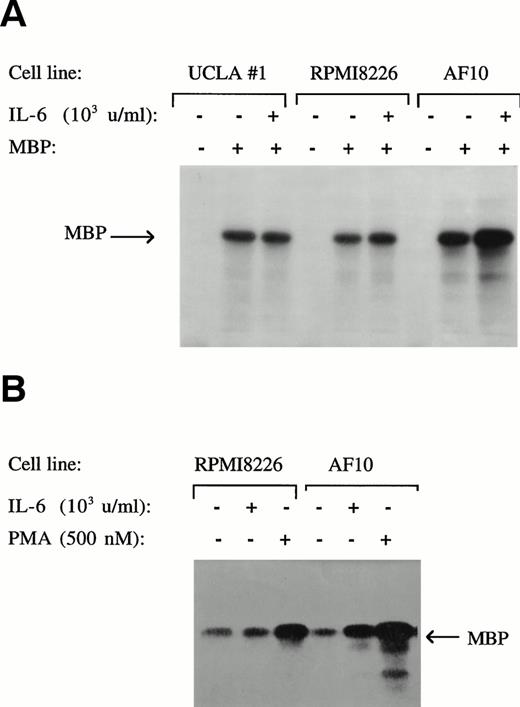
The second method used for testing ERK activity was by in vitro kinase assays using MBP as a substrate (Fig 5). The results obtained (panel A) were consistent with the immunoblot analyses described above. As shown, constitutive ERK activity was detected in UCLA #1, 8226, and AF 10 cells. However, only in AF 10 cells was an IL-6–dependent increase in ERK activity demonstrated. Also shown in Fig 5 (panel B) is the finding that PMA is capable of significantly stimulating ERK activity above the constitutively expressed level in 8226 cells. Thus, these experiments collectively argue against the RAF/MEK/ERK pathway as playing any role in mediating IL-6–induced protection against apoptosis in 8226 and UCLA #1 cells because anti-apoptotic concentrations of the cytokine failed to activate RAF-1 or ERK above baseline levels in these cells, although these signaling proteins were sensitive to activation by PMA and IL-6 was successful in activating them in AF-10 cells.
Protective concentrations of IL-6 do not activate ERK function. 8226, AF-10, and UCLA #1 MM cells cultured with and without IL-6 (1,000 U/mL, upper panel) or with and without PMA (500 nmol/L, lower panel). Whole-cell lysates were then tested for enzymatic activity against the substrate MBP in an in vitro kinase assay.
Protective concentrations of IL-6 do not activate ERK function. 8226, AF-10, and UCLA #1 MM cells cultured with and without IL-6 (1,000 U/mL, upper panel) or with and without PMA (500 nmol/L, lower panel). Whole-cell lysates were then tested for enzymatic activity against the substrate MBP in an in vitro kinase assay.
Effects on jun kinase activation.
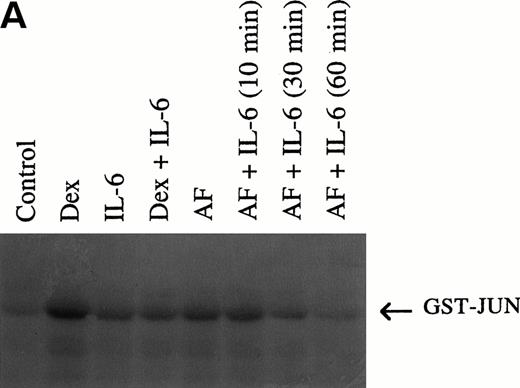
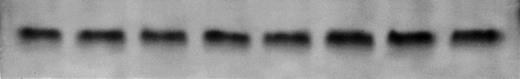
Initial experiments used in vitro kinase assays with c-jun as substrate. As shown in Fig 6A, anti-fas antibody and dexamethasone clearly activate JNK activity in 8226 cells. By densitometric analysis, dexamethasone induced a 5-fold and anti-fas induced a 3.5-fold activation in JNK activity. In a time-course experiment not shown, 10 minutes was the optimal incubation duration with anti-fas or dexamethasone for JNK activation. When cells are co-incubated with IL-6, the anti-fas and dexamethasone-induced enhancement of JNK activity is inhibited. Pretreatment with IL-6 for 30 or 60 minutes was more effective than only 10 minutes on subsequent anti–fas-induced JNK activity. Figure 6B shows equal loading of protein in individual lanes by Coomassie blue staining of the gel. Figure 7A, top band, shows a similar activation of JNK activity in UCLA #1 cells when exposed to anti-fas (3.3-fold increase in activity by densitometry) or dexamethasone (2.8-fold increase in activity) and a similar inhibition of activity by IL-6 when IL-6 was present 60 minutes before addition of anti-fas or dexamethasone. Because p38 MAPK can also phosphorylate c-jun, we also performed the in vitro kinase assay on UCLA #1 cells using anti-JNK antibody to specifically precipitate cellular JNK. The bottom portion of Fig 7A showsjun phosphorylation induced by immunoprecipitated JNK in cells either treated with dexamethasone (lane B, 10−6mol/L, for 10 minutes) or anti-fas (lane C, 0.5 μg/mL for 10 minutes). Figure 7B shows the Coomassie blue–stained gel confirming equal protein loading of Fig 7A's (top panel) experiment.
(A) Anti-fas and dexamethasone activate and IL-6 inhibits jun kinase in 8226 cells. 8226 MM cells incubated in media (control) or dexamethasone alone (10−6 mol/L), IL-6 (1,000 U/mL) alone, dexamethasone + IL-6 (IL-6 present for 60 minutes followed by dexamethasone for 10 minutes, anti-fas alone (0.5 μg/mL for 10 minutes), or anti-fas + IL-6 where IL-6 is present for 10, 30, or 60 minutes before addition of anti-fas. Whole-cell lysates were then tested for enzymatic activity against GST-jun in an in vitro kinase assay. (B) Coomassie blue staining of gel shown in (A) to confirm equal protein loading.
(A) Anti-fas and dexamethasone activate and IL-6 inhibits jun kinase in 8226 cells. 8226 MM cells incubated in media (control) or dexamethasone alone (10−6 mol/L), IL-6 (1,000 U/mL) alone, dexamethasone + IL-6 (IL-6 present for 60 minutes followed by dexamethasone for 10 minutes, anti-fas alone (0.5 μg/mL for 10 minutes), or anti-fas + IL-6 where IL-6 is present for 10, 30, or 60 minutes before addition of anti-fas. Whole-cell lysates were then tested for enzymatic activity against GST-jun in an in vitro kinase assay. (B) Coomassie blue staining of gel shown in (A) to confirm equal protein loading.
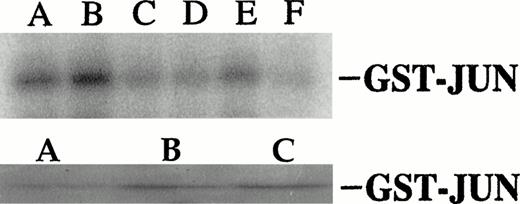
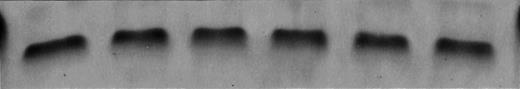
(A) Dexamethasone and anti-fas activate and IL-6 inhibits jun kinase activity in UCLA #1 cells. Top band: UCLA #1 cells were incubated in dexamethasone alone (lane A, 10−6 mol/L, for 10 minutes), anti-fas alone (lane B, 0.5 μg/mL for 10 minutess), media alone (control, lane C), dexamethasone (10−6 mol/L) + IL-6 (lane D, IL-6 at 1,000 U/mL present for 60 minutes before addition of dexamethasone), anti-fas + IL-6 (lane E, IL-6 present for 10 minutes before anti-fas), or anti-fas + IL-6 (lane F, IL-6 present for 60 minutes before anti-fas). In vitro kinase assay performed for jun kinase activity as shown in Fig 6. Bottom band: UCLA #1 cells incubated in media (lane A), dexamethasone (10−6 mol/L, lane B), or anti-fas (0.5 μg/mL, lane C) for 10 minutes. Jun kinase then immunoprecipitated from protein lysates and tested against GST-jun in in vitro kinase assay. (B) Coomassie blue staining of gel shown in upper band of (A).
(A) Dexamethasone and anti-fas activate and IL-6 inhibits jun kinase activity in UCLA #1 cells. Top band: UCLA #1 cells were incubated in dexamethasone alone (lane A, 10−6 mol/L, for 10 minutes), anti-fas alone (lane B, 0.5 μg/mL for 10 minutess), media alone (control, lane C), dexamethasone (10−6 mol/L) + IL-6 (lane D, IL-6 at 1,000 U/mL present for 60 minutes before addition of dexamethasone), anti-fas + IL-6 (lane E, IL-6 present for 10 minutes before anti-fas), or anti-fas + IL-6 (lane F, IL-6 present for 60 minutes before anti-fas). In vitro kinase assay performed for jun kinase activity as shown in Fig 6. Bottom band: UCLA #1 cells incubated in media (lane A), dexamethasone (10−6 mol/L, lane B), or anti-fas (0.5 μg/mL, lane C) for 10 minutes. Jun kinase then immunoprecipitated from protein lysates and tested against GST-jun in in vitro kinase assay. (B) Coomassie blue staining of gel shown in upper band of (A).
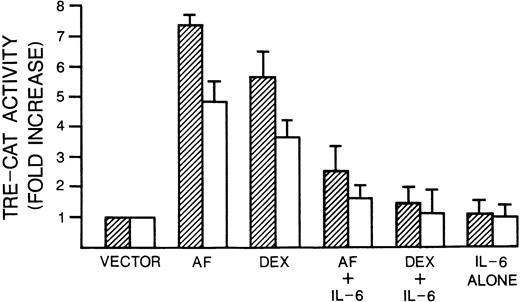
We next tested whether anti-fas and dexamethasone also activate c-jun transcriptional activity downstream of JNK activation and whether IL-6 prevents such activation. After phosphorylation by JNK,jun/fos heterodimers bind to DNA at TRE, the binding site in the jun promoter.28 Thus, we exploited a reporter gene assay which uses a promoter containing three TRE sites fused to the CAT gene (TRE-CAT). As shown in Fig 8, anti-fas and dexamethasone treatment activated TRE-CAT 7.5-and 5.5-fold in 8226 and 5- and 3.5-fold, respectively, in UCLA #1 cells. Thus, these apoptosis-inducing drugs lead to an increase in AP-1 activity in the same cells that demonstrate activated JNK activity. Concurrent exposure to IL-6 significantly inhibited this activation of AP-1 activity induced by both agents in both cell types (Fig 8).
Dexamethasone and anti-fas induce and IL-6 inhibits TRE-CAT activity. 8226 cells (▨) or UCLA #1 cells (□) were transfected with the AP-1–dependent transcriptional reporter 3× TRE-CAT and treated with anti-fas (0.5 μg/mL), dexamethasone (10−6 mol/L), IL-6 alone (1,000 U/mL), or the combinations of dexamethasone + IL-6 or anti-fas + IL-6. Treatments were for 60 minutes and cells were lysed after 24 hours; CAT activity was measured with (14C) chloramphenicol as substrate. Data represent means ± SE of three independent trasnfections and are expressed as fold increase in activity compared with control cells that received the TRE-CAT vector but no drug treatment.
Dexamethasone and anti-fas induce and IL-6 inhibits TRE-CAT activity. 8226 cells (▨) or UCLA #1 cells (□) were transfected with the AP-1–dependent transcriptional reporter 3× TRE-CAT and treated with anti-fas (0.5 μg/mL), dexamethasone (10−6 mol/L), IL-6 alone (1,000 U/mL), or the combinations of dexamethasone + IL-6 or anti-fas + IL-6. Treatments were for 60 minutes and cells were lysed after 24 hours; CAT activity was measured with (14C) chloramphenicol as substrate. Data represent means ± SE of three independent trasnfections and are expressed as fold increase in activity compared with control cells that received the TRE-CAT vector but no drug treatment.
A dominant negative mutant that inhibits c-jun prevents anti–fas-induced MM cell apoptosis.
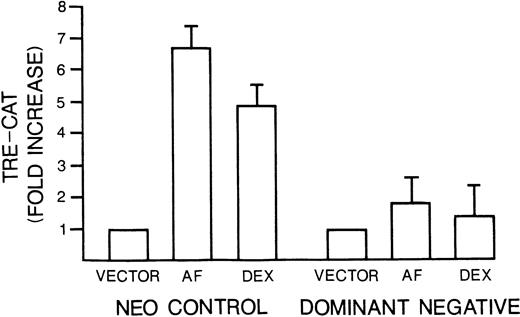
Several prior studies have provided evidence that JNK activation and subsequent c-jun activity play a role in induction of apoptosis. Thus, we considered the possibility that IL-6–dependent protection of MM cells was mediated through the cytokine's inhibitory effects on anti-fas and dexamethasone-induced stimulation of JNK and c-jun. To further test this hypothesis, we examined the effect of a well-characterized jun mutant, Jun In282,23 on apoptosis: Jun In282 is a DNA binding mutant caused by an insertion in the basic region and acts as a dominant negative construct by competing with endogenous c-junas a substrate for JNK activity. We reasoned that if IL-6–dependent protection against apoptosis was mediated via inhibition of JNK activity with subsequent prevention of c-jun phosphorylation and c-jun transactivation, then disruption of c-junfunction by use of the dominant negative construct should also protect against apoptosis. Thus, 8226 cells were transduced to express thejun mutant or neo alone by retroviral infection. After selection in G418, the two polyclonal cell populations were tested for the ability of anti-fas or dexamethasone to stimulate the activation of TRE-CAT. As shown in Fig 9, the c-jun dominant negative line was prohibited in TRE-CAT activation by both apoptosis-inducing agents. The dominant negative c-jun had no significant effect on fas expression or viability of the continuously cultured MM cells (not shown). However, when c-jun mutant-containing MM cells were exposed to anti-fas, they were protected against apoptosis. This was confirmed by testing viability with dye exclusion assays (Table 2), testing DNA fragmentation by the diphenylamine assay (Table 2), and by electrophoresis of extracted DNA (Fig 10). In contrast, the apoptotic response of mutant c-jun–containing MM cells to dexamethasone was, in fact, significantly increased (Table 2 and Fig 10). We also cloned the mutant c-jun–containing MM cells by limiting dilution and generated four clones. After confirming an inhibition of TRE-CAT activation by dexamethasone and anti-fas in these clones, we tested them for sensitivity to apoptosis. The results were comparable to the transduced polyclonal population of cells. Althoughneo control cells exposed to anti-fas (0.5 μg/mL) for 20 hours or dexamethasone (10−6mol/L) for 48 hours resulted in a significant loss of viability (from 87% to 69% for dexamethasone and 83% to 47% for anti-fas) and increase in DNA fragmentation (from 4% to 26% for anti-fas and 3% to 23% for dexamethasone), the clones were relatively resistant to anti-fas (viability, 73% to 89%v control of 87%; DNA fragmentation, 2% to 5% vcontrol of 4%) and showed a modest increase in sensitivity to dexamethasone (viability, 49% to 60% v control of 90%; DNA fragmentation, 30% to 37% v control of 4.7%). Thus, these data provide supportive evidence that IL-6 protects MM cells against anti–fas-induced apoptosis via its inhibitory effects onjun kinase activity and subsequent c-jun function but also indicate that protection against dexamethasone proceeds along different pathways.
Mutant c-jun–containing 8226 cells have a blunted TRE-CAT response to anti-fas and dexamethasone.Neo control or dominant negative c-jun–transduced 8226 cells were transfected with TRE-CAT and treated without or with anti-fas (0.5 μg/mL) or dexamethasone (10−6mol/L) for 10 minutes. TRE-CAT activity is mean ± SE of three separate experiments.
Mutant c-jun–containing 8226 cells have a blunted TRE-CAT response to anti-fas and dexamethasone.Neo control or dominant negative c-jun–transduced 8226 cells were transfected with TRE-CAT and treated without or with anti-fas (0.5 μg/mL) or dexamethasone (10−6mol/L) for 10 minutes. TRE-CAT activity is mean ± SE of three separate experiments.
Effect of Anti-fas and Dexamethasone on Mutant c-jun–Containing MM Cells
| Cell Line . | Drug . | % Viability . | % DNA Fragmentation . |
|---|---|---|---|
| neo control | None-control | 81 ± 5 | 6.4 ± 1 |
| AF, 0.1 μg/mL | 67 ± 4* | 16.4 ± 2* | |
| AF, 0.25 μg/mL | 60 ± 4* | 22 ± 3* | |
| AF, 0.5 μg/mL | 35 ± 2* | 29.7 ± 3* | |
| dom neg | None-control | 83 ± 5 | 3.1 ± 1 |
| AF, 0.1 μg/mL | 82 ± 5 | 4.4 ± 1 | |
| AF, 0.25 μg/mL | 80 ± 3 | 3.5 ± 1 | |
| AF, 0.5 μg/mL | 81 ± 4 | 4.2 ± 1 | |
| neo control | Non-control | 92 ± 4 | 3.9 ± 1 |
| Dex, 10−6 mol/L | 73 ± 3 | 21 ± 3 | |
| Dex, 2 × 10−6mol/L | 61 ± 5 | 29 ± 3 | |
| dom neg | None-control | 90 ± 6 | 4.1 ± 1 |
| Dex, 10−6 mol/L | 54 ± 5† | 33.8 ± 3† | |
| Dex, 2 × 10−6 mol/L | 38 ± 4† | 44.2 ± 4† |
| Cell Line . | Drug . | % Viability . | % DNA Fragmentation . |
|---|---|---|---|
| neo control | None-control | 81 ± 5 | 6.4 ± 1 |
| AF, 0.1 μg/mL | 67 ± 4* | 16.4 ± 2* | |
| AF, 0.25 μg/mL | 60 ± 4* | 22 ± 3* | |
| AF, 0.5 μg/mL | 35 ± 2* | 29.7 ± 3* | |
| dom neg | None-control | 83 ± 5 | 3.1 ± 1 |
| AF, 0.1 μg/mL | 82 ± 5 | 4.4 ± 1 | |
| AF, 0.25 μg/mL | 80 ± 3 | 3.5 ± 1 | |
| AF, 0.5 μg/mL | 81 ± 4 | 4.2 ± 1 | |
| neo control | Non-control | 92 ± 4 | 3.9 ± 1 |
| Dex, 10−6 mol/L | 73 ± 3 | 21 ± 3 | |
| Dex, 2 × 10−6mol/L | 61 ± 5 | 29 ± 3 | |
| dom neg | None-control | 90 ± 6 | 4.1 ± 1 |
| Dex, 10−6 mol/L | 54 ± 5† | 33.8 ± 3† | |
| Dex, 2 × 10−6 mol/L | 38 ± 4† | 44.2 ± 4† |
Neo control or c-jun dominant negative–containing 8226 cells cultured with anti-fas (AF) for 20 hours or dexamethasone (Dex) for 48 hours and viability and DNA fragmentation (diphenylamine assay) was evaluated. Data are means ± SD of three separate experiments.
Significantly different (P < .05) from corresponding control (culture without drug).
Significantly different (P < .05) from corresponding values in neo-control cells.
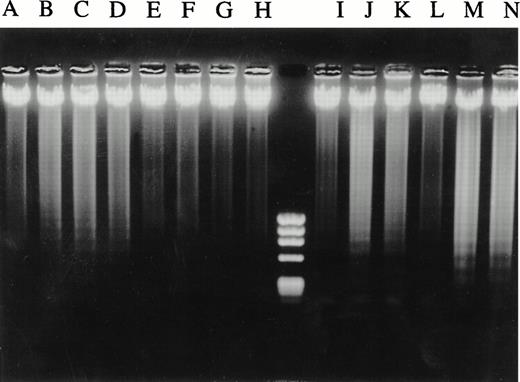
Mutant c-jun–containing 8226 cells are protected from anti–fas-induced DNA laddering but not from dexamethasone. Neo control (lanes A through D and I through K) and mutant c-jun–transduced 8226 cells (lanes E through H and L through N) were cultured in media for 20 hours (lanes A and E) or 48 hours (lanes I and L) or with increasing concentrations of anti-fas (0.1, 0.25, 0.5 μg/mL, lanes B through D and F through H) or with dexamethasone (10−6 mol/L and 2 × 10−6 mol/L, lanes J and K and M and N, respectively). DNA was then extracted and electrophoresed.
Mutant c-jun–containing 8226 cells are protected from anti–fas-induced DNA laddering but not from dexamethasone. Neo control (lanes A through D and I through K) and mutant c-jun–transduced 8226 cells (lanes E through H and L through N) were cultured in media for 20 hours (lanes A and E) or 48 hours (lanes I and L) or with increasing concentrations of anti-fas (0.1, 0.25, 0.5 μg/mL, lanes B through D and F through H) or with dexamethasone (10−6 mol/L and 2 × 10−6 mol/L, lanes J and K and M and N, respectively). DNA was then extracted and electrophoresed.
DISCUSSION
The results of this study indicate that IL-6–dependent protection against anti–fas- and dexamethasone-induced apoptosis of malignant plasma cells is not mediated via alterations in cell-cycle distribution or the RAF-1/MEK/ERK pathway. However, IL-6 prevented anti-fas and dexamethasone-induced activation of junkinase in these cells. Our experiments with mutant c-jun–containing MM cells provide support that these inhibitory effects on jun kinase mediate IL-6–induced protection against death at least for MM cells challenged with anti-fas. Protection against dexamethasone-induced MM cell death appears to be mediated by other pathways.
These results confirm and extend the work of Chauhan et al.20 These investigators also detected a correlation between IL-6–dependent protection against anti–fas-induced apoptosis and the cytokine's ability to inhibit anti–fas-stimulated JNK activity. Furthermore, IL-6 inhibited the downstream events of JNK activation, namely c-juntransactivation of the reporter TRE-CAT. Although these latter data and the study of Chauhan et al20 suggested a similar mechanism whereby IL-6 protects against apoptosis and prevents JNK activation, they did not prove a causal relationship. It was certainly possible that JNK/c-jun activation were either epiphenomena or even occurred downstream of the apoptosis machinery, which has been suggested for TNF29 and anti–fas-induced apoptosis of Jurkat cells.30 Thus, we hypothesized that, if IL-6–induced protection was mediated through inhibition of JNK activation and subsequent c-jun activity, a comparable inhibition of c-jun function, achieved by different means, should also protect against apoptosis. The resistance of dominant negative c-jun–containing MM cells to anti–fas-induced apoptosis thus provides support for this hypothesis. We did not test the effects of IL-6 on p38MAPK in this study because the previous work of Chauhan et al20 clearly showed that anti-apoptotic concentrations of IL-6 had no effect on the activation of p38MAPK induced by anti-fas.
The role of the MEKK/SEK1/JNK pathway in the regulation of apoptosis is controversial. The best evidence that JNK activation can initiate or sustain apoptosis comes from experiments like ours where selective disruption of the pathway by introduction of mutant genes7,31-34 or by use of antisense oligonucleotides35,36 inhibited the apoptotic response induced by TNF,31 ceramide,31,35 growth factor withdrawal,7,36 or anti-fas.32Specifically for myeloma cells and IL-6, two other previous studies support a role for JNK activation and AP-1 activity in apoptosis. One37 showed that cAMP-stimulated AP-1 activity might be involved in inhibiting MM cell growth, and a second36showed that anti-sense oligonucleotides directed to c-junprevented apoptosis of MM cells induced by IL-6 deprivation. However, a critical role for JNK activation and c-jun function implies that gene transactivation would be required for MM cell apoptosis and most models of cellular apoptosis do not require protein synthesis.38 39 We have not been able to test the requirements for protein synthesis in MM cell apoptosis because all our MM cell lines are exquisitely sensitive to the apoptotic effects of cycloheximde, emetine, and actinomycin-D when used alone (not shown). It is also possible that the protection conferred by mutant c-jun is mediated through yet unknown pathways that do not involve its well-known role as a transcription factor.
It is not clear whether all activators of JNK/c-jun will induce MM cell apoptosis. For example, CD40 triggering induce marked JNK activation in other cell types, although previous studies40,41 did not detect apoptosis in CD40-stimulated MM cells. However, these latter cells may also be stimulated to secrete IL-6,40, 41 which might protect them from a CD40-initiated apoptosis program. In fact, in MM cells that are not triggered for IL-6 secretion by CD40, we have detected an induction of apoptosis. Nevertheless, our own data suggest that, though activation of JNK/c-jun is critical, it may not be sufficient for apoptosis. Thus, although dexamethasone activates JNK in these cells, this activation does not mediate apoptosis as shown by experiments with the c-jun mutant. Another possible example of this may be IL-1β, which induces marked JNK activation, but is not known to be an apoptotic stimulus, and, in fact, may even activate MM cells. It is possible that the cellular context within which JNK and c-junbecome activated is important for determining outcome. For example, the duration of JNK activation42 or the presence of other activated pathways working in concert with the JNK cascade, may be critical in determining whether target cells undergo activation or apoptosis.
Chauhan et al,20 who also studied 8226 MM cells, were unable to detect activation of ERK-1 or -2 by IL-6 in plasma cells protected against apoptosis. In similar fashion, we also were unable to detect IL-6–dependent activation of the RAF/MEK/ERK cascade in protected 8226 as well as UCLA #1 MM cells, although PMA successfully activated both proteins. In contrast, IL-6 successfully activated both RAF-1 and ERK in AF-10 plasma cells. This differential effect on RAF/MEK/ERK in these different MM targets may reflect the finding that IL-6 can stimulate proliferation in AF-10 cells but only has anti-apoptotic function in 8226 and UCLA #1 cells without effects on proliferation. This is consistent with previous work26,27that suggested IL-6 signaling through RAF/MEK/ERK was crucial for stimulation of MM cell proliferation. Recent work by Ogata et al43 indicates that lack of SOS phosphorylation and activation in 8226 cells exposed to IL-6 explains the absence of RAF/MEK/ERK activation and loss of proliferative responsiveness in these cells. However, we detected an IL-6–independent constitutive activation of ERK in 8226 and UCLA #1 cells as determined by in vitro kinase assays (Fig 5) and by immunoblotting with antiphosphotyrosine antibodies (Fig 4). Such constitutive activation may be due to the presence of an activating mutation of ras in these tumor cells. Activating ras mutations are relatively common in MM44 and would allow downstream activation of ERKs in the absence of SOS phosphorylation.
Our results that argue against ras-dependent MAPK signaling events being important in IL-6–induced protection against MM cell apoptosis appear inconsistent with results of Billadeau et al.45 These latter investigators showed that activating mutations of ras suppress apoptosis in MM cells when they are deprived of IL-6. The most obvious difference between Billadeau's model and ours is that he studied an IL-6–dependent MM line and we studied MM cell lines that are not IL-6 dependent. Antibodies to IL-6 have no effect on the in vitro growth of our 8226 and UCLA #1 cell lines2 and, furthermore, exogenous IL-6 does not stimulate any proliferation of these two cell lines. The inconsistency between the studies of Billadeau et al and our own could be reconciled by the presence of two separate IL-6–dependent anti-apoptotic pathways in MM cells, only one of which (that studied by Billadeau et al45) being intimately integrated into IL-6–dependent signals that result in proliferation as well.
Although our results provide some insight into the mechanism by which IL-6 protects MM cells against anti-fas, the pathways involved in protection against dexamethasone remain unclear. Although we demonstrated a dexamethasone-induced activation of JNK/SAPK and TRE-CAT reporter gene expression, mutant c-jun did not protect against dexamethasone and, in fact, significantly sensitized MM cells to enhanced apoptosis induced by dexamethasone. This suggests that JNK activation during dexamethasone exposure is a protective response to dexamethasone and that enhanced AP-1 transactivation induces expression of protective proteins. This is consistent with a recent study46 where the immunosuppressant drug rapamycin was found to inhibit jun kinase activity and markedly potentiate dexamethasone-induced apoptosis of lymphoblastoid cells. It should be mentioned that other investigators could not detect SAPK/JNK activation in 8226 cells challenged with dexamethasone.47 The reason for this discrepancy is not readily obvious to us. It could be caused by the need for costimulatory molecules selectively present in our culture media. Other investigators have documented the ability of dexamethasone to induce JNK activation in different cell models.48-50
The results of our cell-cycle analysis confirm that protection against dexamethasone-induced apoptosis by IL-6, at least in 8226 cells, is not accompanied by protection against dexamethasone-induced cytostasis. This shows that IL-6–induced protection against dexamethasone-induced apoptosis is not likely due to inhibition of very proximal events such as dexamethasone receptor expression or binding of dexamethasone to its receptor, but is selective for dexamethasone-induced downstream events that are more specific for apoptosis. However, cell-cycle analysis also showed that, unlike the interaction between IL-6 and activated p53 in M1 cells,24 IL-6 was not protecting MM cells by complementing the antiproliferative effect of dexamethasone and blocking cells in a dormant state. We have also been unable to correlate IL-6–induced protection against dexamethasone-induced MM cell death with any alteration in expression of BCL-2 or BAX proteins.2 In a previous study,51 we detected an IL-6–dependent upregulation of BCL-XL in UCLA #1 cells, which is similar to previous findings in other MM lines.52,53 However, repeated studies with 8226 targets exposed to IL-6 have failed to demonstrate any alteration of BCL-XL expression.51 We have also assessed the ability of IL-6 to phosphorylate BAD protein in protected MM cells because recent work54 suggests BAD phosphorylation is crucial for mediating IL-3–induced protection against apoptosis. However, we detected very little BAD protein in these human MM cells and IL-6 was incapable of phosphorylating BAD. Thus, although there are many more members of the BCL protein family that have not been examined, work to date has not detected consistent alterations of this family of proteins that could conceivably account for IL-6–induced protection.
In summary, this study indicates that inhibition of c-junfunction protects MM cells against apoptosis induced by anti-fas. The results support the hypothesis that IL-6–induced protection against anti-fas is mediated through its inhibition of the JNK/c-jun pathway. Furthermore, it indicates that the ability of IL-6 to protect MM cells against dexamethasone must be mediated by yet unknown mechanisms.
Supported by research funds from the Veteran's Administration.
Address reprint requests to Alan Lichtenstein, MD, Hematology-Oncology, VA West LA Hospital, 691/W111H, 11301 Wilshire Blvd, Los Angeles, CA 90073.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. IL-6 protects against anti-fas and dexamethasone. 8226 (lanes A through F) and UCLA #1 cells (lanes G through K) were cultured in media alone for 48 hours (lanes A and G), IL-6 alone for 48 hours (1,000 U/mL, lane B), anti-fas for 20 hours (0.5 μg/mL, lanes C and H), dexamethasone for 48 hours (10−6 mol/L, lanes E and J) or the combination of anti-fas + IL-6 (lanes D and I) or dexamethasone + IL-6 (lanes F and K). In the anti-fas + IL-6 combination, IL-6 was present for 1 hour before addition of anti-fas. DNA was then extracted and electrophoresed. Culture of both cell lines with a control antibody of identical isotype to the anti-fas antibody resulted in normal, intact, high-molecular-weight DNA (identical to that shown in lanes A and G [not shown]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/1/10.1182_blood.v92.1.241.413k28_241_251/5/m_blod41328001w.jpeg?Expires=1769148273&Signature=lTLlJ25T6Wr2G4iLTE~X0nBWvOdWnLL2s4ftBu2T39SFlcNan2GAuDLjDoY63niQ1GhhrSNfJxhEcuBQd9SrDy2lL2mZfTLcBO3PbfcLY1werGt0wDPvMqqq2S7QPAdl1jI6REEQYJgIaEz2Dex4azxJsyyzYM5tZR6aBfubX7d2azgVdMmm7ZwvgSs8IHIesNmbSCQQsqiAja3pEO-iZjA76dMG2KZ7qHlAeYBvQWzJ8U6v6ax1aUSAy~Am9b5Sw~edWIU4Q~BWLAxoisozWa8ndfvmEWuFAywsCMLdVKQk6Tz7XPphDxeAhIFR66Z46BNf4sfj2cN6rFkVcBK52Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal