Abstract
Ad.CMV-CD is a replication incompetent adenoviral vector carrying a cytomegalovirus (CMV)-driven transcription unit of the cytosine deaminase (CD) gene. The CD transcription unit in this vector catalyzes the deamination of the nontoxic pro-drug, 5-fluorocytosine (5-FC), thus converting it to the cytotoxic drug 5-fluorouracil (5-FU). This adenoviral vector prodrug activation system has been proposed for use in selectively sensitizing breast cancer cells, which may contaminate collections of autologous stem cells products from breast cancer patients, to the toxic effects of 5-FC, without damaging the reconstitutive capability of the normal hematopoietic cells. This system could conceivably kill even the nondividing breast cancer cells, because the levels of 5-FU generated by this system are 10 to 30 times that associated with systemic administration of 5-FU. The incorporation of 5-FU into mRNA at these high levels is sufficient to disrupt mRNA processing and protein synthesis so that even nondividing cells die of protein starvation. To test if the CD adenoviral vector sensitizes breast cancer cells to 5-FC, we exposed primary explants of normal human mammary epithelial cells (HMECs) and the established breast cancer cell (BCC) lines MCF-7 and MDA-MB-453 to the Ad.CMV-CD for 90 minutes. This produced a 100-fold sensitization of these epithelial cells to the effects of 48 hours of exposure to 5-FC. We next tested the selectivity of this system for BCC. When peripheral blood mononuclear cells (PBMCs), collected from cancer patients during the recovery phase from conventional dose chemotherapy-induced myelosuppression, were exposed to the Ad.CMV-CD for 90 minutes in serum-free conditions, little or no detectable conversion of 5-FC into 5-FU was seen even after 48 hours of exposure to high doses of 5-FC. In contrast, 70% of 5-FC was converted into the cytotoxic agent 5-FU when MCF-7 breast cancer cells (BCCs) were exposed to the same Ad.CMV-CD vector followed by 5-FC for 48 hours. All of the BCC lines tested were shown to be sensitive to infection by adenoviral vectors when exposed to a recombinant adenoviral vector containing the reporter gene betagalactosidase (Ad.CMV-βgal). In contrast, less than 1% of the CD34-selected cells and their more immature subsets, such as the CD34+CD38− or CD34+CD33− subpopulations, were positive for infection by the Ad.CMV-βgal vector, as judged by fluorescence-activated cell sorting (FACS) analysis, when exposed to the adenoviral vector under conditions that did not commit the early hematopoietic precursor cells to maturation. When artificial mixtures of hematopoietic cells and BCCs were exposed for 90 minutes to the Ad.CMV-CD vector and to 5-FC for 10 days or more, a greater than 1 million fold reduction in the number of BCCs, as measured by colony-limiting dilution assays, was observed. To test if the conditions were damaging for the hematopoietic reconstituting cells, marrow cells collected from 5-FU–treated male donor mice were incubated with the cytosine deaminase adenoviral vector and then exposed to 5-FC either for 4 days in vitro before transplantation or for 14 days immediately after transplantation in vivo. There was no significant decrease in the reconstituting capability of the male marrow cells, as measured by their persistence in female irradiated recipients for up to 6 months after transplantation. These observations suggest that adenovirus-mediated gene transfer of the Escherichia coli cytosine deaminase gene followed by exposure to the nontoxic pro-drug 5-FC may be a potential strategy to selectively reduce the level of contaminating BCCs in collections of hematopoietic cells used for autografts in breast cancer patients.
THE DISEASE-FREE SURVIVAL of poor prognosis newly resected or advanced disease breast cancer patients1 and those with metastatic disease2can be improved by the delivery of intensive combination chemotherapy treatments followed by hematopoietic rescue with autologous stem cell transplantation. Approximately 8,000 of such treatments are performed every year in the United States.1
Currently, breast cancer patients with gross contamination of the bone marrow by tumor cells are usually excluded from such treatments. Recently, a study based on polymerase chain reaction (PCR) analysis for cytokeratin 19 (CK-19), a marker for metastatic circulating cells, has shown that the majority of advanced disease breast cancer patients already have cancer cells contaminating their bone marrow.3The presence of a positive PCR assay for CK-19 in these patients is associated with a decrease in disease-free interval after transplantation3 and an increased probability of relapse from 19% to greater than 80%.
These data have led to an increased interest in procedures that can selectively remove breast cancer cells (BCCs) from autologous hematopoietic cells to be used for transplant, without damaging the hematopoietic reconstituting cells. Methods applied to this problem include monoclonal antibodies (MoAbs) targeted to breast cancer-associated cell surface markers,4 breast cancer specific MoAbs conjugated to toxins,5 in vitro incubation with cytotoxic drugs,6 lectin agglutination,7phototherapy,8 and biological modifiers.9Unfortunately, many of these systems for eradicating BCCs also lead to the destruction of clonogenic progenitor cells and thereby cause a prolongation of neutropenia after intensive therapy and transplantation. No combination of MoAbs using positive selection of normal hematopoietic cells and negative selection of BCCs has yet to become commercially available for use in reducing the level of neoplastic cells in autologous hematopoietic stem cells.
One promising strategy for removing BCCs from autologous bone marrow or peripheral blood mononuclear cells (PBMCs) is to selectively infect the BCCs with replication-incompetent adenoviral vectors that carry genes that encode for pro-drug activation enzymes. We have studied the feasibility of using a pro-drug activation approach for the eradication of neoplastic epithelial breast cells that contaminate collections of autologous hematopoietic cells. Specifically, we used a replication-incompetent adenoviral vector to selectively introduce the bacterial chemotherapy sensitization gene, cytosine deaminase (CD), into BCCs ex vivo, without introducing this gene into the normal early hematopoietic reconstituting cells.
CD converts the innocuous antibiotic pro-drug 5-fluorocytosine (5-FC) into the cytotoxic chemotherapeutic agent 5-fluorouracil (5-FU), thereby sensitizing the BCCs to 5-FC. The adenoviral vector is one of the most attractive candidates for the delivery of chemosensitization genetic elements, because it can infect epithelial neoplastic cells at high frequency, although it does not efficiently infect the very early normal hematopoietic stem cells,11,12 unless they are induced to mature to later stages of differentiation by exposure to serum and hematopoietic growth factors, such as interleukin-3 (IL-3).13,14 5-FU generated by the CD adenoviral vector and 5-FC is converted into its phosphorylated metabolites, a prerequisite for incorporation into DNA and RNA.12 If the level of 5-FU becomes high enough (>30 μmol/L), as is the case with cells that contain the CD gene, the incorporation of 5-FU into RNA is sufficiently high to disrupt protein synthesis with consequent death of even nondividing cells.15-18 Thus, adenoviral vectors containing the bacterial CD gene could be potentially useful for ex vivo and in vivo killing of even nondividing BCCs and can therefore be considered for human bone marrow purging. Results demonstrating the feasibility of this approach are the subject of this report.
MATERIALS AND METHODS
Cells and cell culture.
The BCC lines MCF-7, MDA-MB-453, MDA-MB-436, MDA-MB-231, MDA-468, T47D, and BT-20 (obtained from American Type Culture Collection [ATCC], Rockville, MD) were grown in DMEM-F12 (GIBCO-BRL, Gaithersburg, MD) containing 10% heat-inactivated fetal bovine serum (FBS; HyClone Laboratories Inc, Logan, UT). The transformed human kidney cell line 293 (obtained from ATCC) was propagated in Dulbecco's modified Eagle's medium (DMEM; GIBCO-BRL) containing 10% heat-inactivated FBS. Human mammary epithelial cells (HMECs), derived from an explant of normal breast epithelium, were obtained from Clonetics Corp (San Diego, CA) and grown according to the manufacturer's specifications. These cells were maintained in a 5% CO2/95% air humidified atmosphere. Mobilized peripheral blood (MPB) cells were obtained from cancer patients undergoing therapeutic autologous stem cell harvest. Mononuclear cells (MNCs) were isolated by density centrifugation over Ficoll (Histopaque-1077; Sigma Chemical Co, St Louis, MO) at 500g for 30 minutes and washed twice with Medium 199/HBSS (JRH Biosciences, Lenexa, KS).
Chemicals.
5-FC and 5-FU were purchased from Sigma Chemical Co and 6-[3H]5-Fluorocytosine (4.1 Ci/mmol) was purchased from Moravek Biochemicals Inc (Brea, CA).
Drug cytotoxicity assays.
To test for drug sensitivity, 1 × 104 MCF-7 breast cancer cells, MDA-MD-453 breast cancer cells, or primary normal breast epithelial cells (HMECs) were plated in triplicate in tissue culture medium supplemented with 5-FC or 5-FU (0 to 10 mmol/L) in 96-well flat-bottom tissue culture plates (Corning Inc, New York, NY). Drugs were added approximately 24 hours after seeding. After 5 to 6 days in culture, the effect on cell proliferation was assessed using a fluorimetric/colorimetric assay (Alamar Blue Assay; Alamar Biosciences Inc, Campbell, CA).
Drug cytotoxicity assays after exposure to the CD adenoviral vector.
To test for adenoviral induced sensitization to 5-FC, 1 × 104 MCF-7 cells, MDA-453 cells, or HMECs were infected with the Ad.CMV-CD at different multiplicities of infection (MOI; 10, 50, and 100) in serum-free medium for 90 minutes. After washing, cells were then plated in triplicate into 96-well flat-bottom tissue culture plates. Different concentrations of 5-FC were added to the cells after infection and, after 5 days, the number of cells remaining was determined as described above.
In vitro testing of the selectivity of Ad.CMV-CD–mediated sensitization of BCCs to 5-FC.
Peripheral blood stem cells, MCF-7 BCCs, and mixtures of the two cell populations (a total of 2.5 × 106 cells) were incubated for 48 hours in 25-cm2 flasks containing 10 mL of medium with 500 μmol/L 5-FC and 20 μCi of [6-3H]5-FC (Moravek Biochemicals, Brea, CA). A 1-mL aliquot of the medium was sampled and mixed with an equal amount of ice-cold methanol, centrifuged at 7,500g for 5 minutes, and stored at −20°C before analysis. The conversion from 5-FC to 5-FU was evaluated by high-performance liquid chromatography (HPLC) using a Microsorb C18 reverse phase column (25 cm × 4.6 mm internal diameter; Rainin, Inc, Woburn, MA) eluted with 50 mmol/L potassium phosphate monobasic (KH2PO4), pH 3.0, at room temperature. Fractions were collected at 1-minute intervals and radioactivity was counted after the addition of scintillation fluid.
To evaluate drug metabolism and incorporation, MCF-7 BCCs were incubated with 500 μmol/L 5-FC containing 40 μCi of [6-3H]5-FC for 48 hours after infection with 100 MOI of Ad.CMV-CD or with 5 μmol/L 5-FU in the presence of 25 μCi [6-3H]5-FU for 24 hours at 37°C and then washed twice with ice-cold phosphate-buffered saline (PBS), and the mono-layer was treated with 0.5 mL of 5% trichloroacetic acid (TCA). The pellet was washed twice with 5% TCA and the incorporated radioactivity was determined after digestion with NCS tissue solubilizer (Amersham, Arlington Heights, IL). To analyze the fluorinated metabolites, TCA cell extracts were neutralized with Freon/trioctylamine extraction and separated on a Spherisorb SAX HPLC column (25 cm × 4.6 mm i.d.) at room temperature.14 The column was eluted with a sodium phosphate (pH 3.3) gradient from 0.02 to 0.3 mol/L for 40 minutes at 0.7 mL/min. The effluent was collected in 1-mL fractions and radioactivity was determined. Unlabeled standards were added to each aliquot assay to positively locate the fluoronucleotides.
Recombinant adenovirus.
A replication-incompetent recombinant adenoviral vector obtained from the laboratory of Dr Ron Crystal (Cornell Medical School, New York, NY) that contained the cytosine deaminase gene (Ad.CMV-CD) in a cytomegalovirus (CMV)-driven transcription unit was used in this series of experiments.19 In this vector, a portion of the E1a and E1b gene region of human adenovirus serotype 5 had been replaced by the bacterial cytosine deaminase gene under the transcriptional control of the human CMV promoter as described previously.19 A similar adenoviral vector (Ad.CMV-βgal) was engineered in our laboratory in which a β-galactosidase transcription unit was inserted into the E1a and E1b region.20 Recombinant adenovirus was purified by a cesium chloride (CsCl) gradient density centrifugation. The final viral band was diluted 1:1 with sterile glycerol and stored at −70°C. The number of adenovirus particles in viral stocks was determined by limiting dilution and plaque formation of 293 cells exposed to various dilutions of the vector (plaque-forming units [pfu]). Absence of replication-competent virus was confirmed by the limiting dilution and plaque formation assays in HeLa cells exposed to the vector.
Fluorescein di-β-D-galactopyranoside (FDG) flow cytometry analysis of infectivity of the β-galactosidase adenoviral vector for BCCs versus hematopoietic cells.
Mobilized peripheral blood or enriched CD34+ cells were exposed for 30 minutes, 90 minutes, or 24 hours to the Ad.CMV-βgal virus (100 MOI) in serum-free medium (Iscove's modified Dulbecco's medium [IMDM]; GIBCO-BRL), washed twice with PBS, and incubated for 24 hours in 24-well flat-bottom plates with IMDM containing 5% heat-inactivated FBS in the absence of growth factors. Twenty-four hours after exposure to Ad.CMV-βgal, the cells were washed once with PBS and prepared for flow cytometry. Cells were stained with CD34 phycoerythrin (PE)-conjugated MoAb alone (HPCA-2; Becton Dickinson, San Jose, CA) or in combination with CD38 Cy5-PE–conjugated or CD33 Cy5-PE–conjugated MoAbs (Caltag Laboratories, Burlingame, CA).
After 30 minutes of incubation in the dark at 4°C, cells were washed with PBS containing 1% bovine serum albumin and stained with fluorescein di-β-D-galactopyranoside (Molecular Probes, Inc, Eugene, OR), as previously described.21 22 A 2 mmol/L solution of FDG (substrate) in 98:1:1 H2O/DMSO/ethanol was mixed with an equal volume (50 μL) of cell suspension that was prewarmed to 37°C. After 1 minute of incubation at 37°C, an equal volume (50 μL) of ice-cold 2× strength PBS was added.
The samples were maintained at 4°C for 3 to 4 hours before analysis by flow cytometry. Cells were treated with Chloroquine to suppress endogenous β-galactosidase activity. Viability studies were performed using propidium iodine. Flow cytometry was performed with a FACStar flow cytometer (Becton Dickinson). The frequency of FDG+cells for each particular immunophenotype was calculated as follows: Frequency (%) = (number of a particular immunophenotype that was FDG+ among vector exposed cells) − (number of a particular immunophenotype that was FDG+ among cells not exposed to vector)/(number of a particular immunophenotype among vector-exposed cells).
BCCs were harvested from monolayer cultures with 0.5 mmol/L EGTA [ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, pH 7.0] and exposed in suspension to the adenoviral vectors for 90 minutes or 24 hours, as specified. After vector exposure, cells were washed twice with PBS and seeded in tissue culture medium. Twenty-four hours after vector exposure, the cells were trypsinized, washed twice in PBS, and stained with FDG.
Integrin analysis.
Analysis of the CD adenoviral vector sensitization of clonogenic cells in BCC lines or hematopoietic cells to 5-FC.
Methods used for this assay have been described in detail previously by our laboratory.25 To determine the proliferative capacity of hematopoietic cells and BCCs, mixtures composed of CD34+cells and MCF-7 cells were exposed in suspension for 90 minutes to the Ad.CMV-CD vector in duplicate assays, washed twice in PBS, and left in culture for a 2-day period in the presence or absence of 5-FC. To determine the proliferative capacity of the hematopoietic cells, granulocyte-macrophage colony-forming unit (CFU-GM) cells were assayed as follows25: 2 to 3 × 103CD34+ selected cells were cultured in 1 mL of 0.9% methylcellulose in IMDM, 30% fetal bovine serum, 1% bovine serum albumin, 10−4 mol/L 2-mercaptoethanol, 2 mmol/L L-glutamine, 5% serum containing phytohemoagglutinin leukocyte conditioned medium (PHA-LCM), and 3 U/mL of recombinant human erythropoietin (MethoCult, StemCell Technologies Inc, Vancouver, British Columbia, Canada). Cells were cultured in 35-mm plates and incubated for 14 days at 37°C in a humidified atmosphere containing 5% CO2 in air. Colonies consisting of 50 cells or more were counted as CFU-GM. A limiting dilution assay was used to determine the effects of the Ad.CMV-CD vector on BCCs. At the end of the 2-day incubation period, all cells were collected and washed twice in PBS. A total of 104 cells were serially diluted 10-fold, and each dilution was plated into a 96-well flat-bottom microtiter plate that contained 200 μL medium DMEM (DMEM-F12, 10% FBS) per well and incubated for 14 days. The clonogenic growth of surviving tumor cells was evaluated by phase-contrast microscopy, scoring the number of wells with at least 1 colony containing 20 or more cells.
Evaluation of the efficacy of the 5-FC/CD adenoviral vector system under conditions that simulate in vitro purging of BCCs from collections of autologous hematopoietic cells.
MCF-7 BCCs (9×107 cells) and 7 × 109 HL60 cells were mixed in a 50 mL volume of PBS and exposed to the cytosine deaminase adenoviral vector (in a ratio of pfu/total cells of 400/1) for 90 minutes. The cells were then diluted and plated overnight in 150-cm2 tissue culture flasks. An aliquot of these cells was subjected to serial 10-fold dilutions up to a 1/106 fold dilution. The cells were plated in 150-cm2 flasks in up to 1/103 dilutions, in 75-cm2 flasks in up to 1/104 dilutions, and in 25-cm2 flasks at higher dilutions. The first dilution was less than 10-fold so that the final 10-fold dilution would produce 1 colony equivalent (20 cells). These cells were then incubated overnight, rinsed free of nonadherent cells, and incubated in medium supplemented with 500 μmol/L 5-FC for 14 days. The colonies were subsequently counted after staining with methylene blue.
Evaluation of the safety of the 5-FC CD adenoviral vector system for hematopoietic reconstituting cells in a mouse transplantation model.
Hematopoietic cells were harvested from the femurs of Balb/C male donor mice 48 hours after treatment with 150 mg/kg of 5-FU. The hematopoietic cells were exposed to the adenoviral vector carrying the cytosine deaminase gene for 90 minutes and rinsed. Between 1 and 2 million of the cells were then transplanted into female recipient mice exposed to 725 to 750 cGy of total body irradiation.25 In some cases, the marrow cells were exposed to 5-FC by either incubating them in QBSF58 serum-free medium (Quality Biologicals Inc, Gaithersburg, MD) supplemented with 100 ng/mL of stem cell factor (Amgen Inc, Thousand Oaks, CA) for 96 hours in suspension culture in the absence of serum at 37°C, with or without supplementation with 500 μmol/L 5-FC before transplantation, or alternatively exposed in vivo after transplantation to 5-FC (peak serum concentrations in the 500 μmol/L range) for 14 days in vivo immediately after the transplantation into the female recipients.
RESULTS
Time course of expression of adenoviral transgenes after infection.
MCF-7 cells were exposed to the β-galactosidase adenoviral vector for 90 minutes, after which the cells were incubated in complete tissue culture medium and assayed for β-galactosidase activity by enzymatic analysis of extracts of cells at 1, 2, 4, 6, 8, 10, and 12 days after infection. As shown in Fig 1, the expression of the transgene quickly reached its maximum and persisted at that level for 4 to 5 days before starting to decrease. β-Galactosidase activity was still detectable well beyond 10 days.
Time course of expression of β-galactosidase gene in MCF-7 BCCs. BCCs were exposed to the β-galactosidase adenoviral vector for 90 minutes, incubated for 24 hours in complete medium, and then tested for expression of the transgene by an enzymatic assay on cell lysates at the indicated times (days) after the infection.
Time course of expression of β-galactosidase gene in MCF-7 BCCs. BCCs were exposed to the β-galactosidase adenoviral vector for 90 minutes, incubated for 24 hours in complete medium, and then tested for expression of the transgene by an enzymatic assay on cell lysates at the indicated times (days) after the infection.
Comparative cytotoxicity effect of 5-FC and 5-FU in normal breast epithelium and BCC lines in the absence of Ad.CMV-CD.
We evaluated the antiproliferative effect of 5-FC or 5-FU at different concentrations of fluoropyrimidines (ranging between 0 and 10 mmol/L), in the absence of Ad.CMV-CD vector, on several BCC lines and on primary HMECs, as shown in Table 1. The results of these experiments showed that 5-FC is 1,000-fold less potent as an inhibitor than 5-FU for HMECs and 10,000-fold less toxic for the two BCC lines than 5-FU.
Differential Cell Growth Inhibition (IC50) of 5-FC and 5-FU in Primary Normal HMECs and in BCC Lines, MCF-7 and MDA-MB-453, Before or After Incubation With the Ad.CMV-CD Vector
| . | Inhibitory Concentration50 (IC50) After Incubation With . | ||
|---|---|---|---|
| 5-FC (μmol/L)* Alone . | 5-FU (μmol/L)* Alone . | 5-FC (μmol/L) + (Ad.CMV-CD) Combined . | |
| HMEC | 3,000 | 1 ± 0.5 | 10 (10 MOI)-151 |
| MDA-MD-453 | >10,000 | 30 ± 5 | 100 (50 MOI)-151 |
| MCF-7 | >10,000 | 4 ± 1 | 10 (10 MOI)-151 |
| CD34 + 0.1% MCF-7 | — | — | 10 (10 MOI)-152 |
| . | Inhibitory Concentration50 (IC50) After Incubation With . | ||
|---|---|---|---|
| 5-FC (μmol/L)* Alone . | 5-FU (μmol/L)* Alone . | 5-FC (μmol/L) + (Ad.CMV-CD) Combined . | |
| HMEC | 3,000 | 1 ± 0.5 | 10 (10 MOI)-151 |
| MDA-MD-453 | >10,000 | 30 ± 5 | 100 (50 MOI)-151 |
| MCF-7 | >10,000 | 4 ± 1 | 10 (10 MOI)-151 |
| CD34 + 0.1% MCF-7 | — | — | 10 (10 MOI)-152 |
*Cells (10,000/well) were plated in the above-noted incubations in triplicate in 96-well plates. Twenty-four hours later, different drug concentrations (see above) were added to the medium and cell proliferation was assessed after 5 days using a colorimetric assay as reported in the Materials and Methods.
Cells were exposed to Ad.CMV-CD for 90 minutes in serum-free medium at different MOI, and then 10,000 cells/well were plated in triplicate in 96-well plates. Twenty-four hours later, 5-FC and 5-FU were added to the medium and cell proliferation was determined after 5 days by a colorimetric assay.
Each of 9 independent wells was seeded with 10,000 cells/well for each condition in the limiting dilution assay. Dilution refers to the total number of cells reseeded.
Increased sensitivity of HMECs and BCCs to 5-FC after exposure to Ad.CMV-CD.
The BCCs were exposed to the Ad.CMV-CD vector for a short period of time (90 minutes) in serum-free conditions and then incubated for 2 days in 5-FC. As shown by the data presented in Table 1, after exposure of the cell lines to the CD adenoviral vector, they became sensitive to concentrations of 5-FC that were nontoxic before exposure to the virus (Table 1). More importantly, even the slowly dividing primary explants of normal breast cells (HMECs) showed growth inhibition between 85% and 100% at concentrations of 5-FC in the range of 100 to 1,000 μmol/L after exposure to the Ad.CMV-CD vector.
These data clearly indicate that the cytosine deaminase vector (Ad.CMV-CD) can efficiently increase the sensitivity of BCC lines or primary explants of normal breast epithelial cells to 5-FC between 100- and 1,000-fold. We also studied the toxicity of 5-FC to the BCCs after they were exposed to varying ratios of the cytosine deaminase adenoviral vector (Table 1). These experiments suggest that even very low ratios of the CD adenoviral vector/cell can generate significant toxicity. These results may underestimate the true potential of the 5-FC/CD adenoviral system, because the cells were exposed to 5-FC for only 2 days before analysis. As will be shown below, longer incubation in 5-FC increases cell kill.
Study of the selectivity of infectivity of BCCs versus hematopoietic cells by Ad.CMV-CD.
The conversion of 5-FC into 5-FU 48 hours after 90 minutes of exposure in serum-free medium to the AD.CMV-CD vector was evaluated as a measure of the infectivity of the adenoviral vector for PBMCs compared with the BCC line MCF-7 (Fig 2). When PBMCs were incubated in 500 μmol/L of 5-FC (2.5 μmol total 5-FC present in the medium) after exposure of the cells to the Ad.CMV-CD vector (100 MOI), almost no detectable conversion of 5-FC to 5-FU occurred in the hematopoietic cells (0.018 μmol). In contrast, when the MCF-7 BCC line was exposed to the Ad.CMV-CD vector (100 MOI) for 90 minutes (or for 24 hours) and then incubated an additional 48 hours in 5-FC, almost 70% of 5-FC was converted into 5-FU (1.74 μmol). Importantly, the adenoviral vector showed infectivity for BCCs even when they were diluted in a 1,000-fold excess of PBMCs, leading to the conversion of 6.4% (0.16 μmol) of 5-FC into 5-FU (Fig 2). The concentration of 5-FU generated in the medium by a culture of 100% MCF-7 cells exposed to 500 μmol/L 5-FC reached a 5-FU concentration of 347 μmol/L, a concentration well above that necessary to kill most of the established cancer cell lines.
Conversion of 5-FC into 5-FU in PBSCs, MCF-7 BCCs, and an 0.1% mixture of MCF-7 cells in PBSCs after exposure to Ad.CMV-CD adenoviral vector. Cells (2.5 × 106) were exposed for 90 minutes to 100 MOI of Ad.CMV-CD and then incubated for 48 hours in a 25-cm2 flask with 10 mL of medium containing 500 μmol/L 5-FC and 40 μCi of [6-3H]5-FC. One milliliter of medium was collected after 24 hours, mixed with an equal amount of ice-cold methanol, and analyzed by HPLC as described in the Materials and Methods. The numbers above each histogram column indicate the total amount of drug in micromoles (either 5-FC or 5-FU) per flask.
Conversion of 5-FC into 5-FU in PBSCs, MCF-7 BCCs, and an 0.1% mixture of MCF-7 cells in PBSCs after exposure to Ad.CMV-CD adenoviral vector. Cells (2.5 × 106) were exposed for 90 minutes to 100 MOI of Ad.CMV-CD and then incubated for 48 hours in a 25-cm2 flask with 10 mL of medium containing 500 μmol/L 5-FC and 40 μCi of [6-3H]5-FC. One milliliter of medium was collected after 24 hours, mixed with an equal amount of ice-cold methanol, and analyzed by HPLC as described in the Materials and Methods. The numbers above each histogram column indicate the total amount of drug in micromoles (either 5-FC or 5-FU) per flask.
We then studied the incorporation of 5-FU into RNA and DNA of MCF-7 cells treated at the known IC50 concentration of the fluoropyrimidine for this adenocarcinoma breast cell line (Fig 3). The IC50 concentration for the MCF-7 breast adenocarcinoma cells was 5 μmol/L of 5-FU generating 15 pmol/106 cells of 5-FU nucleotides with 17 pmol/106 cells of the fluoropyrimidine incorporated into the nucleic acids (RNA and DNA) during a 24-hour period. In contrast, MCF-7 cells exposed to the gene therapy system of Ad.CMV-CD (100 MOI) for 90 minutes followed by 48 hours of incubation in 500 μmol/L 5-FC were able to generate a 320-fold excess of fluorinated nucleotides (FUXP) compared with the same cell line at the IC50concentration of 5-FU, with an incorporation of 170 pmol/106 cells into the nucleic acids, a 10-fold gain in 5-FU incorporation with the vector system over direct treatment with 5-FU (Fig 3). Even when 0.1% of MCF-7 cells were mixed with a population of PBMCs, exposed to Ad.CMV-CD and 50 μmol/L of 5-FC, the presence of 2,500 breast cancer cells (0.1% of the total cells) was able to generate a concentration of 32 μmol/L 5-FU (see Fig 2), well above the 5 μmol/L that is the IC50 value for the MCF-7 cell line for 72 hours of exposure. This is substantially below the IC50 for hematopoietic stem cells, which is 650 μmol/L of 5-FU.26
Comparative metabolism and nucleic acids incorporation of 5-FC and 5-FU in MCF-7 BCCs exposed to the 5-FC/Ad.CMV-CD gene therapy system and 5-FU. MCF-7 cells were incubated for 90 minutes with 100 MOI of Ad.CMV-CD followed by 48 hours of exposure to 500 μmol/L 5-FC with 20 μCi of [6-3H]5-FC or for 24 hours with 5 μmol/L 5-FU in the presence of 25 μCi of [6-3H]5-FU. The conversion of 5-FC into 5-FU was determined after the collection of 1 mL of medium by HPLC as described in the Materials and Methods. Formation of fluorinated nucleotides (FUXP) was determined in the neutralized TCA cell extract by HPLC analysis using a Spherisorb SAX column eluted with a gradient of sodium phosphate, pH 3.3. The incorporation into nucleic acids was determined in the TCA pellets after digestion with NCS tissue solubilizer. The numbers above the histograms represent the micromoles of 5-FU or FUXP measured.
Comparative metabolism and nucleic acids incorporation of 5-FC and 5-FU in MCF-7 BCCs exposed to the 5-FC/Ad.CMV-CD gene therapy system and 5-FU. MCF-7 cells were incubated for 90 minutes with 100 MOI of Ad.CMV-CD followed by 48 hours of exposure to 500 μmol/L 5-FC with 20 μCi of [6-3H]5-FC or for 24 hours with 5 μmol/L 5-FU in the presence of 25 μCi of [6-3H]5-FU. The conversion of 5-FC into 5-FU was determined after the collection of 1 mL of medium by HPLC as described in the Materials and Methods. Formation of fluorinated nucleotides (FUXP) was determined in the neutralized TCA cell extract by HPLC analysis using a Spherisorb SAX column eluted with a gradient of sodium phosphate, pH 3.3. The incorporation into nucleic acids was determined in the TCA pellets after digestion with NCS tissue solubilizer. The numbers above the histograms represent the micromoles of 5-FU or FUXP measured.
BCCs are more sensitive to adenoviral infection than CD34+ cells.
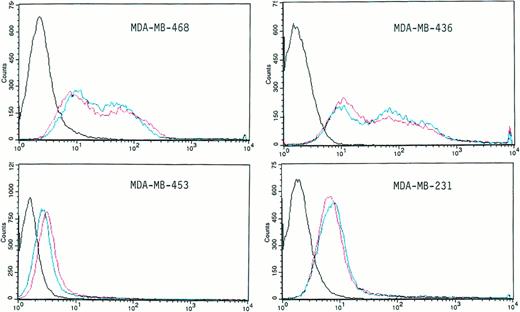
We directly tested the infectivity of several BCC lines (MDA-MB-468, MDA-MB-436, MDA-MB-453, MDA-MB-231, and T-47D) using the adenoviral vector Ad.CMV-βGal (100 MOI) after 90 minutes of exposure and an additional 24 hours of incubation in a fluorescein isothiocyanate (FITC)-conjugated substrate (FDG) for the β-galactosidase reaction. More than 90% of the cells in each of the breast cancer populations studied were infected by the adenoviral vector (Fig 4), as measured by increased fluorescence, because the presence of the β-galactosidase enzyme would generate higher levels of fluorescein (Fig 4).
Infectivity of different human BCC lines by the β-galactosidase Ad.CMV adenoviral vector. BCCs were exposed in suspension for 90 minutes (red line) or 24 hours (blue line) to 100 MOI of the Ad.CMV-βgal vector. Twenty-four hours later, the cells were washed with PBS, stained with FDG, and analyzed by flow cytometry as described in the Materials and Methods. The profile defined by the black line is the control in the absence of virus. The red and the blue profiles are from samples exposed to the vector for 90 minutes and 24 hours, respectively.
Infectivity of different human BCC lines by the β-galactosidase Ad.CMV adenoviral vector. BCCs were exposed in suspension for 90 minutes (red line) or 24 hours (blue line) to 100 MOI of the Ad.CMV-βgal vector. Twenty-four hours later, the cells were washed with PBS, stained with FDG, and analyzed by flow cytometry as described in the Materials and Methods. The profile defined by the black line is the control in the absence of virus. The red and the blue profiles are from samples exposed to the vector for 90 minutes and 24 hours, respectively.
The cells were exposed to the Ad.CMV-βgal vector for 90 minutes (red line) or 24 hours (blue line). There was no difference in the fluorescence intensity between the cells exposed to the vector for 90 minutes or for 24 hours of exposure, demonstrating that a long-term exposure to the vector is not necessary to reach a high frequency of infection in BCC lines. However, the extent of infection, either at 90 minutes or 24 hours, is different for each cell line, ranging between a 0.5-log to a 2-log increase in peak fluorescence intensity.
We used the same method to determine the infectivity of early hematopoietic cells by the Ad.CMV-βgal vector (100 MOI) at 30 minutes, 90 minutes, and 24 hours of exposure. We specifically focused on CD34+ cells and the CD34+CD38− and CD34+CD33− subsets that represent earlier stages of hematopoietic differentiation. The results of these experiments show that less than 1% of the CD34+, CD34+CD38−, and CD34+CD33− hematopoietic cells are infected by the Ad.CMV-βgal vector, even at 24 hours of vector exposure, under our experimental conditions, in which no growth factors are added. Other investigators11,12 have reported similar low frequencies of infection of early hematopoietic precursor cells by the adenoviral vector. If the hematopoietic cells are incubated in growth factors capable of inducing maturation of the early hematopoietic cells, the infectivity by the adenoviral vector increases.13 14
Patterns of integrin expression may explain differences in infectivity of the adenoviral vector observed among BCC lines.
It has been proposed that the integrins αVβ3 and αVβ5play an important role as coreceptors for adenovirus infection.23 24 To test this hypothesis, we studied the level of expression of these integrins in the BCC lines (MDA-MB-453, T47D, MDA-MB-231, MCF-7, MDA-MB-468, BT-20, and MDA-MB-436) and in CD34+ hematopoietic cells. The results of these experiments are shown in Table 2. There was a very low level of expression for αVβ3 in CD34+ cells (4.4%). A low level of αVβ3 integrin was seen in all of the BCC lines (see Table 2) except for the MDA-MB-231 cell line, which was 42.5% positive. In contrast, a substantial but variable level of expression of αVβ5 was detected in all of the BCCs tested (see Table 2). In contrast, the αVβ5 integrin receptor was not detectable in the vast majority CD34+ cells. This integrin has been reported to play a key role in adenoviral vector uptake and release from the post-uptake endosome. BCCs have a 4- to 27-fold higher level of αVβ5 than CD34+ cells, as shown in Table 2. The level of adenoviral infectivity in the BCC lines, as measured by the expression of β-galactosidase from the β-galactosidase adenoviral vector, appeared to be related to the presence of the αVβ5 integrin (see Table2).
Correlation Between Integrin Expression in Different Cell Types, Infectivity by CD Adenoviral Vector as Measured by Adenoviral Vector β-Galactosidase Activity at 72 Hours After Infection, and Functional Expression of the Adenoviral Vector Cytosine Deaminase Gene as Measured by Conversion of 5-FC to 5-FU During a 24-Hour Period at 72 Hours After Infection With CD Adenoviral Vector
| Cell Type . | αVβ3 . | αVβ5 . | β-Galactosidase Activity at 72 h (% of geo. mean) . | 5-FC Conversion (%) . |
|---|---|---|---|---|
| CD34+ | 4.4% | 3.6% | — | — |
| MDA-MB-453 | 0.4% | 15.8% | 38.6 | 15.9 |
| T47D | 0.8% | 96.4% | 586 | 51 |
| MCF-7 | 0.6% | 90.2% | 466.9 | 38.2 |
| MDA-MB-231 | 42.5% | 89.4% | — | — |
| MDA-MB-468 | 3.3% | 90.3% | — | — |
| BT-20 | 0% | 92.5% | — | — |
| MDA-MB-436 | 0% | 33.8% | — | — |
| Cell Type . | αVβ3 . | αVβ5 . | β-Galactosidase Activity at 72 h (% of geo. mean) . | 5-FC Conversion (%) . |
|---|---|---|---|---|
| CD34+ | 4.4% | 3.6% | — | — |
| MDA-MB-453 | 0.4% | 15.8% | 38.6 | 15.9 |
| T47D | 0.8% | 96.4% | 586 | 51 |
| MCF-7 | 0.6% | 90.2% | 466.9 | 38.2 |
| MDA-MB-231 | 42.5% | 89.4% | — | — |
| MDA-MB-468 | 3.3% | 90.3% | — | — |
| BT-20 | 0% | 92.5% | — | — |
| MDA-MB-436 | 0% | 33.8% | — | — |
5-FC conversion: 72 hours after exposure to the CD adenoviral vector, 6-(3H) 5-FC is added to the culture for 24 hours. The cellular extract is then subjected to HPLC to measure conversion by the cells from 5-FC to 5-FU. β-Galactosidase enzymatic assay was assayed 72 hours after exposure to the CD adenoviral vector (72 hours was allowed to elapse to permit time for viral transgene expression).
The pattern of expression of integrins on the BCCs in marrow samples from breast cancer patients was also evaluated by Richard Cote. He found that all the BCCs metastatic to the bone marrow were positive for the αVβ3 integrins (data not shown), as has been previously reported in much larger numbers of patients.27 He also studied the infectivity of primary BCCs present in marrow of advanced breast cancer patients and found them to be infectable with the β-galactosidase adenoviral vector (data not shown).
Correlation between integrin expression, infectivity, and 5-FC conversion in BCC lines.
We have followed the expression of β-galactosidase, as a measure of cell infectivity, over a 72-hour period in BCC lines expressing different levels of αVβ5 after incubation with the Ad.CMV-βgal adenoviral vector. As shown in Table 2, the fluorescence intensity level of FDG, indicating β-galactosidase activity, was 12- to 15-fold higher in the T47D and MCF-7 cell lines that presented high levels (90% to 96%) of αVβ5 integrin expression, as compared with MB-MDA-453 cells that showed only a 15.8% presence of αVβ5. Similarly, the conversion of 5-FC into 5-FU was twofold to threefold higher in the T47D and MCF-7 cell lines with high αVβ5 expression, than with the MDA-MB-453 cell line, which showed low levels of the αVβ5 integrin, after exposure to the Ad.CMV-CD vector and incubation with 500 μmol/L 5-FC for a 24-hour period starting 48 hours after exposure to the adenoviral vector (Table2). These results further confirm the crucial role of integrins in the uptake of the adenoviral vector and ultimately in the expression of the transgene.
Sensitivity of mixtures of BCCs and hematopoietic progenitor cells to treatment with Ad.CMV-CD plus 5-FC.
We used a methylcellulose assay for measuring the effect of the 5-FC/CD adenoviral system on the clonogenic progenitor hematopoietic cells and a limiting dilution colony formation assay to evaluate the growth of colonies from cultures of MCF-7 BCCs after exposure to the CD adenoviral vector and 5-FC. We prepared mixtures of BCCs in a 1,000-fold excess of normal human PBMCs and plated the cells (10,000 cells/well for each condition: virus alone, 5-FC alone, and virus plus 5-FC) in microwell plates as described in the Materials and Methods. We exposed the cells to 5-FC (100 μmol/L) for 2 days in the absence of the Ad.CMV-CD vector and with both 5-FC (100 μmol/L) and Ad.CMV-CD vector (100 MOI). The data from these experiments indicate that vector exposure for 90 minutes, followed by incubation in 5-FC for 48 hours, decreased the proliferative capacity of clonogenic BCCs, even when the BCCs were in a 1,000-fold excess of normal PBMCs. In this low-density culture system, no viable colonies of MCF-7 were seen after incubation to the adenoviral vector plus 5-FC (data not shown). This may again be an underestimation of the true potential of this system, because the target cells were incubated in 5-FC for only 2 days. As shown below, longer periods of incubation of virally exposed cells in 5-FC result in greater levels of cell kill.
The toxicity of the purging system to clonogenic hematopoietic cells was also tested using a methylcellulose colony assay. We measured the number of colonies generated by PBMCs exposed for 90 minutes to the Ad.CMV-CD vector (100 MOI) and then cultured in suspension in the presence of 10 μmol/L and 50 μmol/L 5-FC for 48 hours, when plated in methylcellulose medium. No significant decrease in the numbers of CFU-GM in colonies was detected even when the myeloid cells were mixed with 0.1% of BCCs and then exposed to the Ad.CMV-CD and 5-FC. The data presented in Table 3 show that the early hematopoietic precrusor cells, which give rise to the hematopoietic clonogenic progenitor cells, are resistant to the 5-FC and Ad.CMV-CD vector treatment, even when exposed in the presence of low percentage of BCCs.
Granulocyte-Macrophage Colony-Forming Unit Analysis
| Experimental Conditions . | Ad. CMV-CD (50 MOI) . | 5-FC (μmol/L) . | Colonies . | |
|---|---|---|---|---|
| Exp. 1 . | Exp. 2 . | |||
| CD34 | − | 10 | 734 | 756 |
| CD34 | − | 50 | 744 | 724 |
| CD34 + 0.1% MCF-7 | − | 10 | 750 | 796 |
| CD34 + 0.1% MCF-7 | − | 50 | 761 | 722 |
| CD34 | + | 10 | 766 | 772 |
| CD34 | + | 50 | 880 | 846 |
| CD34 + 0.1% MCF-7 | + | 10 | 824 | 860 |
| CD34 + 0.1% MCF-7 | + | 50 | 684 | 630 |
| Experimental Conditions . | Ad. CMV-CD (50 MOI) . | 5-FC (μmol/L) . | Colonies . | |
|---|---|---|---|---|
| Exp. 1 . | Exp. 2 . | |||
| CD34 | − | 10 | 734 | 756 |
| CD34 | − | 50 | 744 | 724 |
| CD34 + 0.1% MCF-7 | − | 10 | 750 | 796 |
| CD34 + 0.1% MCF-7 | − | 50 | 761 | 722 |
| CD34 | + | 10 | 766 | 772 |
| CD34 | + | 50 | 880 | 846 |
| CD34 + 0.1% MCF-7 | + | 10 | 824 | 860 |
| CD34 + 0.1% MCF-7 | + | 50 | 684 | 630 |
Cells were exposed for 48 hours at the above-described conditions. Three thousand nucleated cells were plated in each 35-mm dish with 1.3 mL methylcellulose and colonies were counted after 12 to 14 days. A colony is defined as 50 cells or greater. (See Hanania et al25 for details of the assay.)
Testing of the potential efficacy of 5-FC/CD adenoviral vector purging of BCC from mixtures with hematopoietic cells.
A mixture of 2 × 106 MCF-7 cells in a 100-fold excess of HL60 cells was exposed to the CD adenoviral vector (400 MOI) for 90 minutes; the cells were then subjected to 10-fold serial dilutions and plated overnight. The nonadherent cells were rinsed away, and the cells were incubated in 500 μmol/L 5-FC for 14 days. In a second experiment, we made a mixture of 9 × 107 MCF-7 cells and 7 × 109 HL60 hematopoietic cells, exposed the cells to the cytosine deaminase vector (200 MOI) for 90 minutes, plated the cells directly or after serial dilution by factors of 10 up to 1/1,000,000 and then incubated overnight, rinsed off the nonadherent cells, and incubated the cells for 14 days in 500 μmol/L 5-FC. Colony counts at the end of this period, even in flask that were inoculated with 107 MCF-7 cells, showed no colonies detectable even in the undiluted cultures, in which the cells have been exposed to the cytosine deaminase vector and incubated in 5-FC (Table 4). This shows that the vector/5-FC system can decrease the level of BCCs by more than 1 million fold when present in a mixture with hematopoietic cells.
MCF-7 Clonogenic Cells After Exposure of a Mixture of 9 × 107 MCF-7 Cells in a 100-Fold Excess of HL60 Cells to the CD Adenoviral Vector (200 MOI) for 90 Minutes
| MCF-7 Cells Plated at Start of Experiment . | Colonies Formed at 10-14 Days of 5-FC Incubation . | |||
|---|---|---|---|---|
| 5-FC/CDAV . | 5-FC Alone . | CDAV Alone . | No 5-FC, No CDAV . | |
| 90 million | None | TMTC | TMTC | TMTC |
| 10 million | None | TMTC | TMTC | TMTC |
| 2 million | None | 1,694 | 2,800 | 2,100 |
| 200,000 | None | 484 | 520 | 600 |
| 20,000 | None | 138 | 111 | 146 |
| 2,000 | None | 34 | 23 | 33 |
| 200 | None | 12 | 0 | 4 |
| 20 | None | 2 | 0 | 4 |
| MCF-7 Cells Plated at Start of Experiment . | Colonies Formed at 10-14 Days of 5-FC Incubation . | |||
|---|---|---|---|---|
| 5-FC/CDAV . | 5-FC Alone . | CDAV Alone . | No 5-FC, No CDAV . | |
| 90 million | None | TMTC | TMTC | TMTC |
| 10 million | None | TMTC | TMTC | TMTC |
| 2 million | None | 1,694 | 2,800 | 2,100 |
| 200,000 | None | 484 | 520 | 600 |
| 20,000 | None | 138 | 111 | 146 |
| 2,000 | None | 34 | 23 | 33 |
| 200 | None | 12 | 0 | 4 |
| 20 | None | 2 | 0 | 4 |
Serial 10-fold dilutions of the cell were followed by plating overnight and rinsing off of the non-adherent cells. The adherent cells were then incubated in 500 μmol/L 5-FC for 10 to 14 days. The colonies were then counted. One colony equals 20 cells.
Abbreviations: TMTC, too many to count; CDAV, cytosine deaminase adenoviral vector.
Testing of the safety of the 5-FC/CD adenoviral vector system through use of an in vivo engraftment transplantation assay after exposure to Ad.CMV-CD and 5-FC.
A potential adverse effect of all ex vivo purging procedures is the toxicity to the 1/10,000 hematopoietic early precursor cells that are responsible for hematopoietic reconstitution after transplantation into lethally irradiated recipients. We have already demonstrated the lack of toxicity of the adenoviral infection, Ad.CMV-CD, and 5-FC treatment to the hematopoietic progenitor cells measured by CFU-GM methylcellulose assay after exposure to the vector and 5-FC in vitro (see Table 4). To test the effect of the 5-FC/CD adenoviral system on the viability of the more primitive reconstituting hematopoietic cells, we used a murine bone marrow transplantation model.
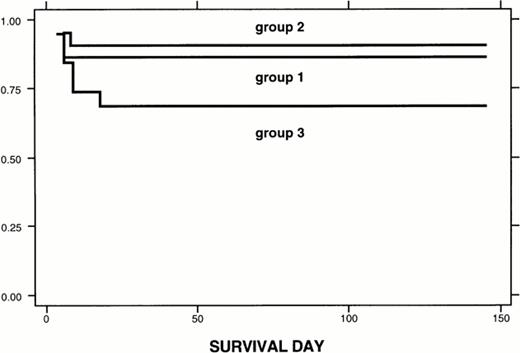
We exposed marrow cells collected from 5-FU–treated male donor mice to the cytosine deaminase adenoviral vector for 90 minutes. We then incubated the marrow cells for 4 days in serum-free medium (QBSF 58 medium) supplemented with 100 ng/mL stem cell factor and 500 μmol/L 5-FC. One to two million of these cells were then used in the transplant into lethally irradiated female mice. Sixty-eight percent of the mice survived the transplant. As shown in Fig 5, most of the deaths occurred before day 10 after transplantation. Deaths that occur before 10 days after transplantation are usually ascribed to infection or radiation toxicity, rather than to failure to engraft. Thus, most of the hematopoietic reconstituting cells survived the in vitro incubation with 5-FC after exposure to the cytosine deaminase adenoviral vector.
Kaplan-Meier analysis of survival. Group 1: 22 mice received 1 to 2 million fresh marrow cells. Group 2: 21 mice transplanted with 1 to 2 million marrow cells cultured in QBSF58 serum-free medium for 4 days, as was group 3, but not exposed to the CD adenoviral vector/5-FC. Group 3: 19 mice transplanted with 1 to 2 million marrow cells exposed to the CD adenoviral vector followed by 5-FC. Survival for each group was estimated by the Kaplan-Meier model. Log-rank test was used to check for equality of survival functions. Sixty-two mice were studied. Follow-up was 83.9 ± 43.4 days (mean ± SD), with a range of 4 to 145 days. Survival functions: Results are given as the mean ± SE, with the 95% confidence interval in parentheses. Group 1: 22 mice were transplanted and 19 survived (from 3 different experiments). Three died at day 6. Survival function: 86.4 ± 7.3 (63.4 to 95.4). Group 2: 21 mice were transplanted, 19 survived and 2 died at days 6 and 8 (2 different experiments). Survival: 90.5 ± 6.4 (67 to 97.5). Group 3: 19 mice were transplanted. Six died at days 4 (1 mouse), 6 (2 mice), 9 (2 mice), and 18 (1 mouse) (4 different experiments total). Survival: 68.4 ± 10.7 (42.8 to 84.4). Log-rank test: P = .16. There was no difference in survival among these 3 groups.
Kaplan-Meier analysis of survival. Group 1: 22 mice received 1 to 2 million fresh marrow cells. Group 2: 21 mice transplanted with 1 to 2 million marrow cells cultured in QBSF58 serum-free medium for 4 days, as was group 3, but not exposed to the CD adenoviral vector/5-FC. Group 3: 19 mice transplanted with 1 to 2 million marrow cells exposed to the CD adenoviral vector followed by 5-FC. Survival for each group was estimated by the Kaplan-Meier model. Log-rank test was used to check for equality of survival functions. Sixty-two mice were studied. Follow-up was 83.9 ± 43.4 days (mean ± SD), with a range of 4 to 145 days. Survival functions: Results are given as the mean ± SE, with the 95% confidence interval in parentheses. Group 1: 22 mice were transplanted and 19 survived (from 3 different experiments). Three died at day 6. Survival function: 86.4 ± 7.3 (63.4 to 95.4). Group 2: 21 mice were transplanted, 19 survived and 2 died at days 6 and 8 (2 different experiments). Survival: 90.5 ± 6.4 (67 to 97.5). Group 3: 19 mice were transplanted. Six died at days 4 (1 mouse), 6 (2 mice), 9 (2 mice), and 18 (1 mouse) (4 different experiments total). Survival: 68.4 ± 10.7 (42.8 to 84.4). Log-rank test: P = .16. There was no difference in survival among these 3 groups.
Because of the data presented in Table 4, in which a 1 million fold reduction of the BCC in an excess of hematopoietic cells was achieved by incubation of the cytosine deaminase vector exposed cells to 10 or more days of 5-FC incubation, we also used the mouse model to test whether CD adenoviral-infected cells, exposed to 14 days of 5-FC immediately after infusion, would maintain the reconstituting capability. One to two million of these cells were used in the transplant into lethally irradiated female recipient mice. As shown by the data in Table 5, the survival of the mice undergoing transplantation with vector-exposed cells with or without the posttransplantation 14-day infusion of 5-FC, at intraperitoneal doses of 5-FC that generate 500 μmol/L peak serum concentrations, was not significantly different from the survival of mice undergoing transplantation with vector-exposed cells without 5-FC treatment (Table 5).
Transplantation of Marrow Cells Exposed Before Transplantation to Cytosine Deaminase Adenoviral Vector Followed by Posttransplantation In Vivo Exposure to 14 Days of 5-FC
| . | Animal Survival Posttransplantation . |
|---|---|
| XRT alone | 32% (8/25) |
| XRT/FBM | 80% (20/25) |
| XRT/FBM/5-FC | 74% (11/15) |
| XRT/FBM/5-FC/AD | 72% (18/25) |
| . | Animal Survival Posttransplantation . |
|---|---|
| XRT alone | 32% (8/25) |
| XRT/FBM | 80% (20/25) |
| XRT/FBM/5-FC | 74% (11/15) |
| XRT/FBM/5-FC/AD | 72% (18/25) |
Results of engraftment (survival) after transplantation with vector-exposed cells followed by 14 days of posttransplant 5-FC (each dose is sufficient to generate a 2-hour peak level of 500 μmol/L).
Abbreviations: XRT, total body irradiation; FBM, fresh bone marrow without incubation; AD, CD adenoviral vector.
Individual posttransplantation white blood cell counts (WBC) and differential counts performed in all animals in each group (transplants with cells exposed to vector followed by 4 days of in vitro 5-FC pretransplantation incubation or 14 days of posttransplantation in vivo 5-FC exposure) showed no differences in leukocytes, lymphocytes, platelets, or hematocrit volumes among the different transplant groups when determined at 2 or 6 months after transplantation (data not shown). These data suggest that the grafts are stable in all of the treatment groups.
To measure specifically whether the male donor cells that were exposed before transplantation in vitro to the cytosine deaminase adenoviral vector followed by 4 days of pretransplantation in vitro exposure to 5-FC or exposed to the vector before transplantation followed by 14 days of posttransplantation 5-FC exposure could stably repopulate the female recipient lethally irradiated mice, lethally irradiated female recipient mice transplanted with Ad.CMV-CD–exposed cells exposed to 5-FC either before or after transplantation were killed 1 to 6 months after transplantation. Then, G-banding cytogenetic analysis for the Y chromosome was performed on the bone marrow cells from these animals. We performed this analysis both for the animals transplanted with vector-exposed cells that either were incubated in vitro for 4 days in QBSF58 serum-free medium supplemented with 500 μmol/L 5-FC (2 animals at 1 to 2 months after transplantation) or were exposed after transplantation in vivo to 5-FC for 14 days (4 animals at 6 months after transplantation).
Because all transplants involved the infusion of male donor cells into lethally irradiated female recipients, we were able to use cytogenetics to test stable and complete engraftment of the Ad.CMV-CD and 5-FC–treated cells. All of the metaphase spreads studied for each of 2 female recipient animals 1 and 2 months after transplantation showed 100% of the cells as donor male cells. In addition, greater than 99% of the metaphases studied at 6 months in both groups (4 days of in vitro 5-FC exposure in QBSF58 serum-free medium before transplantation or 14 days of posttransplantation 5-FC in vivo exposure) showed a male karyotype. These results demonstrate that the engrafting capability of the male reconstituting hematopoietic cells was not affected by the pretransplantation Ad.CMV-CD exposure followed by either 4 days of pretransplantation 5-FC in vitro or 14 days of posttransplantation in vivo 5-FC administration. All of these data show that a sufficient number of the hematopoietic reconstituting cells survived the 5-FC/adenoviral vector exposure to generate complete and stable engraftment in lethally irradiated female recipients.
DISCUSSION
Retroviral marking studies in autologous transplantation used to treat both hematopoietic and solid tumor neoplasms have shown that the presence of neoplastic cells in the infused hematopoietic cells used to restore marrow function after transplantation can contribute to relapse after transplantation.28-30 Fields et al3recently reported that patients infused with autologous hematopoietic cells that are free of contaminating BCCs by PCR assay have a longer disease-free interval than do those patients infused with autologous cells that are positive by PCR assay from BCCs. We therefore decided to test if a replication incompetent adenoviral vector could be used to selectively deliver a chemotherapy sensitization gene, cytosine deaminase, to BCCs without altering the hematopoietic cells. The goal was to sensitize the BCCs to drugs that are nontoxic for the hematopoietic cells.
We chose for this purpose a bacterial gene, cytosine deaminase, that catalyzes the conversion from 5-FC into 5-FU. We showed that there were three types of differences between BCCs and normal immature hematopoietic reconstituting cells that protect the hematopoietic cells and sensitize the BCCs using this system. We first tested whether hematopoietic cells and BCCs show differences to infection by the adenoviral vector. β-Galactosidase assays on breast cancer and CD34-selected cells exposed for 24 hours to an adenoviral vector carrying the Lac-Z gene showed that less than 1% of the CD34-selected early hematopoietic cells were infected by the vector, whereas up to 100% of each of 6 of the BCC lines tested scored positive for infection in the β-galactosidase assay. We also used the cytosine deaminase adenoviral vector to show that conversion of 5-FC to 5-FU, as measured by HPLC, in CD34-selected hematopoietic cells exposed to the cytosine deaminase vector was at the undetectable level, whereas greater than 70% conversion was seen in the MCF-7 BCC line after exposure to the cytosine deaminase vector. Thus, this first difference demonstrated definitively that the CD34-selected cell and its CD34+CD33− and CD34+CD38− subsets exposed to the vector under nondifferentiating conditions were not infectable by the adenoviral vector, whereas the BCC were infectable.
Direct assay of the CD34-selected cells for the presence of the αVβ3 and αVβ5integrins that have been reported to bind the penton proteins of the adenoviral vector,23,24 a step necessary for uptake of the vector and release of adenoviral vectors from the postuptake endosome (which is necessary for high levels of expression of the vector transgenes), were absent from the surface of the early hematopoietic cells. Other workers have shown that the adenoviral vector does not bind efficiently to these hematopoietic cells, presumably due to the absence of the receptor for the fibrillar protein of the vector that mediates vector binding. Our data and those of others25have shown that both the BCC lines and the BCCs metastatic to the marrow have the requisite integrin receptors for adenoviral uptake and are infectable by these vectors.
The conditions of the vector exposure and in vitro purging conditions (serum-free medium [QBSF58] and the absence of late-acting growth factors) were designed to minimize the tendency of the hematopoietic cells to differentiate. Neering et al13 have shown that, during differentiation, the early hematopoietic cells become infectable by the adenoviral vector. Studies in which the adenoviral vector has been mixed with CD34-selected hematopoietic cells in the presence of serum and hematopoietic growth factors13,14 that induce differentiation have shown higher levels of infectability than in our studies or those of others11 12 in which the conditions of exposure to the vector (serum-free medium, no growth factors added) did not induce differentiation of the hematopoietic precursor cells. Thus, it is imperative to use conditions during the 5-FC in vitro incubation after exposure of the hematopoietic cells to the adenoviral vector that do not induce the marrow cells to differentiate to maintain the specificity of infection.
A second difference between BCCs and normal immature hematopoietic cells is the increased ability of BCCs to convert 5-FU into phosphorylated metabolites, a necessary step for 5-FU to exert toxicity to a cell through inhibition of thymidylate synthase or by incorporation into nucleic acids. We found that the levels of orotate-phosphoribosyl transferase, a critical enzyme that catalyzes the conversion of 5-FU to 5-FUMP, is 50- to 100-fold lower in CD34-selected immature normal hematopoietic cells than in neoplastic epithelial cells (data not shown). This second difference between epithelial neoplastic cells and normal immature hematopoietic cells in the capability to metabolize 5-FU so that it will be incorporated into DNA and RNA provides a second level of protection for the early normal hematopoietic cells from 5-FU.
Finally, a third difference between normal immature hematopoietic cells and neoplastic epithelial cells, which protects the immature hematopoietic cells from the toxicity of the cytosine deaminase adenoviral vector/5-FC treatment, is the greater intrinsic resistance of the hematopoietic stem cell to 5-FU. 5-FU exposure is used as a method for fractionating the more sensitive mature hematopoietic cells from the early reconstituting cells for transplantation in mouse models and for isolating human stem cells,26 because the early hematopoietic cells are so resistant to the 5-FU. In contrast, 5-FU is used in the therapy of breast cancer because BCCs are sensitive to 5-FU.
Our direct measurements show that 100% of the BCCs are sensitive to 5-FU at levels (5 μmol/L) that are less than the threshold at which early hematopoietic cells show toxicity from 5-FU (500 μmol/L). This is very important, because 5-FU may diffuse out of the epithelial neoplastic cells before phosphorylation and could potentially destroy the hematopoietic cells not initially infected by the cytosine deaminase vector. The resistance of the immature hematopoietic cells is at least 100-fold greater than that of the BCCs.
Direct analysis of mixtures of BCCs and CD34-selected hematopoietic cells in our studies, using assays for clonogenic BCCs and hematopoietic cells, show that the precursors for the clonogenic progenitor hematopoietic cells are not sensitive to exposure to the 5-FC and cytosine deaminase vector system, whereas a 1 million fold reduction in clonogenic BCC can be produced by the cytosine deaminase vector/5-FC system. In addition, our experiments show that even very slowly growing primary explants of normal breast epithelial cells are sensitive to the effects of the 5-FC and cytosine deaminase adenoviral vector gene therapy method. These results suggest that the nondividing BCCs will be destroyed as well as the dividing BCCs by this treatment. Because a major fraction of BCCs that contaminate collections of hematopoietic cells used for transplantation are nondividing, the mechanism of cell death is probably due to the incorporation of 5-FU into RNA at a level that inhibits protein synthesis.15 16
The ultimate test for the selectivity of the 5-FC/cytosine deaminase adenoviral vector treatment (toxicity for BCCs and the absence of significant toxicity for the early reconstituting hematopoietic cells) is to test for engraftment of the 5-FC/CD adenoviral vector-treated hematopoietic cells in lethally irradiated recipient mice. Our transplantation data in Balb/C mice show stability of engraftment (for up to 6 months) and complete engraftment by the male donor cells exposed to the adenoviral vector and 5-FC (either before transplantation or after transplantation) after transplantation into female recipient mice as determined by cytogenetic analysis and blood counts. A study of the data in Table 5 and Fig 5 suggests that the 14-day in vivo exposure to the 5-FC may be safer in terms of the reconstitutive capacity of the marrow cells used for transplant than is the 4 days of pretransplantation in vitro 5-FC exposure. Clearly, there is an advantage of increased levels ( >1 million-fold) of BCC kill with the posttransplantation 14-day in vivo exposure to 5-FC over the pretransplantation 4-day exposure to 5-FC, due to the longer time of 5-FC exposure. Thus, in a clinical trial already approved at Yale, we will be proposing to use a pretransplantation exposure to the Ad.CMV-CD vector and a posttransplantation in vivo exposure to the 5-FC (for 14 days).
Other investigators have reported studies with adenoviral vectors carrying the P53 gene to correct cells in which mutation in P53 in the BCCs has occurred.11 Induction of cell death directly occurred in only a fraction of the primary BCCs in these studies. Another disadvantage of this latter P53 system is that it will be ineffective for BCCs in which the P53 mutation exerts a transdominant-negative effect on P53 function.
The BCL-X short transcription unit has also been introduced into BCCs in an attempt to directly eliminate them for bone marrow purging purpose.12 We do not yet know whether this system has adverse effects on the early hematopoietic cells. In addition, this vector was only partially suppressive to established BCC lines. Moreover, we do not know what the effect of such a vector would have on slow or nondividing breast cancer cells that are primary explants rather than established rapidly proliferating BCC lines.
Another pro-drug activation system based on the herpes virus thymidine (HVTK) is limited by the fact that the sensitization to ganciclovir generated by incorporation of the HVTK gene into BCCs occurs only if the cells are in S-phase.31 The cytosine deaminase adenoviral vector system we have used in the studies in this report has an obvious advantage, because it is potentially toxic for both dividing and nondividing breast cancer cells. The very high intracellular levels of 5-FU generated in cells infected with the cytosine deaminase adenoviral vector probably results in concentrations of 5-FU necessary for a sufficient incorporation of 5-FU into RNA to kill even nondividing cells.15 16
Another advantage of the cytosine deaminase/5-FC system is that the level of exposure to 5-FU generated in the BCCs is much higher than that which can safely be achieved by infusion of 5-FU in the systemic circulation.15-19 Thus, the vector/5-FC system provides a unique therapeutic advantage over the direct systemic delivery of 5-FU. The cytosine deaminase vector used in our studies has already been approved for use in human cancer gene therapy trials conducted by Ronald Crystal (Cornell Medical School, New York, NY).19 Thus, the availability of this vector, the prevalence of patients with detectable contamination of their marrow and peripheral blood with BCCs, and the selectivity of the 5-FC/cytosine deaminase adenoviral vector system shown in our studies for the BCCs suggest that this method may be useful in the future for improving the overall outcome of autotransplants in advanced breast cancer patients. These findings indicate that the cytosine deaminase vector used to sensitize BCCs to 5-FC, which is nontoxic for hematopoietic cells up to 650 μmol/L, is a method of potential utility for in vitro purging of autologous cells used for transplantation in breast cancer patients.
ACKNOWLEDGMENT
The authors acknowledge Drs Manuel Ramirez and Wing Leung from Johns Hopkins University (Baltimore, MD) for the statistical evaluation of the animal data. Many thanks to Rocco Carbone (Yale Cancer Center FACS Shared Resource Core) for expert flow cytogenetic analysis. Thanks to Ron Brown of Quality Biological, Inc for a gift of QBSF58 medium.
A.B.D. received support from the Anderson Chair for Cancer Treatment and Research and the George and Barbara Bush Leukemia Research Fund at the M.D. Anderson Cancer Center, the Ensign Professorship of Medicine at the Yale University School of Medicine, the Hull Development Fund at the Yale Cancer Center, and the Susan B. Komen Fund for Breast Cancer Research. F.G.-S. was supported by a Fellowship from the Spanish Ministry of Education.
Address reprint requests to A.B. Deisseroth, MD, PhD, Gene Therapy Program, Yale Cancer Center, and Medical Oncology Section, Yale University School of Medicine, WWW 221, 333 Cedar St, New Haven, CT 06520-8032; e-mail: deisseroab@maspo2.mas.yale.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.


![Fig. 2. Conversion of 5-FC into 5-FU in PBSCs, MCF-7 BCCs, and an 0.1% mixture of MCF-7 cells in PBSCs after exposure to Ad.CMV-CD adenoviral vector. Cells (2.5 × 106) were exposed for 90 minutes to 100 MOI of Ad.CMV-CD and then incubated for 48 hours in a 25-cm2 flask with 10 mL of medium containing 500 μmol/L 5-FC and 40 μCi of [6-3H]5-FC. One milliliter of medium was collected after 24 hours, mixed with an equal amount of ice-cold methanol, and analyzed by HPLC as described in the Materials and Methods. The numbers above each histogram column indicate the total amount of drug in micromoles (either 5-FC or 5-FU) per flask.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/2/10.1182_blood.v92.2.672/5/m_blod41419002x.jpeg?Expires=1764974762&Signature=1MYIcUkBD3CAvU~ApVPtgySNr62IWcnQoLUlEpEJLFIp0ZA0s~xJf~-W7c7Kmyvd8JuOow-Fjclhskgv4AuAnYiIg5EjIg4y~tQ6aGw~eEqhMllTTwDbH1xkXBG091WD2eXBcx1PyBY8kjBhpV-mM~-zs3PIRoH-XnOMhi752siVzx8WCUKjbQEQxEtGDwe3vHw4UtPx7gP-eFmWy0Na-kQXIm~yWkUtvLkgZGs~pTQ9T7aecyjyVBUYbgxFbCU6lY7Eukn~N~N2m9rGivglYQFeJ4hyEEEUch1zihgbV~CbxjbFQPEP5Y9PzwF25f9bMGnLfOTBtV7uOgBe9vdDrA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Comparative metabolism and nucleic acids incorporation of 5-FC and 5-FU in MCF-7 BCCs exposed to the 5-FC/Ad.CMV-CD gene therapy system and 5-FU. MCF-7 cells were incubated for 90 minutes with 100 MOI of Ad.CMV-CD followed by 48 hours of exposure to 500 μmol/L 5-FC with 20 μCi of [6-3H]5-FC or for 24 hours with 5 μmol/L 5-FU in the presence of 25 μCi of [6-3H]5-FU. The conversion of 5-FC into 5-FU was determined after the collection of 1 mL of medium by HPLC as described in the Materials and Methods. Formation of fluorinated nucleotides (FUXP) was determined in the neutralized TCA cell extract by HPLC analysis using a Spherisorb SAX column eluted with a gradient of sodium phosphate, pH 3.3. The incorporation into nucleic acids was determined in the TCA pellets after digestion with NCS tissue solubilizer. The numbers above the histograms represent the micromoles of 5-FU or FUXP measured.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/2/10.1182_blood.v92.2.672/5/m_blod41419003x.jpeg?Expires=1764974762&Signature=s0kRTIskWDAF4ksZLMzcNQRaEexRydEuiUFKWIX~NgNev0bbiP2cQcQYa724z6knqll2MyHfkbiDT~5yT2K73BzDIAjAOCpTL1d54HZbDa0F6Zms8etEkakKK-E5OHTyBjoltQr~OW-qRHX7In~2siDqiLAyG~CIu2jgTesmniZZwYzU3A3p25z0Ro2wV04p5G~5QWVtz2RO7kvbiFYEjHObxJIB5aeEA~W-ABxzC8ICIQk8eq6j1B1WYYMz4dA3EXMIEJFSR-R8ypuPtiTdiBcOPzOHbyTeSwOGYinDs79Etks7mmaUdonvE7GkV5CiaPvC~jVpwe4tNeGvKyTBew__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal