Abstract
Previous studies have suggested that multiple myeloma (MM) cells express estrogen receptors (ER). In the present study, we characterized the effects of estrogen agonists and antagonists (anti-estrogens [AE]) on growth of MM cell lines and MM patient cells. In addition to antagonizing estrogen binding to ER, AE can trigger apoptosis. Hence, we also determined whether estrogens or AE altered MM cell survival. Immunoblotting showed that ER- is expressed in 4 of 5 MM cell lines (ARH-77, RPMI 8226, S6B45, and U266, but not OCI-My-5 cells), as well as in freshly isolated MM cells from 3 of 3 patients. 17β-estradiol (E2) did not significantly alter proliferation of MM cell lines or MM patient cells. In contrast, two structurally distinct AE, tamoxifen (TAM) and ICI 182,780 (ICI), significantly inhibited the proliferation of all 5 MM cell lines and MM cells from 2 of 2 patients (IC50, 2 to 4 μmol/L). Proliferation of these cell lines was also inhibited by the hydroxylated TAM derivative, 4-hydroxytamoxifen (4HTAM), although this derivative was less potent than TAM (IC50, 3 to 25 μmol/L). In contrast, the dehalogenated TAM derivative toremifene (TOR) did not inhibit MM cell proliferation. We next examined the effects of these agents on MM cell survival. TAM, ICI, and, to a lesser extent, 4HTAM and TOR triggered apoptosis in both ER-–positive as well as ER-–negative MM cell lines and patient MM cells, evidenced both by fluorescence-activated cell sorting (FACS) analysis using propidium iodide staining and the TUNEL assay. TAM-induced growth inhibition and apoptosis of ER-–positive S6B45 MM cells was not blocked by coculture with excess E2. TAM-induced apoptosis of S6B45 MM cells was also unaffected by addition of exogenous interleukin-6. Importantly, both the inhibition of MM cell proliferation and the induction of MM cell apoptosis were achieved at concentrations of TAM (0.5 and 5.0 μmol/L) that did not significantly alter in vitro growth of normal hematopoietic progenitor cells. Similar plasma levels of TAM have been achieved using high-dose oral TAM therapy, with an acceptable toxicity profile. These studies therefore provide the rationale for trials to define the utility of AE therapy in MM.
© 1998 by The American Society of Hematology.
PREVIOUS STUDIES HAVE suggested that multiple myeloma (MM) cells express estrogen receptors (ER).1,2 Estrogen agonists3,4 and estrogen antagonists (anti-estrogens [AE])5 can downregulate normal B lymphopoiesis; it is therefore possible that these agents have analogous effects on malignant B cells. Anectodal reports of responses in patients with B-cell neoplasms treated with estrogen agonists and the AE tamoxifen (TAM)6,7 support this view. In MM, estrogens and certain AE are of particular interest due to their ability to downregulate interleukin-6 (IL-6) and gp130, the IL-6 receptor β chain.8,9 These agents might therefore inhibit IL-6, which is both a growth and survival factor for MM cells.10-15 However, to date, the functional significance of ERs on MM cells has not been characterized.
The therapeutic use of AE, primarily in breast cancer, has been predicated on their ability to antagonize estrogen binding to ER on tumor cells. However, it is now clear that AE have multiple additional effects on tumor cells, even those cells that lack detectable ERs.16-24 For example, these AE can induce apoptosis in both ER-positive and ER-negative breast cancer cell lines and patient cells, malignant glioma cell lines, and HL60 promyelocytic leukemia cells.18-20 The levels of TAM required to induce apoptosis vary from the nanomolar range for primary breast cancer treated in vivo to 1 to 10 μmol/L for triggering apoptosis of glioma and HL60 cell lines in vitro.21,22 To date, clinical studies have shown that TAM therapy (200 to 700 mg/d) achieves plasma levels of 2 to 7 μmol/L, with minimal toxicity.23-25 Interestingly, long-term use of high-dose oral TAM therapy has achieved responses in patients with recurrent glial tumors, with an acceptable toxicity profile.25
In the present study, we characterized the effects of estrogen as well as AE on human MM cells lines and patient MM cells. Immunoblotting showed the presence of ER-α in both MM cell lines and patient MM cells. Exposure of freshly isolated patient MM cells and MM cell lines to estrogen did not alter growth kinetics or induce apoptosis of these cells. In contrast, TAM and ICI 182,780 (ICI) significantly inhibited proliferation of MM cell lines and patient cells, an effect that was not altered by either estrogen or IL-6. The IC50 of TAM or ICI for MM cell lines and patient MM cells was 2 to 4 μmol/L. AE (TAM, ICI, toremifene [TOR], and 4-hydroxytamoxifen [4HTAM]) also induced apoptosis of MM cell lines and patient MM cells, as evidenced by fluorescence-activated cell sorting (FACS) analysis using propidium iodide (PI) staining and the TUNEL method, which similarly was unaffected by either E2 or IL-6. Importantly, both the inhibition of MM cell proliferation and the induction of MM cell apoptosis were achieved at concentrations of TAM (0.5 and 5.0 μmol/L) that did not significantly alter in vitro growth of normal hematopoietic progenitor cells and correspond to readily achievable plasma levels,25 providing the rationale for clinical trials to define the utility of AE therapy in MM.
MATERIALS AND METHODS
MM cell lines and MM patient cells.
RPMI 8226, U266, ARH-77 MM, and MCF-7 breast cancer cell lines were obtained from the American Type Culture Collection (Rockville, MD). OCI-My-5 cells26 were kindly provided by Dr H.A. Messner (Ontario Cancer Institute, Ontario, Canada). IL-6–transfected S6B45 MM cells27 were kindly provided by Dr T. Kishimoto (Osaka University, Osaka, Japan). Mononuclear cells (MCs) were isolated from MM patients by Ficoll-Hypaque (Pharmacia, Piscataway, NJ) density gradient centrifugation and incubated with HB7 (anti-CD38) monoclonal antibody (MoAb)-biotin-avidin and 2H4 (anti-CD45RA) MoAb-fluorescein isothiocyanate on ice. MM cells (96% ± 2% CD38+, CD45RA−) were isolated from the bone marrow of a patient with Durie Salmon stage III myeloma (MM patient no. 1) and from the blood of a patient with plasma cell leukemia (MM patient no. 2) using an Epics C Cell Sorter (Coulter Electronics, Hialeah, FL). Freshly isolated cells from these 2 patients were used to determine the effects of E2 and AE on both proliferation and apoptosis. In experiments with E2 and AE, OCI-My5 MM cells cells were cultured in phenol red-free Iscove’s Modified Dulbecco’s Medium (GIBCO, Grand Island, NY), whereas the other cell lines and patient MM cells were cultured in phenol red-free RPMI 1640 medium (GIBCO). This was done because phenol red has been shown to act as a weak estrogen agonist.28 All media contained 10% charcoal dextran-treated fetal bovine serum (FBS; Hyclone, Logan, UT), which was analyzed by the manufacturer and determined to be devoid of sex steroids, as well as L-glutamine, penicillin, and streptomycin (GIBCO).
Preparation of estrogens and AE.
E2, TAM, and 4HTAM were obtained from Sigma Chemical (St Louis, MO). ICI, an AE without estrogen agonist activity,29 was the kind gift from Zeneca Pharmaceuticals (Wilmington, DE). TOR, a dehalogenated form of TAM,30 was kindly provided by ORION-FARMOS (Turku, Finland). Stock solutions (10−2mol/L in absolute ethanol) of E2 and AE were stored at −20°C. Cells were cultured for 24 to 72 hours with either E2 or AE (0.5 to 50 μmol/L), which was freshly prepared for each experiment. All treatment groups and controls contained the same concentration (0.5%) of ethanol vehicle.
Immunoblotting assay for estrogen receptor in MM cells.
Cell extracts from 5 MM cell lines (ARH-77, OCI-My-5, RPMI 8226, S6B45, and U266), purified MM cells from 3 patients, ER-α positive MCF-7 breast cancer cells, and ER-α negative DU145 prostatic cells31 were examined by Western blot analysis. Cells were lysed by incubation for 30 minutes in ice-cold lysis buffer (50 mmol/L Tris-HCl [pH 8.0], 150 mmol/L NaCl, 0.1% Nonidet P-40, 1 mmol/L EDTA, 5 μg/mL phenylmethyl sulfonyl fluoride [PMSF], 1 mmol/L Na3VO4, 2 μg/mL aprotinin, 2 μg/mL leupeptin, and 50 mmol/L NaF). Aliquots of lysates were electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Protein was then transferred onto nitrocellulose membranes, and nonspecific binding was blocked by overnight incubation with 5% skim milk. Subsequently, the membranes were washed with TBS-Tween and incubated with the TE1115011 (kind gift of Dr Myles Brown, Dana-Farber Cancer Institute, Boston, MA) anti–ER-α MoAb. Immunoblotting with anti–α-tubulin MoAb (Sigma) assured equivalent protein loading. An enhanced chemiluminescence (ECL) kit (Amersham, Arlington Heights, IL) was used to visualize bound antibodies.
Effect of AE on growth and apoptosis in MM cells.
The effect of AE on growth and survival of MM cells was examined by (1) measuring DNA synthesis using tritiated thymidine (3H-TdR) uptake, as in previous studies32; (2) cell cycle analyses using PI staining, as in prior reports33; and (3) enumeration of viable cells with trypan blue exclusion. For DNA synthesis assays, 2 × 104 MM cells/well were incubated in triplicate in 96-well plates. Experiments involving MM cell lines were performed three times, and those involving fresh patient MM cells were performed twice, the latter due to limitations on obtainable fresh MM cells. Cells were pulsed with 3H-TdR (Dupont, Wilmington, DE; 0.5 μCi/well) during the last 6 hours of 2-day cultures, harvested onto glass filters by use of a HARVESTAR 96 MACH II (Tomtec, Orange, CT) cell harvester, and counted on a 1205 Betaplate (Gaithersburg, MD) scintillation counter. For cell cycle analysis, 1 × 106 MM cells were washed twice with phosphate-buffered saline (PBS) and then incubated with PI solution (0.5 mL of 3.4 mmol/L sodium citrate, 10 mmol/L NaCl, 0.1% NP-40, and 50 μg/mL of PI) for 30 minutes. Fluorescence intensity was measured using the Epics 753 Cell Sorter (Coulter). For each sample, 10,000 cells were analyzed using program M cycle software (Coulter).
To assay for apoptosis, MM cells were treated with varying concentrations of E2, TAM, ICI, 4HTAM, and TOR. In assays to determine the effect of IL-6 on AE-induced apoptosis, S6B45 MM cells were incubated for 72 hours with either TAM (0.5 or 5.0 μmol/L) or equimolar amounts of dexamethasone (DEX) in the presence or absence of IL-6 at a dose (1 ng/mL), which previously has been shown to inhibit apoptosis in MM cells that, like S6B45 cells, constitutively produce IL-6 in picogram concentrations (<10 pg/mL).34 S6B45 MM cells were chosen for these experiments, because this cell line underwent DEX-induced apoptosis that is inhibited with IL-6. IL-6 was not detectable in supernatants from S6B45 cells using an enzyme-linked immunosorbent assay (ELISA) for human IL-6, with a limit of detection of 10 pg/mL.35 Apoptosis of MM cells was assayed using two methods. PI staining and FACS analysis was used, with gating for cells in the sub-G1 region. Induction of apoptosis was confirmed in these studies by miscroscopic examination of cells with acridine orange and ethidium bromide staining. Apoptosis was also measured by the TUNEL method,36 which assays for early apoptosis, using the MEBSTAIN Apoptosis kit (Immunotech, Westbrook, ME). In these studies, MM cells exposed to AE were washed three times with PBS containing 0.2% bovine serum albumin (PBS-BSA). Cells were then fixed with buffered paraformaldehyde at 4°C for 30 minutes, followed by washing twice with PBS-BSA. Proteinase K (1 mL of 1:400 in PBS) was subsequently added to the cell pellet and incubated for 30 minutes at room temperature (RT). After incubation, cells were pelleted and the supernatant was discarded. Cells were then permeabilized by the addition of 1 mL of PBS containing 0.5% of Tween 20 and 0.2% BSA for 10 minutes at RT. After washing twice with PBS-BSA, 30 μL of terminal deoxynucleotidyl transferase (TdT) reaction reagent was added to the cell pellet for 1 hour at 37°C. Cells were next washed twice with PBS-BSA and 10 μL of blocking reagent was added for 10 minutes at RT. Subsequently, 30 μL of avidin-fluorescein isothiocyanate (FITC) reagent was added for 30 minutes at RT. Cells were washed twice with PBS-BSA and resuspended in 500 μL of PBS-BSA. Fluorescence intensity was measured using the Coulter Epics 753 Cell Sorter.
Effect of AE on hematopoietic progenitor cells.
Hematopoietic progenitor cell assays were performed as follows. Mononuclear cells recovered after Ficoll-Hypaque (Pharmacia) gradient centrifugation were plated at 2 × 105 cells/mL in Iscove’s methylcellulose medium containing recombinant human cytokines (MethoCult GF H4434; Stem Cell Technologies, Vancouver, British Columbia, Canada). This medium contains 50 ng/mL recombinant human (rh) stem cell factor, 10 ng/mL rh granulocyte-macrophage colony-stimulating factor, 10 ng/mL rhIL-3, and 3 U/mL rh erythropoietin. After 14 days of incubation at 37°C in a humidified incubator with 5% ambient CO2, colonies composed of 40 cells were enumerated using indirect microscopy and classified on the basis of morphology as either colony-forming unit–granulocyte-macrophage (CFU-GM) or colony-forming unit granulocyte, erythroid, monocyte, megakaryocyte (CFU-GEMM) using an inverted microscope, as previously described.37 Burst-forming unit-erythroid (BFU-E) colonies consisted of at least three erythroid clusters.
RESULTS
Expression of estrogen receptors on MM cell lines and patient MM cells.
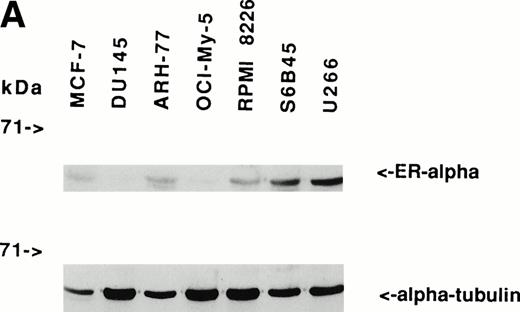
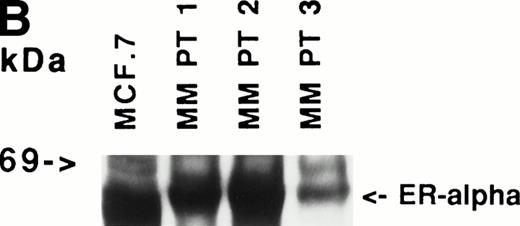
Immunoblotting of cell lysates was used to assay for ER-α expression on MM cell lines and patient MM cells. As can be seen in Fig 1A, Western blotting showed that ARH-77, RPMI 8226, S6B45, and U266 MM cells expressed ER-α. In contrast, OCI-My-5 MM cells did not express ER-α. Freshly purified MM cells from 3 of 3 patients also expressed ER-α (Fig 1B). MCF-7 breast cancer cells and DU145 prostatic cancer cells served as positive and negative controls, respectively, for ER-α expression.31
Expression of estrogen receptor on MM cell lines and MM patient cells. Lysates from ARH-77, OCI My-5, RPMI 8226, S6B45, and U266 MM cells (A) as well as purified MM cells from 3 patients (B) were examined by Western immunoblotting for the presence of ER- using the TE1115011 MoAb. MCF-7 breast cancer cells and DU145 prostatic cancer cells served as positive and negative controls, respectively, for ER- expression. Immunoblotting with anti–tubulin- MoAb assured equivalent protein loading.
Expression of estrogen receptor on MM cell lines and MM patient cells. Lysates from ARH-77, OCI My-5, RPMI 8226, S6B45, and U266 MM cells (A) as well as purified MM cells from 3 patients (B) were examined by Western immunoblotting for the presence of ER- using the TE1115011 MoAb. MCF-7 breast cancer cells and DU145 prostatic cancer cells served as positive and negative controls, respectively, for ER- expression. Immunoblotting with anti–tubulin- MoAb assured equivalent protein loading.
Effects of estrogens and AE on proliferation of MM cell lines and patient MM cells.
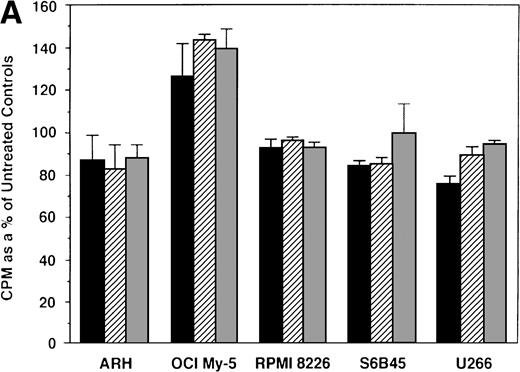
We first examined the responsiveness of MM cells to E2. Proliferation of 5 MM cell lines cultured in the presence or absence of E2 (0.5, 5.0, and 50 μmol/L) for 48 hours was measured by 3H-TdR uptake. A slight (20%) increase in DNA synthesis of OCI My-5 cells cultured with E2 was observed relative to cultures in media alone. However, no significant increase in DNA synthesis was noted in any MM cell lines in response to E2 (Fig 2A). Similarly, E2 (0.5 to 50 μmol/L) did not significantly alter the proliferation of patient MM cells (Fig 2B).
Effect of 17β estradiol on proliferation of MM cell lines and MM patient cells. ARH 77, OCI My-5, RPMI 8226, S6B45, and U266 MM cell lines (A) as well as purified MM cells from 2 patients (B) were cultured with 0.5 (▪), 5.0 (▨), and 50 (▧) μmol/L of E2 for 48 hours. 3H-TdR uptake was compared with DNA synthesis of cells cultured in media alone. Mean CPM and SEM for cultures with media alone were as follows: ARH (3,501 ± 148), OCI-My-5 (8,618 ± 1,081), RPMI 8226 (329,241 ± 11,774), S6B45 (118,811 ± 16,543), U266 (35,304 ± 6,033), MM patient no. 1 (2,949 ± 981), and MM patient no. 2 (12,965 ± 3,799).
Effect of 17β estradiol on proliferation of MM cell lines and MM patient cells. ARH 77, OCI My-5, RPMI 8226, S6B45, and U266 MM cell lines (A) as well as purified MM cells from 2 patients (B) were cultured with 0.5 (▪), 5.0 (▨), and 50 (▧) μmol/L of E2 for 48 hours. 3H-TdR uptake was compared with DNA synthesis of cells cultured in media alone. Mean CPM and SEM for cultures with media alone were as follows: ARH (3,501 ± 148), OCI-My-5 (8,618 ± 1,081), RPMI 8226 (329,241 ± 11,774), S6B45 (118,811 ± 16,543), U266 (35,304 ± 6,033), MM patient no. 1 (2,949 ± 981), and MM patient no. 2 (12,965 ± 3,799).
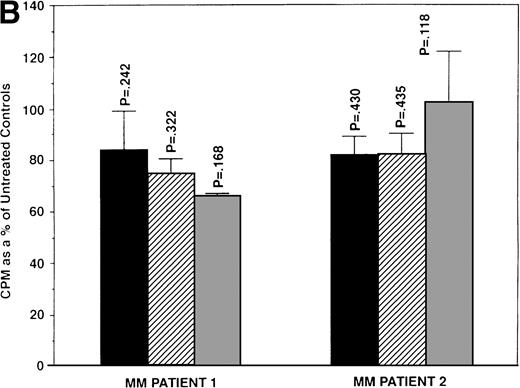
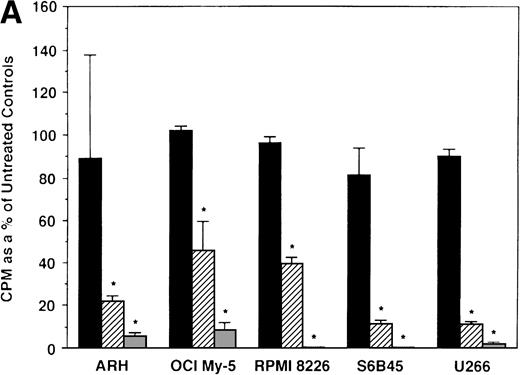
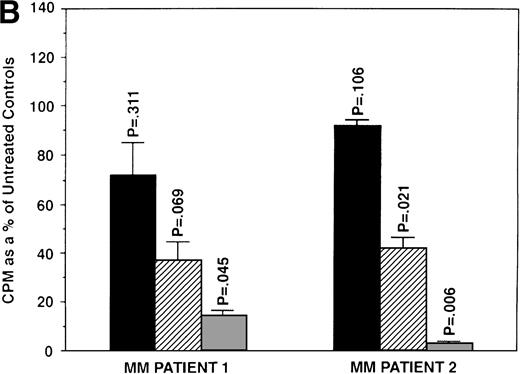
We next determined whether equimolar concentrations of TAM and ICI inhibited proliferation of MM cells. TAM (5.0 and 50 μmol/L) significantly decreased 3H-TdR uptake (n = 3, P < .05) in 48-hour cultures of all 5 MM cell lines evaluated (Fig 3A). As can be seen in Fig 3B, both 5 and 50 μmol/L TAM significantly reduced proliferation in the MM cells of patient no. 2; although a dramatic reduction in the proliferation of the MM cells of patient no. 1 was also evident at 5 and 50 μmol/L, statistical significance was reached only in 50 μmol/L TAM cultures. TAM at 0.5 μmol/L did not significantly alter DNA synthesis in the cells of either MM patient.
Effect of tamoxifen on proliferation of MM cell lines and MM patient cells. ARH 77, OCI My-5, RPMI 8226, S6B45, and U266 MM cell lines (A) as well as purified MM cells from 2 patients (B) were cultured with 0.5 (▪), 5.0 (▨), and 50 (▧) μmol/L of TAM for 48 hours. 3H-TdR uptake was compared with DNA synthesis of cells cultured in media alone. *P < .05. Mean CPM and SEM for media alone cultures were as follows: ARH (11,528 ± 3,423), OCI-My-5 (11,315 ± 593), RPMI 8226 (329,161 ± 6,448), S6B45 (90,722 ± 3,887), U266 (29,748 ± 3,778), MM patient no. 1 (3,277 ± 1,246), and MM patient no. 2 (16,316 ± 2,329).
Effect of tamoxifen on proliferation of MM cell lines and MM patient cells. ARH 77, OCI My-5, RPMI 8226, S6B45, and U266 MM cell lines (A) as well as purified MM cells from 2 patients (B) were cultured with 0.5 (▪), 5.0 (▨), and 50 (▧) μmol/L of TAM for 48 hours. 3H-TdR uptake was compared with DNA synthesis of cells cultured in media alone. *P < .05. Mean CPM and SEM for media alone cultures were as follows: ARH (11,528 ± 3,423), OCI-My-5 (11,315 ± 593), RPMI 8226 (329,161 ± 6,448), S6B45 (90,722 ± 3,887), U266 (29,748 ± 3,778), MM patient no. 1 (3,277 ± 1,246), and MM patient no. 2 (16,316 ± 2,329).
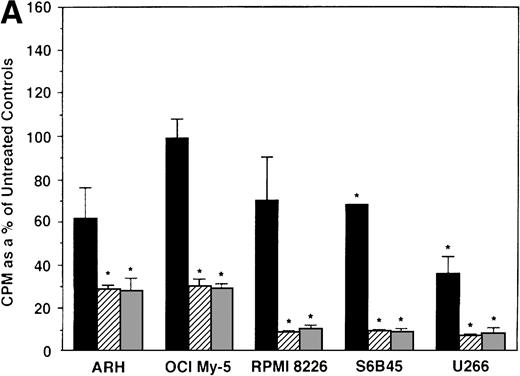
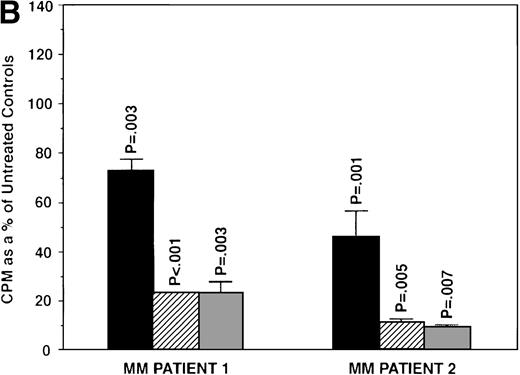
To further examine the effects of AE on MM cells, we next cultured MM cells with ICI, 4HTAM, and TOR at these concentrations. As can be seen in Fig 4A, ICI (5 and 50 μmol/L) significantly decreased (n = 3, P < .05) 3H-TdR uptake in all 5 MM cell lines relative to cultures of media alone. Moreover, S6B45 and U266 MM cells were more sensitive to ICI, because 0.5 μmol/L ICI also significantly decreased proliferation. In a parallel fashion, significant decreases in proliferation were observed in cultures of both patient MM cells treated at all three dose levels of ICI (Fig 4B). As shown in Fig 5, 4HTAM also inhibited proliferation of all 5 MM cell lines and patient cells, but was less potent than TAM. In contrast, TOR did not inhibit MM cell line proliferation (data not shown).
Effect of ICI 182,780 on proliferation of MM cell lines and MM patient cells. ARH 77, OCI My-5, RPMI 8226, S6B45, and U266 MM cell lines (A) as well as purified MM cells from 2 patients (B) were cultured with 0.5 (▪), 5.0 (▨), and 50 (▧) μmol/L ICI 182,780 (ICI) for 48 hours. 3H-TdR uptake was compared with DNA synthesis of cells cultured in media alone. *P < .05. Mean CPM and SEM for media alone cultures were as follows: ARH (8,626 ± 2,511), OCI-My-5 (11,020 ± 965), RPMI 8226 (354,146 ± 10,439), S6B45 (99,331 ± 3,461), U266 (35,349 ± 4,037), MM patient no. 1 (2,121 ± 65), and MM patient no. 2 (14,059 ± 2,006).
Effect of ICI 182,780 on proliferation of MM cell lines and MM patient cells. ARH 77, OCI My-5, RPMI 8226, S6B45, and U266 MM cell lines (A) as well as purified MM cells from 2 patients (B) were cultured with 0.5 (▪), 5.0 (▨), and 50 (▧) μmol/L ICI 182,780 (ICI) for 48 hours. 3H-TdR uptake was compared with DNA synthesis of cells cultured in media alone. *P < .05. Mean CPM and SEM for media alone cultures were as follows: ARH (8,626 ± 2,511), OCI-My-5 (11,020 ± 965), RPMI 8226 (354,146 ± 10,439), S6B45 (99,331 ± 3,461), U266 (35,349 ± 4,037), MM patient no. 1 (2,121 ± 65), and MM patient no. 2 (14,059 ± 2,006).
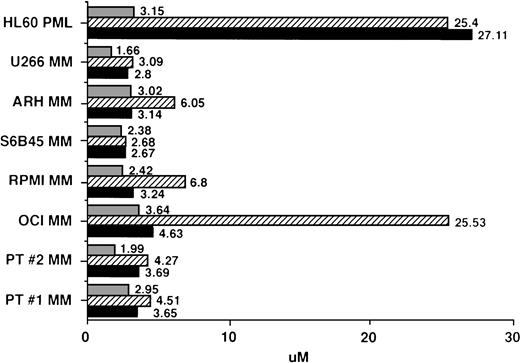
IC50 values for MM cells cultured with AE. Regression plot analysis was used to calculate the dose of TAM (▪), 4HTAM (▨), and ICI (▧) required to inhibit 3H-TdR uptake of patient MM cells, MM cell lines, and HL60 promyelocytic leukemia cells by 50% (IC50 dose).
IC50 values for MM cells cultured with AE. Regression plot analysis was used to calculate the dose of TAM (▪), 4HTAM (▨), and ICI (▧) required to inhibit 3H-TdR uptake of patient MM cells, MM cell lines, and HL60 promyelocytic leukemia cells by 50% (IC50 dose).
Regression plot analysis was used to determine those concentrations of TAM, 4HTAM, and ICI that resulted in 50% growth inhibition (IC50) of MM cell lines, patient MM cells, and HL60 promyelocytic leukemia cells. These studies showed that 2 to 4 μmol/L TAM or ICI was capable of inhibiting by 50% the growth of MM cell lines and freshly isolated patient MM cells (Fig 5). Although 4HTAM was also capable of inhibiting the growth of most MM cell lines and patient MM cells, the concentrations of 4HTAM were higher than the doses of TAM required to achieve this effect. ARH-77, RPMI 8226, and OCI My-5 MM cell lines were relatively resistant to the effects of 4HTAM, with a twofold to fivefold higher IC50 relative to TAM. HL60 cells displayed a 10-fold higher IC50 for both TAM and 4HTAM than did MM cells; however, the sensitivity of HL60 cells to ICI (IC50 of 3.15 μmol/L) was similar to that of MM cells.
Effect of 17β estradiol on the tamoxifen-related growth suppression of S6B45 MM cells.
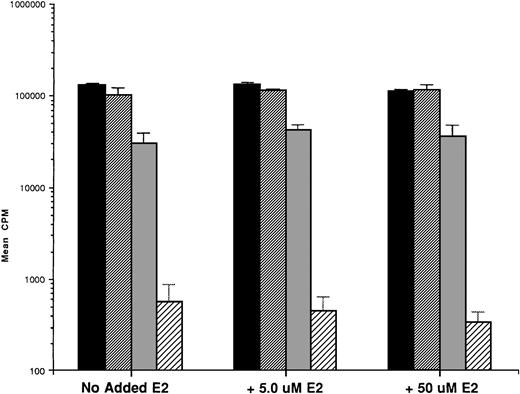
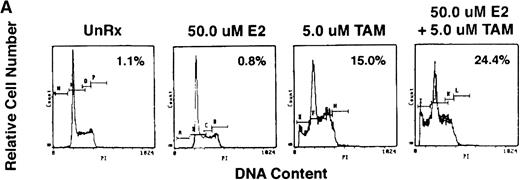
To assess whether E2 could abrogate the growth-inhibitory effects of AE, we next cultured ER-α–positive SB645 MM cells in media alone or with TAM (0.5, 5.0, and 50.0 μmol/L) for 48 hours in the presence or absence of equimolar and of excess E2 (5.0 and 50 μmol/L). As can be seen in Fig 6, the addition of E2 did not abrogate the downregulatory effects of TAM on S6B45 MM cell growth.
Effect of 17β estradiol on the tamoxifen-related growth suppression of S6B45 MM cells. ER-–positive S6B45 MM cells were cultured for 48 hours in media alone (▪) or 0.5 (▨), 5.0 (▧), and 50 (░) μmol/L of TAM with or without E2 (5.0 and 50 μmol/L).3H-TdR uptake was compared for cells cultured with TAM and E2 versus those cultured in TAM alone.
Effect of 17β estradiol on the tamoxifen-related growth suppression of S6B45 MM cells. ER-–positive S6B45 MM cells were cultured for 48 hours in media alone (▪) or 0.5 (▨), 5.0 (▧), and 50 (░) μmol/L of TAM with or without E2 (5.0 and 50 μmol/L).3H-TdR uptake was compared for cells cultured with TAM and E2 versus those cultured in TAM alone.
Effect of AE on the survival of MM cell lines and patient MM cells.
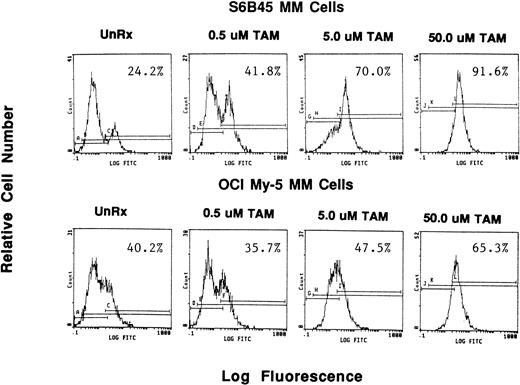
Because we demonstrated effects of AE on proliferation of MM cells, we next wanted to determine whether these agents also altered survival of these tumor cells. OCI My-5, RPMI 8226, S6B45, and U266 MM cells were treated for 72 hours with TAM, TOR, or ICI (0.5, 5.0, and 50 μmol/L). Induction of apoptosis was assayed both by the TUNEL method (MEBSTAIN) and PI staining. TUNEL analysis showed that TAM induced apoptosis of S6B45 and OCI My-5 MM cells (Fig 7) and U266 MM cells, but not of RPMI 8226 cells. Time course studies with S6B45 and OCI-My-5 MM cells showed induction of apoptosis occurring as early as 24 hours after TAM exposure. TOR also induced apoptosis of S6B45 and OCI-My-5 MM cells; however, higher concentrations (50 μmol/L) were required to achieve similar effects. In contrast, ICI did not induce apoptosis of these MM cell lines. Similar results were obtained by FACS analysis using PI staining (not shown).
Effect of tamoxifen on apoptosis of MM cell lines. S6B45 and OCI-My-5 MM cells were cultured for 24 hours in the absence or presence of TAM (0.5, 5.0, and 50 μmol/L), and the TUNEL technique was used to detect early apoptosis. The percentages of cells within the apoptosis gates are shown.
Effect of tamoxifen on apoptosis of MM cell lines. S6B45 and OCI-My-5 MM cells were cultured for 24 hours in the absence or presence of TAM (0.5, 5.0, and 50 μmol/L), and the TUNEL technique was used to detect early apoptosis. The percentages of cells within the apoptosis gates are shown.
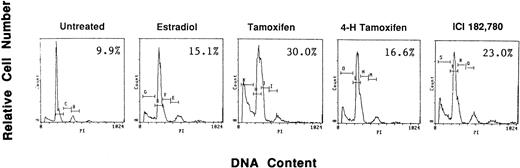
To examine if AE could induce apoptosis of freshly isolated patient MM cells, we cultured tumor cells with TAM, 4HTAM, ICI, or E2 (5.0 μmol/L). These patient MM cells were greater than 97% viable by trypan blue staining at 72 hours in cultures with media alone. Apoptosis was determined by FACS analysis using PI staining and was confirmed by examination under fluorescent microscopy after acridine orange and ethidium bromide staining. The percentage of cells in the apoptotic gate for MM patient no. 2 is shown in Fig 8. AE induced apoptosis of patient MM cells with the following relative potencies: TAM>ICI>>4 HTAM; in contrast, E2 did not trigger significant apoptosis.
Effect of AE on apoptosis of patient MM cells. Freshly isolated MM cells were exposed to the AE TAM, 4HTAM, or ICI (5.0 μmol/L) as well as to E2 (5.0 μmol/L) for 72 hours. Apoptosis was determined by PI staining and FACS analysis. The percentages of cells within the apoptosis gate are shown.
Effect of AE on apoptosis of patient MM cells. Freshly isolated MM cells were exposed to the AE TAM, 4HTAM, or ICI (5.0 μmol/L) as well as to E2 (5.0 μmol/L) for 72 hours. Apoptosis was determined by PI staining and FACS analysis. The percentages of cells within the apoptosis gate are shown.
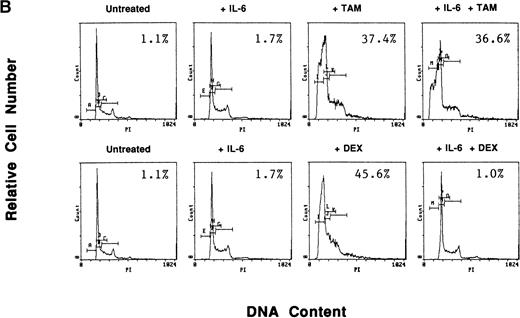
To determine whether exogenous estrogen could inhibit apoptosis triggered by AE, we cultured ER-α–positive S6B45 MM cells with TAM (5 μmol/L), E2 (50 μmol/L), or both. E2 did not abrogate TAM-induced apoptosis of S6B45 MM cells (Fig 9A). Because IL-6 is known to inhibit apoptosis of MM cells induced by DEX,38 we next determined whether IL-6 could similarly block the apoptotic effect of AE on MM cells. S6B45 MM cells were treated with either DEX or TAM (0.5 and 5.0 μmol/L) in the presence or absence of IL-6 (1 ng/mL). As seen in Fig9B, DEX and TAM (0.5 μmol/L) induced apoptosis of S6B45 MM cells; IL-6 inhibited DEX-induced apoptosis but did not affect apoptosis triggered by TAM. At higher doses of TAM and DEX (5.0 μmol/L), apoptosis triggered in S6B45 MM cells was not blocked by IL-6.
Effect of estrogens or IL-6 on tamoxifen-induced apoptosis of S6B45 MM cells. S6B45 MM cells were cultured for 72 hours in media alone or with TAM (5.0 μmol/L), E2 (50 μmol/L), or both (A). These cells were also cultured for 72 hours in media alone, IL-6 (1 ng/mL), TAM (0.5 μmol/L), DEX (0.5 μmol/L), and either TAM or DEX with IL-6 (B). Apoptosis was determined by PI staining and FACS analysis. The percentages of cells within the apoptosis gate are shown.
Effect of estrogens or IL-6 on tamoxifen-induced apoptosis of S6B45 MM cells. S6B45 MM cells were cultured for 72 hours in media alone or with TAM (5.0 μmol/L), E2 (50 μmol/L), or both (A). These cells were also cultured for 72 hours in media alone, IL-6 (1 ng/mL), TAM (0.5 μmol/L), DEX (0.5 μmol/L), and either TAM or DEX with IL-6 (B). Apoptosis was determined by PI staining and FACS analysis. The percentages of cells within the apoptosis gate are shown.
Effect of AE on normal hematopoietic progenitor cell growth.
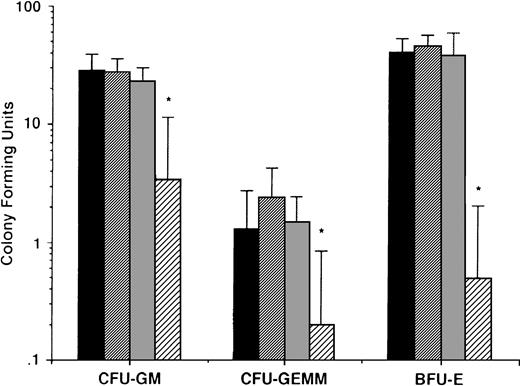
To determine whether the effects described above of AE on MM cells were nonspecific and in anticipation of the potential clinical use of these agents, we also delineated the effects of AE on normal hematopoietic progenitor cells. Peripheral blood CD34+ stem cells from 5 normal donors were cultured in long-term cultures in the presence or absence of TAM (0.5, 5.0, and 50 μmol/L). After culture for 2 weeks, colony-forming units of granulocyte-macrophage (CFU-GM), granulocyte, erythroid, monocyte, megakaryocyte (CFU-GEMM), and burst forming units of erythroid lineage (BFU-E) were enumerated. As can be seen in Fig 10, there was no significant difference in number of CFU-GM, CFU-GEMM, and BFU-E in cultures with media alone, 0.5 μmol/L TAM, or 5.0 μmol/L TAM. In contrast, TAM at 50 μmol/L significantly decreased colony stem cell growth of all lineages.
Effect of AE on growth of normal hematopoietic progenitor cells. CD34+ stem cells from 5 normal donors were cultured with media (▪) or with 0.5 (▨), 5.0 (▧), and 50 (░) μmol/L TAM. After 2 weeks, colonies of granulocyte-macrophages (CFU-GM), granulocyte erythrocyte monocyte macrophages (CFU-GEMM), and blood-forming units of erythroid lineage (BFU-E) were enumerated.P values denote comparison with cultures in media alone. *P < .05.
Effect of AE on growth of normal hematopoietic progenitor cells. CD34+ stem cells from 5 normal donors were cultured with media (▪) or with 0.5 (▨), 5.0 (▧), and 50 (░) μmol/L TAM. After 2 weeks, colonies of granulocyte-macrophages (CFU-GM), granulocyte erythrocyte monocyte macrophages (CFU-GEMM), and blood-forming units of erythroid lineage (BFU-E) were enumerated.P values denote comparison with cultures in media alone. *P < .05.
DISCUSSION
In the present study, we demonstrate by immunoblotting that most human MM cell lines and patient MM cells express ER-α. The presence of ER-α on MM cells suggested to us that estrogens might modulate MM cell proliferation, analogous to their actions in B-cell lymphopoeisis.3,4 Our studies of both MM cell lines and patient MM cells demonstrated that estrogen does not alter MM cell proliferation in vitro. Given the expression of ER-α on MM cells, we next examined whether AE could effect MM cell growth. Of great interest, TAM and ICI both significantly suppressed DNA synthesis in MM cell lines and patient MM cells. TAM (5.0 μmol/L and 50 μmol/L) significantly decreased tumor cell proliferation, whereas lesser concentrations of ICI (0.5 μmol/L) inhibited patient MM cell proliferation. This inhibitory effect occured even in OCI-My-5 MM cells that did not express detectable ER-α and was not significantly inhibited by excess estrogen in ER-α–positive S6B45 MM cells, suggesting that it may not be mediated via ER-α. Moreover, it occurred in MM cell lines that were both IL-6 responsive and IL-6 nonresponsive, suggesting that it is not primarily mediated via IL-6. Most importantly, the IC50 of TAM for MM cell lines and patient MM cells was 2 to 4 μmol/L, well within the plasma levels that are safely achievable using high-dose oral TAM therapy.23-25
The most impressive effect of AE in our study was the induction of MM cell apoptosis. Apoptosis was triggered in MM cell lines by TAM and, to a lesser extent, by 4HTAM and TOR. The mechanism whereby AE induce apoptosis of these MM cells remains undefined. It is possible that AE might antagonize estrogen binding to ER and thereby induce apoptosis analogous to breast cancer cells.39-43 However, the growth-inhibitory and apoptotic effects of AE in MM were not inhibited by excess E2 in ER-α–positive S6B45 MM cells and occurred in OCI My-5 MM cells that lacked detectable ER-α, suggesting that these actions were independent of both estrogen and ER-α. AE binding sites (AEBS) distinct from ER binding sites have been described in many cells types that can mediate binding to both TAM and ICI.44 45These AEBS are more abundant than ER binding sites, and it is therefore likely that such AEBS may mediate the growth-inhibitory and apoptosis-inducing effects of AE in MM cells.
Our previous studies have shown that apoptosis in MM cells induced by γ irradiation (IR), DEX, and anti-Fas MoAb appear to be mediated via distinct signaling cascades.46-48 All three stimuli induce apoptosis associated with cleavage of caspase 3 (CPP32), protein kinase C δ (PKC-δ), and poly (ADP ribose) polymerase (PARP). IR and anti-Fas MoAb, but not DEX, induced apoptosis is associated with activation of stress-activated protein kinase (SAPK) and p38 kinase; in contrast, DEX-triggered apoptosis is associated with downregulation of mitogen-activated protein kinase (MAPK) and p70S6K growth kinases, but not activation of SAPK or p38 kinase.46Furthermore, IR-induced apoptosis is associated with an increase in cytosolic cytochrome-c (cyto-c) levels, whereas apoptosis induced by DEX or anti-Fas MoAb has no detectable effect on cyto-c release.48 Finally, apoptosis induced by these stimuli are differentially inhibited by IL-6: DEX-triggered apoptosis is completely inhibited and anti-Fas MoAb-induced apoptosis is partially inhibited by IL-6, whereas IR-induced apoptosis is not altered by IL-6.38,46 47 Because the apoptotic effect of AE is not altered by IL-6, these agents would appear to be more analogous to IR than to either DEX or anti-Fas MoAb, but further studies are needed to elucidate their signaling mechanisms.
In the present study, induction of MM cell apoptosis was achieved at concentrations of TAM (0.5 and 5.0 μmol/L) that did not significantly alter in vitro growth of normal hematopoietic progenitor cells, providing the rationale for clinical trials. In our studies at concentrations of 50 μmol/L TAM, which correlate to 10-fold higher plasma levels than are achievable in patients on high-dose TAM therapy, cell death was seen in all cell types tested. This nonspecific toxicitiy may be a consequence of genotoxic effects of TAM related to DNA adduct formation, as previously reported in normal human lymphocytes,49 although controversy exists on whether TAM-induced DNA adduct formation occurs in normal human cells even at high (>10 μmol/L) concentrations.50
In our study, the concentration of TAM required to achieve either growth suppression or apoptosis of MM cells in vitro is equivalent to TAM plasma levels that have been safely achieved in clinical trials using high-dose oral TAM therapy.23-25 To date, ICI studies in humans have used conventional doses that result in nanomolar plasma levels51; hence, it is not possible to relate the ICI concentrations used in our in vitro studies to those safely achievable in clinical trials. Finally, TOR induced apoptosis of MM cells in vitro only at concentrations (50 μmol/L) that correspond to higher doses than have been clinically evaluated.52 Therefore, the studies provide the feasibility and rationale for the initiation of clinical trials to define the therapeutic utility of AE, particularly TAM, in MM.
ACKNOWLEDGMENT
The authors thank Dr Myles Brown for providing advice and antibodies used in these studies and thank Mary Beach (Dana-Farber Cryopreservation Laboratory, Boston, MA) for technical support with hematopoietic progenitor cell assays.
Supported by National Institutes of Health Grant No. CA50947 and by the Kraft Family Research Fund.
Address reprint requests to Kenneth C. Anderson, MD, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02215; e-mail:Kenneth-Anderson@dfci.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal