Abstract
In population-based studies it has been established that inherited deficiency of erythrocyte (E) glucose-6-phosphate dehydrogenase (G6PD) confers protection against severe Plasmodium falciparum (P falciparum) malaria. Impaired growth of parasites in G6PD-deficient E in vitro has been reported in some studies, but not in others. In a systematic analysis, we have found that with five different strains ofP falciparum (FCR-3, KI, C10, HB3B, and T9/96), there was no significant difference in either invasion or maturation when the parasites were grown in either normal or G6PD-deficient (Mediterranean variant) E. With all of these strains and at different maturation stages, we were unable to detect any difference in the amount of P falciparum–specific G6PD mRNA in normal versus deficient parasitized E. The rate of 14C-CO2 production from D-[1-14C] glucose (which closely reflects intracellular activity of G6PD) contributed by the parasite was very similar in intact normal and deficient E. By contrast, in studies of phagocytosis of parasitized E by human adherent monocytes, we found that when the parasites were at the ring stage (ring-stage parasitized E [RPE]), deficient RPE were phagocytosed 2.3 times more intensely than normal RPE (P = .001), whereas there was no difference when the parasites were at the more mature trophozoite stage (trophozoite-stage parasitized E [TPE]). Phagocytic removal markers (autologous IgG and complement C3 fragments) were significantly higher in deficient RPE than in normal RPE, while they were very similar in normal and deficient TPE. The level of reduced glutathione was remarkably lower in deficient RPE compared with normal RPE. We conclude that impaired antioxidant defense in deficient RPE may be responsible for membrane damage followed by phagocytosis. Because RPE, unlike TPE, are nontoxic to phagocytes, the increased removal by phagocytosis of RPE would reduce maturation to TPE and to schizonts and may be a highly efficient mechanism of malaria resistance in deficient subjects.
THE PROTECTIVE ROLE of erythrocyte (E) glucose-6-phosphate dehydrogenase (G6PD) deficiency against infection with Plasmodium falciparum (P falciparum) malaria (see Luzzatto1 and Greene2 for reviews) is supported by case-control studies performed in Africa3-5 and Southeast Asia.6,7 Some studies showed protection for both hemi/homozygous and heterozygous subjects,6-8 while other studies indicated that only heterozygous females were protected, while hemi/homozygous subjects with a more severe degree of G6PD deficiency were not protected.5,6 In vitro studies have yielded conflicting results. Parasite growth in hemizygous G6PD-deficient E in short-term cultures without oxidant stress was not inhibited in some studies9-11 and inhibited in other studies,12-14 whereas oxidant stress constantly inhibited growth in deficient E.10,11 Considering those discrepancies, one wonders whether protection against malaria mortality and the consequent selective advantage can be accounted for merely by less favorable growth conditions of the parasite in the deficient host E.15 16
In this study, we first attempted to reconcile some of the contradictory data in the literature. In carefully controlled experiments performed with several strains of P falciparum, we found that invasion and maturation of the parasite in both the first and second growth cycle were quantitatively indistinguishable in normal and deficient E, provided exclusively freshly drawn E were used. Similarly, in all strains and at all maturation stages studied, P falciparum G6PD mRNA was not significantly different in normal and deficient parasitized E. Next, we investigated directly how the G6PD status of the host cell impacts on the parasite's metabolism: we found that the parasite's contribution to 14C-CO2production from D-[1-14C] glucose (which closely reflects intracellular activity of G6PD) again was not significantly different in normal and deficient parasitized E. In an early study of malaria in deficient heterozygotes, suicidal infection was mentioned as one possible mechanism of protection.4 As a specific test of this possibility, we investigated quantitatively the susceptibility of parasitized E to phagocytosis by periferal blood monocytes. We found that deficient, ring-stage parasitized E (RPE) had on their surface a higher density of phagocytic removal markers (such as autologous IgG and complement C3 fragments) than normal RPE, and that in keeping with this finding, deficient RPE were phagocytosed more effectively and almost as effectively as E with more mature forms of the parasite. Preferential phagocytosis at an early stage of the schizogonic cycle offers an attractive explanation for the selective advantage of deficient individuals against P falciparum malaria.
MATERIALS AND METHODS
Materials.
Buffers, culture media, substrates, enzymes and coenzymes, mannitol, heparin, gentamicin, methylene blue, guanidine thiocyanate, cesium chloride, saponin, Triton X-100, 5-amino-2,3-dihydro-1,4-phthalazinedione (luminol), 5,5'-dithiobis(2-nitrobenzoic acid) (DTNB) were from Sigma (St Louis, MO); Percoll was from Pharmacia (Uppsala, Sweden); D-[1-14C] glucose, specific activity 53.4 mCi/mmol, Megaprime DNA labeling system, Hybond-N nylon filters, protein A, [125I]-labeled with Bolton and Hunter reagent, and [125I] were from Amersham International (Amersham, UK); Iodo-beads iodination reagent was from Pierce (Rockford, IL); goat antibody to human C3c (affinity purified, polyclonal) was from The Binding Site (Birmingham, UK); Diff-Quik parasite stain was from Baxter Dade AG (Dudingen, Switzerland); sterile plastics were from Costar (Cambridge, MA). All other reagents were purchased from common commercial sources. Sendai virus was a kind gift of Prof H. Ginsburg (Jerusalem, Israel).
Erythrocyte preparation, P falciparum cultivation, stage-separation of parasitized E, and assessment of parasite growth.
Control E were obtained from hematologically healthy subjects with G6PD B. Deficient blood was obtained from healthy hemizygote male deficient (Mediterranean variant) subjects. In all cases informed consent was obtained. Blood anticoagulated with citrate-phosphate-dextrose with adenine (CPDA-1) was kept for 12 to 24 hours at +4°C for parasite growth experiments and metabolic studies, and for 1 to 10 days for mRNA studies. Normal and deficient E were isolated from plasma and white blood cells by 85% Percoll gradient in phosphate-buffered saline (PBS) centrifugation and three washings in wash medium (RPMI 1640 medium containing 25 mmol/L Hepes, 20 mmol/L glucose, and 32 mg/L gentamicin, pH 7.30). P falciparum strains FCR-3, KI, C1O, HB3B, and T9/96 were cultivated and synchronized as described17 at 0.5% hematocrit (parasite growth experiments and metabolic studies) and at 5% hematocrit (mRNA studies). Briefly, normal schizont-stage parasitized E (SPE) separated on Percoll-mannitol gradient17 (parasitemia >95% SPE) were mixed with normal or deficient E suspended in growth medium (RPMI 1640 medium containing 25 mmol/L Hepes, 20 mmol/L glucose, 2 mmol/L glutamine, 24 mmol/L NaHCO3, 32 mg/L gentamicin, and 10% AB or A human serum, pH 7.30) to start synchronous cultures at indicated hematocrit values. For all studies except growth studies, parasitemia of inoculum was adjusted to 20% normal SPE for isolation of RPE and to 5% normal SPE for isolation of trophozoite-stage parasitized E (TPE). Fourteen to 18 hours after inoculum (ring-stage in the first cycle), 34 to 38 hours (trophozoite-stage in the first cycle), and 40 to 44 hours after inoculum (schizont-stage in the first cycle), RPE, TPE, and SPE were separated on Percoll-mannitol gradients.17 The parasitemia was usually 30% to 45% RPE, >95% TPE and >95% SPE. For parasite growth studies, parasitemia of inoculum was adjusted to 1% to 20% normal SPE as indicated. Parasite growth in normal and deficient E was expressed as parasite invasion and parasite maturation. Parasite invasion was defined as ratio between ring-stage parasitemia measured 14 to 18 hours after inoculum and inoculum parasitemia (first cycle invasion), or as ratio between ring-stage parasitemia measured 62 to 66 hours after inoculum and schizont-stage parasitemia measured 40 to 44 hours after inoculum (second cycle invasion). Parasite maturation was defined as ratio between trophozoite-stage parasitemia measured 34 to 38 hours after inoculum and ring-stage parasitemia measured 14 to 18 hours after inoculum (first cycle maturation), or as ratio between trophozoite-stage parasitemia measured 82 to 86 hours after inoculum and ring-stage parasitemia measured 62 to 66 hours after inoculum (second cycle maturation). Nonparasitized and parasitized E were counted electronically. To assess total parasitemia and relative contribution of RPE, TPE, and SPE, slides were prepared from cultures at indicated times, stained with Diff-Quik parasite stain, and 400 to 1,000 cells examined microscopically. Significance of differences in parasitemia between normal and deficient parasitized E was assessed byt-test for paired samples.
Opsonization of erythrocytes.
Washed nonparasitized and stage-separated parasitized E were incubated in Hepes-glucose wash medium (10 mmol/L Hepes, 10 mmol/L glucose, 140 mmol/L NaCl, pH 7.30) supplemented with 50% fresh autologous serum at 10% hematocrit for 15 minutes at 37°C. Cells were then washed twice in the same medium, resuspended at 10% hematocrit, and used for phagocytosis assay or for measurement of bound autologous IgG and complement C3 fragment.
Extraction of P falciparum total RNA.
Total RNA was extracted from synchronized cultures 14 to 18 hours (RPE), 34 to 38 (TPE), and 40 to 44 hours (SPE) after inoculum during the first cycle of parasite growth using the guanidine thiocyanate method.18 For the extraction of total RNA from the peripheral blood of patients infected by P falciparummalaria, 2 to 3 mL of blood was collected in CPDA-1 and RNA was extracted as mentioned above.18
Northern blot analysis and quantitation.
Total RNA was fractionated on a 1.2% agarose formaldehyde denaturing gel and blotted.19 The P falciparum G6PD probe used was K2O2, a 1.6-kb fragment of the P falciparumG6PD gene.20 A 2.2-kb EcoR1 fragment ofP falciparum calmodulin gene21 was used as an internal control to normalize the expression of P falciparumG6PD gene, as the regulation of the calmodulin gene is considered to be independent of the G6PD status of host E. Probes were labeled using the Amersham Megaprime DNA labeling system and hybridized as described previously.20 Quantitation values for P falciparumG6PD and calmodulin mRNA were obtained from autoradiographs of the same Northern blot filters hybridized with both probes. The hybridization signals obtained with both probes from the same lane were quantified by densitometry using a 2D analytical scanner (Biomed Instruments, Fullerton, CA). The K2O2 signals were normalized using calmodulin signals, and G6PD mRNA levels were then compared in normal and deficient parasitized E.
Measurement of 14C-CO2 production from D-[1-14C] glucose.
14C-CO2 production from D-[1-14C] glucose was measured in nonparasitized and parasitized E according to Pescarmona et al.22 Maximal activation of14C-CO2 production was achieved by adding methylene blue (MB) dissolved in PBS-glucose, pH 7.40 (final concentration, 135 μmol/L). 14C-CO2production in samples incubated at 37°C was linear for 60 minutes under all conditions studied. Nonparasitized E were submitted to the same culture conditions and to the same treatment as parasitized E.14C-CO2 production values obtained with cultures that contained 30% to 45% RPE were extrapolated to 100% RPE parasitemia using the following calculation: I = (TOT − N × n)/(1 − n), where I = amount of 14C-CO2produced by 100% RPE; TOT = amount of 14C-CO2produced by the whole culture; N = amount of14C-CO2 produced by nonparasitized E; n = fraction of nonparasitized E.
Sendai virus treatment of RPE and TPE.
Sendai virus specifically lyses E membrane leaving the parasite membrane intact.23 RPE were treated with Sendai virus as follows: RPE-enriched pellet was resuspended in wash medium at 2% hematocrit and incubated with Sendai virus (40 to 100 μg protein/mL, equivalent to 800 to 1,200 hemagglutination units) for 20 minutes at 37°C. Virus-treated and nontreated RPE were then washed twice and resuspended in the same volume of wash medium. Nontreated RPE were counted electronically. Because microscopic inspection of virus-treated samples indicated extensive lysis, reference was done to counts of nontreated cells. Residual 14C-CO2 production observed in virus-treated E was subtracted. Isolated TPE and nonparasitized E were treated with half the amount of Sendai virus used to lyse RPE. Nontreated TPE were counted electronically; for virus-treated TPE, reference was done to counts of nontreated cells, as indicated above. In all cases, a known amount of cells was used for measurement of 14C-CO2 production. When applied to nonparasitized E, virus lysis eliminated hexokinase and lactate dehydrogenase totally and hemoglobin to 98%. G6PD and glyceraldehyde 3-phosphate dehydrogenase were consistently retained (approximately 40% and 50% retained, not shown). Because D-[1-14C] glucose must be first phosphorylated by parasite hexokinase and adenosine triphosphate (ATP) to give D-[1-14C] glucose-6-phosphate, the presence of retained host G6PD alone without hexokinase should not alter our results. Only the recycling of labeled glucose-6-phosphate to host cytoplasm or loss of nicotinamide adenine dinucleotide phosphate (NADP), ATP, or Mg++ from the parasite compartment could artefactually increase or decrease 14C-CO2production, respectively. We checked those possible artifacts by supplementing virus-treated TPE with hexokinase/ATP, NADP, Mg++, or with various combinations of the above substances. As expected, supplemented hexokinase/ATP alone increased14C-CO2 production, while none of the other additions was effective (not shown).
Measurement of reduced glutathione (GSH).
GSH levels were measured as described24 in nonparasitized E and in stage-separated parasitized E. GSH levels obtained with separated RPE that contained 30% to 45% RPE were extrapolated to 100% RPE parasitemia using the calculation described above.
Preparation of monocytes and measurement of phagocytosis.
Human mononuclear cells were separated from fresh normal or deficient (Mediterranean variant) blood and plated as indicated.25Phagocytosis was quantified as monocyte-ingested hemoglobin by measuring heme-enhanced luminescence as described.26Phagocytosis was expressed as number of ingested E per monocyte making use of a calibration curve prepared with each specific E sample as indicated.26
Binding of autologous IgG and complement C3 fragments.
Erythrocyte-bound autologous IgG and complement C3 fragment was measured as described25 in nonparasitized E and in stage-separated parasitized E. Data obtained with separated RPE that contained 30% to 45% RPE were extrapolated to 100% RPE parasitemia using the calculation described above.
RESULTS
Parasite growth in normal and deficient E.
In a first series of experiments performed with the FCR-3 strain, invasion and maturation were measured in the first and second cycle of parasite growth in normal and deficient E, with 4% parasitemia at the time of inoculum and 0.5% final hematocrit. We found no difference in both parameters during the first cycle (Table 1). In the second cycle, we see some degree of inhibition of both invasion and maturation in deficient E. However, t-test for paired samples indicates that differences were not significant. Similar results were obtained with strains KI, C10, HB3B, and T9/96 (data not shown). In a second series of experiments performed with the FCR-3 strain, a larger number of growth experiments was performed at higher (10% to 20%) parasitemia of the inoculum-parasitized culture and 0.5% final hematocrit. At-test for paired samples of those experiments again indicated no significant difference in invasion (significance of difference,P = .79, n = 8) and maturation (significance of difference,P = .40, n = 7) between normal and deficient parasitized E during the first cycle of parasite growth.
Parasite Invasion and Maturation During the First and Second Cycle of Growth in Normal and Deficient E
| . | First Cycle . | Second Cycle . | ||
|---|---|---|---|---|
| Invasion . | Maturation . | Invasion . | Maturation . | |
| Normal E | 2.22 ± 0.96 | 0.71 ± 0.26 | 4.52 ± 0.91 | 0.74 ± 0.20 |
| n | 6 | 6 | 6 | 6 |
| Deficient E | 2.48 ± 0.83 | 0.67 ± 0.27 | 3.16 ± 1.03 | 0.34 ± 0.34 |
| n | 6 | 6 | 6 | 6 |
| P | .39 | .49 | .05 | .06 |
| . | First Cycle . | Second Cycle . | ||
|---|---|---|---|---|
| Invasion . | Maturation . | Invasion . | Maturation . | |
| Normal E | 2.22 ± 0.96 | 0.71 ± 0.26 | 4.52 ± 0.91 | 0.74 ± 0.20 |
| n | 6 | 6 | 6 | 6 |
| Deficient E | 2.48 ± 0.83 | 0.67 ± 0.27 | 3.16 ± 1.03 | 0.34 ± 0.34 |
| n | 6 | 6 | 6 | 6 |
| P | .39 | .49 | .05 | .06 |
Inoculum was performed mixing separated normal schizont-stage parasitized (strain FCR-3) E (SPE), with normal or deficient E suspended in growth medium. Parasitemia of inoculum was adjusted to 4% normal SPE, final hematocrit of inoculum was 0.5%. Parasite invasion: ratio between ring-stage parasitemia measured 14 to 18 hours after inoculum and inoculum parasitemia (first cycle invasion), or ratio between ring-stage parasitemia measured 62 to 66 hours after inoculum and schizont-stage parasitemia measured 40 to 44 hours after inoculum (second cycle invasion). Parasite maturation: ratio between trophozoite-stage parasitemia measured 34 to 38 hours after inoculum and ring-stage parasitemia measured 14 to 18 hours after inoculum (first cycle maturation), or ratio between trophozoite-stage parasitemia measured 82 to 86 hours after inoculum and ring-stage parasitemia measured 62 to 66 hours after inoculum (second cycle maturation). Nonparasitized and parasitized E were counted electronically. To assess total parasitemia and relative contribution of RPE, TPE, and SPE, slides were prepared from cultures at indicated times, stained with Diff-Quik parasite stain, and 400 to 1,000 cells examined microscopically. Significance of differences between normal and deficient parasitized E was assessed by t-test for paired samples. For details on P falciparum cultivation and stage separation of parasitized E, see Materials and Methods.
P falciparum G6PD mRNA in normal and deficient RPE and TPE.
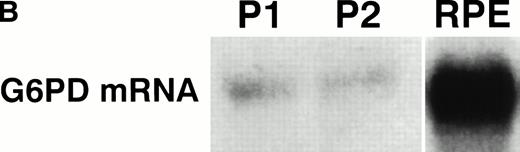
Total RNA was harvested from cultures of several strains (FCR-3, KI, C10, HB3B, T9/96) of P falciparum grown in either normal or deficient E, and analyzed by Northern blot using K202 as a P falciparum G6PD probe. As shown in Fig1A, G6PD gene was found to be constitutively expressed in normal and deficient RPE and TPE. Total RNA was extracted from peripheral blood of two G6PD-normal patients infected with P falciparummalaria and probed with the P falciparum G6PD K202 probe. A single mRNA was revealed, similar in size to the one detected in RPE (Fig 1B). To compare the amount of P falciparum G6PD mRNA in parasites grown in normal and deficient E, the densitometry values obtained for G6PD mRNA were normalized with those of calmodulin mRNA. The ratio of normal to deficient G6PD mRNA was then calculated for ring and trophozoite stages for all strains studied, and schizont stage for FCR-3. At all maturation stages studied, the amount of P falciparum G6PD mRNA was similar in normal and deficient parasitized E (Table 2). The size of trophozoite G6PD mRNA corresponded to the size of G6PD mRNA of 5.1 kb previously reported.20 However, the size of G6PD mRNA in normal and deficient RPE was approximately 200 bp longer than that found in normal or deficient TPE (Fig 1).
(A) Expression of the P falciparum G6PD gene in parasites grown in normal and deficient E. Total P falciparumRNA (strain HB3B) was analyzed by Northern blot using K2O2 as a probe (see Materials and Methods). Approximately 4 μg of total RNA extracted from parasites grown in normal (N) and deficient (D) E at ring (RPE) and trophozoite (TPE) stage of the first growth cycle. Illustrative lanes are labeled at the top with the respective G6PD status. (B) Expression of the P falciparum G6PD gene in peripheral blood of patients infected with P falciparummalaria. Total RNA was extracted from the peripheral blood of two P falciparum–infected G6PD normal patients and analyzed as indicated above. Patient 1: Total RNA extracted from 2 mL of peripheral blood from a patient with a parasitemia of 0.25% RPE, corresponding to approximately 1.8 × 107 parasitized E. Patient 2: Total RNA extracted from 3 mL of peripheral blood from a patient with a parasitemia of 0.6% RPE, corresponding to approximately 8 × 107 parasitized E. RPE: 4.5 μg of total RNA extracted from in vitro culture (ring stage) of strain C1O.
(A) Expression of the P falciparum G6PD gene in parasites grown in normal and deficient E. Total P falciparumRNA (strain HB3B) was analyzed by Northern blot using K2O2 as a probe (see Materials and Methods). Approximately 4 μg of total RNA extracted from parasites grown in normal (N) and deficient (D) E at ring (RPE) and trophozoite (TPE) stage of the first growth cycle. Illustrative lanes are labeled at the top with the respective G6PD status. (B) Expression of the P falciparum G6PD gene in peripheral blood of patients infected with P falciparummalaria. Total RNA was extracted from the peripheral blood of two P falciparum–infected G6PD normal patients and analyzed as indicated above. Patient 1: Total RNA extracted from 2 mL of peripheral blood from a patient with a parasitemia of 0.25% RPE, corresponding to approximately 1.8 × 107 parasitized E. Patient 2: Total RNA extracted from 3 mL of peripheral blood from a patient with a parasitemia of 0.6% RPE, corresponding to approximately 8 × 107 parasitized E. RPE: 4.5 μg of total RNA extracted from in vitro culture (ring stage) of strain C1O.
Comparison of P falciparum G6PD mRNA Expression in Different Parasite Strains Grown in Normal and Deficient E
| Strain . | Maturation Stage . | N/D . |
|---|---|---|
| FCR-3 | Ring | 1.119 |
| Trophozoite | 0.869 | |
| Schizont | 1.050 | |
| KI | Ring | 0.955 |
| Trophozoite | 1.141 | |
| C10 | Ring | 1.163 |
| Trophozoite | 1.154 | |
| HB3B | Ring | 1.082 |
| Trophozoite | 0.972 | |
| T9/96 | Trophozoite | 0.976 |
| Strain . | Maturation Stage . | N/D . |
|---|---|---|
| FCR-3 | Ring | 1.119 |
| Trophozoite | 0.869 | |
| Schizont | 1.050 | |
| KI | Ring | 0.955 |
| Trophozoite | 1.141 | |
| C10 | Ring | 1.163 |
| Trophozoite | 1.154 | |
| HB3B | Ring | 1.082 |
| Trophozoite | 0.972 | |
| T9/96 | Trophozoite | 0.976 |
Northern blots were probed with K2O2, a 1.6-kb fragment ofP falciparum G6PD gene and with a 2.2-kb fragment ofP falciparum calmodulin gene. The hybridization signals obtained with both probes were quantified by densitometry. The K2O2 signals were normalized using calmodulin signals and the ratios: Normal (N)/Deficient (D) calculated. N, P falciparum (grown in normal E) normalized K2O2 signal; D, P falciparum (grown in deficient E) normalized K2O2 signal.
Production of 14C-CO2 from D-[1-14C] glucose in normal and deficient RPE.
Production of 14C-CO2 from D-[1-14C] glucose, which closely reflects intracellular activity of G6PD, is remarkably stimulated by MB and other endogenous or exogenous redox compounds.27 Because P falciparum produces oxidant metabolic products,28 host14C-CO2 production is likely to be activated in parasitized E. Thus, we preferred to compare net maximal parasite-dependent 14C-CO2 production (briefly, net maximal parasite production) obtained after MB stimulation. Net maximal parasite production (Table 3, column D-B) was calculated by subtracting14C-CO2 production of MB-stimulated (normal or deficient) nonparasitized E from 14C-CO2production of MB-stimulated (normal or deficient) parasitized E. Net maximal parasite production was very similar in normal and deficient RPE. Thus, ring forms appear to possess the same maximal G6PD activity irrespective of the G6PD activity of their host cell. Basal14C-CO2 production in deficient RPE was half that in normal RPE (Table 3, column C), indicating lower protection against oxidative stress compared with normal RPE.14C-CO2 production in Sendai virus-treated normal and deficient RPE was considerably reduced in both (−80% and −85%, respectively). However, residual activity was very similar in normal and deficient RPE (not shown).
Production of 14C-CO2 From D-[1-14C] Glucose in Normal and Deficient RPE
| . | NPE . | NPE + MB . | RPE . | RPE + MB . | . |
|---|---|---|---|---|---|
| Column | A | B | C | D | D-B |
| Normal E | 0.15 ± 0.06 | 3.92 ± 0.56 | 0.68 ± 0.21 | 5.27 ± 0.98 | 1.35 |
| n | 16 | 18 | 3 | 5 | |
| P→ | .001 | .001 | |||
| P→→ | .001 | .001 | |||
| P↓ | .001 | .001 | .166 | .028 | |
| Deficient E | 0.05 ± 0.002 | 0.07 ± 0.04 | 0.33 ± 0.29 | 1.39 ± 0.37 | 1.32 |
| No. | 11 | 11 | 3 | 5 | |
| P→ | .001 | .006 | |||
| P→→ | .001 | .001 |
| . | NPE . | NPE + MB . | RPE . | RPE + MB . | . |
|---|---|---|---|---|---|
| Column | A | B | C | D | D-B |
| Normal E | 0.15 ± 0.06 | 3.92 ± 0.56 | 0.68 ± 0.21 | 5.27 ± 0.98 | 1.35 |
| n | 16 | 18 | 3 | 5 | |
| P→ | .001 | .001 | |||
| P→→ | .001 | .001 | |||
| P↓ | .001 | .001 | .166 | .028 | |
| Deficient E | 0.05 ± 0.002 | 0.07 ± 0.04 | 0.33 ± 0.29 | 1.39 ± 0.37 | 1.32 |
| No. | 11 | 11 | 3 | 5 | |
| P→ | .001 | .006 | |||
| P→→ | .001 | .001 |
Production of 14C-CO2 is expressed as μmol/1010 cells/hour at 37°C. Mean values ± SD of 3 of 18 separate experiments (n) as indicated. RPE were separated from synchronized P falciparum cultures as detailed in Materials and Methods 14 to 18 hours after inoculum. 14C-CO2production values obtained with cultures containing 30% to 45% RPE were extrapolated to 100% RPE parasitemia as indicated in Materials and Methods. Nonparasitized E (NPE) were kept in culture and processed in the same way as parasitized E. MB, 135 μmol/L final, was used to maximally stimulate 14C-CO2 production. Column D-B: net maximal 14C-CO2 production by RPE after MB stimulation (see Results for details). P→ = significance to the first column to the right;P→→ = significance to the second column to the right; P↓ = significance within the same column. Significance of differences was assessed by t-test for paired samples.
Reduced GSH in normal and deficient RPE.
As shown in Table 4, culture conditions per se led to a decrease in GSH levels. After 14 to 18 hours in culture, GSH decrease was more pronounced in nonparasitized deficient E (−33%) compared with nonparasitized normal E (−25%). The presence of ring-stage parasites caused a further decrease in GSH level. In absolute terms, the level of GSH in deficient RPE was very low (0.21 ± 0.25 μmol/1010 cells, n = 6), corresponding to 21% of time-matched nonparasitized deficient E.
Levels of Reduced GSH in Normal and Deficient RPE
| . | NPE . | RPE . |
|---|---|---|
| Normal E | (A) 2.15 ± 0.56, n = 4 | |
| (B) 1.88 ± 0.27, n = 6 | 1.01 ± 0.65, n = 4 | |
| Deficient E | (A) 1.43 ± 0.18, n = 4 | |
| (B) 1.02 ± 0.32, n = 6 | 0.21 ± 0.25, n = 6 |
| . | NPE . | RPE . |
|---|---|---|
| Normal E | (A) 2.15 ± 0.56, n = 4 | |
| (B) 1.88 ± 0.27, n = 6 | 1.01 ± 0.65, n = 4 | |
| Deficient E | (A) 1.43 ± 0.18, n = 4 | |
| (B) 1.02 ± 0.32, n = 6 | 0.21 ± 0.25, n = 6 |
Levels of reduced GSH are expressed as μmol/1010cells. Mean values ± SD of 4 to 6 separate experiments as indicated. NPE: (A) kept in culture and subjected to the same treatment as parasitized E; (B) not kept in culture. RPE: parasitized E were fractionated from asynchronous cultures by the Percoll-mannitol method. GSH level values obtained with cultures containing 30% to 45% RPE were extrapolated to 100% RPE parasitemia as indicated in Materials and Methods. Significance of differences: Normal E: (A) versus RPE,P = .021; (B) versus RPE, P = .013. Deficient E: (A) versus RPE, P = .001; (B) versus RPE, P = .001. Significance of differences was assessed by t-test for paired samples.
Production of 14C-CO2 from D-[1-14C] glucose in normal and deficient TPE and SPE.
As shown in Table 5, basal production of14C-CO2 was remarkably increased in TPE compared with RPE. By contrast, basal production of14C-CO2 was decreased in deficient SPE (−44%) and in normal SPE (−31%) compared with TPE. Differences in basal or MB-stimulated 14C-CO2production between normal and deficient mature forms (TPE, SPE) were not significant. Virus-treated normal and deficient TPE retained 84% and 89% of 14C-CO2 production, respectively. Net maximal parasite 14C-CO2 production (see Table 3, column D-B) was not calculated in TPE because of the good retention after virus treatment. As observed in RPE, addition of MB to virus-treated TPE increased 14C-CO2 production by 38% in normal TPE and by 54% in deficient TPE.
Production of 14C-CO2 From D-[1-14C] Glucose in Normal and Deficient TPE and SPE
| . | NPE . | NPE + MB . | TPE . | TPE + MB . | SPE . |
|---|---|---|---|---|---|
| Intact PE/SPE | |||||
| Normal E | 0.15 ± 0.06 | 3.92 ± 0.56 | 4.33 ± 0.52 | 5.98 ± 1.60 | 2.44 |
| n | 16 | 18 | 6 | 6 | 2 |
| P→ | .001 | .129 | .037 | ||
| P→→ | .001 | .014 | |||
| P↓ | .001 | .001 | .91 | .45 | |
| Deficient E | 0.05 ± 0.002 | 0.07 ± 0.04 | 3.95 ± 0.87 | 6.85 ± 2.19 | 2.74 |
| n | 11 | 11 | 6 | 6 | 2 |
| P→ | .113 | .001 | .013 | ||
| P→→ | .001 | .001 | |||
| NPE | NPE + MB | TPE | TPE + MB | ||
| Sendai virus- treated TPE | |||||
| Normal E | nil | nil | 3.51 ± 0.42 | 4.85 ± 1.29 | |
| n | 8 | 8 | 8 | 8 | |
| P→ | .014 | ||||
| P↓ | .97 | .15 | |||
| Deficient E | nil | nil | 3.50 ± 0.77 | 6.10 ± 1.95 | |
| n | 8 | 8 | 8 | 8 | |
| P→ | .003 |
| . | NPE . | NPE + MB . | TPE . | TPE + MB . | SPE . |
|---|---|---|---|---|---|
| Intact PE/SPE | |||||
| Normal E | 0.15 ± 0.06 | 3.92 ± 0.56 | 4.33 ± 0.52 | 5.98 ± 1.60 | 2.44 |
| n | 16 | 18 | 6 | 6 | 2 |
| P→ | .001 | .129 | .037 | ||
| P→→ | .001 | .014 | |||
| P↓ | .001 | .001 | .91 | .45 | |
| Deficient E | 0.05 ± 0.002 | 0.07 ± 0.04 | 3.95 ± 0.87 | 6.85 ± 2.19 | 2.74 |
| n | 11 | 11 | 6 | 6 | 2 |
| P→ | .113 | .001 | .013 | ||
| P→→ | .001 | .001 | |||
| NPE | NPE + MB | TPE | TPE + MB | ||
| Sendai virus- treated TPE | |||||
| Normal E | nil | nil | 3.51 ± 0.42 | 4.85 ± 1.29 | |
| n | 8 | 8 | 8 | 8 | |
| P→ | .014 | ||||
| P↓ | .97 | .15 | |||
| Deficient E | nil | nil | 3.50 ± 0.77 | 6.10 ± 1.95 | |
| n | 8 | 8 | 8 | 8 | |
| P→ | .003 |
Production of 14C-CO2 is expressed as μmol/1010 cells/hour at 37°C. Mean values ± SD of 2 of 18 separate experiments (n) as indicated. TPE and SPE were separated from synchronized P falciparum cultures as detailed in Materials and Methods 34 to 38 and 40 to 44 hours after inoculum, respectively. Parasitemia was 95% to 100% TPE or SPE. NPE were kept in culture and processed in the same way as parasitized E. MB, 135 μmol/L final, was used to maximally stimulate14C-CO2 production. P = significance to the first column to the right; P→→ = significance to the second column to the right; P↓ = significance within the same column. Significance of differences was assessed by t-test for paired samples.
Phagocytosis of normal and deficient RPE and TPE by adherent human monocytes.
As shown in Fig 2, deficient RPE were phagocytosed 2.3 times more intensely than normal RPE (9.25 ± 1.25 deficient RPE per monocyte, n = 5 v 4 ± 0.75 normal RPE per monocyte, n = 5, P = .001). The number of phagocytosed RPE per monocyte was close to the maximal erythrophagocytic capacity of cultured monocytes according to the method used here.26 The transition of ring-forms to trophozoite-forms was accompanied by irrelevant increase in phagocytosis in the case of parasitized deficient E (from 9.25 ± 1.25 RPE per monocyte to 10.5 ± 1.5 TPE per monocyte, n = 5, P = .190). By contrast, normal TPE were phagocytosed 2.2 times as intensely compared with normal RPE (8.8 ± 2.5 normal TPE per monocyte v 4 ± 0.75 normal RPE per monocyte, n = 5, P = .003). Normal and deficient TPE were phagocytosed maximally and similarly (8.8 ± 2.5 normal TPE per monocyte, n = 5; 10.5 ± 1.5 deficient TPE per monocyte, n = 5, difference not significant). Phagocytosis of nonparasitized and parasitized E was also tested with monocytes prepared from deficient blood and found to be very similar to phagocytosis by normal monocytes (not shown).
Phagocytosis of normal and deficient nonparasitized (NPE), RPE, and TPE by adherent human monocytes. Parasitized E were fractionated from asynchronous cultures by the Percoll-mannitol method. After opsonization with fresh autologous serum and washings, parasitized E and nonparasitized controls were subjected to phagocytosis. Phagocytosis is expressed as number of ingested cells per monocyte. Mean values ± SD of five separate experiments. Significance of differences between normal and deficient RPE: P = .001; between normal and deficient TPE: P = .229. Significance of differences was assessed by t-test for paired samples.
Phagocytosis of normal and deficient nonparasitized (NPE), RPE, and TPE by adherent human monocytes. Parasitized E were fractionated from asynchronous cultures by the Percoll-mannitol method. After opsonization with fresh autologous serum and washings, parasitized E and nonparasitized controls were subjected to phagocytosis. Phagocytosis is expressed as number of ingested cells per monocyte. Mean values ± SD of five separate experiments. Significance of differences between normal and deficient RPE: P = .001; between normal and deficient TPE: P = .229. Significance of differences was assessed by t-test for paired samples.
Binding of autologous IgG and complement C3 fragments to normal and deficient RPE and TPE.
As shown previously,25 phagocytosis of parasitized E is dependent on opsonization with fresh autologous serum and is mediated by binding of autologous IgG and complement factors. As shown in Fig 3, binding of autologous IgG (expressed as number of [125I] Protein A molecules bound per cell) and complement C3 fragments (expressed as cpm of [125I] anti-C3c antibodies bound per 1,000 cells) was significantly higher in deficient RPE compared with normal RPE (P = .001 and P= .006, respectively). As for phagocytosis, deposition of autologous IgG was not significantly different in normal and deficient TPE.
Binding of autologous IgG (A) and complement C3 fragments (B) in normal and deficient NPE, RPE, and TPE. Normal and deficient parasitized E were fractionated from asynchronous cultures by the Percoll-mannitol method. After opsonization with fresh autologous serum and washings, parasitized E and nonparasitized controls were incubated with [125I] Protein A, or with [125I] anti-C3c antibodies and their binding assayed as indicated. Binding of molecules is expressed as number of [125I] Protein A molecules per cell (as measure of autologous IgG binding) or as cpm of [125I] anti-C3c antibodies bound per 1,000 cells (as measure of complement C3 fragments binding). Mean values ± SD of five separate experiments. Data obtained with separated RPE fractions that contained 30% to 45% RPE were extrapolated to 100% RPE parasitemia as detailed in Materials and Methods. Data obtained with separated TPE fractions (>95% TPE) were not extrapolated to 100% TPE parasitemia. Binding of autologous IgG binding: difference of normal RPE versus deficient RPE: P = .001; normal TPE versus deficient TPE:P = .39. Binding of complement C3 fragments: difference of normal RPE versus deficient RPE: P = .006; normal TPE versus deficient TPE: P = .004. Significance of differences was assessed by t-test for paired samples.
Binding of autologous IgG (A) and complement C3 fragments (B) in normal and deficient NPE, RPE, and TPE. Normal and deficient parasitized E were fractionated from asynchronous cultures by the Percoll-mannitol method. After opsonization with fresh autologous serum and washings, parasitized E and nonparasitized controls were incubated with [125I] Protein A, or with [125I] anti-C3c antibodies and their binding assayed as indicated. Binding of molecules is expressed as number of [125I] Protein A molecules per cell (as measure of autologous IgG binding) or as cpm of [125I] anti-C3c antibodies bound per 1,000 cells (as measure of complement C3 fragments binding). Mean values ± SD of five separate experiments. Data obtained with separated RPE fractions that contained 30% to 45% RPE were extrapolated to 100% RPE parasitemia as detailed in Materials and Methods. Data obtained with separated TPE fractions (>95% TPE) were not extrapolated to 100% TPE parasitemia. Binding of autologous IgG binding: difference of normal RPE versus deficient RPE: P = .001; normal TPE versus deficient TPE:P = .39. Binding of complement C3 fragments: difference of normal RPE versus deficient RPE: P = .006; normal TPE versus deficient TPE: P = .004. Significance of differences was assessed by t-test for paired samples.
DISCUSSION
Epidemiological studies (reviewed by Luzzatto1 and Greene2) clearly show that G6PD deficiency confers protection against P falciparum malaria. Clinical field work performed in Africa4,5 has demonstrated protection in heterozygotes for G6PD deficiency, but other studies have shown protection in heterozygotes and in homo/hemizygous subjects.6-8
Growth of P falciparum in deficient E.
Culture studies seemed to provide a rationale for protection based on impaired growth in E with the African A-12,13 and the Mediterranean G6PD variant14 during the first cycle of growth. However, data presented here show that growth of P falciparum in synchronized cultures was not significantly impaired in fresh deficient E with the Mediterranean variant during the first and second cycle of growth. Differences in terms of invasion and maturation were never significant over a wide range of parasitemias of the inoculum-parasitized culture. Methodologic differences may explain discrepancies in the culture results. For instance, we found that blood samples stored for longer than 24 hours were frequently unable to sustain optimal parasite growth possibly in relation to E membrane damage (Turrini F., unpublished results, 1997).
Expression and activity of parasite G6PD in parasitized deficient E.
It has been shown that P falciparum after some cycles in G6PD-deficient E seems to adapt to this type of host cells.13 It has been hypothesized therefore that inhibition and adaptation might be related to induction of the parasite's own G6PD.13,20 As to the level of P falciparum G6PD mRNA, in all five strains studied (FCR-3, KI, C10, HB3B, and T9/96), no difference was found between normal and deficient parasitized E within each maturation stage during the first cycle of growth (Table 2). This finding was in agreement with data showing that the P falciparum G6PD activity could be detected in normal and deficient parasitized E at all stages of the intraerythrocytic cycle.29 30
To ascertain activity of parasite G6PD in normal and deficient E, we compared basal and MB-stimulated 14C-CO2production in stage-separated normal and deficient parasitized E and used Sendai virus permeabilized parasitized E. In late forms (TPE and SPE), both approaches indicated that basal and MB-stimulated14C-CO2 production was very similar in normal and deficient TPE and SPE and was largely accounted for by parasite G6PD, whereby host G6PD contributed to approximately 1% of total14C-CO2 production. In RPE, assessment of parasite G6PD activity by the Sendai virus technique was not possible, because Sendai virus treatment abrogated 80% to 85% of14C-CO2 production. Thus, parasite G6PD activity in RPE was inferred by comparing net maximal14C-CO2 production in normal and deficient RPE. Using this indirect approach, net maximal14C-CO2 production was very similar in normal and deficient RPE (Table 3, column D-B). Taken together, these data suggest that the expression of the parasite G6PD gene is comparable in normal and deficient E at all maturation stages examined and the same amount of 14C-CO2 is produced.
Preferential phagocytosis of deficient RPE: An alternative mechanism of protection in G6PD deficiency.
The explanation we are offering for protection against malaria by G6PD deficiency is based on preferential phagocytosis of deficient RPE. This appears to be the ultimate consequence of very low parasite G6PD activity in RPE, accompanied by inherently low G6PD activity and very low GSH levels in the deficient host E. This combination makes deficient RPE peculiarly prone to oxidative membrane modifications leading to opsonization and phagocytic removal. Specifically, we suggest that as a consequence of vulnerability to oxidants, deficient RPE bind higher amounts of removal markers and are therefore phagocytosed more effectively than normal RPE. Previous work has determined that deposition of autologous IgG with specificity to aggregated or clustered band 3, and deposition of complement C3 fragments are essential for phagocytosis.25 It has also been shown that oxidative clustering of band 3 is originated by oxidation of specific cysteine residues located on the cytoplasmic domain,31 whereby the process is more likely to occur in deficient E, because of their inability to regenerate GSH and thiols.
We have shown previously that phagocytosed ring-forms are digested rapidly by cultured monocytes, and the process repeated without loss of efficiency.32 By contrast, more mature forms of the parasite, although actively phagocytosed, hinder repetition of the process because the malarial pigment hemozoin, abundantly present in mature parasite forms, is indigestible and severely affects functions of the monocyte such as oxidative burst and ability to perform multiple cycles of phagocytosis.32 Increased phagocytosis of ring-forms is certainly advantageous to the host, because it will keep parasitemia down (see Luzzatto et al4 and Bienzle et al5), and because less parasitized E mature to trophozoite or schizont, which adhere to cerebral and renal postcapillary venules.33 Adhesion to cerebral endothelia may cause cerebral malaria, a major cause of malaria mortality.34Also, if deficient RPE in the double E population in heterozygotes are preferentially removed, we may expect to find less parasitized deficient E in circulation, in agreement with the findings of Luzzatto et al.4 Other methods of host defense may depend on direct parasite killing by cytokines, oxygen, or nitrogen radicals, produced by adherent or adjacent host cells, such as leuko/monocytes, endothelial cells, and platelets.35 Due to oxidant vulnerability of deficient RPE, enhanced intracellular killing of early parasite forms may cooperate with increased phagocytosis to elicit better resistance.
Preferential phagocytosis of RPE: Common mechanism of protection in widespread protective E mutations.
The three major E mutations conferring protection against malaria36 are characterized by increased generation of noxious oxygen radicals (thalassemias, sickle cell anemia37,38) or by decreased ability to cope with oxidant damage (G6PD deficiency). Because the presence of developing parasite with its remarkable metabolic demands in a limited time period imposes an additional oxidative stress upon the host cell leading to its enhanced demise,25,28 it is conceivable that similar oxidant-mediated membrane modifications leading to enhanced phagocytosis occur in RPE in all three mutations in question. Data obtained by some of us (Ayi K., Turrini F., Mannu F., Arese P., unpublished results, 1998) indicate that heterozygous beta-thalassemic E behave quite similarly as deficient E, showing the same parasite invasion and maturation as nonthalassemic controls, enhanced deposition of removal markers, and enhanced phagocytosis at ring-stage. Other studies have also indicated increased opsonization39 and enhanced susceptibility to phagocytosis in parasitized E with thalassemic trait.40 In sickle cell anemia heterozygotes, a suicidal infection, ie, preferential phagocytosis of parasitized E with sickle cell trait has been previously described by one of the authors.41
The present model does not exclude and is compatible with other defense mechanisms possibly operating in parallel in vivo: preferential damage of parasites harbored in G6PD-deficient parasites by cytokines, toxic oxygen, or nitrogen metabolites secreted by activated phagocytes or endothelia; preferential pitting of the parasite from G6PD-deficient cells in the spleen42 reduced rosetting.43 The recently proposed model of protection in alpha+-thalassemia,44 based on increased susceptibility to P vivax and subsequent induction of cross-protection against P falciparum malaria, also appears to be compatible with this model, as increased splenomegaly was present in alpha+-thalassemics with P falciparum infection, indicating enhanced phagocytic removal in those subjects. Finally, we cannot exclude, despite present clear-cut results and absence of ex vivo evidence, that parasite growth might be impaired in mutant E under in vivo conditions of fluctuating oxygenation and pH values, mechanical and osmotical stress, or in the presence of increased amounts of fetal hemoglobin.45 Future research will allow investigators to dissect the relative weight of outlined mechanisms of resistance and, possibly, suggest new ones.
In conclusion, we can visualize the mechanism of relative resistance against P falciparum as resulting from the following stepwise sequence: (1) Invasion of G6PD-normal and deficient E by the parasite is equally efficient; apparently the level of G6PD activity is not relevant for this process. (2) During its ring stage of intraerythrocytic development, the parasite has very little G6PD activity compared with the host cell (although production of parasite-specific G6PD mRNA has started). Therefore, in deficient host cells, the total G6PD (from host plus parasite) is very low; this is associated with GSH depletion, which makes the deficient parasitized cell very vulnerable to damage even in the absence of any deliberately imposed oxidative stress. These damaged cells are highly susceptible to phagocytosis. (3) By the time the parasite has developed into a trophozoite (and subsequently into a schizont), its own G6PD activity is high; in a deficient host cell it is, in fact, much higher than that of the host cell itself. At this stage of development the physical properties of the parasitized E are sufficiently different from those of nonparasitized E that they are susceptible to phagocytosis regardless of their G6PD status. (4) As a result of the above, the stage at which parasitized E are likely to undergo phagocytosis is brought forward in the case of deficient E, thus limiting the level of parasitemia. The above model of resistance against malaria is applicable, in principle, to both males who are hemizygous and females who are heterozygous for G6PD deficiency.
Supported by World Health Organization/United Nations Development Programme/World Bank Special Programme for Research and Training in Tropical Diseases (WHO TDR IMMAL); Compagnia di San Paolo, Torino; Italian Ministry of University, Roma; and British Council, Roma. M.C. was a recipient of a research grant from the European Community (Contract No. BIO2-CT93-6917).
M.C. and G.G. contributed equally to this work.
Address reprint requests to Paolo Arese, MD, PhD, Dipartimento di Genetica, Biologia, Biochimica, Via Santena 5 bis, 10126 Torino, Italy; e-mail: arese@molinette.unito.it.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



![Fig. 3. Binding of autologous IgG (A) and complement C3 fragments (B) in normal and deficient NPE, RPE, and TPE. Normal and deficient parasitized E were fractionated from asynchronous cultures by the Percoll-mannitol method. After opsonization with fresh autologous serum and washings, parasitized E and nonparasitized controls were incubated with [125I] Protein A, or with [125I] anti-C3c antibodies and their binding assayed as indicated. Binding of molecules is expressed as number of [125I] Protein A molecules per cell (as measure of autologous IgG binding) or as cpm of [125I] anti-C3c antibodies bound per 1,000 cells (as measure of complement C3 fragments binding). Mean values ± SD of five separate experiments. Data obtained with separated RPE fractions that contained 30% to 45% RPE were extrapolated to 100% RPE parasitemia as detailed in Materials and Methods. Data obtained with separated TPE fractions (>95% TPE) were not extrapolated to 100% TPE parasitemia. Binding of autologous IgG binding: difference of normal RPE versus deficient RPE: P = .001; normal TPE versus deficient TPE:P = .39. Binding of complement C3 fragments: difference of normal RPE versus deficient RPE: P = .006; normal TPE versus deficient TPE: P = .004. Significance of differences was assessed by t-test for paired samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/7/10.1182_blood.v92.7.2527/3/m_blod41907003ax.jpeg?Expires=1765927008&Signature=zwkvtBtGNmxnQUdiYzTmfTYp6kqksiNZyhGSz9TIQhFjQbUdOOi7MGAGMk1n5I6GRrF1f0wNqh7xCbjSIl6IXo0ErEq1YQiGpsQGtPeY2VoNCR-2lIM9Un1XW6M8HU1CkZzX5gy7qHCWv3pyX5AJWi9CZnsG-59RJ~mQT7vf6OtBngwsxKPCKT6OT7Se97kI5aIHAGM6BhToRr5MC7L0sUpbBRPjvLZvJ4O2~sF5OGW~6bxOmAT1yDBabCyf8abMxqbGmwgdJH05yoMNoTu65e17kvgInVdRTPaw7GgtfPNSxzF6gyjcX5QADYyJeTbd0B9At9dJueGNFdwtPJcgRQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Binding of autologous IgG (A) and complement C3 fragments (B) in normal and deficient NPE, RPE, and TPE. Normal and deficient parasitized E were fractionated from asynchronous cultures by the Percoll-mannitol method. After opsonization with fresh autologous serum and washings, parasitized E and nonparasitized controls were incubated with [125I] Protein A, or with [125I] anti-C3c antibodies and their binding assayed as indicated. Binding of molecules is expressed as number of [125I] Protein A molecules per cell (as measure of autologous IgG binding) or as cpm of [125I] anti-C3c antibodies bound per 1,000 cells (as measure of complement C3 fragments binding). Mean values ± SD of five separate experiments. Data obtained with separated RPE fractions that contained 30% to 45% RPE were extrapolated to 100% RPE parasitemia as detailed in Materials and Methods. Data obtained with separated TPE fractions (>95% TPE) were not extrapolated to 100% TPE parasitemia. Binding of autologous IgG binding: difference of normal RPE versus deficient RPE: P = .001; normal TPE versus deficient TPE:P = .39. Binding of complement C3 fragments: difference of normal RPE versus deficient RPE: P = .006; normal TPE versus deficient TPE: P = .004. Significance of differences was assessed by t-test for paired samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/7/10.1182_blood.v92.7.2527/3/m_blod41907003bx.jpeg?Expires=1765927008&Signature=3tB3qM2TWFNYzDYyJwr2c9DH3Nv7tDctLMuCyPw35t3DehfM4vTnjw~XO7kgIjghAQC6hq6r5f8pg7JDESnb6nx~mHB2bhfJpiANh-7NZzyh2q7pHUoz6TBNW3R0dB-Ja6ovbnkkujxSUkwVBoyXVX9ZeG7JCI8GgfBoKyiRbbeW7EbbnCj5D7we6046PzEL4qikzu7hTpt7soKKxEwWm9OENuqEzRUe4AUzS7xCDXpqc7OxoYJ~71-8aoUP5me0cOIVYDq-vGsxbZB-mxAPOfrbT66nqn9G7fue28wZ95jxTWWPWvmDfldtymK-axM-qCeFH~R57wrAdzigvbylgA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal