To the Editor:
Congenital dyserythropoietic anemia type II (CDA-II, CDAN2, or HEMPAS) (MIM224100) is an autosomal recessive trait and it represents the most frequent form of congenital dyserythropoiesis.1 It is characterized by normocytic anemia, variable jaundice, and hepatosplenomegaly. Gallbladder disease and secondary hemochromatosis are frequent complications. Bone marrow histology shows binucleated and multinucleated (10% to 40%) erythroblasts with karyorrehexis. Electron microscopy of these cells shows the presence of the so-called double membrane, ie, peripheral cisternae running parallel to and beneath the plasma membrane.2 The diagnosis could be confirmed by Positive Acidified Serum test (Ham test) and by presence of enhanced agglutination with anti-i antibodies. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of red blood cell membrane proteins shows a narrower aspect and a faster migration of band 3 (anion exchange transporter), and Western blot of membrane proteins shows the presence of reticulum-endotelial proteins (calreticulin, glucose regulated protein 78, protein disulphide isomerase).3
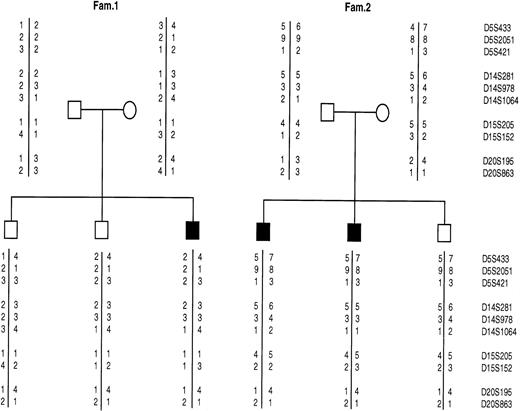
Recently we have recruited a panel of well-characterized CDA II families and used them to search for CDA II gene by linkage analysis. We first excluded three candidate genes (α-Mannosidase II, α-Mannosidase x, and N-Acetylglucosaminyltransferase II) (MANA, MANAx, Gnt-II),4 and obtained conclusive evidence for linkage of CDA II to microsatellite markers on the long arm of chromosome 20 (20q11.2).5 Strong evidence of allelic association with the disease was also detected with marker D20S863. Here we describe two unrelated families in which CDA-II disease was not linked with the CDAN2 locus, demonstrating for the first time the presence of genetic heterogeneity.
The first family comes from a little town on the Ionian sea (southern Italy). The parents could be related because three of the grandparents had the same family name. The propositus was born in 1982; at 3 days of age a severe icterus demanded an exchange transfusion. A severe thrombocytopenia was observed. During the next years anemia (range, 61 to 92 g/L) seldom required transfusions and the platelet count was always low (15 to 50 × 109/L). Bone marrow observation and electronic microscopy showed the characteristic feature of CDA-II associated with severe reduction of megakaryocytes, which did not show double membranes. Analysis of SDS-PAGE of red blood cell membrane protein and the Western blot showed the characteristic feature of CDA-II.3
The second family comes from Lecce province (southern Italy) and it consisted of two affected and one unaffected sibs (Fig1). The mother and the sons (II-2 and II-3) had β-thalassemic trait. The probands' (II-1 and II-2) anemia was documented since the newborn period when jaundice required exchange-transfusion. During infancy and childhood the patients received an unknown number of red blood cell transfusions, until splenectomy. Physical examination showed moderate hepatomegaly. After splenectomy the affected brothers had a very mild anemia requiring one transfusion only during an episode of infection. Bone marrow observation showed characteristic pattern of CDA-II as well as SDS-PAGE and Western blotting.
Allele segregation for microsatellite markers in two CDA-II families (1 and 2): the solid squares indicate the affected individuals. The microsatellite markers are tightly linked to the following genes: MANA, mapping on chromosome 5 (5q2.1-2.2); MANAx isozyme, mapping on chromosome 15q25; Gnt-II mapping on chromosome 14q21 and CDAN2 mapping on chromosome 20q11.2.
Allele segregation for microsatellite markers in two CDA-II families (1 and 2): the solid squares indicate the affected individuals. The microsatellite markers are tightly linked to the following genes: MANA, mapping on chromosome 5 (5q2.1-2.2); MANAx isozyme, mapping on chromosome 15q25; Gnt-II mapping on chromosome 14q21 and CDAN2 mapping on chromosome 20q11.2.
Linkage analysis by means of microsatellite markers localized at long arm of chromosome 20 performed in these families showed that the CDA-II locus was not linked to chromosome 20 (Fig 1). As a matter of fact, there is no segregation of alleles of each marker with the disease.
Furthermore, in literature a reduced activity of Gnt-II has been shown in two cases and of MANA in another CDA-II case.6,7Since it was suggested that these alterations could be directly involved in CDA-II, we performed a linkage analysis with these genes. For this purpose, highly informative markers located at the same chromosomal region where MANA-II and Gnt-II genes mapped were used as previously described.4 Negative results obtained indicated that none of the investigate regions contains the gene involved in determining CDA-II in these families (Fig1).
The CDA-II anemia is often mild to moderate but may be severe and, rarely, causes fetal distress. Approximately 300 cases are present in the literature, but the prevalence of this disorder is almost certainly higher because it is likely that many asymptomatic cases with little or no anemia are underdiagnosed.1 The clinical heterogeneity could be caused by genetic heterogeneity or by the association with another red blood cell disorder (ie, β-talassemia). The presented clinical cases are severe forms. In one case β-thalassemia could account for the increased clinical feature; in the other case no other red blood cell abnormality was demonstrated, but the coinheritance of thrombocytopenia in a consanguineous tree suggested the presence of two hematopoietic defects. CDA-II diagnosis was confirmed by bone marrow observation, electron microscopy, and laboratory data. From the biochemical point of view the disease appears very homogeneous. All the subjects showed the characteristic pattern of SDS-PAGE and the presence of reticulum endotelial proteins exposed on the surface. These two new families demonstrated for the first time that in these severe cases, although in the presence of an identical biochemical pattern, the disease is caused by a different gene and, thus, that genetic heterogeneity is present. Moreover, the biochemical defect most likely could present into a different step of the same pathway. A complementation assay could confirm this data.
ACKNOWLEDGMENT
We thank the families who collaborated in this work. This work is supported in part by Ministero Italiano della Sanita' (to P.G.) and by Telethon (to A.I.) project E-645.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal