To the Editor:
Interactions between circulating blood cells and the vascular endothelium are tightly regulated to maintain the integrity of a functional circulatory system. Endothelial cells, which are bipolar, provide the vascular system with a nonthrombogenic surface on the lumenal side and perform a number of specialized metabolic and transport functions while in contact with the subendothelial matrix on the basal side.1 Injury to the vascular endothelium can expose the thrombogenic subendothelium and upset the delicate balance between blood flow and hemostasis. In sickle cell (SS) disease, injury to the vascular endothelium has been shown to result from increased adherence of SS red blood cells (RBCs) to endothelial cells and by vaso-occlusion of abnormally shaped SS RBCs.2,3Vaso-occlusion by SS RBCs can produce prolonged hypoxia to local areas of the microvasculature resulting in endothelial cell damage.4 In addition, vaso-occlusion can contribute to the adherence of activated polymorphonuclear neutrophils, which can further damage endothelial cells by release of reactive oxygen metabolites.5
One endothelial-cell–derived component extremely sensitive to cell injury is the vasoconstrictor peptide, endothelin-1 (ET-1), which has been found to be increased in the plasma of patients with diabetes,6 uremia,7 myocardial infarction,8 cardiogenic shock,9 and in patients after hemodialysis.10 Damage to endothelial cells in SS disease may be another system in which plasma ET-1 is increased. If this were the case, the local vasoconstriction produced by ET-1 could decrease the diameter of some blood vessels and cause slower microvasculature transit times hypothesized to be necessary for cell sickling in vivo.11 If ET-1 were elevated in SS disease, it would not only be a marker for endothelial cell damage but could also be a factor in exacerbating a vaso-occlusive event.
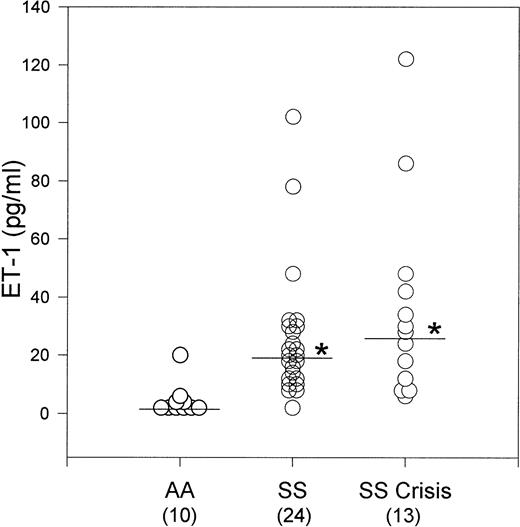
We measured the plasma levels of immunoreactive ET-1 in patients with SS disease in both steady state and crisis, and in normal age- and race-matched controls (AA) using an enzyme-linked immunosorbent assay (ELISA) method (Amersham Pharmacia Biotech, Arlington Heights, IL). Thirty-seven homozygous SS patients, (13 in crisis and 24 in steady state) from the Bronx Comprehensive Sickle Cell Center and 10 hematologically normal (AA) controls participated in the study. Individuals with hypertension or renal disease were excluded from the study. Plasma from 1 mL of heparinized blood was acidified with 0.25 mL 1 N HCl and loaded onto Sep-Pak C18 columns (Waters Associates, Milford, MA). ET-1 was eluted from the columns with 2 mL 80% methanol, 0.1% trifluoroacetic acid; the eluant lyophilized; and the pellet reconstituted in 0.25 mL assay buffer. The median ET-1 plasma level for SS patients in steady state was 18.79 pg/mL (n = 24), which was significantly higher than the median ET-1 plasma level of 0.58 pg/mL (n = 10) for AA control subjects (P < .0001). Because the distribution of data points was not symmetrical, we used a nonparametric analysis, the Kruskal-Wallis One-Way Analysis of Variance on Ranks. There was no significant difference in median plasma ET-1 levels in SS patients in steady state (18.79 pg/mL) and those in crisis (26.16 pg/mL, n = 13) (Fig 1). Asterisks indicate that the median plasma ET-1 levels for SS steady state and crisis were significantly different than the AA control median plasma ET-1 level at P < .0001.
Plasma ET-1 levels in SS patients in steady state and crisis (asterisks) were significantly increased compared to control AA subjects. There was no significant difference in plasma ET-1 levels between SS patients in steady state and crisis.
Plasma ET-1 levels in SS patients in steady state and crisis (asterisks) were significantly increased compared to control AA subjects. There was no significant difference in plasma ET-1 levels between SS patients in steady state and crisis.
Our data indicate that ET-1 is elevated in SS disease; however, the mechanism by which this occurs has not been determined. Direct evidence for damage to the endothelium in SS disease comes from studies by Sowemino-Coker et al,12 who measured circulating endothelial cells in plasma from SS patients and found increased circulating endothelial cells during crisis. An alternative mechanism to account for increased plasma levels of ET-1 in SS disease is the upregulation of specific endothelial cell genes by circulating plasma factors and/or hypoxic conditions.13,14 In a recent report, Phelan et al15 found that SS RBCs that have previously undergone sickling induce a fourfold to eightfold increase in the transcription of the ET-1 gene as well as a fourfold to sixfold increase in ET-1 peptide production. The induction by sickled SS RBCs is specific for ET-1 and unsickled SS RBCs had no effect. Now that animal models for SS disease have been developed,16 17it should be possible to determine which mechanism is responsible for increased plasma ET-1. Specific inhibitors of endothelin converting enzyme (ECE) that prevent the conversion of proET-1 to ET-1 are available18; increased synthesis of ET-1 would be blocked by these ECE inhibitors.
The physiological significance of increased ET-1 in SS disease is also an open question. So many factors contribute to the pathophysiology of SS disease (eg, tissue hypoxia, vaso-occlusion, increased blood flow) that it is difficult to assess the contribution of one specific factor. A mouse model for SS disease where one of the autocrine functions of ET-1, such as endothelial cell proliferation, can be assessed will be an invaluable system in which to study the role of ET-1 in SS disease.
ACKNOWLEDGMENT
This research was supported by National Institutes of Health Sickle Cell Center Grant No. HL-38655. We thank Sylvia Musto for excellent technical assistance, Gwendolyn Swinson, RN, for procurement of patient blood samples, Dr Robert Schwartz for statistical analysis of the data, and Dr Ronald Nagel for helpful discussions. A preliminary report of this work was published in Blood 78:202a, 1991 (abstr, suppl 1).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal