Abstract
Because sphingosine (Sph) is actively incorporated into platelets and rapidly converted to sphingosine 1-phosphate (Sph-1-P), which is then released extracellularly, it is important to study the effects of Sph and Sph-1-P on endothelial cells from the viewpoint of platelet-endothelial cell interaction. In this study, we found that Sph, as well as ceramide, induces apoptosis in human umbilical vein endothelial cells (HUVECs). In contrast, Sph-1-P acts as a HUVEC survival factor; this bioactive lipid was shown to protect HUVECs from apoptosis induced by the withdrawal of growth factors and to stimulate HUVEC DNA synthesis. In metabolic studies, [3H]Sph, incorporated into HUVECs, was converted to [3H]Cer and further to [3H]sphingomyelin in a time-dependent manner, whereas [3H]Sph-1-P formation from [3H]Sph was weak and transient. These findings in HUVECs are very different from those of platelets, which possess a highly active Sph kinase but lack Sph-1-P lyase. As a result, platelets abundantly store Sph-1-P, whereas HUVECs contain much less Sph-1-P. Finally, HUVECs, in contrast to platelets, failed to release Sph-1-P extracellularly, indicating that HUVECs themselves are not able to supply the survival factor Sph-1-P, but receive it from activated platelets. Our results suggest that platelets may maintain the integrity of endothelial cells by incorporating Sph and releasing Sph-1-P.
SIGNAL TRANSDUCTION pathways that use glycerophospholipid metabolites have been very well characterized.1-3 Recently, sphingolipids, another major class of membrane lipids, have also emerged as signal-transducing lipids.4-7 Functionally, a distinguishing characteristic of the sphingolipids is their apparent participation in pro- or anti-proliferative cell regulation pathways. For example, ceramide (Cer)4,5,8 and sphingosine (Sph)7,9,10 are important regulatory participants in programmed cell death (apoptosis), whereas sphingosine 1-phosphate (Sph-1-P) induces mitogenesis and has been implicated as a second messenger in cellular proliferation induced by platelet-derived growth factor and serum.11 It has been reported that the balance between the intracellular levels of Cer and Sph-1-P and their regulatory effects on different family members of mitogen-activated protein kinase may determine cell fate.12
To clarify the involvement of sphingolipids in hemostasis, thrombosis, and vascular biology, we have studied the metabolism and functional effects of Sph derivatives in human platelets. We found that, in platelets, Sph-1-P is rapidly formed from Sph by Sph kinase, abundantly stored intracellularly, and released into the extracellular environment upon stimulation.13,14 Furthermore, exogenously added Sph-1-P induces platelet aggregation,13 suggesting that Sph-1-P acts as an autocrine platelet stimulator.
Because exogenous Sph is actively incorporated into platelets and rapidly converted to Sph-1-P, which is then released extracellularly, it is important to study the effects of Sph and Sph-1-P on endothelial cells from the viewpoint of platelet-endothelial cell interaction. In this study, we investigated the effects of sphingolipids, including Sph and Sph-1-P, on the cell fate of human umbilical vein endothelial cells (HUVECs). Furthermore, we also examined the metabolism of HUVEC sphingolipids in detail.
MATERIALS AND METHODS
Materials.
The following materials were obtained from the indicated suppliers: D-erythro-Sph, 12-O-tetradecanoylphorbol 13-acetate (TPA), lysophosphatidic acid (LPA), sphingomyelin, and sphingomyelinase (fromStaphylococcus aureus) (Sigma, St Louis, MO); Sph-1-P, N,N-dimethylsphingosine (DMS), and C2-Cer (Biomol, Plymouth Meeting, PA); thrombin (Mochida Pharmaceutical, Tokyo, Japan); interleukin-1β (IL-1β; Boehringer Mannheim Biochemica, Mannheim, Germany); tumor necrosis factor-α (TNF-α; Genzyme, Cambridge, MA); angiotensin II (Bachem California, Torrance, CA); [Arg8]-vasopressin (AVP; Seikagaku Corp, Tokyo, Japan); platelet-activating factor (PAF; Avanti Polar Lipids, Alabaster, AL); staurosporine (Kyowa Medex, Tokyo, Japan); and D-erythro-[3-3H]Sph (22.0 Ci/mmol), [3-3H]C6-Cer (22.3 Ci/mmol), and [methyl-3H]thymidine (20.0 Ci/mmol) (Du-Pont NEN, Boston, MA).
Cell preparation.
HUVECs were isolated from human umbilical cords with trypsin treatment, plated onto 0.2% gelatin-coated dishes, and cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 20% fetal calf serum (FCS; ICN Biomedicals, Aurora, OH), 10 ng/L of recombinant human basic fibroblast growth factor (bFGF; Becton Dickinson Labware, Lincoln Park, NJ), penicillin G (100 U/mL), and streptomycin sulfate (100 μg/mL) at 37°C under an atmosphere of 5% CO2 and 95% room air. FCS and bFGF were removed to deprive HUVEC growth factors. Human platelets were prepared as described previously.13
Fluorescence microscopy.
After the reactions indicated, cells (floating cells and adhesive cells) were collected and washed twice with phosphate-buffered saline (PBS). They were then fixed overnight in 1% glutaraldehyde (Nacalai, Kyoto, Japan) and washed in PBS. Cells were stained with bisbenzimide trihydrochloride (1 mmol/L in 30% glycerol/PBS; Hoechst 33258; Calbiochem, San Diego, CA) in darkness for 10 minutes and visualized using fluorescence microscopy. Apoptotic cells were identified by the findings of condensation and fragmentation of chromatin. A total of 20 random microscope fields were examined under each of the experimental conditions. The total number of cells and the number of apoptotic cells were counted in each field.
Analysis of DNA fragmentation by electrophoresis.
After the reactions indicated, cells were harvested, washed, resuspended in TTE buffer (1 mol/L Tris HCl, 0.5 mol/L EDTA, 10% Triton X-100), and treated with 400 μg/mL of RNase A for 1 hour at 37°C. Then, 400 μg/mL of proteinase K was added and the incubation was continued for 2 hours at 37°C. DNA was extracted with phenol/chloroform/isoamyl alcohol, washed in ethanol, resuspended in TE buffer, and separated by electrophoresis in a 2% agarose gel. Then, the DNA was stained with ethidium bromide. The gels were photographed with UV transillumination.
Measurement of DNA synthesis in HUVECs.
HUVECs were cultured in 35-mm dishes until the cells were confluent and quiescent in 10% FCS plus bFGF. Cells were washed and preincubated in 10% FCS for 20 minutes and then treated with Sph-1-P for 24 hours; [3H]thymidine (2 μCi/mL) was present for the last 2 hours of the incubation. This time period was chosen because it gave maximum incorporation of the radiolabel at 10 μmol/L Sph-1-P in preliminary experiments (data not shown). After the incubation, HUVEC morphology was checked; cells firmly adhered to dish and were of normal size and shape. HUVECs were washed twice with Hanks’ balanced salt solution and then with 5% trichloroacetic acid. The acid-insoluble material was then redissolved in 0.1 N sodium hydroxide, and an aliquot was taken to measure the radioactivity levels by liquid scintillation counting.
Metabolism of [3H]Sph and [3H]C6-Cer in HUVECs.
HUVECs were incubated with 1% FCS containing 1 μmol/L (0.2 μCi) [3H]Sph or [3H]C6-Cer. At the indicated time points, the reaction was terminated by the addition of 1 mL of ice-cold methanol, and lipids were extracted from the cell and medium separately by the method of Bligh and Dyer15 and then analyzed for [3H]Sph or [3H]C6-Cer metabolism as described previously.13 Portions of lipids obtained from the lower chloroform phase were applied to silica gel high-performance thin layer chromatography (TLC) plates (Merck, Darmstadt, Germany), and the plates were then developed in butanol/acetic acid/water (3:1:1), followed by autoradiography. Each autoradiogram shown is a typical one from at least three experiments. When indicated, silica gel areas containing radiolabeled sphingolipids were scraped off and counted by liquid scintillation counting. The radioactivity counts were corrected by recovery rates of the sphingolipids into the lower phase. Under our conditions, recovery rates in the lower phase of Sph, Sph-1-P, Cer, and sphingomyelin were 78%, 47%, 73%, and 71%, respectively.
Sphingomyelinase treatment of the extracted lipids.
The lower phase samples of the lipid extract were dried completely and recovered in a solution of 0.1 mol/L Tris-HCl (pH 7.4), 0.01% Triton X-100, and 40 mmol/L MgCl2, with sonication. Sphingomyelinase (5 U/mL) was added to the reaction mixture (200 μL in all). After 1 hour at 37°C, the reaction was terminated by the addition of 800 μL of ice-cold chloroform/methanol/concentrated HCl (100:200:1), and the lipids were extracted and the phases separated by the method of Bligh and Dyer.15 The resultant lower chloroform phase samples were analyzed for sphingomyelin degradation by TLC developed in butanol/acetic acid/water (3:1:1), followed by autoradiography.
Quantitative measurement of Sph-1-P.
Sph-1-P was extracted from platelets and HUVECs and quantitated by N-acylation with [3H]acetic anhydride into [3H]C2-Cer-1-P (N-[3H]acetylated Sph-1-P), as described previously.16 The phospholipid amounts were also measured, as described previously,16 17 to normalize the sample amounts for Sph-1-P quantitation.
RESULTS
Induction of HUVEC apoptosis by sphingolipids.
Of sphingolipids, Cer, formed via the sphingomyelin cycle, has been shown to be a lipid second messenger or biomodulator of stress-related responses, including apoptosis, in a variety of systems.4,5,8,18 In contrast, the effects of Sph on cell fates seem to be cell-type specific. Sph shows strong mitogenic effects in some cells,6,19,20 whereas the role of Sph in apoptosis induction has been reported in others.7,9,10 To examine the possible role(s) of sphingolipids in HUVEC fate, we first examined the effects of various Sph derivatives on HUVEC apoptosis. In HUVECs challenged with Sph, phase-contrast microscopy showed morphological changes of apoptosis; when exposed to 20 μmol/L Sph for 4 hours, HUVECs shrank and retracted from neighboring cells, and floating apoptotic cells appeared in the culture medium (data not shown). When HUVECs were stained with Hoechst 33258 and assessed by fluorescence microscopy, cells with condensed chromatin or fragmented nuclei and blebbing of the plasma membrane were clearly visualized (Fig 1A). The induction of apoptosis by Sph was confirmed by demonstrating DNA fragmentation through agarose gel electrophoresis (Fig 1B). Sph-induced HUVEC apoptosis was a concentration-dependent process (Fig 2). Under identical conditions, DMS, a methylated derivative of Sph,7 induced apoptosis in a stronger manner than Sph (Fig2).
Induction of HUVEC apoptosis by Sph. (A) Morphological features of HUVEC apoptosis induced by Sph. HUVECs were treated without (a and c) or with (b and d) 20 μmol/L Sph for 4 hours in the presence (a and b) or absence (c and d) of growth factors. Apoptotic cells were detected using Hoechst 33258 staining and visualized with fluorescence microscopy. (B) DNA fragmentation in Sph-treated HUVECs. HUVECs were treated without (lanes a and c) or with (lanes b and d) 20 μmol/L Sph for 4 hours in the presence (a and b) and absence (c and d) of growth factors. Fragmented DNA was isolated and electrophoresed on a 2% agarose gel. DNA was then visualized with ethidium bromide staining. Lane m is a 200-bp DNA ladder.
Induction of HUVEC apoptosis by Sph. (A) Morphological features of HUVEC apoptosis induced by Sph. HUVECs were treated without (a and c) or with (b and d) 20 μmol/L Sph for 4 hours in the presence (a and b) or absence (c and d) of growth factors. Apoptotic cells were detected using Hoechst 33258 staining and visualized with fluorescence microscopy. (B) DNA fragmentation in Sph-treated HUVECs. HUVECs were treated without (lanes a and c) or with (lanes b and d) 20 μmol/L Sph for 4 hours in the presence (a and b) and absence (c and d) of growth factors. Fragmented DNA was isolated and electrophoresed on a 2% agarose gel. DNA was then visualized with ethidium bromide staining. Lane m is a 200-bp DNA ladder.
Dose-dependent induction of HUVEC apoptosis by Sph or DMS. HUVECs were treated with various concentrations of Sph (○, •) or DMS (□, ▪) for 4 hours in the presence (○, □) or absence (•, ▪) of growth factors. Apoptotic cells were detected using Hoechst 33258 staining and visualized with fluorescence microscopy. Results were expressed as the percentage of apoptotic cells (apoptotic cells/total cells × 100).
Dose-dependent induction of HUVEC apoptosis by Sph or DMS. HUVECs were treated with various concentrations of Sph (○, •) or DMS (□, ▪) for 4 hours in the presence (○, □) or absence (•, ▪) of growth factors. Apoptotic cells were detected using Hoechst 33258 staining and visualized with fluorescence microscopy. Results were expressed as the percentage of apoptotic cells (apoptotic cells/total cells × 100).
It is well known that growth factor deprivation of HUVEC induces apoptosis with characteristic morphological and biochemical features.21 22 We confirmed this under our conditions (Figs1 and 2). Furthermore, the percentage of apoptotic cells increased when HUVECs were challenged with Sph or DMS in the absence of growth factors, although the effects of these sphingolipids themselves became less clear (Figs 2 and 3).
C2-Cer is a synthetic cell-permeable Cer and has been shown to induce apoptosis in endothelial cells, which was confirmed under our conditions (Fig 3). When the induction of apoptosis was compared among sphingolipids, the order of potency was DMS > Sph = C2-Cer; DMS induced statistically significant apoptosis in the absence of growth factors as well as in their presence, whereas Sph and C2-Cer did not (Fig 3). Under all conditions, Sph-1-P did not induce apoptosis at all (data not shown). Staurosporine is known to induce apoptosis possibly through its protein kinase C inhibition. This inhibitor, used as a control in our experiments, was found to induce marked HUVEC apoptosis (Fig 3).
Induction of HUVEC apoptosis by various sphingolipids and staurosporine. HUVECs were treated without (C) or with 20 μmol/L Sph, DMS, or C2-Cer or 1 μmol/L staurosporine (Ssp) for 4 hours in the presence (left panel) or absence (right panel) of growth factors. Apoptotic cells were detected using Hoechst 33258 staining and visualized with fluorescence microscopy. Results were expressed as the percentage of apoptotic cells (apoptotic cells/total cells × 100). Columns and error bars represent the mean ± SD (n = 3). *Statistically significant (t-test, P < .05) compared with the control cells (without treatment).
Induction of HUVEC apoptosis by various sphingolipids and staurosporine. HUVECs were treated without (C) or with 20 μmol/L Sph, DMS, or C2-Cer or 1 μmol/L staurosporine (Ssp) for 4 hours in the presence (left panel) or absence (right panel) of growth factors. Apoptotic cells were detected using Hoechst 33258 staining and visualized with fluorescence microscopy. Results were expressed as the percentage of apoptotic cells (apoptotic cells/total cells × 100). Columns and error bars represent the mean ± SD (n = 3). *Statistically significant (t-test, P < .05) compared with the control cells (without treatment).
DNA synthesis and suppression of apoptosis by Sph-1-P in HUVECs.
It is established that, in some systems, Sph-1-P, formed from Sph by Sph kinase-catalyzed phosphorylation, is involved in cell survival.6,11 12 To investigate the possible role of Sph-1-P in HUVEC proliferation, we examined the effect of Sph-1-P on DNA synthesis in quiescent HUVECs cultured in a 10% FCS medium. Sph-1-P was found to stimulate proliferation of quiescent HUVECs as measured by [3H]thymidine incorporation (Fig 4, left panel). A marked mitogenic effect was observed at 1 to 10 μmol/L. However, Sph-1-P at 20 μmol/L did not induce significant [3H]thymidine uptake, possibly because DNA synthesis was complete before the [3H]thymidine addition.
Stimulation of DNA synthesis and suppression of apoptosis by Sph-1-P. (Left panel) Incorporation of [3H]thymidine into HUVEC DNA. HUVECs were treated with the indicated concentrations of Sph-1-P for 22 hours in the presence of 10% FCS, followed by incubation in the presence of [3H]thymidine for 2 hours. DNA-associated radioactivity was measured using a liquid scintillation counter. Columns and error bars represent the mean ± SD (n = 3). (Right panel) Inhibition of apoptosis induced by HUVEC growth factor deprivation. HUVECs were treated with indicated concentrations of Sph-1-P for 4 hours in the absence of growth factors. Apoptotic cells were detected using Hoechst 33258 staining and visualized with fluorescence microscopy. The protection percentage from apoptosis was calculated as ([apoptotic cells in the absence of growth factors] − [apoptotic cells in the presence of Sph-1-P])/ ([apoptotic cells in the absence of growth factors] − [apoptotic cells in the presence of growth factors]) × 100.
Stimulation of DNA synthesis and suppression of apoptosis by Sph-1-P. (Left panel) Incorporation of [3H]thymidine into HUVEC DNA. HUVECs were treated with the indicated concentrations of Sph-1-P for 22 hours in the presence of 10% FCS, followed by incubation in the presence of [3H]thymidine for 2 hours. DNA-associated radioactivity was measured using a liquid scintillation counter. Columns and error bars represent the mean ± SD (n = 3). (Right panel) Inhibition of apoptosis induced by HUVEC growth factor deprivation. HUVECs were treated with indicated concentrations of Sph-1-P for 4 hours in the absence of growth factors. Apoptotic cells were detected using Hoechst 33258 staining and visualized with fluorescence microscopy. The protection percentage from apoptosis was calculated as ([apoptotic cells in the absence of growth factors] − [apoptotic cells in the presence of Sph-1-P])/ ([apoptotic cells in the absence of growth factors] − [apoptotic cells in the presence of growth factors]) × 100.
As described above, deprivation of growth factor induced apoptosis in HUVECs. Sph-1-P was found to counteract the apoptosis in a concentration-dependent manner (Fig 4, right panel). The percentage of apoptosis suppression by 20 μmol/L Sph-1-P was as high as 75%. Accordingly, Sph-1-P plays an important role in HUVEC survival, not only by stimulation of cellular proliferation, but also by protection from apoptosis.
Metabolism of sphingolipids in HUVECs.
To study metabolism of sphingolipids in HUVECs, we first examined the metabolic fate of Sph in HUVECs by exogenous addition of [3H]Sph into the cell culture. The added [3H]Sph was incorporated into the HUVECs and rapidly (within 20 minutes) converted to [3H]Sph-1-P (Fig 5, upper panel). However, this conversion was transient and the [3H]Sph-1-P band could not be observed 4 hours after the label addition (Fig 5, upper panel), possibly due to degradation by Sph-1-P lyase. This is completely different from [3H]Sph-1-P formation in platelets labeled with [3H]Sph; we previously reported that the high percentage of the [3H]Sph originally added remains as [3H]Sph-1-P, even after a long incubation, indicating the stability of Sph-1-P in platelets, which lack Sph-1-P lyase activity.14
Metabolism of [3H]Sph in HUVECs. (Upper panel) HUVECs were incubated with [3H]Sph for various durations. Lipids were then extracted from cells or media and analyzed for [3H]sphingolipids by TLC autoradiography. Locations of standard lipids are indicated on the left. SM, sphingomyelin; O, origin. (Lower panel) HUVECs and human platelets were incubated with [3H]Sph for 4 hours and, after the autoradiography, silica gel areas containing radiolabeled sphingolipids were scraped off and counted using a liquid scintillation counter. Radioactivity was expressed as a percentage of the value of [3H]Sph at time 0. Columns and error bars represent the mean ± SD (n = 3).
Metabolism of [3H]Sph in HUVECs. (Upper panel) HUVECs were incubated with [3H]Sph for various durations. Lipids were then extracted from cells or media and analyzed for [3H]sphingolipids by TLC autoradiography. Locations of standard lipids are indicated on the left. SM, sphingomyelin; O, origin. (Lower panel) HUVECs and human platelets were incubated with [3H]Sph for 4 hours and, after the autoradiography, silica gel areas containing radiolabeled sphingolipids were scraped off and counted using a liquid scintillation counter. Radioactivity was expressed as a percentage of the value of [3H]Sph at time 0. Columns and error bars represent the mean ± SD (n = 3).
In sharp contrast to the weak and transient formation of [3H]Sph-1-P, the formation of [3H]sphingomyelin, possibly through [3H]Cer, was active and time-dependent (Fig 5, upper panel). We also examined whether [3H]DMS could be formed, because DMS was found to induce marked apoptosis in HUVECs (see above) and because Sph N-methyltransferase activity exists in some tissues and cells.23 24 However, DMS was not formed in HUVECs labeled with [3H]Sph both in the resting (Fig 5) and activated (see Fig 7) states.
Quantitative comparison was made between [3H]Sph metabolism in HUVECs and platelets (Fig 5, lower panel). In HUVECs, [3H]Sph was mainly converted to [3H]Cer by N-acylation and further to [3H]sphingomyelin through the action of sphingomyelin synthase.25 On the other hand, in platelets, [3H]Sph-1-P is overwhelmingly the main product of [3H]Sph, which can be best explained by the fact that platelets possess very active Sph kinase and practically no lyase activity for degradation of Sph-1-P to a fatty aldehyde and ethanolamine phosphate.14
We previously developed an assay for quantification of Sph-1-P by its N-acylation with [3H]acetic anhydride into [3H]C2-Cer-1-P (N-acetylated Sph-1-P).16 When the platelet extract containing Sph-1-P was N-acylated with [3H]acetic anhydride, a highly radioactive C2-Cer-1-P was formed (Fig 6, left lane). Under identical conditions, only a trace amount of [3H]C2-Cer-1-P was formed from the HUVEC extract (Fig 6, right panel). These results confirm our previous finding that platelets abundantly store Sph-1-P16 26 and further indicate that HUVECs contain much less Sph-1-P.
Detection of Sph-1-P in the extracts from human platelets and HUVECs. The extracts from platelets (Plt) and HUVECs were N-acylated with [3H]acetic anhydride into [3H]C2-Cer-1-P to quantify Sph-1-P. The extracts analyzed were those obtained from the cells containing 5 μmol phospholipid.
Detection of Sph-1-P in the extracts from human platelets and HUVECs. The extracts from platelets (Plt) and HUVECs were N-acylated with [3H]acetic anhydride into [3H]C2-Cer-1-P to quantify Sph-1-P. The extracts analyzed were those obtained from the cells containing 5 μmol phospholipid.
As described above, [3H]Sph-1-P was transiently formed in HUVECs incubated with [3H]Sph (Fig 5). This conversion into [3H]Sph-1-P was not affected by IL-1β, thrombin, or TPA (Fig 7). Furthermore, [3H]Sph-1-P was not detected in the medium under these conditions (Fig 7), indicating that stimulation-dependent Sph-1-P release does not occur in HUVECs. This is in contrast with platelets, which release Sph-1-P extracellularly, possibly in a protein kinase C-dependent manner.14
Effects of various agents on [3H]Sph metabolism in HUVECs. HUVECs were incubated with [3H]Sph for 1 minute and then challenged without (C) or with 100 U/mL of IL-1β (IL-1), 1 U/mL of thrombin (Thr), or 1 μmol/L TPA for 20 minutes in the presence of a 1% FCS medium. Lipids were extracted from cells or media and analyzed for [3H]Sph metabolism. Locations of standard lipids are indicated on the left.
Effects of various agents on [3H]Sph metabolism in HUVECs. HUVECs were incubated with [3H]Sph for 1 minute and then challenged without (C) or with 100 U/mL of IL-1β (IL-1), 1 U/mL of thrombin (Thr), or 1 μmol/L TPA for 20 minutes in the presence of a 1% FCS medium. Lipids were extracted from cells or media and analyzed for [3H]Sph metabolism. Locations of standard lipids are indicated on the left.
We finally examined the metabolism of the radiolabeled C6-Cer. One radioactive band was detected in the extract of HUVECs incorporating [3H]C6-Cer (Fig 8A). This band was found to be located just below sphingomyelin on TLC and was specifically eliminated by treatment with sphingomyelinase (data not shown). These results indicate that [3H]C6-sphingomyelin is formed from [3H]C6-Cer in HUVECs. This is consistent with the fact that [3H]sphingomyelin was formed later than [3H]Cer when HUVECs were incubated with [3H]Sph (Fig 5). [3H]C6-sphingomyelin production from [3H]C6-Cer was not affected by treatment with thrombin, TPA, LPA, angiotensin II, AVP, or PAF (Fig 8B).
Metabolism of [3H]C6-Cer in HUVECs. (A) HUVECs were incubated with [3H]C6-Cer for various durations. Lipids were then extracted from cells and analyzed for [3H]C6-Cer metabolism. Locations of standard lipids are indicated on the left. (B) HUVECs were incubated with [3H]C6-Cer for 1 minute and then challenged without (C) or with 1 U/mL of thrombin (Thr), 1 μmol/L TPA, 10 μmol/L LPA, 1 μmol/L angiotensin II (A II), 1 μmol/L AVP, or 1 μmol/L PAF for 20 minutes in the presence of a 1% FCS medium.
Metabolism of [3H]C6-Cer in HUVECs. (A) HUVECs were incubated with [3H]C6-Cer for various durations. Lipids were then extracted from cells and analyzed for [3H]C6-Cer metabolism. Locations of standard lipids are indicated on the left. (B) HUVECs were incubated with [3H]C6-Cer for 1 minute and then challenged without (C) or with 1 U/mL of thrombin (Thr), 1 μmol/L TPA, 10 μmol/L LPA, 1 μmol/L angiotensin II (A II), 1 μmol/L AVP, or 1 μmol/L PAF for 20 minutes in the presence of a 1% FCS medium.
DISCUSSION
Regulation by sphingolipids of HUVEC apoptosis and growth.
It is now acknowledged that the branching pathways of sphingolipid metabolism may determine whether a cell survives or dies. Whereas Cer has been shown to be an important regulatory component of apoptosis,4,5,8 Sph-1-P is reportedly involved in cellular survival as a signaling molecule.6,11,12 In contrast, Sph has been reported to mediate either apoptotic or mitogenic responses, depending on the cell type.6,7,9-11,19,20 In this study, we first investigated the ability of various sphingolipids to induce apoptosis in HUVECs. We found that not only Cer, but also Sph and its methylated derivative DMS, induced HUVEC apoptosis. The effect of DMS on apoptosis induction was more potent than that of Sph, which may be explained by the fact that DMS, but not Sph, is metabolically stable when incubated with intact cells.27
Both Sph and DMS have an inhibitory effect on protein kinase C28,29; a pharmacological protein kinase C inhibitor, staurosporine,30 also induced apoptosis. These observations suggest that the induction of apoptosis by Sph and DMS may be related to protein kinase C inhibition. However, recent reports have shown that the mechanism(s) by which Sph and DMS induces apoptosis is complex and cannot be explained only by protein kinase C inhibition.7Induction of apoptosis by Sph is strongly correlated with inhibition of mitogen-activated protein kinase, and this is unrelated to protein kinase C inhibition.31 Furthermore, Sph downregulates the expression of the antiapoptotic proteins, Bcl-2 and Bcl-XL.32,33 Recently, inhibition of DNA primase by Sph/DMS was also reported.34 There is also the possibility that a Sph/DMS-dependent protein kinase may be involved in induction of apoptosis.35 The mechanism by which Sph (and DMS) induces apoptosis remains to be determined.
It is well known that deprivation of growth factor induced apoptosis in HUVECs.21,22 In this study, we demonstrated that Sph-1-P protects HUVECs from apoptosis induced by withdrawal of growth factors. Furthermore, Sph-1-P stimulates DNA synthesis in HUVECs, indicating that this bioactive lipid acts as a HUVEC survival factor both by protection from apoptosis and by stimulation of proliferation. The results were as we expected, because Sph-1-P reportedly enhances cell survival in some cells6,11,12 and because HUVECs express the cell surface Sph-1-P receptor, Edg-1.36
Metabolism of sphingolipids in HUVECs.
Although the physiological roles of sphingolipids have been suggested, current evidence for the involvement of sphingolipids in endothelial cell function(s) consists largely of data on the cellular effects caused by the exogenous addition of sphingolipids. Few studies have reported the metabolic analysis of sphingolipids in endothelial cells. We found that [3H]Sph, incorporated into HUVECs, is converted to [3H]Cer and further to [3H]sphingomyelin in a time-dependent manner, whereas [3H]Sph-1-P formation from [3H]Sph is weak and transient. The transient [3H]Sph-1-P formation can be explained by its degradation to ethanolamine phosphate and fatty aldehyde by Sph-1-P lyase, as is the case with most cells.6,11,37 The findings for HUVECs are very different from those of platelets. Platelets possess a highly active Sph kinase but lack Sph-1-P lyase; hence, [3H]Sph-1-P formation from [3H]Sph is very strong and long-lasting.14Accordingly, it is not surprising that platelets abundantly store Sph-1-P and that HUVECs contain much less Sph-1-P. Furthermore, platelets release Sph-1-P extracellularly upon stimulation, possibly through a mechanism dependent on protein kinase C,14 which platelets abundantly express,38 whereas HUVECs do not. It is unlikely that endothelial cells may be the source of plasma Sph-1-P; these cells themselves are not able to supply the survival factor Sph-1-P, but receive it from activated platelets.
Platelet-endothelial cell interaction.
Hemostasis, thrombosis, and atherosclerosis are considered an integrated group of multicellular events, of which reactions between endothelial cells and platelets are very important.39 Some metabolic systems that regulate platelet reactivity are present in endothelial cells: eicosanoids (cyclooxygenase metabolites), endothelium-derived relaxing factor/nitric oxide, and ecto-nucleotidase(s).39
On the other hand, maintenance of vascular endothelium integrity can be attributed to secreted products of platelets. During activation, platelets release a variety of vasoactive substances, and their role in the repair of damaged vascular intima is established.40,41Although the repair of the thin endothelial lining of the intima requires the appearance of endothelial mitogens and the platelet would be a logical source of such mitogen release, not much is known regarding the endothelial mitogens or survival factors within platelets. It has been reported that platelets store the angiogenic factor platelet-derived endothelial cell growth factor, but this was later confirmed to be identical to thymidine phosphorylase.42,43 Furthermore, platelets were found to store, but not to secrete, this angiogenic factor.43 Just recently, vascular endothelial growth factor, a potent endothelial growth factor and permeability mediator, was found to be released by activated platelets.44 45
We previously showed that stimulated platelets release Sph-1-P in a protein kinase C-dependent manner.14 Furthermore, Sph-1-P was found to be a normal constituent of human plasma and serum; serum Sph-1-P levels were elevated compared with plasma, indicative of Sph-1-P release during whole blood coagulation.26 In the present study, we found that Sph-1-P was a survival factor for endothelial cells. Accordingly, Sph-1-P should be added to the list of endothelial survival factors released from platelets. Furthermore, we believe that Sph-1-P is the most potent lipid mediator released from platelets as an endothelial survival factor, at least of those currently known.
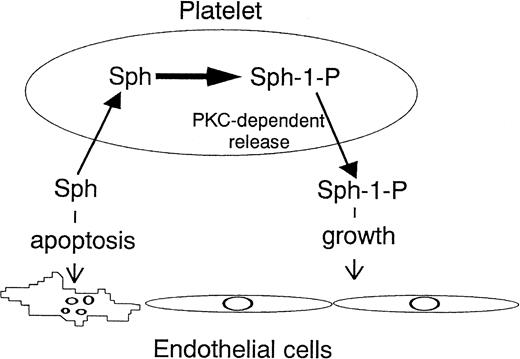
In summary, Sph acts as an inducer of endothelial cell apoptosis, whereas Sph-1-P acts as a survival factor. Platelets may maintain the integrity of endothelial cells by incorporating Sph and releasing Sph-1-P (Fig 9).
Platelet-endothelial cell interaction from the viewpoint of sphingolipids. Platelets incorporate Sph and release Sph-1-P in a protein kinase C (PKC)-dependent manner. Sph acts as an inducer of endothelial cell apoptosis, whereas Sph-1-P acts as a survival factor. This may be one of the mechanisms by which platelets maintain the integrity of endothelial cells.
Platelet-endothelial cell interaction from the viewpoint of sphingolipids. Platelets incorporate Sph and release Sph-1-P in a protein kinase C (PKC)-dependent manner. Sph acts as an inducer of endothelial cell apoptosis, whereas Sph-1-P acts as a survival factor. This may be one of the mechanisms by which platelets maintain the integrity of endothelial cells.
ACKNOWLEDGMENT
The authors thank Dr Yasuyuki Igarashi (Hokkaido University) for helpful discussions and Drs Y. Fukada and K. Hoshi (Yamanashi Medical University) for providing us with human umbilical cords.
Supported by the Clinical Pathology Research Foundation of Japan, Uehara Memorial Foundation, and Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Yutaka Yatomi, MD, PhD, Department of Laboratory Medicine, Yamanashi Medical University, Nakakoma, Yamanashi 409-3898, Japan.




![Fig. 4. Stimulation of DNA synthesis and suppression of apoptosis by Sph-1-P. (Left panel) Incorporation of [3H]thymidine into HUVEC DNA. HUVECs were treated with the indicated concentrations of Sph-1-P for 22 hours in the presence of 10% FCS, followed by incubation in the presence of [3H]thymidine for 2 hours. DNA-associated radioactivity was measured using a liquid scintillation counter. Columns and error bars represent the mean ± SD (n = 3). (Right panel) Inhibition of apoptosis induced by HUVEC growth factor deprivation. HUVECs were treated with indicated concentrations of Sph-1-P for 4 hours in the absence of growth factors. Apoptotic cells were detected using Hoechst 33258 staining and visualized with fluorescence microscopy. The protection percentage from apoptosis was calculated as ([apoptotic cells in the absence of growth factors] − [apoptotic cells in the presence of Sph-1-P])/ ([apoptotic cells in the absence of growth factors] − [apoptotic cells in the presence of growth factors]) × 100.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4293/4/m_blod41226004x.jpeg?Expires=1765907824&Signature=qwh1GSPfRYFMceksl5K4zGxSTcZhy4z-McyC3K3bDquSr08nZmRp3iIJZGUhTaCNVHVXRtDdaKe9sBvhpi3eBhRZMTuN-WYJ~8-6ewY0XDAbCf6AdPHopbOf6OvbdwPGQVcnEu-hJqTQ7T7MwhuasL9cQqTx4PnFfuCPweNKd0e9VZwE0Z7zcDaMCiWOTD18XQAUHWi-KmMkD3BOqFw3oVYbjqOo~sq4s9dQ2W2aslLYxbJ8Rdyu3HJqTBHHBT6qwQ1Kr4NjpyAiIZ0rDy8ObSJvOHQn~eLXQcIW-pHHTqQa2MYm73mBFsieDgf67tzZbe58KQO5uJUlJ4PlTxSAPw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Metabolism of [3H]Sph in HUVECs. (Upper panel) HUVECs were incubated with [3H]Sph for various durations. Lipids were then extracted from cells or media and analyzed for [3H]sphingolipids by TLC autoradiography. Locations of standard lipids are indicated on the left. SM, sphingomyelin; O, origin. (Lower panel) HUVECs and human platelets were incubated with [3H]Sph for 4 hours and, after the autoradiography, silica gel areas containing radiolabeled sphingolipids were scraped off and counted using a liquid scintillation counter. Radioactivity was expressed as a percentage of the value of [3H]Sph at time 0. Columns and error bars represent the mean ± SD (n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4293/4/m_blod41226005w.jpeg?Expires=1765907824&Signature=bqBw8PFaiyWZroisuwdNVaeX~qBjzKsFnYUj-QjuW64MwM7qi63H23G4A2chheY5um6NlsE9oF6n~Jw9qs79FuL7V3o8mnnKr0alxLdbzcwtq8fDI~umqcgSUbSQHw-PbWY4oiEUHH6dOiJlL-lQdE8zvDldcwLX1hHvUvUDJNLhKVKVIWqyG5ZpEeG2g3DVt16RQM1voGy5Wad7t2fD4pPNF8wvLdv2d2hJlbDc4AH~STHTtIjMIBmQ1dyy9Qg1uwrMuLRwnIpss0k4qyrS0ZRHkPOT106X~XkbGIb8zh1cwWl-ZwrTlYxPA1r2lC2UgAIUgUjXhILpq-PMYEv9KQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Detection of Sph-1-P in the extracts from human platelets and HUVECs. The extracts from platelets (Plt) and HUVECs were N-acylated with [3H]acetic anhydride into [3H]C2-Cer-1-P to quantify Sph-1-P. The extracts analyzed were those obtained from the cells containing 5 μmol phospholipid.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4293/4/m_blod41226006w.jpeg?Expires=1765907824&Signature=fgLqfZgVxoqqolw~6fPMmrRGC8S81iUi-AgMeceqHTMFF4a~lLbmpRZ~AVzoLUrNPELvFwdF4A~zuj~WU7ZwlXhVCmXX-HTF7DWky~DnLUpzOOVBRaGgqk6HYeMSYn2OhmWih6MlAT--UsW1Kcg8xtnEnOSwvY20AN5T80JJgiOsLv8rFuLHx0dXWEOJpCK64eKRkwudjPh7xJ4qfxcrmDxDW1aj5YFXnoltjwyQ6JB3bEVuSYppG2n~vJ12Zclkohjb6Q4vUpRWkhwwT3AufhY-Vyo2gGCBmYYTsRzVQCZgMSVNXED0du1WsXGtpNT1lmiycfKtY51DwDdJASoYCQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Effects of various agents on [3H]Sph metabolism in HUVECs. HUVECs were incubated with [3H]Sph for 1 minute and then challenged without (C) or with 100 U/mL of IL-1β (IL-1), 1 U/mL of thrombin (Thr), or 1 μmol/L TPA for 20 minutes in the presence of a 1% FCS medium. Lipids were extracted from cells or media and analyzed for [3H]Sph metabolism. Locations of standard lipids are indicated on the left.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4293/4/m_blod41226007w.jpeg?Expires=1765907824&Signature=RoddhfOQpY~ux3E6VUPnW36BovpcvY355s7Ry4L2dYMstDjymLQ6ISwkHMCVSAQmnvTfTMsWYFDwO5MMycLFGNM70lFnX1iBFv0drTMd2vYdWmD7qsJQQp5R-aEtbYL4QYF1O9z9EtM4DB5NvBH50kfzGufHM96ibdJCIuNhid1Yb~BvHuAhjYXUqogSkOg8Bho9HiOijv2~r4C~XltR1Ufcr32epImyrMQVSAXY~WtOpDOxCBA~-bkQ9-7zHFFAl9pYCespT9aOMFgy3inn2wKet~dBOxS1yUXLL0Qxp1afVYRwNJkdntstNOgBbKiZrpPQv7eVK3baV~c9IYM2vw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Metabolism of [3H]C6-Cer in HUVECs. (A) HUVECs were incubated with [3H]C6-Cer for various durations. Lipids were then extracted from cells and analyzed for [3H]C6-Cer metabolism. Locations of standard lipids are indicated on the left. (B) HUVECs were incubated with [3H]C6-Cer for 1 minute and then challenged without (C) or with 1 U/mL of thrombin (Thr), 1 μmol/L TPA, 10 μmol/L LPA, 1 μmol/L angiotensin II (A II), 1 μmol/L AVP, or 1 μmol/L PAF for 20 minutes in the presence of a 1% FCS medium.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4293/4/m_blod41226008w.jpeg?Expires=1765907824&Signature=Po7MBJpUx6j6r9g-YN26kK-g8TZaVA64ZAzJdTvnMVvJ87pt2cW3xhMUyBhrf64nWvmeDpR3oQf~1-YNNkMhHT81~ffp6nr5ovlm0ycUMUa2E6zTrESIn3HYbCLgof9NlNg-CxLZlUIGA6QhQJ~BmIFhpGq82qtX7GoDfhtiebC7b0HafBf8hLEdi5YiXje7VI4Yx0wn2EJsrmXKP4SLvIykLx5oc9OcrETNXGV32YkK8sWmUkuH8eseUmiW3KmJucdO~9xw8GiVoYtRJw0lYUlw-uS6GSQdHnkkvmLyjMfOOYGFMDldyCnK8QytkpZuT1CaoFv4ww4wjpd28m2KXA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal