Abstract
Dendritic cells (DC) are critically involved in the initiation of primary immune processes, including tumor rejection. In our study, we investigated the effect of interleukin-10 (IL-10)–treated human DC on the properties of CD8+ T cells that are known to be essential for the destruction of tumor cells. We show that IL-10–pretreatment of DC not only reduces their allostimulatory capacity, but also induces a state of alloantigen-specific anergy in both primed and naive (CD45RA+) CD8+ T cells. To investigate the influence of IL-10–treated DC on melanoma-associated antigen-specific T cells, we generated a tyrosinase-specific CD8+ T-cell line by several rounds of stimulation with the specific antigen. After coculture with IL-10–treated DC, restimulation of the T-cell line with untreated, antigen-pulsed DC demonstrated peptide-specific anergy in the tyrosinase-specific T cells. Addition of IL-2 to the anergic T cells reversed the state of both alloantigen- or peptide-specific anergy. In contrast to optimally stimulated CD8+ T cells, anergic tyrosinase-specific CD8+ T cells, after coculture with peptide-pulsed IL-10–treated DC, failed to lyse an HLA-A2–positive and tyrosinase-expressing melanoma cell line. Thus, our data demonstrate that IL-10–treated DC induce an antigen-specific anergy in cytotoxic CD8+ T cells, a process that might be a mechanism of tumors to inhibit immune surveillance by converting DC into tolerogenic antigen-presenting cells.
DENDRITIC CELLS (DC) are highly specialized antigen-presenting cells (APC) of the immune system.1 Their strategic positioning in nonlymphoid tissue and their ability to circulate via blood and lymph to lymphoid organs after antigen stimulation demonstrate their important role in the induction of immune responses against invading pathogens.2,3 During their migration, DC such as Langerhans cells (LC) are thought to undergo characteristic modulations of function and phenotype.4,5 Locally produced inflammatory cytokines and the encounter with an antigen promote the maturation and migration of DC to regional lymph nodes. During this process DC undergo a differentiation from a processing to a presenting functional cell type, characterized by the expression of costimulatory molecules, cytokine production, and a typical morphology.5-7 In contrast to other types of APC, fully mature DC are potent activators of naive T cells and are regarded as important initiators of primary specific immune responses.1
Tumors use various strategies for escape from immunologic recognition or destruction.8 One of the potential escape mechanism is the production of the immunosuppressive cytokine interleukin-10 (IL-10) by the tumor cells themselves or the induction of such factors in tumor-infiltrating cells. The release of IL-10 from many tumors, such as human renal, colon, ovarian, lung, and basal cell carcinomas, brain neoplasms, and Epstein-Barr virus–transformed B lymphomas, has been reported.9-14 Furthermore, in malignant melanoma cells, the expression of IL-10 mRNA or protein was demonstrated.10,15-17 Studies in progressing tumors showed a correlation of an advanced course of the disease with elevated IL-10 serum levels, a preferential detection of IL-10 secreting tumor cells in metastatic lesions, and a higher release of IL-10 by metastatic cells from patients with progressive melanoma metastases compared with metastases responding to therapy.18-20
The immunosuppressive properties of IL-10 have been well documented in several studies. An inhibitory effect on the function of APC and T cells has been described. The inhibitory influence of IL-10 on the APC function of DC and macrophages is due to the downregulation of major histocompatibility complex (MHC) class II and several costimulatory molecules and the reduction of a variety of secreted inflammatory cytokines.21-28 With regard to tumor rejection, it was demonstrated that IL-10 inhibits tumor antigen presentation by epidermal APC in the murine system.29 More importantly, it was shown that IL-10–treated LC induce an antigen-specific tolerance in Th1 cells, but not in Th2 cell clones.30
Recently, we demonstrated that IL-10–treated human DC, generated from peripheral blood, induce a state of antigen-specific anergy in various populations of CD4+ T cells.31
In the present study, we investigated the effect of IL-10–treated DC on the function of CD8+ T cells to evaluate a potential mechanism of tumor cells to inhibit the cytotoxic capacity of T cells by anergy induction. We demonstrate that IL-10–treated DC show a reduced capacity to stimulate the proliferation of both primed and naive CD8+ T cells in allogeneic MLR and anti-CD3 assays. More interestingly, IL-10–treated DC induce a state of alloantigen-specific anergy in CD8+ T cells. To investigate the influence on melanoma-associated antigen-specific T cells, the effect of IL-10–treated DC on a tyrosinase-specific T-cell line was observed. Restimulation of the specific T cells with untreated, antigen-pulsed DC demonstrated a peptide-specific anergy in the tyrosinase-specific CD8+ T cells. In contrast to optimally stimulated CD8+ T cells, tyrosinase-specific anergic CD8+ T cells failed to lyse an HLA-A2+, tyrosinase-expressing melanoma cell line. In conclusion, production of IL-10 by tumor cells may reflect an escape mechanism from immune surveillance by converting DC into tolerogenic APC.
MATERIALS AND METHODS
Preparation of DC.
Blood-derived DC were prepared according to a modified protocol originally described by Romani et al.32 Briefly, whole blood was heparinized and separated by a Ficoll gradient. Peripheral blood mononuclear cell fractions were then depleted of T and B cells using immunomagnetic beads coated with anti-CD2 and anti-CD19 monoclonal antibody (MoAb; Dynal, Oslo, Norway). The remaining cells were cultured in X-VIVO 15 (Biowhittaker, Walkersville, MD) in 6-well-plates (Costar, Cambridge, MA) for 7 days. Cultures were supplemented with 1,000 U/mL human IL-4 (hIL-4; PBH, Hannover, Germany), 800 U/mL human granulocyte-macrophage colony-stimulating factor (hGM-CSF; Leukomax; Sandoz, Basel, Switzerland), and 1% autologous plasma. Cells were fed with fresh medium every 2 days. On day 7, nonadherent cells were rinsed off the plates and resuspended in fresh complete medium with GM-CSF and IL-4 and additionally stimulated with IL-1β (10 ng/mL), tumor necrosis factor-α (TNF-α; 10 ng/mL; PBH), IL-6 (1,000 U/mL; R&D Systems, Wiesbaden, Germany), and prostaglandin E2(PGE2; 1 μg/mL; Sigma, München, Germany) to induce and stabilize the maturation of DC. DC were harvested for fluorescence-activated cell sorting (FACS) analysis or functional tests 3 to 5 days after resuspension and stimulation. Simultaneously to the addition of the stimulating mixture of cytokines and PGE2, IL-10 (40 ng/mL; DNAX, Palo Alto, CA) was added to the culture for the last 2 days of culture. For the study of kinetics, IL-10 was added at various time points (as indicated).
T-cell purification, allogeneic proliferation, and anti-CD3 assay.
T cells were prepared from human blood using Ficoll gradients and subsequent purification by antibody-coated immunomagnetic beads (MACS Systems; Miltenyl, Bergisch Gladbach, Germany) according to standard protocols (purity of >95% CD8+ T cells and >90% CD45RA+ T cells). In some experiments, cord blood was used as the source of naive CD8+ T cells. Purity was tested using FACS analysis.
DC were prepared as described above and cocultured with 2 × 105 T cells per well in 96-well plates (Costar). For anti-CD3 assays anti-CD3 MoAb (OKT3, ATCC, CRL 8001) was used at a titrated dilution of a hybredoma supernatant (1:20). After 2 days (anti-CD3 assay) or 4 days (allogeneic MLR), the cells were pulsed with 1 μCi of [3H] TdR ([methyl-3H]thymidine)/well for 16 hours, harvested, and counted. Tests were performed in triplicate, and results were expressed as the mean cpm ± standard deviation (SD).
Tyrosinase-specific T-cell lines (CTL).
Naive CD8+ T cells (2 × 105) from HLA-A2+ donors (purity of >95% CD8+ T cells, generated as described above) were cultured in X-vivo 20 (Biowhittaker) and stimulated with mature, HLA-A2+, autologous DC (2 × 104) pulsed with the specific tyrosinase peptide (YMDGTMSQV; 20 μg/mL). After several restimulations (5 to 8 times) every 7 days and expansion of the cell number by addition of IL-2 (10 U/mL), the peptide-specific proliferation was tested using specific (tyrosinase) and unspecific (MART-1 [EAAGIGLTV], MAGE-1 [EADPTGHSY]) melanoma-associated antigens. Before their use in experiments, T cells were rested 7 to 8 days after the last addition of antigen and stimulator cells. Three tyrosinase-specific CD8+ T-cell lines from 2 unrelated HLA.A2+donors were generated and used for the experiments. MART-1–specific T-cell lines served as controls.
Anergy assay.
Allogeneic CD8+ T cells or tyrosinase-specific CD8+ T cells were prepared as described above. T cells were cocultured during the first incubation at a density of 2 × 105 (allogeneic CD8+) or 2 × 104 (tyrosinase-specific CD8+ T cells) with 1 × 104 or 1 × 103 DC, pretreated with IL-10 (40 ng/mL), or untreated. In some experiments, anti-CD3 MoAb was added at a titrated dilution of a supernatant (as described above). Thirty-six hours later, T cells were separated by Histopaque and rested for 1 to 7 days in culture medium containing 2 U/mL IL-2. Subsequently, T cells were restimulated with DC generated from the same donor as used for the first culture in experiments with CD8+ T cells or with DC generated from an HLA-A2+ donor. Proliferation was measured 48 hours later by thymidine incorporation. Tests were carried out in triplicates, and results were expressed as mean cpm ± SD. Additionally, cytokine production was measured by enzyme-linked immunosorbent assay (ELISA) in supernatants of restimulated cultures 48 hours after the beginning of culture.
Cytotoxicity assay.
Cytotoxic activity was measured in a standard 4-hour assay using the51Cr-labeled tyrosinase-expressing and HLA.A2+melanoma cell line SK-MEL 28 (provided by Dr T. Wölfel, Mainz, Germany) as targets. Briefly, 3 × 10351Cr-labeled tumor cells were cultured with tyrosinase-specific, HLA.A2+ CD8+ CTL, precultured with mature or IL-10–treated DC, in effector:target ratios as indicated for 4 hours at 37°C. Tyrosinase-specific CD8+ T cells cocultured with the unrelated peptide MART-1 and untreated or IL-10–treated DC or HLA-mismatched DC (HLA.A1+) during the primary culture were used as controls.
Additional control experiments were performed using DC (HLA.A2+) as target cells pulsed with various peptides: tyrosinase (HLA.A2-restricted presentation), MART-1 (HLA.A2-restricted presentation), or MAGE-1 (HLA.A1-restricted presentation). Similar to the experimental setting described above, these51Cr-labeled target cells were cocultured with the tyrosinase-specific, HLA.A2+ CD8+CTL, precultured with mature or IL-10–treated DC, for 4 hours at 37°C.
The percentage of specific lysis was calculated from the average of triplicates as 100 × (51Cr-release into supernatant − spontaneous release)/(total release in detergent − spontaneous release). All synthetic petides were tested for nonspecific lysis of target cells in the absence of cytotoxic T lymphocytes.
Cytokine analysis.
For assessment of cytokine production, supernatants were collected 48 hours after restimulation of allogeneic-specific/tyrosinase-specific CD8+ T cells with mature, untreated DC and stored at −70°C. Amounts of IL-2, IL-4, IL-10, transforming growth factor-β(TGF-β), and interferon-γ (IFN-γ) were measured by ELISA using commercially available antibodies and standards according to the manufacturer’s protocols (Pharmingen, Hamburg, Germany).
RESULTS
Inhibition of the alloantigen-induced or anti-CD3–induced proliferation of CD8+ T cells after coculture with IL-10–treated DC.
To evaluate the effect of IL-10 on the stimulatory capacity of DC, precursors of DC, generated from peripheral blood, were cultured for 7 days as described above and subsequently stimulated for the last 2 days with the defined cytokine cocktail (IL-1β, TNF-α, and IL-6) and PGE2 alone or additionally treated with IL-10 (40 ng/mL) and used as APC in variable numbers in alloantigen-induced or anti-CD3–induced proliferation assays (Fig1A and B). Human allogenic CD8+ T cells, purified from peripheral blood or in some experiments from cord blood, were used as responder cells. A significant inhibition of the proliferation was demonstrated in all T-cell:DC ratios, if IL-10–treated DC were set in as APC both in alloantigen-induced and in anti-CD3–induced proliferation assays. The reduced proliferation was demonstrated, if naive CD8+/CD45RA+ T cells (Fig 1A and B) or activated CD8+/CD45RO+ T cells (data not shown) were cocultured with IL-10–treated DC.
Inhibitory effects of IL-10–pretreated DC on alloantigen-or anti-CD3–induced proliferation of naive CD8+ T cells: dependence on the state of maturation of DC. DC were generated from peripheral progenitors as described above, resuspended at day 7, and stimulated with IL-1β, IL-6, TNF-, and PGE2 to induce and stabilize the maturation of the DC. IL-10 was added for the last 2 days of culture at various time points after the stimulation: simultaneous additon at day 7 and harvested of the DC at day 9 (A and B) or addition at a later stage of maturation (day 10) and subsequently harvest at day 12 (C and D) . Control and IL-10–treated DC were cocultured with naive CD45 RA+/CD8+ T cells (2 × 105) in allogeneic MLR (A and C) or anti-CD3 assays (B and D). Proliferation was measured by [3H] TdR uptake. The results are representative of five experiments.
Inhibitory effects of IL-10–pretreated DC on alloantigen-or anti-CD3–induced proliferation of naive CD8+ T cells: dependence on the state of maturation of DC. DC were generated from peripheral progenitors as described above, resuspended at day 7, and stimulated with IL-1β, IL-6, TNF-, and PGE2 to induce and stabilize the maturation of the DC. IL-10 was added for the last 2 days of culture at various time points after the stimulation: simultaneous additon at day 7 and harvested of the DC at day 9 (A and B) or addition at a later stage of maturation (day 10) and subsequently harvest at day 12 (C and D) . Control and IL-10–treated DC were cocultured with naive CD45 RA+/CD8+ T cells (2 × 105) in allogeneic MLR (A and C) or anti-CD3 assays (B and D). Proliferation was measured by [3H] TdR uptake. The results are representative of five experiments.
The effect of IL-10 on the DC was inhibited by the simultaneous addition of an anti–IL-10 antibody (10 μg/mL; R&D Systems, Weisbaden, Germany) to the culture (data not shown).
Mature DC are resistant to the effect of IL-10.
Prior experiments had shown that fully mature DC were generated after 11 to 14 days of culture after stimulation with IL1-β, IL-6, TNF-α, and PGE2 at day 7 and that these cells induced the most effective proliferative T-cell response.33 In our experiments, IL-10 was added at various time points after the stimulation of the DC to determine whether IL-10 would affect the function of DC at all stages of their differentiation. The addition of IL-10 was most effective if DC at days 7 to 9 of culture were used, whereas fully mature DC at days 10 to 13 of culture were completely resistant to the effect of IL-10 (Fig 1). As an example, no inhibition of proliferation was observed if DC at day 12 of culture, after treatment with IL-10 at day 10, were cocultured with CD8+ T cells both in alloantigen-induced or anti-CD3–induced proliferation assays (Fig 1C and D).
Induction of alloantigen-specific anergy in CD8+ T cells by IL-10–treated DC.
To test whether IL-10–treated DC would induce a state of alloantigen-specific anergy in CD8+ T cells, we performed a two-step anergy assay. In these experiments, allogeneic CD8+ T cells were cocultured with untreated or IL-10–treated DC. After the first coculture, T cells were rescued, cultured for 36 hours in the presence of IL-2 (10 U/mL), and subsequently restimulated with untreated, fully mature DC.
CD8+ T cells, cultured with untreated DC during the first coculture, showed a vigorous proliferation to restimulation with mature DC during the second coculture (Fig 2). In contrast, CD8+ T cells cocultured with IL-10–treated DC were unresponsive to further stimulation with APC, if these cells were generated from the same donor as used in the first coculture (Fig 2). To determine whether this induction of anergy was antigen-specific, DC from a second, unrelated donor were used for restimulation. In these experiments, an unrestricted proliferation of the CD8+ T cells was observed independently of a pretreatment with IL-10 (Fig 2).
Induction of alloantigen-specific anergy in CD8+ T cells by IL-10–treated DC. Purified naive CD8+ T cells were cultured in medium alone; with untreated, control DC; or with DC pretreated with IL-10 (first coculture). After 36 hours, T cells were rescued, cultured for 1 to 7 days in medium containing low levels of IL-2 (2 U/mL), and subsequently restimulated (second coculture) with untreated, mature DC generated from the same donor (░) or a second unrelated donor (▪). [3H] TdR incorporation was determinated after 48 hours. The left bars show the antigen-specific response and the right bars show the T-cell response to IL-2 (100 U/mL).
Induction of alloantigen-specific anergy in CD8+ T cells by IL-10–treated DC. Purified naive CD8+ T cells were cultured in medium alone; with untreated, control DC; or with DC pretreated with IL-10 (first coculture). After 36 hours, T cells were rescued, cultured for 1 to 7 days in medium containing low levels of IL-2 (2 U/mL), and subsequently restimulated (second coculture) with untreated, mature DC generated from the same donor (░) or a second unrelated donor (▪). [3H] TdR incorporation was determinated after 48 hours. The left bars show the antigen-specific response and the right bars show the T-cell response to IL-2 (100 U/mL).
All experimental groups of T cells responded vigorously to IL-2, a cytokine that has been described to overcome the state of anergy (Fig2).30
To assess the kinetics of the induction of this T-cell anergy, CD8+ T cells rescued after the first coculture were cultured for various time periods (up to 7 days) in the presence of IL-2 (10 U/mL) before restimulation with untreated mature DC. In all experiments performed, the T cells showed a markedly reduced proliferation if IL-10–treated DC were used in the primary culture and the APC for both cultures were derived from the same donor (data not shown).
These experiments demonstrate that IL-10 converts the function of DC to anergy-inducing cells and that this state of T-cell anergy is alloantigen-specific.
Inhibition of peptide-specific proliferation of a tyrosinase-specific cytotoxic CD8+ T-cell line.
Cytotoxic CD8+ T cells are important for the lysis and elimination of tumors cells. To analyze the effect of IL-10–treated DC on the function of cytotoxic, melanoma-associated antigen-specific CD8+ T cells, we established three tyrosinase-peptide–specific CD8+ T-cell lines from two unrelated HLA.A2+ donors. Cytotoxic CD8+ T cells were used after 5 to 8 weekly restimulations with peptide-pulsed autologous DC in the presence of low levels of IL-2 (10 U/mL).
We assessed the proliferation of the tyrosinase-specific CD8+ T-cell line after coculture with untreated versus IL-10–treated tyrosinase peptide-pulsed DC (Fig 3). Strong peptide-specific proliferation was observed in cultures stimulated with untreated tyrosinase-peptide pulsed DC. In contrast, DC generated in the presence of IL-10 induced a markedly impaired proliferation of the T-cell line (Fig 3).
Inhibition of the proliferation of cytotoxic tyrosinase-specific CD8+ T cells by IL-10–treated DC. Tyrosinase-specific, cytotoxic CD8+ T cells were cultured with the specific tyrosinase peptide, either in combination with HLA.A2+ DC pretreated with IL-10 or untreated. In control experiments, the unspecific peptide MART-1 was added to the culture. Proliferation was measured by incorporation of [3H] TdR after 3 days of culture. The results represent one of three experiments.
Inhibition of the proliferation of cytotoxic tyrosinase-specific CD8+ T cells by IL-10–treated DC. Tyrosinase-specific, cytotoxic CD8+ T cells were cultured with the specific tyrosinase peptide, either in combination with HLA.A2+ DC pretreated with IL-10 or untreated. In control experiments, the unspecific peptide MART-1 was added to the culture. Proliferation was measured by incorporation of [3H] TdR after 3 days of culture. The results represent one of three experiments.
No proliferation was observed if a second unrelated melanoma-associated antigen MART-1 was added to the culture medium independently of the pretreatment of the DC with IL-10, indicating a peptide-specific proliferation of the CD8+ T-cell line.
Induction of peptide-specific anergy in a tyrosinase-specific CD8+ T-cell line.
In the following experiments we evaluated whether tyrosinase-specific CD8+ T-cell lines were anergized by the stimulation with IL-10–pretreated DC. In a first coculture, the peptide-specific cytotoxic CD8+ T cells were cultured with autologous, IL-10–treated, or untreated (HLA.A2+) DC in combination with the specific tyrosinase peptide. Subsequently, T cells were rescued, cultured for 1 day (in some kinetic experiments up to 7 days; data not shown), and restimulated with mature, untreated DC in the presence of specific tyrosinase peptide in a second coculture (Fig 4).
Induction of melanoma antigen-specific anergy in tyrosinase-specific, cytotoxic CD8+ T cells. In a first coculture, tyrosinase-specific HLA.A2+ CD8+T cells were used as responder cells for HLA.A2+ DC additionally pretreated with IL-10 or untreated (cultured in complete medium alone). Cultures were set up in the presence of the specific tyrosinase peptide or an unspecific control peptide (MART-1). Subsequently, the T cells were rescued, cultured for 1 to 7 days in the presence of low levels of IL-2 (2 U/mL), and restimulated with mature HLA.A2+ DC during the second coculture. After 2 days, the proliferation was measured by [3H]TdR incorporation. The left bars show the specific response and the right bars show the T-cell response to IL-2 (100 U/mL). The results are representative for three experiments.
Induction of melanoma antigen-specific anergy in tyrosinase-specific, cytotoxic CD8+ T cells. In a first coculture, tyrosinase-specific HLA.A2+ CD8+T cells were used as responder cells for HLA.A2+ DC additionally pretreated with IL-10 or untreated (cultured in complete medium alone). Cultures were set up in the presence of the specific tyrosinase peptide or an unspecific control peptide (MART-1). Subsequently, the T cells were rescued, cultured for 1 to 7 days in the presence of low levels of IL-2 (2 U/mL), and restimulated with mature HLA.A2+ DC during the second coculture. After 2 days, the proliferation was measured by [3H]TdR incorporation. The left bars show the specific response and the right bars show the T-cell response to IL-2 (100 U/mL). The results are representative for three experiments.
After restimulation, a vigorous proliferative response was observed in CD8+ T cells precultured with untreated DC in the presence of the tyrosinase peptide. In contrast, coculture with IL-10–pretreated DC in the presence of the specific antigen induced a significant inhibition of the T-cell proliferation, indicating a tyrosinase-specific (melanoma-associated antigen-specific) anergy.
To show that the induction of anergy is antigen-specific, we stimulated the tyrosinase-specific CD8+ T-cell line during the first coculture with the unrelated peptide MART-1. After the second coculture, an unrestricted T-cell proliferation was observed, independent of a primary stimulation with untreated or IL-10–treated DC (Fig 4).
Additional control experiments were performed using untreated and IL-10–treated HLA-mismatched DC (HLA.A1+) stimulated with the tyrosinase peptide during the first coculture. After restimulation, an unhibited proliferation of the CD8+ tyrosinase-specific T cells was demonstrated, indicating an HLA-restricted antigen presentation and anergy induction (data not shown).
The addition of IL-2 (100 U/mL) to the second coculture reversed the state of peptide-specific anergy in the CD8+ T cells (Fig 4).
To analyze the cytokine pattern of the anergic, peptide-specific CD8+ T cells after restimulation, cytokines in the supernatants were detected by ELISA. The anergic T cells showed a markedly reduced secretion of the cytokines IL-2 and IFN-γ, but no production of IL-4 or IL-10. These data indicate a block in Tc1 cytokine production but no shift to a Tc2 pattern in the anergic T cells (Table 1).
Suppression of Cytokine Production of Anergic Tyrosinase-Specific CD8+ T Cells After Restimulation
| First Coculture . | Cytokine Production After Second Coculture (pg/mL) . | ||||
|---|---|---|---|---|---|
| IL-2 . | IFN-γ . | IL-4 . | IL-10 . | TGF-β . | |
| Tyrosinase-specific CD8+ T cells and | |||||
| DC | 987 ± 23 | 3,765 ± 124 | ND | ND | ND |
| DC (IL-10) | 1,102 ± 76 | 4,087 ± 218 | ND | ND | ND |
| DC + tyrosinase peptide | 1,154 ± 205 | 3,867 ± 134 | ND | ND | ND |
| DC (IL-10) + tyrosinase peptide | 105 ± 20 | 258 ± 87 | ND | ND | ND |
| DC + MART-1 | 1,287 ± 156 | 3,841 ± 434 | ND | ND | ND |
| DC (IL-10) + MART-1 | 1,087 ± 187 | 4,187 ± 128 | ND | ND | ND |
| DC [HLA.A1+] + tyrosinase peptide | 1,376 ± 231 | 4,283 ± 349 | ND | ND | ND |
| DC (IL-10) [HLA.A1+] + tyrosinase peptide | 1,498 ± 327 | 3,762 ± 297 | ND | ND | ND |
| First Coculture . | Cytokine Production After Second Coculture (pg/mL) . | ||||
|---|---|---|---|---|---|
| IL-2 . | IFN-γ . | IL-4 . | IL-10 . | TGF-β . | |
| Tyrosinase-specific CD8+ T cells and | |||||
| DC | 987 ± 23 | 3,765 ± 124 | ND | ND | ND |
| DC (IL-10) | 1,102 ± 76 | 4,087 ± 218 | ND | ND | ND |
| DC + tyrosinase peptide | 1,154 ± 205 | 3,867 ± 134 | ND | ND | ND |
| DC (IL-10) + tyrosinase peptide | 105 ± 20 | 258 ± 87 | ND | ND | ND |
| DC + MART-1 | 1,287 ± 156 | 3,841 ± 434 | ND | ND | ND |
| DC (IL-10) + MART-1 | 1,087 ± 187 | 4,187 ± 128 | ND | ND | ND |
| DC [HLA.A1+] + tyrosinase peptide | 1,376 ± 231 | 4,283 ± 349 | ND | ND | ND |
| DC (IL-10) [HLA.A1+] + tyrosinase peptide | 1,498 ± 327 | 3,762 ± 297 | ND | ND | ND |
Tyrosinase-specific CD8+ T cells were cocultured with untreated or IL-10–treated DC in combination with the specific tyrosinase peptide, with an unspecific peptide (MART-1), or without antigen during the first coculture. Additional control experiments were performed using HLA-mismatched (HLA.A1+) untreated and IL-10–treated DC and the specific tyrosinase peptide. After 2 days, the T cells were rescued, cultured for 2 days in the presence of low levels of IL-2 (2 U/mL), and restimulated with untreated DC and the specific tyrosinase peptide. Subsequently, the supernatants of the T-cell culture were harvested and assessed for IL-2, IL-4, IL-10, VTGF-β, and IFN-γ content by ELISA.
Abbreviation: ND, not detectable.
Anergic CD8+ T cells fail to lyse melanoma cells.
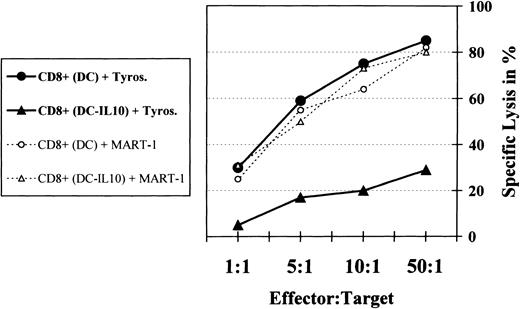
The release of immunosuppressive factors such as IL-10 has been described for many tumors, including malignant melanoma.9-19 To test whether this process might be one possible escape mechanism of tumor cells by inhibiting the stimulatory function of DC, we analyzed the cytotoxic function of the anergic, tyrosinase-specific CD8+ T cells after coculture with untreated or IL-10–treated DC. The cytotoxic activity was measured using the 51Cr-labeled tyrosinase-expressing and HLA.A2+ melanoma cells SK-MEL 28 as target cells. Tyrosinase-specific control and anergic CD8+ T cells were cocultured with the melanoma cells for 4 hours in effector-target ratios, as indicated (Fig 5).
Anergic tyrosinase-specific CTL fail to lyse tumor cells. Control (precultured with untreated DC) and anergic (precultured with IL-10–treated DC) tyrosinase-specific HLA.A2+CD8+ T cells were cocultured with the HLA.A2+ tyrosinase-expressing melanoma cell line SK-MEL 28 in a 51Cr-release assay for 4 hours. Experiments with specific CD8+ T cells cocultured with MART-1 during the primary culture served as controls. Various effector:T-cell ratios were used in the experimental setting. The percentage of specific lysis was calculated from the average of triplicates as 100 × (51Cr-release into supernatant − spontaneous release)/(total release in detergent − spontaneous release). All synthetic peptides were tested for nonspecific lysis of target cells in the absence of CTL.
Anergic tyrosinase-specific CTL fail to lyse tumor cells. Control (precultured with untreated DC) and anergic (precultured with IL-10–treated DC) tyrosinase-specific HLA.A2+CD8+ T cells were cocultured with the HLA.A2+ tyrosinase-expressing melanoma cell line SK-MEL 28 in a 51Cr-release assay for 4 hours. Experiments with specific CD8+ T cells cocultured with MART-1 during the primary culture served as controls. Various effector:T-cell ratios were used in the experimental setting. The percentage of specific lysis was calculated from the average of triplicates as 100 × (51Cr-release into supernatant − spontaneous release)/(total release in detergent − spontaneous release). All synthetic peptides were tested for nonspecific lysis of target cells in the absence of CTL.
The tyrosinase-specific CD8+ T cells, precultured with untreated DC, showed a strong cytotoxic activity at all effector:target ratios used, with up to 85% lysis of the target tumor cells (Fig 5). In contrast, after prior coculture with IL-10–treated DC, tyrosinase-specific anergic CD8+ T cells induced a significantly decreased lysis of the melanoma cells.
Antigen specifity was demonstrated by control experiments. No reduction of the cytotoxicity was shown if the unrelated peptide MART-1 was added to the tyrosinase-specific CD8+ T cells during the primary culture, independent of a coculture with untreated or IL-10–treated DC (Fig 5).
To show the HLA-dependent restriction of the anergy induction, control experiments with HLA-mismatched (HLA.A1+) untreated and IL-10–treated DC pulsed with the tyrosinase peptide during the primary culture were performed. The stimulated tyrosinase-specific CD8+ T cells demonstrated an unhibited lysis of the tyrosinase-expressing, HLA.A2+ melanoma cells, independent of the use of untreated or IL-10–treated, HLA.A1+ DC (data not shown).
Similar results were observed if peptide-pulsed mature DC were used as target cells. Coculture of anergic tyrosinase-specific CD8+T cells with tyrosinase-pulsed DC in a 51Cr-release assay led to a markedly reduced lysis of the target cells compared with control tyrosinase-specific CD8+ T cells. Peptide-specifity was demonstrated by the use of DC pulsed with the unrelated peptide MART-1 or MAGE-1 (data not shown).
DISCUSSION
The release of immunosuppressive factors such as IL-10 has been described for many tumors, including malignant melanoma.9-19 This immunologic process might be a mechanism of tumor cells to inhibit immune surveillance by converting DC from potent stimulatory cells of the immune system to tolerogenic APC.
In the present study, we investigated the effect of human IL-10–treated DC on the properties of cytotoxic CD8+ T cells that are known to be involved in tumor rejection. We demonstrate that IL-10–treated DC induce an alloantigen-specific anergy in naive and activated CD8+ T cells and a tyrosinase-specific anergy in cytotoxic CD8+ T cells, resulting in a failure to lyse melanoma cells.
The escape mechanism of tumor cells regulated by the secretion of IL-10 may be due either to inhibition of recognition by immune cells or to inhibition of destruction of tumor cells by effector cells. In support of the first possibility, it was shown that IL-10 dowregulates MHC class I expression by tumor cells and prevents their lysis by cytotoxic T cells.34,35 Although in these models a decreased expression of MHC class I molecules is described, significant residual levels of class I antigens that remain on the tumor targets argue against this alteration of tumor cells being the sole mechanism responsible for resistance to CTL lysis.34 35
Alternatively, IL-10 might inhibit the antigen-presenting function of APC-like DC or macrophages and thus prevent a T-cell–mediated antitumor response. Experiments have shown that IL-10 downregulates the stimulatory capacity of APC such as macrophages and monocytes, but not that of B cells. This inhibitory influence of IL-10 is due to the downregulation of MHC class II molecules and the costimulatory molecules B7-1/-2 and intercellular adhesion molecule (ICAM-1).21,22,24,25 Furthermore, IL-10 reduces the release of a variety of inflammatory cytokines by monocytes/macrophages, including IL-1, IL-6, IL-8, TNF-α, and GM-CSF.23 26-28
We recently demonstrated that IL-10–treated human DC induce an alloantigen- or peptide-specific anergy in CD4+ T cells, if IL-10 was added to immature DC.31 The pretreatment with IL-10 results in a reduced expression of the costimulatory molecules CD58 and CD86 and MHC class II molecules.31 The induction of anergy in various populations of T cells in this system might be due to a lack of costimulatory molecules and can be partially overcome by stimulatory CD28 MoAbs. Additionally, analysis of the supernatants of the IL-10–treated DC demonstrated an inhibited production of the proinflammatory cytokines IL-1β, IL-6, and TNF-α and a lack of IL-12 production by the DC.
In our present study, we demonstrate that IL-10–treated human DC are able to induce a state of antigen-specific anergy also in CD8+ T cells. In the case of the tyrosinase-specific CD8+ T cells, a significantly decreased lysis of melanoma cells was observed after coculture with the anergic CD8+ T cells and the tumor cells in a 51Cr-release assay. Analysis of the cytokine pattern of the anergic T cells showed a diminished secretion of IL-2 and IFN-γ. Because IL-10–treated DC are known to have a reduced expression of costimulatory molecules such as CD86 and show a reduced production of IL-12, this might result in a defect to activate effector function of CTL characterized by the inhibition of IL-2 and IFN-γ production.
As a consequence, our findings support earlier data showing that production of IL-10 by tumor and/or tumor-infiltrating lymphoid cells might serve as a mechanism for tumor-induced anergy. This induction of anergy was described in various tumor models, including malignant melanoma.20,36 In these systems, IL-10 can act directly on the properties of cytotoxic CD8+ T cells as shown as a significantly reduced lysis of murine lymphoma cells in an in vitro model.36 On the other hand, IL-10 is able to modulate the stimulatory characteristics of the APC by altering the surface expression of various MHC class I/II, costimulatory molecules, or the pattern of the cytokines secreted.21-28Investigations of human DC of responding or progressing melanoma metastases demonstrated a markedly increased production of IL-10 in tumor cells of progressively growing melanomas.20Furthermore, in a costimulation-dependent anti-CD3 tolerance assay, DC of the progressive tumor cells but not of the responding cells induce a state of antigen-specific anergy in cocultured T lymphocytes.20
Additional studies have shown that IL-10 inhibits tumor antigen presentation by epidermal antigen-presenting cells in a murine squamous cell carcinoma model,29 and in a murine plasmocytoma model it was demonstrated that, in the initiation but not in the effector phase of the immune response, IL-10 prevents DC accumulation in the tumor and inhibits rejection of the tumor if IL-10 was simultaneously expressed in the GM-CSF–transfected tumor cells.37Furthermore, IL-10 transgenic mice, in which the expression of IL-10 was expressed under the control of the IL-2 promotor, were unable to limit the growth of Lewis lung tumor cells.38 The injection of anti–IL-10 antibodies significantly reduced the tumor progression, demonstrating a direct effect of IL-10 transgene to IL-10 action.38
Paradoxically, in some experimental models, IL-10 has an immunostimulatory activity. Systemic injection of IL-10, transfection of tumor cells with IL-10, or the high physiological expression of IL-10 in certain tumors resulted in an increased immunogenicity and rejection of the tumor cells.39-43
These contrasting results might be due to the different tumor models used, the varying amounts of IL-10 used, and the different forms of IL-10 (virus IL-10 v cellular IL-10) applied. It was demonstrated that the antitumor effect of IL-10 was dose-dependent and that only very high levels of IL-10 were effective in tumor rejection.40-43 Furthermore, the amount of IL-10 required for immunosuppression may change for different tumors.
In our model, we demonstrate that a pretreatment of human DC with IL-10 induces a state of antigen-specific anergy in cytotoxic CD8+ T cells. When a tyrosinase-specific CD8+T-cell line was used, the anergic T cells failed to lyse melanoma cells. Therefore, the secretion of IL-10 in the environment of tumor cells might be one mechanism of tumors to inhibit immune surveillance by reversing the properties of human DC from potent stimulatory cells of the immune system to tolerance of inducing cells.
ACKNOWLEDGMENT
The authors thank L. Paragnik for excellent technical assistance and Dr T. Tüting for critical reading of the manuscript.
Supported by the DFG and the BMBF. K.S. was supported by a fellowship of the Deutsche Forschungsgemeinschaft.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kerstin Steinbrink, MD, Department of Dermatology, University of Mainz, Langenbeckstr. 1, D-55131 Mainz, Germany; e-mail:steinbrink@hautklinik.klinik.uni-mainz.de.

![Fig. 1. Inhibitory effects of IL-10–pretreated DC on alloantigen-or anti-CD3–induced proliferation of naive CD8+ T cells: dependence on the state of maturation of DC. DC were generated from peripheral progenitors as described above, resuspended at day 7, and stimulated with IL-1β, IL-6, TNF-, and PGE2 to induce and stabilize the maturation of the DC. IL-10 was added for the last 2 days of culture at various time points after the stimulation: simultaneous additon at day 7 and harvested of the DC at day 9 (A and B) or addition at a later stage of maturation (day 10) and subsequently harvest at day 12 (C and D) . Control and IL-10–treated DC were cocultured with naive CD45 RA+/CD8+ T cells (2 × 105) in allogeneic MLR (A and C) or anti-CD3 assays (B and D). Proliferation was measured by [3H] TdR uptake. The results are representative of five experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1634/4/m_blod40511001ax.jpeg?Expires=1769104988&Signature=2JY2D~UzfRYFY~Mv9DonKvHH5nEKOuF3Q2TG5E0afFItun-FqBxrPfGLJwaPET~o8qH2OLJ9MpBmMRDqfS0M~yBXuFcPouUxaRNTgSdRxSYDqK4s9ZKv-UfAsICj9Z1XPN3xomfBOwYK5TNYPEXqLNksqr4ZYPwHkytGsVByePKCaUUi000XDmpBBRG9h3aKdZcAf9GeAa6b7V5anGtJXtzMO-cLmNBXVxaPRV2qsm5aIzNy5jVJG4WDrTSzex8hX3zDww27cjz3hpg9Orr4qy1u1z6p82NUlZZt51mQWFtFfGwu3LqmmpQdDJznnzuCGjUPFvcmXFbon15bZcXfOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Inhibitory effects of IL-10–pretreated DC on alloantigen-or anti-CD3–induced proliferation of naive CD8+ T cells: dependence on the state of maturation of DC. DC were generated from peripheral progenitors as described above, resuspended at day 7, and stimulated with IL-1β, IL-6, TNF-, and PGE2 to induce and stabilize the maturation of the DC. IL-10 was added for the last 2 days of culture at various time points after the stimulation: simultaneous additon at day 7 and harvested of the DC at day 9 (A and B) or addition at a later stage of maturation (day 10) and subsequently harvest at day 12 (C and D) . Control and IL-10–treated DC were cocultured with naive CD45 RA+/CD8+ T cells (2 × 105) in allogeneic MLR (A and C) or anti-CD3 assays (B and D). Proliferation was measured by [3H] TdR uptake. The results are representative of five experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1634/4/m_blod40511001bx.jpeg?Expires=1769104988&Signature=yp7l8ZB3~YZk7G27WVvAH4BVBKr8B78rPASAQylxom2tzxwfh-~ktNPJ7L~XZ8aIUgEmAsAPHv9eQ2sKbNsFMgqN23TsdCcmkPcVLse9ydT12~EGyl6R50rMsrMU98xsFzAWn1bxRvYhwG3ZZh6jn4f~qiR7j7T2QbiEQpXZWOk-rBtRDGOoA8tlMvk1eymEKtRiGI1Vk2mR3GcVFhCOTGIS84pi75Ue8kPwWDgS2GNritzkFES608LWw4yJqdqx0VAHDmvCeRoQX9DKdN3AVnuXbZXDVgJl2APajmGLEWU3buJJj6hJFib3cuUHykVXn4F4tJ3QdPvyvjQ3iA4eqw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Inhibitory effects of IL-10–pretreated DC on alloantigen-or anti-CD3–induced proliferation of naive CD8+ T cells: dependence on the state of maturation of DC. DC were generated from peripheral progenitors as described above, resuspended at day 7, and stimulated with IL-1β, IL-6, TNF-, and PGE2 to induce and stabilize the maturation of the DC. IL-10 was added for the last 2 days of culture at various time points after the stimulation: simultaneous additon at day 7 and harvested of the DC at day 9 (A and B) or addition at a later stage of maturation (day 10) and subsequently harvest at day 12 (C and D) . Control and IL-10–treated DC were cocultured with naive CD45 RA+/CD8+ T cells (2 × 105) in allogeneic MLR (A and C) or anti-CD3 assays (B and D). Proliferation was measured by [3H] TdR uptake. The results are representative of five experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1634/4/m_blod40511001cx.jpeg?Expires=1769104988&Signature=vSoB48gdYzBb4-m-VdnBUspNweeXUZTUsfKbneLx47n-1Opg7fe5Z7omCNV5oDIgMQYdwjPGl3C5ncSheCMHi9hJvVVeuMBVhu2G-dpcwHXde~9j9EqEUM4KDjWhtdxYVjdcpmQYiXeEnYH4YgGtQr-L~Rt9pWH4R6LfjSG0fv~phU6deG7hHZGXumgw6-FlRdnJtvyuDsUUJMsYboqt7LsazaH1nExkCU-sOockkJ5e9Ctdmfve-1jbBrx5c77N55ichR4Ke3BoeUi9Ji2-J1Aa7X6D6xb8gkVVwWWDvJCwkHzKl70LEzZuALgK6kGUWSRd9xzh18mrFovdqJMukA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Inhibitory effects of IL-10–pretreated DC on alloantigen-or anti-CD3–induced proliferation of naive CD8+ T cells: dependence on the state of maturation of DC. DC were generated from peripheral progenitors as described above, resuspended at day 7, and stimulated with IL-1β, IL-6, TNF-, and PGE2 to induce and stabilize the maturation of the DC. IL-10 was added for the last 2 days of culture at various time points after the stimulation: simultaneous additon at day 7 and harvested of the DC at day 9 (A and B) or addition at a later stage of maturation (day 10) and subsequently harvest at day 12 (C and D) . Control and IL-10–treated DC were cocultured with naive CD45 RA+/CD8+ T cells (2 × 105) in allogeneic MLR (A and C) or anti-CD3 assays (B and D). Proliferation was measured by [3H] TdR uptake. The results are representative of five experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1634/4/m_blod40511001dx.jpeg?Expires=1769104988&Signature=fYT89vWWTpXGU4gzpgoc94gEJ6srx88z1krPuStbhh9kuoU0mHoOrIVSaXmzVyIp8mxeRcBTS6Cz54ocVht-Yldy-fazi1w~6NrnwDS4l6YH27GhA1qduuQTuhBPk1gMu2lje-JUiHXgdkGZGAqmazEN5kgwZVOzX0qt9gUwwzLBu054mG5suqeL2JgSNDi1TvcXE0ZTdyGjqc5N0me1tj2gd1bjaxlSlMkj0ydGqEP3bOCuLIqOsAvnK6YbyMBZBsujXgrgee0e-J082Uy9BvabmSM5HPASqP~dlPCIU-pPSMp8yIersz5bTDhMzf6SwhX5AQvp1gEK839510H2KA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Induction of alloantigen-specific anergy in CD8+ T cells by IL-10–treated DC. Purified naive CD8+ T cells were cultured in medium alone; with untreated, control DC; or with DC pretreated with IL-10 (first coculture). After 36 hours, T cells were rescued, cultured for 1 to 7 days in medium containing low levels of IL-2 (2 U/mL), and subsequently restimulated (second coculture) with untreated, mature DC generated from the same donor (░) or a second unrelated donor (▪). [3H] TdR incorporation was determinated after 48 hours. The left bars show the antigen-specific response and the right bars show the T-cell response to IL-2 (100 U/mL).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1634/4/m_blod40511002x.jpeg?Expires=1769104989&Signature=CNGLznmVgMx3ek~2IHm43yAykPt3GrAki~QsScaFjtDw5pg4AZtQqiwTwLEJKiRxCO8JGxHvJ1izt1IUs7i8fh7LrlOkxdvWPUb2nhiWow6JGsm8fG~VZEv0xc73PLuajnSoF-yQ~TBO-7CyOPxyg3l1g2bF8LF1XgNprKpdyKtoeRE2blkxTA2xk72VpQDefxqwMTCdsCEAJ0vwdVDho37nxmqkglSjQefbgRdIpFBD-CKxClp4HZJBTJ91Mp2iePbQtHYOEpwdPGAeh5liLgy8DoxLjlQDW0pYIZ2sVNY1Uw8Sxmnk54MXObMN5nrTO1saurxZdE8WWxs4ZO7GPA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Inhibition of the proliferation of cytotoxic tyrosinase-specific CD8+ T cells by IL-10–treated DC. Tyrosinase-specific, cytotoxic CD8+ T cells were cultured with the specific tyrosinase peptide, either in combination with HLA.A2+ DC pretreated with IL-10 or untreated. In control experiments, the unspecific peptide MART-1 was added to the culture. Proliferation was measured by incorporation of [3H] TdR after 3 days of culture. The results represent one of three experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1634/4/m_blod40511003x.jpeg?Expires=1769104989&Signature=Er1DWAru90uQMxuFyjhtiTQvKZ6qdLLf7ycb6opfYtgaOvFfepQcfogEYXPvxcZKlbT5SqiTs2KXn~tHAuWP8xEPOZ4-Bdc11EW1lMrxTZiVwJLWWGFIj9kD0VHprcUxRb413elUthcSPEgaOYwtFSf0cU6Y9kd8pSMRbOddqp0Ug2hKkpiZSsjxigY2TaGFCVz-8l5phic5WDaAMpgdKWFtH-fTZZ-HDkfNeIu2d6TD9x2DXX~KKr-PTajxz7dnNdluR98KUiSRZ~UQibEBZE9LJLDs3hkK8ISRjZEXP~t74qEwfDCnApThlXiA6MFiS1Ar0f9CbXnP~6PCqHEGBA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Induction of melanoma antigen-specific anergy in tyrosinase-specific, cytotoxic CD8+ T cells. In a first coculture, tyrosinase-specific HLA.A2+ CD8+T cells were used as responder cells for HLA.A2+ DC additionally pretreated with IL-10 or untreated (cultured in complete medium alone). Cultures were set up in the presence of the specific tyrosinase peptide or an unspecific control peptide (MART-1). Subsequently, the T cells were rescued, cultured for 1 to 7 days in the presence of low levels of IL-2 (2 U/mL), and restimulated with mature HLA.A2+ DC during the second coculture. After 2 days, the proliferation was measured by [3H]TdR incorporation. The left bars show the specific response and the right bars show the T-cell response to IL-2 (100 U/mL). The results are representative for three experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/5/10.1182_blood.v93.5.1634/4/m_blod40511004x.jpeg?Expires=1769104989&Signature=0ruWxubfCfQXbrPkMcDksm0k3ysRaLPKn9RPan6E312q0b02DgJy9G1S~t3TKFJjWKNQ-PF4uZiqeHSqigt8q5KyagVd5JyXzo2pwQJsrgsh9yUk7Y6JPS0ice0UkXm9klE33hT~JvFy6V6YKsHxjegLXvK5f2yx7AsHBkGZwrZtHlljNQxB1l0jf1pVtv4bM3FwwWLwJVGarYexv503v3KbPqMEiPRdZ3yMK4-EPHh6~USWNX1MCy9lhTiShJyZdMJvmXT4pqxIuhdvekjwww~MN3cMOgufcr38gJ98-fOlowtauzuW82tYXJ-VLKVPNsZxzH4eIj9eBujSTbx7Mg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal