Analysis of 15 cases of T-cell acute lymphoblastic leukemia with spectral karyotyping (SKY), which can identify all chromosomes simultaneously, clarified the chromosome rearrangements in 3 cases and confirmed them in 11 others; no abnormal cells were identified in 1 case, which had only 10% abnormal cells. Five of the latter cases had a normal karyotype. Thus, the use of SKY substantially improves the precision of karyotype analysis of malignant cells, which in turn leads to a more accurate assessment of the genotypic abnormalities in those cells.
ACUTE LYMPHOBLASTIC leukemia (ALL) affecting T cells has been associated with a normal karyotype in 30% to 40% of patients.1-3 This is in contrast to B-cell ALL, in which about 10% to 25% of patients have a normal karyotype in their leukemic cells.2 One concern regarding such data is the possibility that a recurring translocation may have been overlooked. This concern is reinforced by the observation only a few years ago of the presence of the t(12;21) in 25% of childhood pre–B-cell ALL. This translocation was identified by Berger et al,4 using a chromosome 12 painting probe. The translocation breakpoints were subsequently cloned and shown to involveTEL (ETV6) on chromosome 12 and AML1(CBFA2) on chromosome 21.5 6
We therefore undertook a study of T-cell ALL to determine whether there was an overlooked recurring translocation analogous to the t(12;21). We used spectral karyotyping (SKY) to perform this study because we wanted to examine the entire genome in one hybridization.7-9 In our present study, SKY clarified the karyotype abnormalities in a number of cases, but it did not detect any unexpected recurring aberrations.
MATERIALS AND METHODS
We have karyotyped 33 patients with T-cell ALL from 1988 to 1997. Of these, 7 had a normal karyotype in the bone marrow sample obtained at diagnosis, 4 had known recurring abnormalities [t(8;14), t(11;14)], and 22 had random, often complex, karyotype rearrangements. We had material for SKY analysis for 17 patients, 5 normal and 12 abnormal. Cells from 2 of the abnormal samples had such poor morphology that precise analysis was impossible, and these samples are excluded from the summary that follows. Therefore, this report is based on 15 patients. There were 11 males and 4 females; 11 were children 16 years of age or under, and 4 were older than 16. The diagnosis of T-cell ALL was based on immunophenotypic markers, including positivity for CD2, CD5, and/or CD7 and absence of B-cell markers (eg, HLA-DR or CD19) or myeloid markers. The samples were obtained with informed consent. They were obtained at diagnosis in 12 cases and at relapse in 3 cases; the analysis was performed using bone marrow in 12 cases and peripheral blood in 3 cases.
We used previously prepared slides from 13 patients; for 2 patients, we used freshly prepared slides from material stored in fixative for up to 8 years. We hybridized one or two slides for each case, using the SKY fluorescence in situ probe according to the protocol recommendations by the manufacturer (Applied Spectral Imaging, Carlsbad, CA). For each case, between 4 and 12 (average, 8) metaphase cells were captured and analyzed, using the SD200 system (Applied Spectral Imaging). Each case that presented questionable or nonobvious G-banded chromosome rearrangements was then analyzed using the appropriate single- or dual-color chromosome paints (WCPs; Vysis, Downers Grove, IL). Chromosome probes for 2, 5, 8, 12, 14, 16, and 21 were labeled with Spectrum Orange™ (SO); those for 4, 7, 13, X, and Y were labeled with Spectrum Green™ (SG), and both fluorochromes were used for chromosomes 1, 6, and 9. Coatasome 17 (Oncor, Gaithersburg, MD) was labeled with digoxigenin.
RESULTS
The clinical data, as well as the conventional cytogenetic analysis and the summary of the SKY results, are included in Table1.
Summary of Clinical and Cytogenetic Data
| Case . | Age/ Sex . | Status/ Source* . | Conventional Karyotype . | SKY Karyotype . | Comments† . |
|---|---|---|---|---|---|
| 1 | 2/F | DX/BM | 46,X,inv(X)(p22q28),add(1)(q32),der(5) t(1;?;5)(q32;?;q23),add(6)(q27), del(9) (p2?1p24)[25]/46,XX[5] | 46,X,inv(X),t(1;5)(q3?2;q3?1),der(6)t(5;6)(q31;q2?7),del(9p)[12] | Vysis WCP 1 sg+5so, 1sg+6so, and 5so+6sg confirmed t(1;5) and der(6) t(5;6). SKY CLARIFIED ANALYSIS |
| 2 | 50/M | DX/BCore | 43,X,−Y,der(12)t(12;13)(p11.2;q11),−13,add(14)(q32),add(17) (q25),−19, add(21) (p13)[9]/43,idem,del(6)(q13q25)[3]/46,XY[8] | 43,X,−Y,t(6;17),der(12)t(12;13), −13,−13,dup(14)(q24q32), der(21)t(Y;21)[1]/42,X,−Y,t(6;17), der(12)t(12;13),−13−13,−19, der(21)t(Y;21)[1]/42,X,−Y,t(6;17), der(12)t(12;13),−13,−13, dup(14q),−16, der(21)t(Y;21)[1]/39,X,−Y,−5,t(6;17), −7,−11,der(12)(12;13),−13,−13, dup(14q),der(21)t(Y;21)[1]/41,X,−Y,−3,t(6;17), −12,der(12)t(12;13),−13, −19,?+20,der(21)t(Y;21)[1]/45,X,−Y,?+3,?+6,t(6;17),der(12)t(12;13),−13, −13,dup (14q),der(21)t(Y;21)[1] | All 14’s classified as normal; Vysis WCP6 +Oncor COatasome 17 dig confirmed t(6;17); Ysg+21so confirmed der(21) t(Y;21) 12so+13sg confirmed der(12) t(12;13); SKY CLARIFIED ANALYSIS |
| 3 | 36/M | RD/BM | 47,XY,+?Y,dup(1)(p22p36.1),t(7;18) (p13;p11),?add(14)(q32)[10]/46,idem, add(19)(p13 or q13)[3]/46,XY[7] | 47,XY,+Y,dup(1p),der(14)t(7;14) (p13;q32),der(18)t(4;18)(p1?2;p1?2), der(19)t(9;19)(q34;p or q13)[7]/46,XY[3] | Vysis WCP 7sg+14so confirmed der(14) t(7;14) der(19) t(9;19); 4sg+6so confirmed der(18) t(4;18). 7sg+WCP 14so confirmed 7on 14 but no evidence for 14 on 7; SKY CLARIFIED ANALYSIS |
| 4 | 15/F | DX/PB | 46,X,t(X;7)(q13;q32),t(8;9)(q21;p2?4),del(9)(p13p22)[16]/46,idem, del(6) (q1?5q2?3)[9]/45,idem,−6[4]/46,XX[2] | 46,X,t(X;7),t(8;9),del(9p)[6]/46,idem,del(6q)[4] | Vysis WCP 8so+9sg confirmed t(8;9); SKY CONFIRMED ANALYSIS |
| 5 | 9/F | DX/PB | 46,XX,t(1;16)(q21;q24)[29] | 46,XX,t(1;16)[6]/46,XX[1] | Vysis WCP 1sg+16so confirmed t(1;16). SKY CONFIRMED ANALYSIS |
| 6 | 0/F | DX/BM | 46,XX,t(7;11)(q34;p11)[19]/46,XX[4] | 46,XX,(7;11)[6]/46,XX[2] | SKY CONFIRMED ANALYSIS |
| 7 | 5/M | DX/PB | 46,XY,del(9)(p13p22 or p22p24)[5]/46,idem, del(6)(q15q25),[3]/46,XY[24] | 46,XY,del(9p)[2]/46,XY,idem,del(6q)[2]/46,XX[1] | SKY CONFIRMED ANALYSIS |
| 8 | 25/M | DX/BM | 46,XY,del(1)(p32p34)[1]/46,idem,inv(7)(p15q34)[17]/46,XY[13] | 46,XY,del(1)(p),inv(7)[2]/46,XY[2] | SKY CONFIRMED ANALYSIS |
| 28 | Relapse BM (post BMT)‡ | 46,XY,del(1),add(1)(p21),t(2;15)(q37;q15),del(5)(q13q35),del(6)(q23q25), inv(7),der(17)t(1;17)(q23;p11)[9]/46,XY,[11] | 46,XY,del(1)(p),ins(1)(p34;q2?3q32),t(2;15)(q3?3;q1?5),del(5),del(6),inv(7),t(12;14)(q24;q3?2),der(17)t(1;17)(q3?2;p11)[6]/46,XY[1] | A painting probe for chromosome 12 and a telomere probe for 14q confirmed the t(12;14) SKY CLARIFIED ANALYSIS | |
| 9 | 3/M | DX/BM | 46,XY,t(9;9)(p11;q11),del(14)(q21q32) [14]/90,XXYY,t(9;9)(p11;q11)X2, del(14)(q21q32)X2,−15,−?15[3]/46,XY[3] | 46,XY,t(9;9),del(14)[4]/89-92[2] | SKY CONFIRMED ANALYSIS |
| 10 | 6/M | DX/BM | 46,XY,t(11;14)(p13;q11)[2]/46,XY[21] | 46,XY[7] | No abnormal cells identified. |
| 11 | 7/M | RL?/BM‡ | 46,XY[23] | 46,XY[8] | SKY CONFIRMED ANALYSIS |
| 12 | 10/M | DX/BM | 46,XY,inv(9)c[45] | 46,XY[4] | SKY CONFIRMED ANALYSIS |
| 13 | 16/M | DX/BM | 46,XY[30] | 46,XY[9] | SKY CONFIRMED ANALYSIS |
| 14 | 30/M | RD‡/BM | 46,XY[20] | 46,XY[7] | SKY CONFIRMED ANALYSIS |
| 15 | 6/M | DX/BM | 46,XY[22] | 46,XY[5] | SKY CONFIRMED ANALYSIS |
| Case . | Age/ Sex . | Status/ Source* . | Conventional Karyotype . | SKY Karyotype . | Comments† . |
|---|---|---|---|---|---|
| 1 | 2/F | DX/BM | 46,X,inv(X)(p22q28),add(1)(q32),der(5) t(1;?;5)(q32;?;q23),add(6)(q27), del(9) (p2?1p24)[25]/46,XX[5] | 46,X,inv(X),t(1;5)(q3?2;q3?1),der(6)t(5;6)(q31;q2?7),del(9p)[12] | Vysis WCP 1 sg+5so, 1sg+6so, and 5so+6sg confirmed t(1;5) and der(6) t(5;6). SKY CLARIFIED ANALYSIS |
| 2 | 50/M | DX/BCore | 43,X,−Y,der(12)t(12;13)(p11.2;q11),−13,add(14)(q32),add(17) (q25),−19, add(21) (p13)[9]/43,idem,del(6)(q13q25)[3]/46,XY[8] | 43,X,−Y,t(6;17),der(12)t(12;13), −13,−13,dup(14)(q24q32), der(21)t(Y;21)[1]/42,X,−Y,t(6;17), der(12)t(12;13),−13−13,−19, der(21)t(Y;21)[1]/42,X,−Y,t(6;17), der(12)t(12;13),−13,−13, dup(14q),−16, der(21)t(Y;21)[1]/39,X,−Y,−5,t(6;17), −7,−11,der(12)(12;13),−13,−13, dup(14q),der(21)t(Y;21)[1]/41,X,−Y,−3,t(6;17), −12,der(12)t(12;13),−13, −19,?+20,der(21)t(Y;21)[1]/45,X,−Y,?+3,?+6,t(6;17),der(12)t(12;13),−13, −13,dup (14q),der(21)t(Y;21)[1] | All 14’s classified as normal; Vysis WCP6 +Oncor COatasome 17 dig confirmed t(6;17); Ysg+21so confirmed der(21) t(Y;21) 12so+13sg confirmed der(12) t(12;13); SKY CLARIFIED ANALYSIS |
| 3 | 36/M | RD/BM | 47,XY,+?Y,dup(1)(p22p36.1),t(7;18) (p13;p11),?add(14)(q32)[10]/46,idem, add(19)(p13 or q13)[3]/46,XY[7] | 47,XY,+Y,dup(1p),der(14)t(7;14) (p13;q32),der(18)t(4;18)(p1?2;p1?2), der(19)t(9;19)(q34;p or q13)[7]/46,XY[3] | Vysis WCP 7sg+14so confirmed der(14) t(7;14) der(19) t(9;19); 4sg+6so confirmed der(18) t(4;18). 7sg+WCP 14so confirmed 7on 14 but no evidence for 14 on 7; SKY CLARIFIED ANALYSIS |
| 4 | 15/F | DX/PB | 46,X,t(X;7)(q13;q32),t(8;9)(q21;p2?4),del(9)(p13p22)[16]/46,idem, del(6) (q1?5q2?3)[9]/45,idem,−6[4]/46,XX[2] | 46,X,t(X;7),t(8;9),del(9p)[6]/46,idem,del(6q)[4] | Vysis WCP 8so+9sg confirmed t(8;9); SKY CONFIRMED ANALYSIS |
| 5 | 9/F | DX/PB | 46,XX,t(1;16)(q21;q24)[29] | 46,XX,t(1;16)[6]/46,XX[1] | Vysis WCP 1sg+16so confirmed t(1;16). SKY CONFIRMED ANALYSIS |
| 6 | 0/F | DX/BM | 46,XX,t(7;11)(q34;p11)[19]/46,XX[4] | 46,XX,(7;11)[6]/46,XX[2] | SKY CONFIRMED ANALYSIS |
| 7 | 5/M | DX/PB | 46,XY,del(9)(p13p22 or p22p24)[5]/46,idem, del(6)(q15q25),[3]/46,XY[24] | 46,XY,del(9p)[2]/46,XY,idem,del(6q)[2]/46,XX[1] | SKY CONFIRMED ANALYSIS |
| 8 | 25/M | DX/BM | 46,XY,del(1)(p32p34)[1]/46,idem,inv(7)(p15q34)[17]/46,XY[13] | 46,XY,del(1)(p),inv(7)[2]/46,XY[2] | SKY CONFIRMED ANALYSIS |
| 28 | Relapse BM (post BMT)‡ | 46,XY,del(1),add(1)(p21),t(2;15)(q37;q15),del(5)(q13q35),del(6)(q23q25), inv(7),der(17)t(1;17)(q23;p11)[9]/46,XY,[11] | 46,XY,del(1)(p),ins(1)(p34;q2?3q32),t(2;15)(q3?3;q1?5),del(5),del(6),inv(7),t(12;14)(q24;q3?2),der(17)t(1;17)(q3?2;p11)[6]/46,XY[1] | A painting probe for chromosome 12 and a telomere probe for 14q confirmed the t(12;14) SKY CLARIFIED ANALYSIS | |
| 9 | 3/M | DX/BM | 46,XY,t(9;9)(p11;q11),del(14)(q21q32) [14]/90,XXYY,t(9;9)(p11;q11)X2, del(14)(q21q32)X2,−15,−?15[3]/46,XY[3] | 46,XY,t(9;9),del(14)[4]/89-92[2] | SKY CONFIRMED ANALYSIS |
| 10 | 6/M | DX/BM | 46,XY,t(11;14)(p13;q11)[2]/46,XY[21] | 46,XY[7] | No abnormal cells identified. |
| 11 | 7/M | RL?/BM‡ | 46,XY[23] | 46,XY[8] | SKY CONFIRMED ANALYSIS |
| 12 | 10/M | DX/BM | 46,XY,inv(9)c[45] | 46,XY[4] | SKY CONFIRMED ANALYSIS |
| 13 | 16/M | DX/BM | 46,XY[30] | 46,XY[9] | SKY CONFIRMED ANALYSIS |
| 14 | 30/M | RD‡/BM | 46,XY[20] | 46,XY[7] | SKY CONFIRMED ANALYSIS |
| 15 | 6/M | DX/BM | 46,XY[22] | 46,XY[5] | SKY CONFIRMED ANALYSIS |
White blood cell count (109/L) with percent blasts in parentheses is as follows: case 4, 234 (96%); case 5, 32.5 (80%); case 7, 950; case 10, 14.4; case 12, 110 (99.5%); case 13, 17.6 (94%); case 15, 13.1.
sg and so are Spectrum Green and Spectrum Orange, respectively (Vysis, Downers Grove, IL).
Two years before this sample, patient had a bone marrow transplant. A subsequent sample 13 months later had multiple clones with many abnormalities.
Case 1.
The G-banded karyotype showed complex rearrangements including an interchange involving chromosomes 1, 5, and 6. SKY was not informative with regard to the inverted X chromosome, but in cells with adequate DAPI banding, it could be identified because of the abnormal position of the centromere. The del(9p) was confirmed. SKY was very helpful in sorting out the 1, 5, 6 rearrangement (Fig1, upper portion). The der(1) had part of chromosome 5 translocated to the long arm; the der(5) had part of chromosome 1, and these could represent a balanced t(1;5)(q31;q32). One chromosome 6 also had material from 5, most likely from 5q, because it seemed to be too large to be from 5p. In addition, there is one normal chromosome 5. The chromosome 1, 5, and 6 abnormalities were confirmed using painting probes for these three chromosomes. The genetic consequences of these changes are loss of part of 6q and 9p and gain of 5, possibly 5q.
Examples of the analysis of cells from 2 patients showing the DAPI, SKY, and classified images as well as the results using painting probes. (Top two rows, Case 1) (Left) Reverse DAPI image with the der(1), der(5), and der (6) chromosomes as well as normal 9 and del (9p) identified by arrows. (Middle) SKY image; (right) classified image. (Second row right) The classified karyotype of this cell. (Second row) Three panels show the results with painting probes for chromosomes 1, 5, and 6, confirming (left) that chromosome 5 material is on both the der(1) and der(6), (middle left) that the t(1;5) is reciprocal, and (middle right) that there is no material from chromosome 6 on either the der(1) or the der(5). (Bottom two rows, Case 3) (Left) Reverse DAPI image with the abnormal chromosomes including two Y chromosomes and dup(1p), del(7p), der(14), der(18), and der(19), are identified by arrows. (Middle) SKY image of same cell; (right) classified image. (Bottom row right) Classified karyotype. (Bottom row) Three panels showing the results of painting probes for chromosomes 4, 7, and 9, confirming (left) that part of chromosome 4 is on the der(18), (middle left) that part of 7, presumably 7p, is on the der(14), and (middle right) that part of 9 is on the der(19).
Examples of the analysis of cells from 2 patients showing the DAPI, SKY, and classified images as well as the results using painting probes. (Top two rows, Case 1) (Left) Reverse DAPI image with the der(1), der(5), and der (6) chromosomes as well as normal 9 and del (9p) identified by arrows. (Middle) SKY image; (right) classified image. (Second row right) The classified karyotype of this cell. (Second row) Three panels show the results with painting probes for chromosomes 1, 5, and 6, confirming (left) that chromosome 5 material is on both the der(1) and der(6), (middle left) that the t(1;5) is reciprocal, and (middle right) that there is no material from chromosome 6 on either the der(1) or the der(5). (Bottom two rows, Case 3) (Left) Reverse DAPI image with the abnormal chromosomes including two Y chromosomes and dup(1p), del(7p), der(14), der(18), and der(19), are identified by arrows. (Middle) SKY image of same cell; (right) classified image. (Bottom row right) Classified karyotype. (Bottom row) Three panels showing the results of painting probes for chromosomes 4, 7, and 9, confirming (left) that part of chromosome 4 is on the der(18), (middle left) that part of 7, presumably 7p, is on the der(14), and (middle right) that part of 9 is on the der(19).
Case 2.
A complex pattern of abnormalities was noted with G-banding, which included loss of the Y chromosome, a 12;13 rearrangement, and additional unidentified material on chromosomes 14, 17, and 21, as well as a second clone with deletion of 6q added to these abnormalities. Using SKY, we confirmed the 12;13 rearrangement and clarified the origin of the other rearrangements (Fig 2, upper right). The large chromosome 14 is probably a duplication, because there was no evidence for a translocation of other chromosomal material. The add(21) was a derivative (Y;21); the origin of the centromere was unclear. In addition, there was an apparently balanced undetected translocation involving chromosomes 6 and 17, with material from 17q translocated to 6q and 6q to 17q, resulting in the der(17) chromosome. The involvement of chromosomes 6, 17, 12, 13, 21, and the Y was confirmed with the appropriate sets of painting probes. The consequences of the rearrangements were loss of 12p, probable duplication of the telomeric portion of 14q, and loss of the centromere-short arm region of 13 and material from either the Y or chromosome 21.
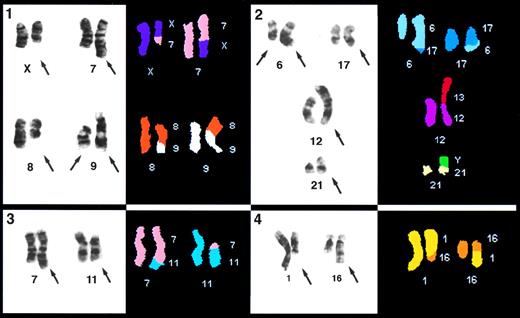
Partial karyotypes of cells with rearranged chromosomes; the involved chromosomes from the G-banded karyotype on the left and the classified images on the right. The chromosome origin of the classified image is listed for each chromosome. In panel 1, note that for Case 4, the left chromosome 9 has a deletion of the p arm. Panel 2 is Case 2, panel 3 is Case 6, and panel 4 is Case 5.
Partial karyotypes of cells with rearranged chromosomes; the involved chromosomes from the G-banded karyotype on the left and the classified images on the right. The chromosome origin of the classified image is listed for each chromosome. In panel 1, note that for Case 4, the left chromosome 9 has a deletion of the p arm. Panel 2 is Case 2, panel 3 is Case 6, and panel 4 is Case 5.
Case 3.
Standard G-banding identified a hyperdiploid karyotype with a probable gain of a Y chromosome, a duplication of 1p, possibly additional material on 14q, a 7;18 translocation, and a second clone with an add (19p or 19q) in addition to these abnormalities. SKY confirmed that the extra small chromosome was a Y and the duplication 1p contained only material from chromosome 1 (Fig 1, lower portion). The add(14) was shown to result from a translocation from 7p. The short arm of chromosome 18 was involved with chromosome 4, possibly the short arm of chromosome 4. Involvement of chromosomes 4, 7, 9, and 14 was confirmed with the appropriate painting probes; we could find no evidence of chromosome 14 on chromosome 7. Therefore, the consequences of these rearrangements is an extra Y, duplication of 1p, loss of part of 14q, and an extra copy of some portion of chromosomes 4 and 9, both as yet undefined.
SKY confirmed conventional G-banded karyotype analysis in 6 abnormal cases. In one case (Case 10), no abnormal cells were detected; however, abnormal cells were identified in fewer than 10% (2 of 23) of the cells in the bone marrow (BM) sample analyzed in the clinical laboratory. G-banded analysis of Case 4 showed t(X;7), t(8;9), and del(9p), all of which were confirmed by SKY; no additional abnormalities were identified (Fig 2, upper left). The 8;9 translocation was also confirmed with painting probes. The genetic consequence was loss of 9p. With G-banding, 29 cells of Case 5 had a t(1;16), which was confirmed using SKY (Fig 2, lower right). The translocation was also confirmed with painting probes for chromosomes 1 and 16. The G-banded karyotype in Case 6 showed a t(7;11) in 19 of 23 cells. This was confirmed by SKY, which also confirmed that it was a balanced translocation (Fig 2, lower left). The G-banded analysis of Case 7 showed a del(9p) in all five cells and a del(6q) in three of them. Thus, the genetic consequence was loss of part of 9p in all cells and of 6q in some cells.
The G-banded karyotype in Case 8 showed a deletion of 1p in all abnormal cells and an inv(7) in most of the abnormal cells. SKY confirmed del(1p) and inv(7) in two cells.
We studied a sample from case 8 obtained in first relapse, 3.5 years after the initial sample. The original abnormalities were present as well as several new ones. The cells had a t(2;15)(q37;q15) that was confirmed by SKY. The breakpoints were modified based on SKY to 2q3?3 and 15q1?5. The karyotype contained an add(1)(p21) as well as a der(17)t(1;17)(q23;p11). SKY showed that the add(1) contained only chromosome 1 material. The banding pattern of the dark band at the end of the short arm appeared to be 1q31. In addition, the der(17) lacked any evidence of 1q31. Therefore, a plausible explanation is that there was a break in 1q23 with 1q23 to 1q32 being translocated to the add(1p) and 1q32 to 1qter being translocated to the der(17). Deletions of 5q and 6q were confirmed by SKY. In addition, an undetected translocation involving the long arm of both chromosomes 12 and 14 was observed; this rearrangement was confirmed using a painting probe for chromosome 12 and a telomere probe for 14q.
This relapse sample was obtained after extensive chemotherapy, radiotherapy, and a bone marrow transplant; a deletion of 5q is common in this situation. The additional structural rearrangements could also be secondary to exposure to these mutagenic agents. The results of the chromosome rearrangements are loss of material from 5q, 6q, and 17p and triplication of genetic material from most of 1q. The genetic consequence of the 12;14 translocation is unclear.
An interchange between both chromosomes 9 as well as a deletion of one chromosome 14 was seen on conventional karyotyping in Case 9. These abnormalities were confirmed with SKY. Case 10 had a t(11;14) seen in 2 of 23 cells. Only 7 cells were analyzed using SKY, and all had a normal karyotype.
We examined cells from 5 patients (Cases 11 through 15) said to have a normal karyotype; four of these patients were younger than 17 years of age. Based on SKY analysis of about 7 cells per patient (range, 4 to 9 cells), all of the cases seemed to have a normal karyotype. This number of cells is too low for our results to be considered more than preliminary. However, considering that all 5 were bone marrow samples at diagnosis, which for Cases 12 and 13 contained 99.5% and 94% blasts, respectively, our data suggest that leukemia cells of some patients may have a normal karyotype.
DISCUSSION
SKY clarified the abnormalities in three patients with complex karyotypes. In each case, the standard G-banded analysis showed chromosomes that had added unidentified material. SKY confirmed the analysis of G-banded chromosomes in 8 cases that had balanced translocations or deletions alone or in combination. SKY also confirmed the presence of a normal karyotype in five patients. We did not identify any recurring translocations in the patients with a normal karyotype analogous to the recently discovered t(12;21) in B-cell ALL. However, the number of patients with normal karyotypes examined, especially of children, was small; moreover, only about seven cells were analyzed for each of these patients.
The most common abnormality seen in three of our patients was a del(9p), accompanied in all three by a del(6q); these abnormalities were identified by standard G-banding. We identified two balanced and one unbalanced translocations involving chromosome 9, one on the short arm and two on the long arm. All of these occurred in cells with 6q deletions, and in two patients, the del(6q) was clearly a secondary event. One patient had an unbalanced translocation involving 12p, leading to a deletion of part of 12p. Deletions of 6q, 9p, and 12p are well recognized recurring abnormalities in ALL of both the B- and T-cell lineages.1-3 In the recent report from CCG,2 31 of 169 children with T-cell ALL had del(6q), and 15 had del(9p). In the past, we and others have suggested that, in fact, del(9p) may be more common in T-cell ALL than in B-cell ALL.10-12 The analysis of karyotype and survival of children with T-cell ALL showed that there was no statistically significant difference among various cytogenetic subgroups.2
There has been a significant improvement in the software used to analyze the spectral images, although there are still technical problems that can interfere with an optimal analysis. For example, the results vary considerably depending on the amount of cytoplasm remaining after the hybridization, which may lead to background and misclassification of some chromosomes. In addition, new software that produces an improved reverse DAPI image allows more accurate G-banded analysis and thus, better identification of deletions, duplications, and inversions. However, at present, SKY should be used as an adjunct to classical cytogenetics. No matter how good the resolution may seem, it is still not as precise as a G-banded metaphase cell because of the harsh treatment of the chromosomes during the hybridization process.
At present, the type of therapy administered to patients is often based on the genetic aberrations identified in the patient’s cells, as defined by specific chromosome abnormalities. There seems to be no correlation between cytogenetic subgroups using conventional cytogenetic analysis and survival in children with T-cell ALL.2 Accurate cytogenetic analysis is the best, and in fact, for some aberrations, it is the only means of detecting these changes. Thus, for the foreseeable future, any technique that enhances our ability to define the genetic changes in cells, and therefore, results in more accurate karyotypic diagnosis, may potentially have a measurable impact on treatment. As we have shown in this report, the cytogenetic findings in 3 of 15 patients were modified based on SKY. All 3 patients had abnormalities that were recognized by the cytogeneticists as being incompletely characterized. Except for one case (No. 10) which had only 10% abnormal cells, SKY confirmed the original analysis in the other 11 cells. Thus, SKY provides an additional tool that will be important to clarify the karyotype in patients in whom complex and incompletely defined abnormalities were identified on standard karyotype analysis.
ACKNOWLEDGEMENT
The authors thank Dr Michelle LeBeau for providing the conventional cytogenetic data, Margie Isaacson for data management and Elizabeth Davis and Raphael Espinosa for assistance with SKY hybridization and preparation of the figures.
Supported by National Cancer Institute Grant No. CA42557 (J.D.R.); Purchase of the SKY equipment was partially funded by Amgen, Inc, Thousand Oaks, CA.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Janet D. Rowley, MD, DSc, University of Chicago, Medical Center, Section of Hematology/Oncology, 5841 S Maryland Ave, MC 2115, Chicago, IL 60637-1470; e-mail:jdrowley@mcis.bsd.uchicago.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal