Abstract
The flt3 ligand (FL) is a growth factor for primitive hematopoietic cells. Serum levels of FL are inversely related to the number and proliferative capacity of early hematopoietic progenitors. We sought to elucidate the molecular mechanism underlying this regulation. Expression of FL was examined in peripheral blood (PB) and bone marrow (BM) cells under normal steady-state hematopoiesis and during transient BM failure induced by chemoradiotherapy in 16 patients with hematological malignancies. Using anti-FL antibodies in Western analysis, flow cytometry, and confocal microscopy, we detected high levels of preformed FL inside but not on the surface of T lymphocytes in steady-state hematopoiesis. Intracellular FL colocalized with giantin and ERGIC-53, indicating that it is stored within and close to the Golgi apparatus. After chemotherapy-induced hematopoietic failure, FL rapidly translocated to the surface of T lymphocytes and the levels of FL released to serum increased approximately 100-fold. Expression of FL mRNA was enhanced only about sevenfold; a similar, twofold to sixfold increase in mRNA was observed in the thymus and BM of mice with irradiation-induced aplasia. Upregulation of FL mRNA was delayed when compared with the appearance of cell surface-associated and soluble protein isoforms. The described changes in FL expression in response to chemotherapy-induced aplasia were observed in all patients, irrespective of the diagnosis and treatment regimen. Our data demonstrate that mobilization of preformed FL from intracellular stores rather than de novo synthesis is responsible for increased FL levels in BM failure.
HEMATOPOIESIS is a multistep cell proliferation and differentiation process sustained by a pool of hematopoietic stem cells located primarily in the bone marrow (BM). Pathways that lead from individual stem cells to the various terminally differentiated blood cells are tightly regulated and involve complex interactions between soluble and membrane-bound growth factors and their corresponding cell surface receptors.1,2 The flt3 ligand (FL) belongs to the small family of hematopoietic cytokines, including also stem cell factor (SCF) and macrophage colony-stimulating factor (M-CSF), that are specific for tyrosine kinase receptors.3-5 The flt3 receptor is predominantly expressed on primitive hematopoietic progenitors, an indication that signaling through this receptor is important in the very early steps of hematopoiesis.6 Indeed, FL has proven to be a potent cytokine for in vitro expansion and in vivo mobilization of stem cells (reviewed in Lyman and Jacobsen7). It also increases the yield of dendritic cells generated from hematopoietic precursors8,9 and effectively enhances antitumor immune responses in mice.10 So far, most studies have focused on elucidating the biological properties of FL and less attention has been paid to the mechanisms regulating expression of the cytokine in human hematopoiesis. In contrast to the restricted distribution offlt3 receptors, FL mRNA is ubiquitously expressed in hematopoietic and nonhematopoietic tissues.4,11 Analysis of multiple splice variants of human FL mRNA indicates that the predominant form of FL is a cell surface transmembrane protein, which presumably generates soluble FL upon proteolytic cleavage.12 The protease responsible for this cleavage has not yet been identified, and neither is it known whether the two FL isoforms have different functions in hematopoiesis.
Serum levels of FL increase dramatically, from an average of 14 up to 7,000 pg/mL, in diseases characterized by a deficiency of primitive hematopoietic progenitors, such as congenital and acquired aplastic anemia, and during transient pancytopenia induced by high-dose chemotherapy.13,14 FL levels do not increase in diseases affecting single blood lineages,13 in contrast to lineage-specific growth factors such as erythropoietin, thrombopoietin, and granulocyte colony-stimulating factor, which are elevated in pure red blood cell aplasia, congenital thrombocytopenia, and severe congenital neutropenia, respectively.15-17 Moreover, response to multilineage hematopoietic damage is specific for FL, because serum levels of a related early acting cytokine, SCF, do not change in chemotherapy-induced aplasia or aplastic anemia.18 19 These results suggest that FL has a nonredundant role in the compensatory hematopoietic response in BM failure aimed at replenishment of the stem cell compartment and restoration of normal hematopoiesis.
The mechanism responsible for the dramatic increase in circulating FL levels in patients with BM failure has not been identified. In this study, we investigated the regulation of FL expression in peripheral blood (PB) and BM cells during chemotherapy-induced transient aplasia. The results indicate that, under physiological steady-state conditions, FL is produced constitutively but retained intracellularly within and close to the Golgi apparatus. In hematopoietic failure, FL is rapidly translocated to the cell surface of T lymphocytes. The existence of such a regulatory mechanism securing a rapid supply of FL in hematopoietic deficiency argues for the importance of this cytokine in reconstitution of BM function in primary and iatrogenic BM failure.
MATERIALS AND METHODS
Patients and controls.
Sixteen patients with different hematological malignancies who underwent myeloablative therapy were included in the study; 7 patients underwent chemotherapy and 9 patients were transplanted. For the detailed characteristics of the disease and therapy, see Table 1. All PB and BM samples were obtained with informed consent in compliance with the guidelines of the Ethical Committee of the University Hospitals of Basel (Basel, Switzerland). Control PB and BM from normal donors were used after informed consent was received.
Patient Characteristics
| Patient . | Age/ Sex . | Diagnosis . | Duration of Disease (mo) . | Clinical Status . | Treatment . |
|---|---|---|---|---|---|
| M.E. | 55/F | MDS, RAEB | 14 | Transition into AML | CT |
| F.B. | 58/M | NHL | 21 | Rel | Cond/syn-PBSCT |
| A.R. | 52/F | CML | 4 | CP | Cond/syn-PBSCT |
| L.P. | 65/F | AML, M5 | 2 | CR | CT |
| B.G. | 30/F | BL | 2 | PR | CT |
| L.B. | 27/F | NHL | 3 | CR | Cond/syn-PBSCT |
| M.T. | 38/F | PRCA | 210 | CP | Cond/syn-PBSCT |
| C.L. | 42/M | AML, M2 | 7 | PR | Cond/allo-BMT |
| C.B. | 34/F | AML, M4 | 4 | PR | CT |
| R.M. | 45/F | ALL | 1 | CR | Cond/allo-PBSCT |
| C.H. | 38/F | CML | 2 | AP | Cond/allo-PBSCT |
| H.P. | 61/M | AML, M2 | 59 | PR | CT |
| L.J. | 62/M | AML, M2 | 5 | PR | CT |
| K.B. | 31/M | CML | 7 | PR | Cond/allo-BMT |
| M.J. | 35/F | MM | 8 | CP | Cond/allo-PBSCT |
| Y.M. | 65/F | AML, M4 | 7 | PR | CT |
| Patient . | Age/ Sex . | Diagnosis . | Duration of Disease (mo) . | Clinical Status . | Treatment . |
|---|---|---|---|---|---|
| M.E. | 55/F | MDS, RAEB | 14 | Transition into AML | CT |
| F.B. | 58/M | NHL | 21 | Rel | Cond/syn-PBSCT |
| A.R. | 52/F | CML | 4 | CP | Cond/syn-PBSCT |
| L.P. | 65/F | AML, M5 | 2 | CR | CT |
| B.G. | 30/F | BL | 2 | PR | CT |
| L.B. | 27/F | NHL | 3 | CR | Cond/syn-PBSCT |
| M.T. | 38/F | PRCA | 210 | CP | Cond/syn-PBSCT |
| C.L. | 42/M | AML, M2 | 7 | PR | Cond/allo-BMT |
| C.B. | 34/F | AML, M4 | 4 | PR | CT |
| R.M. | 45/F | ALL | 1 | CR | Cond/allo-PBSCT |
| C.H. | 38/F | CML | 2 | AP | Cond/allo-PBSCT |
| H.P. | 61/M | AML, M2 | 59 | PR | CT |
| L.J. | 62/M | AML, M2 | 5 | PR | CT |
| K.B. | 31/M | CML | 7 | PR | Cond/allo-BMT |
| M.J. | 35/F | MM | 8 | CP | Cond/allo-PBSCT |
| Y.M. | 65/F | AML, M4 | 7 | PR | CT |
Abbreviations: MDS, myelodysplastic syndrome; RAEB, refractory anemia with excess blasts; AML, acute myeloid leukemia; M2, M4, and M5, AML variants according to the French-American-British classification system; ALL, acute lymphocytic leukemia; NHL, non-Hodgkin’s lymphoma; CML, chronic myeloid leukemia; MM, multiple myeloma; BL, Burkitt’s lymphoma; PRCA, pure red blood cell aplasia; Rel, relapse; CP, chronic phase; AP, accelerated phase; CR, complete remission; PR, partial remission; CT, chemotherapy; Cond, conditioning before transplantation (high-dose CT and total body irradiation); PBSCT, peripheral blood stem cell transplantation; allo-, allogeneic; syn-, syngeneic; BMT, bone marrow transplantation.
Cell purification.
Mononuclear cells from heparinized PB or BM (PBMC and BMMC, respectively) were isolated by Ficoll-Hypaque density gradient centrifugation (d = 1.077). For flow cytometry (fluorescence-activated cell sorting [FACS]), confocal microscopy, RNA preparation, and protein extraction (see below), cells were used immediately without prior freezing. T cells were purified from PBMC of normal donors. For confocal microscopy, they were rosetted with sheep erythrocytes. For in vitro cultures, negative selection using super-paramagnetic MACS MicroBeads (Miltenyi Biotech, Auburn, CA) was used. Purified T cells were cultured in Iscove’s modified Dulbecco’s medium under serum-free conditions, in the absence or presence of 10 μg/mL of brefeldin A and cycloheximide (both from Sigma Immunochemicals, St Louis, MO).
Preparation of PB serum and measurement of soluble FL.
Serum from patients undergoing chemotherapy was collected daily from native PB after centrifugation for 10 minutes at 3,000 rpm. Samples were aliquoted and stored at −70°C. Soluble FL was measured by an enzyme-linked immunosorbent assay (ELISA); the limit of detection was 100 pg/mL.13
FACS analysis of membrane-bound FL.
PBMC and BMMC, washed in a FACS buffer containing phosphate-buffered saline (PBS), 0.5% bovine serum albumin (BSA), and 0.02% NaN3, were incubated for 20 minutes on ice with monoclonal antibody (MoAb) M5 against human FL (rat IgG2a13), followed by fluoresceine isothiocyanate (FITC)-conjugated goat antirat IgG (Caltag Lab, Burlingame, CA). M5 antibody specifically recognizes an epitope in the extracellular domain of FL.13 For double-color FACS analysis, MoAb M5 followed by goat antirat IgG-FITC with minimal species cross-reactivity (Jackson Immunoresearch Laboratories, West Grove, PA) and phycoerythrin (PE)-labeled mouse antihuman CD3 MoAb (Becton Dickinson, San Jose, CA) were used. Control staining was with goat antirat IgG-FITC (Jackson Immunoresearch Laboratories) and PE-labeled mouse IgG1 (Becton Dickinson). Acquisition was performed with FACScan (Becton Dickinson). The lymphocyte gate was set according to forward and sideward light scatter; dead cells were stained with propidium iodide and excluded from the analysis. Ten thousand to 25,000 events were acquired. Analysis was performed using CellQuest software (Becton Dickinson).
FACS analysis of intracellular FL.
The analysis was performed as decribed.20 Briefly, 2 × 105 PBMC or BMMC were washed in a FACS buffer and preincubated with an excess of MoAb M5 (100 μg/mL for 1 hour) to prevent further detection of any membrane-bound FL. After fixation in 4% paraformaldehyde, cells were permeabilized for 1 hour in PBS containing 0.5% BSA and 0.1% saponin (PBS-S buffer) and stained with MoAb M5 that had been conjugated with FITC using FluoroTag FITC conjugation kit (Sigma Immunochemicals). To confirm the specificity of detection of intracellular FL, control experiments were performed using FITC-conjugated MoAb M5 preincubated with a 100-fold molar excess of recombinant human FL (rhFL) or BSA. Five thousand to 10,000 events were acquired and analyzed as described above.
Confocal microscopy.
PBMC or T cells purified from PBMC by rosetting were allowed to settle onto Poly-L-Lysin–coated coverslips (Sigma), fixed with 4% paraformaldehyde, and permeabilized with 0.1% saponin. Unspecific staining was blocked with 0.5% sodium borohydride (Merck, Darmstadt, Germany) and 5% goat serum. Cells were incubated for 1 hour at room temperature with MoAb M5 or control rat IgG2a (PharMingen, San Diego, CA) and mouse antihuman CD3, washed in PBS-S, and stained for 1 hour in the dark with secondary antibodies (goat antirat IgG-FITC [Jackson Immunoresearch Laboratories] and goat antimouse IgG-Cy3 [Amersham, Pittsburgh, PA]). For intracellular localization of FL, T cells were stained with MoAb M5 as described above and with antibodies against human giantin, ERGIC-53, transferrin receptor, lamp-1 (all mouse IgG1; gift from Hans-Peter Hauri, Biozentrum, Basel, Switzerland) or rabbit antihuman cathepsin D (Accurate Chemicals, San Francisco, CA), followed by goat antimouse or goat antirabbit IgG-Cy3 (Amersham). Slides were mounted with Mowiol (Hoechst, Frankfurt, Germany) containing 20 mg/mL 1,4-diazabicyclooctane (DABCO; Sigma). Confocal microscopy was performed with TCS4D (Leica, Glattbrugg, Switzerland) operating in the sequential acquisition mode with 488 (FITC) and 568 (Cy3) excitation lines. Images were adjusted for brightness and contrast and were analyzed for colocalization using the IMARIS (Bitplane AG, Zürich, Switzerland) software package.
Induction of aplasia in mice.
Ten-week-old C57BL/6 mice were subjected to 8 Gy total body irradiation. Groups of three mice were killed at 0, 3, 6, and 10 days and tissue RNA was prepared after determination of BM cellularity. The experiments were approved by the local animal welfare committee.
RNA preparation and reverse transcription-polymerase chain rection (RT-PCR).
Total cellular RNA was extracted.21 Murine tissues were rinsed in PBS upon excision, immediately transferred to guanidinum isothiocyanate, and homogenized using Polytrone PT 1200 (Kinematica AG, Luzern, Switzerland). One microgram of RNA was reverse transcribed into complementary DNA (cDNA) in 20 μL reactions using 2.5 μmol/L random hexamers (Perkin Elmer, Branchburg, NJ) as primers and 2.5 U/μL Superscript II RNase H−-reverse transcriptase (GIBCO, Gaithersburg, MD). Four microliters of the cDNA product and 0.15 μmol/L of each oligonucleotide primer specific for FL or β-actin sequences were amplified by PCR using AmpliTaq DNA polymerase at 0.025 U/μL (Perkin Elmer). A control reaction containing water instead of RNA was performed. The following programs were used for both FL and β-actin: denaturing at 94°C for 1 minute, annealing at 60°C for 1 minute, and extending at 72°C for 1 minute; for 25 cycles (human probes) or 27 cycles (murine probes) in Perkin Elmer Cetus DNA termal cycler 8930 (Norwalk, CT). Sense and antisense primers corresponded to the following nucleotide positions: human FL, 60-81 and 345-366 in exons 2-411; murine FL, 139-160 and 411-431 in exons 2-422; human β-actin, 2038-2058 and 2447-246623; and murine β-actin, 530-553 and 738-761.24 PCR products were separated in 1.2% agarose gels, examined by staining with ethidium bromide, transferred onto nylon membranes, and hybridized with the (γ-32P)ATP-labeled oligonucleotide probes corresponding to the internal sequences of the amplified PCR products: human FL, 173-190; murine FL, 202-222; human β-actin, 2058-2075, and murine β-actin, 639-659. Radioactivity in each band was quantified in PDUnits using Phosphoimager analysis (Bio-Rad, Hercules, CA). FL mRNA levels were determined based on the ratio of signals given by FL and β-actin PCR products. Autoradiography was performed using X-OMAT Kodak x-ray film and amplifying screens (Eastman Kodak, Rochester, NY).
Protein extraction and Western analysis.
PBMC or BMMC were lysed in extraction buffer containing 1% Triton-X, 50 mmol/L Tris, pH 7.5, 150 mmol/L NaCl, 5 mmol/L EDTA, 3 mmol/L phenylmethylsulfonylfluoride, 20 μg/mL aprotinin, and 50 μg/mL leupeptin (all protease inhibitors from Boehringer Mannheim, Mannheim, Germany). Extracts from 1 × 106 cells or 5 ng of rhFL in Laemmli’s sample buffer were electrophoresed on reducing sodium dodecyl sulfate (SDS)-polyacrylamide gels (12%) and transferred to nitrocellulose. Western analysis of FL was performed with MoAb M5 followed by secondary horseradish peroxidase-conjugated rabbit antirat IgG (Southern Biotechnology, Birmingham, AL), and detection was with enhanced chemiluminesce (Pierce, Rockford, IL). For the control immunoblotting, isotype-matched rat IgG2a (PharMingen) or secondary antibody only were used. For the control antibody-blocking experiments, MoAb M5 was preincubated with 50-fold molar excess of rhFL for 1 hour on ice.
Statistical analysis.
The Student’s t-test was used to compare the time course of expression of FL mRNA and FL protein isoforms. Spearman Rank Correlation test was used to analyze the relationship between FL serum levels and PB leukocyte counts.
RESULTS
Expression of FL during normal hematopoiesis.
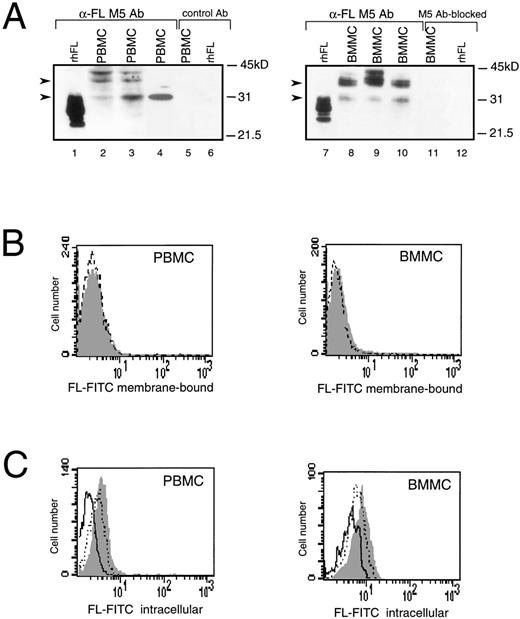
In humans, the highest levels of constitutively expressed FL mRNA have been reported in PBMC,4,11 but expression of FL protein in these cells has not been analyzed. Western analysis of PBMC and BMMC showed multiple FL-specific signals with some heterogeneity in their pattern in cells from individual healthy donors (Fig 1A, lanes 2 through 4 and 8 through 10). The size of detected proteins was larger than that of the recombinant soluble FL. The major products of about 30 and 36 kD were similar in size to proteins expressed in COS cells transfected with FL cDNA, which are thought to represent the intermediate and fully glycosylated transmembrane forms of FL, respectively.25Additional bands may correspond to heterogenously glycosylated proteins and/or products of alternatively spliced variants of FL mRNA.
Expression of FL in PBMC or BMMC. (A) Immunoblot analysis of FL. Recombinant human CHO-derived FL (rhFL; 5 ng; lanes 1 and 7) or cell lysates from 1 × 106 PBMC (lanes 2 through 4) or BMMC (lanes 8 through 10) from 3 healthy donors were analyzed by Western blotting with anti-FL MoAb M5. Controls: lanes 5 and 6, PBMC and rhFL, respectively, analyzed with rat IgG2a (control Ab); lanes 11 and 12, BMMC and rhFL, respectively, analyzed with M5 preincubated with 50-molar excess of rhFL before probing (M5 Ab-blocked). Migration of protein size markers is indicated; arrowheads point to 30- and 36-kD proteins. The amount of FL can be estimated as approximately 1 to 5 ng/106 mononuclear cells, because signals are weaker than those given by 5 ng of rhFL (lanes 1 and 7) and no signal is given by 1 ng of loaded rhFL (not shown). (B) Flow cytometric analysis of cell surface FL. PBMC or BMMC were stained with FITC-conjugated MoAb M5 (shaded area) or with FITC-conjugated control rat IgG2a (broken line). (C) Flow cytometric analysis of intracellular FL. PBMC or BMMC were preincubated with MoAb M5 to block cell surface FL, permeabilized with 0.1% saponin, and stained with FITC-conjugated M5 (shaded area). Control staining was with FITC-conjugated M5 preincubated for 30 minutes with a 100-fold excess of rhFL (solid line) or BSA (dotted line).
Expression of FL in PBMC or BMMC. (A) Immunoblot analysis of FL. Recombinant human CHO-derived FL (rhFL; 5 ng; lanes 1 and 7) or cell lysates from 1 × 106 PBMC (lanes 2 through 4) or BMMC (lanes 8 through 10) from 3 healthy donors were analyzed by Western blotting with anti-FL MoAb M5. Controls: lanes 5 and 6, PBMC and rhFL, respectively, analyzed with rat IgG2a (control Ab); lanes 11 and 12, BMMC and rhFL, respectively, analyzed with M5 preincubated with 50-molar excess of rhFL before probing (M5 Ab-blocked). Migration of protein size markers is indicated; arrowheads point to 30- and 36-kD proteins. The amount of FL can be estimated as approximately 1 to 5 ng/106 mononuclear cells, because signals are weaker than those given by 5 ng of rhFL (lanes 1 and 7) and no signal is given by 1 ng of loaded rhFL (not shown). (B) Flow cytometric analysis of cell surface FL. PBMC or BMMC were stained with FITC-conjugated MoAb M5 (shaded area) or with FITC-conjugated control rat IgG2a (broken line). (C) Flow cytometric analysis of intracellular FL. PBMC or BMMC were preincubated with MoAb M5 to block cell surface FL, permeabilized with 0.1% saponin, and stained with FITC-conjugated M5 (shaded area). Control staining was with FITC-conjugated M5 preincubated for 30 minutes with a 100-fold excess of rhFL (solid line) or BSA (dotted line).
Although detected in Western analysis, FL levels were unmeasurable or very low on the surface of BM and PB cells when analyzed by flow cytometry (Fig 1B). However, immunostaining for FL was positive after permeabilization of cells with saponin (Fig 1C). The intracellular signal was abrogated after preincubation of anti-FL antibody with recombinant human FL, but not with bovine serum albumin, confirming that recognition of the ligand inside the cells was specific. Dual-staining of permeabilized cells with anti-FL antibodies and antibodies against mononuclear cell surface markers detected FL inside peripheral CD3+ and CD19+ lymphocytes and in CD34+ hematopoietic progenitors purified from BM, but not in purified PB monocytes (data not shown). These results of the Western and FACS analyses demonstrate that, during normal steady-state hematopoiesis, FL is produced by PB and BM cells but is undetectable or very sparse on their surface.
Expression of FL during aplasia.
We have previously demonstrated that stem cell deficiency in cancer patients receiving high-dose chemoradiotherapy leads to an approximately 100-fold increase in serum levels of FL.14 To examine the mechanism by which FL levels are upregulated, 16 patients with various hematological malignancies were recruited for the study (Table 1). From day 8 or 9 after initiation of chemotherapy, all patients developed severe aplasia lasting for at least 2 weeks, with peripheral leukocyte counts decreasing from 5 to 10.0 × 109/L to as little as 0.1 × 109/L.
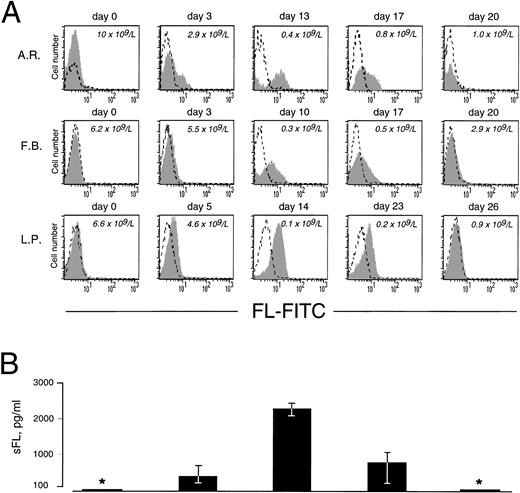
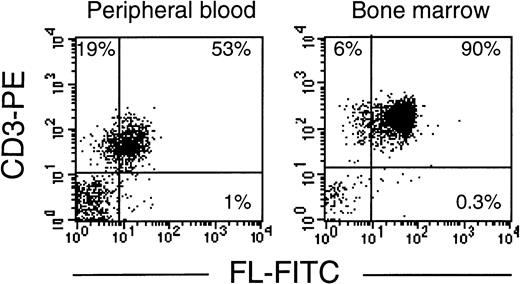
As in cells from healthy donors, surface expression of FL in patients’ cells before treatment was very low or undetectable (Fig 2A). However, 3 to 5 days after initiation of therapy, membrane-bound FL appeared on the cell surface and remained high throughout the period of aplasia. Surface expression of the ligand then gradually declined to barely detectable pretreatment levels along with hematopoietic recovery. The kinetics of the appearance of membrane-bound FL paralleled changes in serum levels of soluble FL (Fig 2B). As measured by ELISA, FL was undetectable in serum before treatment, increased to 200 to 700 pg/mL after 3 to 5 days and to 2,500 pg/mL during severe aplasia, and then decreased upon normalization of blood cell counts. It should be emphasized that the initial increase in both membrane-bound and soluble FL occurred when leukocyte counts were still within the normal range (see Fig 2A). To identify the cell population expressing surface FL, antibodies specific for mononuclear cell surface markers were used in flow cytometry. In severe aplasia, the vast majority of CD3+ cells in both PB and BM expressed high levels of cell surface-bound FL (Fig 3). Persistent cell surface expression of FL in T cells during aplasia was observed with all analyzed patients undergoing myeloablative chemotherapy, independent of the underlying disease and treatment regimen.
Expression of cell surface and soluble FL in chemotherapy-induced aplasia. (A) Flow cytometric analysis of cell surface FL. PBMC were stained with FITC-conjugated MoAb M5 (shaded area) or with FITC-conjugated control rat IgG2a (broken line). Analysis was performed with 9 patients; representative results for 3 patients are shown. Days of treatment and peripheral leukocyte count are indicated. (B) Serum concentrations of soluble FL (sFL) determined by ELISA. *Below the detection limit of 100 pg/mL. Results refer to the same 3 patients and same days as the FACS data shown in (A). The standard deviation is indicated. Correlation between sFL levels and leukocyte count was −.51 (P < .0001).
Expression of cell surface and soluble FL in chemotherapy-induced aplasia. (A) Flow cytometric analysis of cell surface FL. PBMC were stained with FITC-conjugated MoAb M5 (shaded area) or with FITC-conjugated control rat IgG2a (broken line). Analysis was performed with 9 patients; representative results for 3 patients are shown. Days of treatment and peripheral leukocyte count are indicated. (B) Serum concentrations of soluble FL (sFL) determined by ELISA. *Below the detection limit of 100 pg/mL. Results refer to the same 3 patients and same days as the FACS data shown in (A). The standard deviation is indicated. Correlation between sFL levels and leukocyte count was −.51 (P < .0001).
T lymphocytes express FL on the cell surface in chemotherapy-induced aplasia. Flow cytometric analysis of membrane-bound FL in PBMC or BMMC stained with PE-conjugated anti-CD3 MoAb and FITC-conjugated anti-FL MoAb M5. Analysis was performed with 4 patients; representative results for patients B.G. and K.B. are shown.
T lymphocytes express FL on the cell surface in chemotherapy-induced aplasia. Flow cytometric analysis of membrane-bound FL in PBMC or BMMC stained with PE-conjugated anti-CD3 MoAb and FITC-conjugated anti-FL MoAb M5. Analysis was performed with 4 patients; representative results for patients B.G. and K.B. are shown.
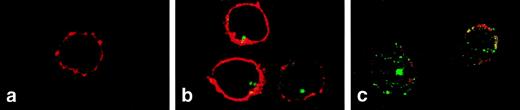
Confocal microscopy was used to visualize changes in FL distribution during steady-state and compromised hematopoiesis. In normal peripheral T cells, FL was detected intracellularly as a strong clustered signal (Fig 4B). In T cells from patients during aplasia, FL signals became scattered in the typical vesicular distribution pattern of a secreted protein, visible also in the outer rim of the cytoplasm, partly overlapping with staining of the CD3 surface antigen (Fig 4C). These results confirm that FL is present inside T cells during steady-state hematopoiesis and is translocated to the cell surface in hematopoietic failure.
Two-color immunofluorescence confocal microscopy analysis of FL expression in T lymphocytes. PBMC were settled on the slides, fixed with paraformaldehyde, permeabilized with 0.1% saponin, and stained with anti-FL MoAb M5 followed by FITC-labeled goat antirat IgG and with anti-CD3 antibody followed by Cy3-labeled goat antimouse IgG. (a) Donor cells, staining with anti-CD3 (red) and control rat IgG2a followed by secondary FITC-labeled goat antirat IgG (no signal). (b) Donor cells stained for CD3 (red) and FL (green). (c) Patient’s cells (M.T.) stained for CD3 (red) and FL (green). CD3 staining of patient’s cells was partly destroyed during handling due to drug-related fragility of cell membranes. Areas of overlap are highlighted in yellow.
Two-color immunofluorescence confocal microscopy analysis of FL expression in T lymphocytes. PBMC were settled on the slides, fixed with paraformaldehyde, permeabilized with 0.1% saponin, and stained with anti-FL MoAb M5 followed by FITC-labeled goat antirat IgG and with anti-CD3 antibody followed by Cy3-labeled goat antimouse IgG. (a) Donor cells, staining with anti-CD3 (red) and control rat IgG2a followed by secondary FITC-labeled goat antirat IgG (no signal). (b) Donor cells stained for CD3 (red) and FL (green). (c) Patient’s cells (M.T.) stained for CD3 (red) and FL (green). CD3 staining of patient’s cells was partly destroyed during handling due to drug-related fragility of cell membranes. Areas of overlap are highlighted in yellow.
Intracellular localization of FL in T cells.
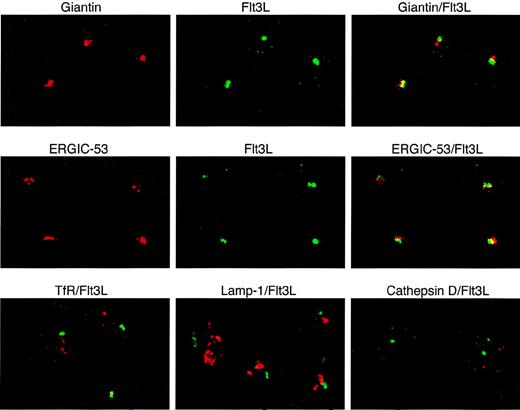
To define more precisely the localization of intracellular FL in T lymphocytes during normal hematopoiesis, we performed dual immunofluorescence confocal microscopy using anti-FL antibodies and antibodies recognizing organelle-specific proteins (Fig 5). FL colocalized to a large degree with giantin, a Golgi marker, and ERGIC-53, a marker for the endoplasmic reticulum/Golgi intermediate compartment. FL did not colocalize with the transferrin receptor, a marker for early endosomes; lamp-1, a marker for late endosomes and lysosomes; or cathepsin D, a marker for lysosomes (reviewed in Farquhar and Hauri26 and Rothman and Wieland27). These results indicate that preformed FL in peripheral T lymphocytes is localized in the Golgi apparatus and in nearby structures of the endoplasmatic reticulum.
Localization of intracellular FL (Flt3L) in T lymphocytes. Two-color immunofluorescence confocal microscopy of T cells purified from PB of a healthy donor was performed after staining with anti-FL MoAb M5 followed by FITC-labeled goat antirat IgG (green) and with antibodies against giantin, ERGIC-53, transferrin receptor (TfR), lamp-1 (all mouse IgG1), and cathepsin D (rabbit IgG), followed by secondary Cy3-labeled goat antimouse IgG or goat antirabbit IgG, as appropriate (red signals). Areas of exact overlap of green FL signal with red signals of giantin or ERGIC-53 are highlighted in yellow.
Localization of intracellular FL (Flt3L) in T lymphocytes. Two-color immunofluorescence confocal microscopy of T cells purified from PB of a healthy donor was performed after staining with anti-FL MoAb M5 followed by FITC-labeled goat antirat IgG (green) and with antibodies against giantin, ERGIC-53, transferrin receptor (TfR), lamp-1 (all mouse IgG1), and cathepsin D (rabbit IgG), followed by secondary Cy3-labeled goat antimouse IgG or goat antirabbit IgG, as appropriate (red signals). Areas of exact overlap of green FL signal with red signals of giantin or ERGIC-53 are highlighted in yellow.
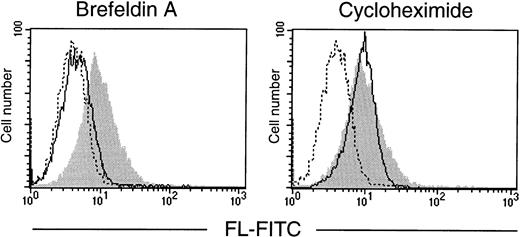
To verify that it is preexisting FL that is translocated from the Golgi to the cell surface, T cells were treated with brefeldin A, a reagent that blocks protein secretion by disrupting the Golgi apparatus,28 or with cycloheximide to inhibit translation. In vitro cultures of purified T lymphocytes were established and analyzed by flow cytometry (Fig 6). After 72 hours in serum-free conditions, T cells spontaneously expressed FL on their surface, indicating that mere exposure to in vitro conditions is sufficient to induce externalization of the ligand. Importantly, the surface appearance of FL in cultured cells was fully prevented by brefeldin A, but not by cycloheximide. The clearly pronounced difference between the effects of the two drugs demonstrates the requirement for a functional protein transport machinery, but not de novo protein synthesis, and confirms that the control of FL expression is fundamentally posttranslational.
Effect of brefeldin A and cycloheximide on expression of FL by cultured T lymphocytes. T lymphocytes, purified from PB of a healthy donor, were cultured for 72 hours in serum-free medium in the absence or presence of brefeldin A or cycloheximide (both at 10 μg/mL). Flow cytometric analysis of cell surface FL was performed with anti-FL MoAb M5 followed by FITC-labeled goat antirat IgG. (Shaded area) FL expression in untreated cells; (solid line) FL expression in brefeldin A- or cycloheximide-treated cells, as indicated; (broken line) control staining with FITC-labeled goat antirat IgG.
Effect of brefeldin A and cycloheximide on expression of FL by cultured T lymphocytes. T lymphocytes, purified from PB of a healthy donor, were cultured for 72 hours in serum-free medium in the absence or presence of brefeldin A or cycloheximide (both at 10 μg/mL). Flow cytometric analysis of cell surface FL was performed with anti-FL MoAb M5 followed by FITC-labeled goat antirat IgG. (Shaded area) FL expression in untreated cells; (solid line) FL expression in brefeldin A- or cycloheximide-treated cells, as indicated; (broken line) control staining with FITC-labeled goat antirat IgG.
FL mRNA expression in aplasia.
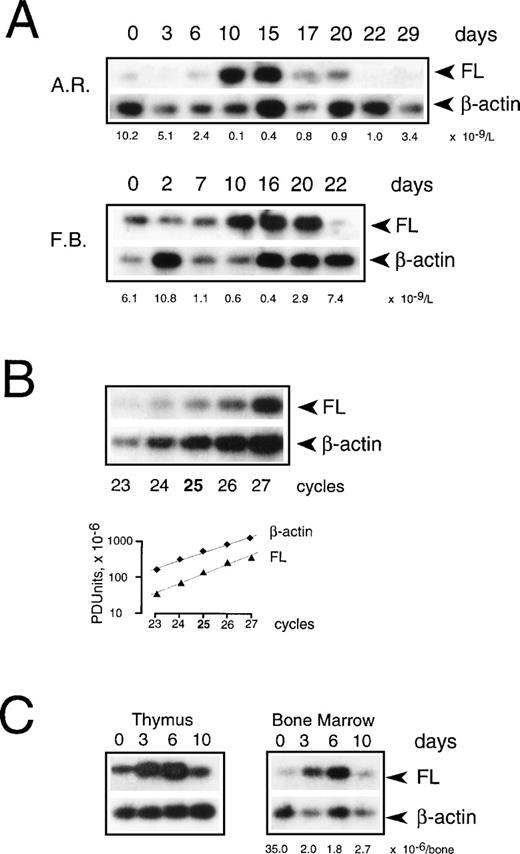
To examine if transcriptional regulation contributes to the increase of FL in aplasia, we measured FL mRNA levels in PBMC collected during chemotherapy (Fig 7A). Using semiquantitative RT-PCR under conditions of the linear accumulation of PCR products (Fig 7B), FL-specific transcripts were detected before treatment, and their level increased approximately sevenfold during severe aplasia and returned to pretreatment values upon hematopoietic recovery. An increase in mRNA level was observed between days 8 and 10 after the start of chemotherapy. Thus, the initial appearance of FL on the cell surface and in serum (see Fig 2) preceded the initial increase of FL mRNA (4.6 ± 0.7 and 4.3 ± 1.4 v 9.0 ± 1.5 days, respectively; P < .05). Furthermore, mRNA levels increased only when pancytopenia was very severe, as judged by an at least 50-fold reduction in white blood cells, whereas externalization of FL started when leukocyte counts were still within the normal range. In parallel with a delayed increase in FL mRNA levels, an approximately threefold increase of total cell content of FL protein was observed by Western analysis (not shown). These data argue in favor of rapidly and massively elevated membrane-bound and soluble forms of FL being derived from the protein present in patients’ cells before the beginning of treatment rather than from de novo expression.
Expression of FL mRNA during aplasia. (A) Semiquantitative RT-PCR analysis in PBMC from patients during chemotherapy treatment. Twenty-five PCR cycles were performed under conditions of the linear accumulation of FL and β-actin PCR products (B). Southern blots were hybridized with 32P-labeled internal probes, and radioactivity in each band was quantified by phosphorimaging analysis. Autoradiography was for 4 hours (FL) and 30 minutes (β-actin). Three analyses were performed for each of 4 analyzed patients; representative results for 2 patients are shown. Days of treatment are indicated above and peripheral leukocyte counts below the autoradiograms. Methods more accurate than RT-PCR could not be applied given the extremely limited availability of patients’ cells at the time of treatment. (C) Semiquantitative RT-PCR analysis in mouse tissues during irradiation-induced aplasia. Mice received 8.0 Gy total body irradiation. RNA was isolated before irradiation (day 0), during aplasia (days 3 and 6), and at the beginning of recovery (day 10), and FL mRNA expression was analyzed as described above. Three analyses were performed for 2 animals; representative results are shown. The BM nucleated cell count per femur plus tibia is indicated below the autoradiogram.
Expression of FL mRNA during aplasia. (A) Semiquantitative RT-PCR analysis in PBMC from patients during chemotherapy treatment. Twenty-five PCR cycles were performed under conditions of the linear accumulation of FL and β-actin PCR products (B). Southern blots were hybridized with 32P-labeled internal probes, and radioactivity in each band was quantified by phosphorimaging analysis. Autoradiography was for 4 hours (FL) and 30 minutes (β-actin). Three analyses were performed for each of 4 analyzed patients; representative results for 2 patients are shown. Days of treatment are indicated above and peripheral leukocyte counts below the autoradiograms. Methods more accurate than RT-PCR could not be applied given the extremely limited availability of patients’ cells at the time of treatment. (C) Semiquantitative RT-PCR analysis in mouse tissues during irradiation-induced aplasia. Mice received 8.0 Gy total body irradiation. RNA was isolated before irradiation (day 0), during aplasia (days 3 and 6), and at the beginning of recovery (day 10), and FL mRNA expression was analyzed as described above. Three analyses were performed for 2 animals; representative results are shown. The BM nucleated cell count per femur plus tibia is indicated below the autoradiogram.
To test the possibility that BM failure leads to an increase in FL mRNA levels in other tissues, we induced transient aplasia in mice by sublethal irradiation with 8.0 Gy and isolated RNA from several hematopoietic and nonhematopoietic organs. FL mRNA increased moderately, up to sixfold, only in the BM and thymus (Fig 7C), but remained unchanged in spleen, kidney, lymph nodes, brain, liver, lung, and heart (not shown). It thus appears unlikely that organs other than the lymphohematopoietic system contribute significantly, at least by assessment of mRNA content, to the increase in FL levels seen in response to chemotherapy and irradiation in humans.
DISCUSSION
The restricted range of hematopoietic cells carrying flt3receptors6 in conjunction with widespread expression of FL mRNA4 11 led to the suggestion that expression of the receptor and not the ligand is the key to understanding the role of FL in hematopoiesis. In the work described here, FL expression was examined by Western analysis, flow cytometry, and confocal microscopy in PB and BM cells from healthy donors and patients with multilineage BM failure caused by high-dose chemoradiotherapy. Our results show that the supply of FL for hematopoiesis in humans is tightly regulated by a process specific for this cytokine. This regulation is based on intracellular retention of preformed FL and its release from intracellular stores, dependent on the status of the stem cell compartment.
During normal hematopoiesis, FL is produced constitutively but little of the cytokine is released by cells: it is undetectable or very sparse on the surface of cells and in the circulation. Instead, high levels of preformed FL are present inside T and B lymphocytes and CD34+ hematopoietic precursors. In T cells, intracellular FL colocalized with ERGIC-53 and giantin, the proteins resident in the endoplasmic reticulum/Golgi intermediate compartment and Golgi apparatus, respectively.27 This finding is surprising because, as a cytokine designed for an extracellular function, FL is devoid of amino acid sequences serving as typical intracellular retrieval/retention signals.11 The intracellular accumulation of FL argues for the existence of a negative regulator controlling the availability of the ligand in steady-state hematopoiesis. This mechanism of intracellular retention of FL does not function when cells are maintained outside their natural microenvironment, because T lymphocytes cultured in vitro spontaneously express FL at their surface. In vivo, release of the ligand from intracellular stores may be triggered by stem cell deficiency in BM failure. Indeed, the highest expression of membrane-bound FL after myeloablative therapy was observed in patients with profound aplasia (Fig 2A). The nature of inductive signal(s) generated by BM failure remains unknown. In analogy to a heme-containing oxygen sensor involved in feedback regulation of expression of erythropoietin,28 a putative stem cell sensor may be controlling the level of FL by counteracting the retention signal and mobilizing FL in response to stem cell deficiency.
Intracellular storage and release upon demand distinguish FL regulation from known mechanisms regulating the production of hematopoietic cytokines by T cells and other blood cell types. Expression levels of interleukin-2, interleukin-3, interferon-γ, and granulocyte-macrophage colony-stimulating factor are primarily determined by the rate of mRNA transcription and/or decay,29-31 and these factors have not been found to accumulate in human PB cells.32 In contrast, transcriptional control plays a secondary role in regulating the expression of FL. FL mRNA is expressed constitutively, and the increase in its levels in chemotherapy-induced marrow failure is less pronounced and delayed compared with the increase in FL protein levels. No difference in the proportion of the FL splice variants encoding transmembrane and soluble FL in patients’ as compared with normal PB cells was found (results not shown). Transcript stability is not likely to play a major regulatory role either, because FL mRNA is highly stable, with a half-life of about 24 hours (results not shown). Intracellular storage of the preformed protein also distinguishes FL from SCF and M-CSF, two other ligands for hematopoietic tyrosine kinase receptors. SCF and M-CSF, produced predominantly by BM stroma cells, are constitutively expressed but also rapidly secreted, resulting in remarkably high (in nanograms per milliliter) serum levels of these cytokines during normal hematopoiesis as well as hematopoietic failure.18,19,33,34 Interestingly, expression of ligands for Fas and CD40 may be regulated in some cell types in a manner similar to FL. In primary resting human monocytes and platelets and in Jurkat T cells, prestorage of these ligands by an unknown mechanism and secretion in response to cell-specific stimuli have been recently reported.35-37 Therefore, posttranslational control by prestorage and regulated release, rather than de novo synthesis, is emerging as a novel mechanism regulating the availability of biologically relevant molecules.
During chemotherapy, surface expression of membrane-bound FL and serum levels of FL increase in parallel. The mechanism by which soluble FL is generated awaits identification of a putative protease that, by analogy to the metalloproteinase-processing tumor necrosis factor-α,38 39 may release FL from the membrane. The simultaneous increase of both ligand forms in response to BM failure and their simultaneous decrease upon hematopoietic recovery suggest that circulating FL in serum is derived from cleavage of the ligand translocated to the T-cell surface from intracellular stores. The amounts of preformed intracellular FL, estimated as 1 to 5 ng/106 cells (see Fig 1, legend), would be sufficient to elevate serum FL to the observed concentrations, if secreted by T cells that are still present in normal numbers during the first days of treatment. Alternative cell or tissue sources of FL cannot be ruled out, although we could show that, in mice with experimentally induced aplasia, levels of FL mRNA increased only in BM and thymus, suggesting that lymphohematopoietic organs are the major site of FL upregulation in response to BM failure also in humans.
Our results point to a role for T-cell surface-associated FL in the reconstitution of hematopoiesis. During myeloablation caused by the severe toxicity of chemotherapy, T lymphocytes remain as a predominant population of circulating cells. Membrane-bound FL, expressed in high amounts throughout aplasia, may stimulate and enhance the level of hematopoietic progenitors by direct cell-to-cell contact. Signaling by membrane-bound and soluble FL isoforms may not be redundant, in analogy to signaling by membrane-bound SCF on stroma cells having an essential function for normal hematopoiesis.40 41 Cell-surface expression of FL by T lymphocytes in aplasia suggests that not only interactions between stroma and progenitor cells, but also those between immune and hematopoietic cells may be involved in a compensatory growth factor response to overcome BM failure.
ACKNOWLEDGMENT
The authors thank A. Gratwohl, J. Passweg, I. Turkalij, and C. Pino for patients’ material; A. Rolink for irradiated mice; H.-P. Hauri for MoAbs towards organellar markers; M. Bürk for help with intracellular FACS; and N. Hynes, A. Lanzavecchia, G. De Libero, and R.C. Skoda for critically reviewing the manuscript.
Supported by grants from the Swiss National Science Foundation (32-045926.95 and 7GUPJ041426), Basler Krebsliga (17/96), Schweizerische Akademie der Medizinischen Wissenschaften, and Stiftung zur Krebsbekämpfung to C.N., A.W.-F., and E.C.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Aleksandra Wodnar-Filipowicz, PhD, Department of Research, University Hospital Basel, Hebelstr. 20, CH-4031 Basel, Switzerland; e-mail: Filipowicz@ubaclu.unibas.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal