Abstract
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is an important hematopoietic cytokine that exerts its effects by interaction with the GM-CSF receptor (GMR) on the surface of responsive cells. The GM-CSF receptor consists of two subunits: GMR, which binds GM-CSF with low affinity, and GMRβ, which lacks intrinsic ligand-binding capability but complexes with GMR to form a high-affinity receptor (GMR/β). We conducted dynamic kinetic analyses of GM-CSF receptors to define the role of GMRβ in the interaction of ligand and receptor. Our data show that GMR/β exhibits a higher kon than GMR, indicating that GMRβ facilitates ligand acquisition to the binding pocket. Heterogeneity with regard to GM-CSF dissociation from GMR/β points to the presence of loose and tight ligand-receptor complexes in high-affinity binding. Although the loose complex has a koff similar to GMR, the lower koffindicates that GMRβ inhibits GM-CSF release from the tight receptor complex. The two rates of ligand dissociation may provide for discrete mechanisms of interaction between GM-CSF and its high-affinity receptor. These results show that the β subunit functions to stabilize ligand binding as well as to facilitate ligand acquisition.
GRANULOCYTE-MACROPHAGE colony-stimulating factor (GM-CSF) is a hematopoietic cytokine that stimulates myeloid precursor cell growth and differentiation, and enhances the function of mature granulocytes and mononuclear phagocytes.1 GM-CSF functional activity is initiated by binding to its cognate receptor (GMR) on the cell surface consisting of two transmembrane subunits, GMRα and GMRβ. GMRα is an 84-kD polypeptide that binds GM-CSF with low affinity (kd 1 to 10 nmol/L).2-4Although GMRβ has no intrinsic ligand-binding ability, it interacts with GMRα to form a high-affinity receptor with a kd of 20 to 100 pmol/L.5-7 Both α and β subunits belong to the hematopoietin receptor superfamily characterized by conserved extracellular motifs and the absence of an intrinsic tyrosine kinase domain.8 GMRβ is a common subunit shared by the interleukin-3 and interleukin-5 receptors, which have unique α subunits.9,10 Receptors for GM-CSF are expressed on myeloid progenitors and mature neutrophils, eosinophils, mononuclear phagocytes,11-14 and on nonhematopoietic cells and tissues including placenta, endothelium, prostate, melanocytes, and oligodendrocytes.15-19
Activation of the β subunit is critical to initiating intracellular signal transduction mediated by protein phosphorylation pathways.11,20-23 The mechanism by which GMRβ confers high-affinity ligand binding to the duplex receptor is not well understood. A previous study suggested that the β subunit slows GM-CSF dissociation in comparison with the low-affinity receptor, but does not affect ligand association.6 It is therefore commonly believed that the β subunit is mainly involved in inhibiting ligand release from the receptor complex.
To clarify the role of GMRβ in the ligand-receptor interaction, we performed a detailed kinetic analysis of high-affinity and low-affinity GM-CSF receptors at 23°C and 4°C. Our results indicate that the interactions between GM-CSF and its receptor subunit components are more complex than was previously thought. We found that the β subunit enhances GM-CSF association to the GMRα/β receptor complex in the initial ligand acquisition event. In contrast, dissociation kinetic analysis reveals two phases of ligand release from the high-affinity receptor, suggesting the formation of distinct “loose” and “tight” ligand-receptor complexes after binding. Although the rate of ligand dissociation from the loose complex is similar to that of GMRα alone, the β subunit substantially retards GM-CSF dissociation from the tight complex. Our results indicate that the β subunit functions to enhance ligand association to the high-affinity GM-CSF receptor and inhibits dissociation in the formation of stable (tight) receptor-ligand complexes.
MATERIALS AND METHODS
Cell culture.
Human HL-60 promyelocytic cells and COS-1 cells were maintained in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% glutamine, and antibiotics.
Expression of membrane-bound low-affinity human GM-CSF receptor in COS cells.
The eukaryotic expression vector pMX (a gift from Genetics Institute, Inc, Cambridge, MA24) carrying the cDNA for human GMRα was transfected into COS cells using a diethyl aminoethyl (DEAE)–dextran method.25 The transfected COS cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS, 1% glutamine, and antibiotics. COS cells were harvested 40 to 60 hours after transfection by incubation with 40 mmol/L EDTA in IMDM at 37°C for 30 minutes followed by addition of an equal volume of IMDM containing 200 μg/mL chondroitin sulfate and 10% FBS. The mixture was then incubated at 37°C for an additional 40 minutes.2 The detached and disaggregated COS cells were washed twice in phosphate-buffered saline (PBS) before measuring GM-CSF binding.
GM-CSF receptor binding assay.
Kinetic binding assays were performed at pH 7.4 on HL-60 promyelocytic cells expressing human high-affinity GMRα/β or transfected COS cells expressing human low-affinity GMRα using125I-labeled GM-CSF (DuPont, NEN, Boston, MA) in 60 μL of assay medium (IMDM containing 10% FBS, 20 mmol/L EDTA, and 50 μg/mL chondroitin sulfate). Nonspecific binding was determined by addition of 3.3 μmol/L unlabeled human recombinant GM-CSF (a gift from Amgen, Inc, Thousand Oaks, CA) to the assay mixtures. For equilibrium binding studies, aliquots of cells were incubated with increasing concentrations of 125I-labeled GM-CSF at 23°C for 5 hours or at 4°C overnight in the presence or absence of 0.1% sodium azide. HL-60 promyelocytic cells were treated with or without 10 mIU/mL heparinase (Sigma-Aldrich, St Louis, MO) and heparitinase (Sigma-Aldrich) in PBS at 34°C and 43°C for 2 hours, respectively, before kinetic assay. Receptor binding was determined by centrifugation of assay mixtures through 0.5 mL calf serum at 10,000g for 3 minutes and measuring cell pellet-associated radioactivity in a γ-counter (Packard, Downers Grove, IL). Equilibrium kinetic values were determined by Scatchard analysis.
Association kinetics were determined by the addition of125I-labeled GM-CSF (50 to 150 pmol/L) to parallel cell suspensions in assay medium as above. At each time point of incubation, an aliquot was collected, and cells were washed by centrifugation through calf serum to measure GM-CSF binding. To take into account the depletion of free ligand and receptor as well as ligand dissociation from the ligand-receptor complex during the association reaction, the on rate constant, kon, was determined by fitting the association-binding data of the entire time course into the general second-order Equation26:
where
and
In Equations 2 and 3, [L], [R], and [RL] are the concentrations of ligand, receptor, and receptor-ligand complex, respectively. The subscript “total” denotes the total concentration of each component, and “e” and “t” denote values at equilibrium and those at time t, respectively. When the association reaction follows Equation 1, a plot of (LnA)/B versus time is linear with a slope of kon.
In dissociation kinetic assays, cells expressing high- or low-affinity GM-CSF receptor were pre-equilibrated with 125I-labeled GM-CSF at ligand concentrations twofold to fivefold more than the kd at 23°C for 5 hours or 4°C overnight. The time course of dissociation was measured by adding 2 μmol/L nonradioactive GM-CSF to the assay mixture as a competitor. The amount of radioactive GM-CSF retained on the cell surface was measured by washing the cells through calf serum as above. Dissociation rate kinetics were analyzed by Equation 427:
where Co is the initial binding observed at the start of the dissociation phase and Ct is the residual binding at time t. The off rate constant, koff, was determined from the slope of the linear plot of Ln(Ct/Co) versus time.
In all kinetic assays, duplicate samples were measured for each experimental point, and the average values were used for data analysis. The equilibrium dissociation constants, on rate and off rate constants were determined by at least three independent experiments.
RESULTS
Equilibrium kinetics of GM-CSF binding to high- and low-affinity receptors.
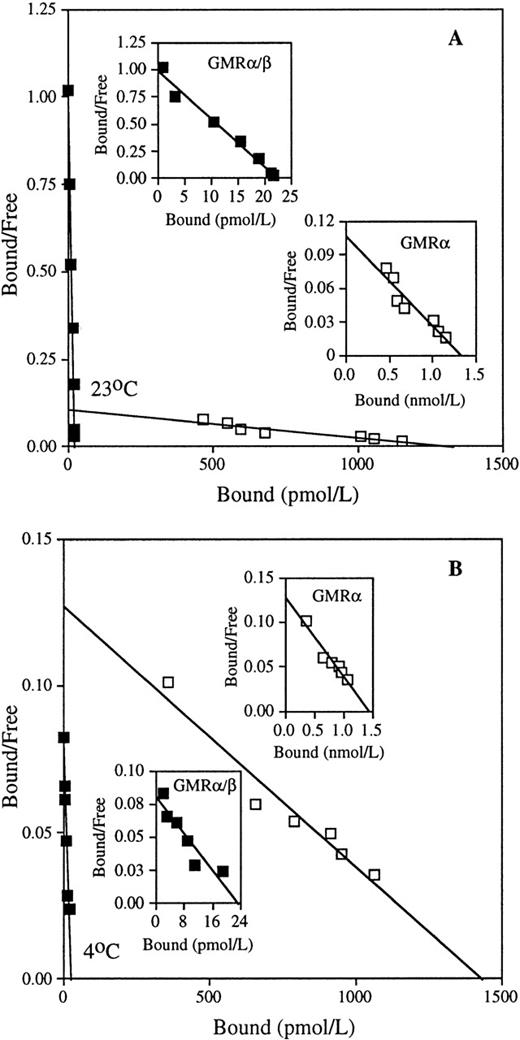
To study the mechanism of interaction between GM-CSF and its receptor subunit components, we first evaluated the equilibrium binding kinetics of GM-CSF on low- and high-affinity receptors at 23°C and 4°C. At both temperatures, COS cells transfected with human GMRα alone exhibited low-affinity GM-CSF binding with a kd of approximately 14 nmol/L (Fig 1 and Table 1). Human HL-60 promyelocytic leukemia cells displayed only high-affinity binding sites with kd values of approximately 34 pmol/L at 23°C and 234 pmol/L at 4°C (Fig 1 and Table 1). These results are consistent with previous studies.2-7 Mean values of equilibrium kd of low- and high-affinity GM-CSF receptors determined at 23°C and 4°C are presented in Table 1. Although GMRα had a similar kd at both temperatures, GMRα/β showed higher GM-CSF binding affinity at 23°C than at 4°C. Because no low-affinity binding sites could be detected on HL-60 cells at either temperature, this cell line was used in all subsequent experiments for functional analyses of the high-affinity GM-CSF receptor. Because intracellular internalization of ligand-receptor complexes might be expected to affect binding kinetics, we sought to evaluate equilibrium binding at 4°C and 23°C with sodium azide, a potent inhibitor of internalization.28 29 We found that incubation with sodium azide affected neither the kd nor the number of binding sites/cell (Table 2). This result indicates that even at 23°C, internalization plays little role in GM-CSF binding kinetics in HL-60 cells. Subsequent experiments were conducted in the absence of sodium azide.
Equilibrium binding of GM-CSF to the low- and high-affinity GM-CSF receptors. Binding assays were performed on COS cells transfected with cDNA encoding human low-affinity GMR (□), and on HL-60 promyelocytic cells expressing human high-affinity GMR/β (▪), with various concentrations of125I-labeled GM-CSF at 23°C (A) and 4°C (B), as described in Materials and Methods. Representative Scatchard analyses of the binding data are shown. The kd values of low-affinity GMR are 12.5 nmol/L (23°C) and 11.3 nmol/L (4°C), and the kd values of high-affinity GMR/β are 22.9 pmol/L (23°C) and 291.5 pmol/L (4°C) in the presented data.
Equilibrium binding of GM-CSF to the low- and high-affinity GM-CSF receptors. Binding assays were performed on COS cells transfected with cDNA encoding human low-affinity GMR (□), and on HL-60 promyelocytic cells expressing human high-affinity GMR/β (▪), with various concentrations of125I-labeled GM-CSF at 23°C (A) and 4°C (B), as described in Materials and Methods. Representative Scatchard analyses of the binding data are shown. The kd values of low-affinity GMR are 12.5 nmol/L (23°C) and 11.3 nmol/L (4°C), and the kd values of high-affinity GMR/β are 22.9 pmol/L (23°C) and 291.5 pmol/L (4°C) in the presented data.
Kinetic Parameters of GM-CSF Receptors at 23°C and 4°C
| . | Low-Affinity GM-CSF Receptor . | |||
|---|---|---|---|---|
| 23°C . | Mean Ratio . | 4°C . | Mean Ratio . | |
| kd | (15 ± 5.6) nmol/L | 1.1 | (14 ± 7.0) nmol/L | 1.0 |
| kon | (2.5 ± 1.3) × 10−6 pmol/L−1min−1 | 2.8 | (8.9 ± 2.7) × 10−7 pmol/L−1min−1 | 1.0 |
| koff | (5.5 ± 1.7) × 10−2 min−1 | 1.5 | (3.8 ± 1.0) × 10−2min−1 | 1.0 |
| High-Affinity GM-CSF Receptor | ||||
| 23°C | Mean Ratio | 4°C | Mean Ratio | |
| kd | (34 ± 5.2) pmol/L | 0.0025 | (234 ± 85) pmol/L | 0.017 |
| kon | (1.5 ± 0.27) × 10−4 pmol/L−1min−1 | 171 | (6.2 ± 1.8) × 10−5 pmol/L−1min−1 | 69 |
| koff (loose) | (4.5 ± 0.71) × 10−2 min−1 | 1.2 | (2.7 ± 0.24) × 10−2min−1 | 0.73 |
| koff (tight) | (2.1 ± 0.60) × 10−3 min−1 | 0.056 | (3.6 ± 0.32) × 10−3min−1 | 0.097 |
| . | Low-Affinity GM-CSF Receptor . | |||
|---|---|---|---|---|
| 23°C . | Mean Ratio . | 4°C . | Mean Ratio . | |
| kd | (15 ± 5.6) nmol/L | 1.1 | (14 ± 7.0) nmol/L | 1.0 |
| kon | (2.5 ± 1.3) × 10−6 pmol/L−1min−1 | 2.8 | (8.9 ± 2.7) × 10−7 pmol/L−1min−1 | 1.0 |
| koff | (5.5 ± 1.7) × 10−2 min−1 | 1.5 | (3.8 ± 1.0) × 10−2min−1 | 1.0 |
| High-Affinity GM-CSF Receptor | ||||
| 23°C | Mean Ratio | 4°C | Mean Ratio | |
| kd | (34 ± 5.2) pmol/L | 0.0025 | (234 ± 85) pmol/L | 0.017 |
| kon | (1.5 ± 0.27) × 10−4 pmol/L−1min−1 | 171 | (6.2 ± 1.8) × 10−5 pmol/L−1min−1 | 69 |
| koff (loose) | (4.5 ± 0.71) × 10−2 min−1 | 1.2 | (2.7 ± 0.24) × 10−2min−1 | 0.73 |
| koff (tight) | (2.1 ± 0.60) × 10−3 min−1 | 0.056 | (3.6 ± 0.32) × 10−3min−1 | 0.097 |
Data represent the results of at least three individual experiments. Mean ratios were normalized by dividing the mean measured kinetic constant by the corresponding value of the low-affinity GM-CSF receptor obtained at 4°C.
Effect of Sodium Azide on GM-CSF Binding Activity of HL-60 Promyelocytic Cells
| Temperature . | kd ± SD (pmol/L) . | Binding Sites/Cell . | Na Azide . |
|---|---|---|---|
| 23°C | 41.8 ± 8.6 | 255 ± 10 | − |
| 23°C | 38.5 ± 5.3 | 237 ± 28 | + |
| 4°C | 220.4 ± 35.4 | 251 ± 41 | − |
| 4°C | 226.8 ± 39.9 | 261 ± 29 | + |
| Temperature . | kd ± SD (pmol/L) . | Binding Sites/Cell . | Na Azide . |
|---|---|---|---|
| 23°C | 41.8 ± 8.6 | 255 ± 10 | − |
| 23°C | 38.5 ± 5.3 | 237 ± 28 | + |
| 4°C | 220.4 ± 35.4 | 251 ± 41 | − |
| 4°C | 226.8 ± 39.9 | 261 ± 29 | + |
Data represent the average results of three individual experiments.
Abbreviation: SD, standard deviation.
GMRβ facilitates association of GM-CSF to the high-affinity receptor.
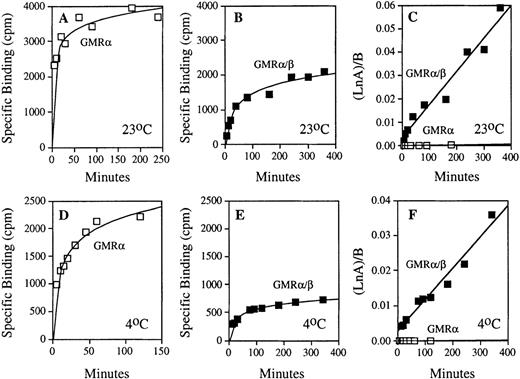
To determine the contribution of GMRβ in the binding of GM-CSF to the high-affinity receptor, dynamic ligand binding was measured at both 23°C and 4°C in cells expressing high- and low-affinity receptors (Fig 2). The high level of binding measured in COS cells expressing low-affinity GM-CSF receptor reflects the higher level of receptor expression attainable in transfected cells compared with endogenous high-affinity receptor expression in hematopoietic cell lines. To adjust for these differences, total receptor concentration in each experiment was determined separately by Scatchard analysis, and the binding data from each time course were then used to determine the association rate constants (see Materials and Methods Eq. 1). By this method, the kon determined for the low-affinity GMRα and the high-affinity GMRα/β receptors were markedly different, reflecting an important contribution of GMRβ in the ligand association step of the ligand-receptor interaction (Fig 2C and F). The linear nature of GM-CSF association by this analysis (r2 = 0.907 at 23°C and r2 = 0.955 at 4°C for GMRα, r2 = 0.963 at 23°C and r2 = 0.966 at 4°C for GMRα/β) strongly supports a second-order kinetic model of ligand-receptor interaction. The average values of kon determined for both high- and low-affinity receptors at 23°C and 4°C are shown in Table 1, indicating that the association rate constant of GMRα/β is substantially higher than that of low-affinity GMRα at both temperatures. Thus, the β subunit increases GM-CSF binding affinity to GMRα/β by acceleration of ligand binding to the α/β receptor complex.
Comparison of the association kinetics of GM-CSF binding with the low- and high-affinity GM-CSF receptors. Time courses of specific 125I-labeled GM-CSF binding to the COS cells expressing human low-affinity GMR and HL-60 promyelocytic cells expressing only human high-affinity GMR/β were measured as described in Materials and Methods. Results of representative experiments performed at 23°C and 4°C are shown. (A) GM-CSF association with low-affinity GMR at 23°C. (B) GM-CSF association with high-affinity GMR/β at 23°C. (C) Analyses of association rate constants (see Eq. 1 in Materials and Methods) for low-affinity (□) and high-affinity (▪) GM-CSF receptors shown in (A) and (B); kon (GMR) = 1.48 × 10−6pmol/L−1 min−1 and kon(GMR/β) = 1.41 × 10−4 pmol/L−1min−1. (D) GM-CSF association with low-affinity GMR at 4°C. (E) GM-CSF association with high-affinity GMR/β at 4°C. (F) Analyses of association rate constants (see Eq. 1 in Materials and Methods) for low-affinity (□) and high-affinity (▪) GM-CSF receptors shown in (D) and (E); kon (GMR) = 8.44 × 10−7 pmol/L−1 min−1and kon (GMR/β) = 8.89 × 10−5pmol/L−1 min−1.
Comparison of the association kinetics of GM-CSF binding with the low- and high-affinity GM-CSF receptors. Time courses of specific 125I-labeled GM-CSF binding to the COS cells expressing human low-affinity GMR and HL-60 promyelocytic cells expressing only human high-affinity GMR/β were measured as described in Materials and Methods. Results of representative experiments performed at 23°C and 4°C are shown. (A) GM-CSF association with low-affinity GMR at 23°C. (B) GM-CSF association with high-affinity GMR/β at 23°C. (C) Analyses of association rate constants (see Eq. 1 in Materials and Methods) for low-affinity (□) and high-affinity (▪) GM-CSF receptors shown in (A) and (B); kon (GMR) = 1.48 × 10−6pmol/L−1 min−1 and kon(GMR/β) = 1.41 × 10−4 pmol/L−1min−1. (D) GM-CSF association with low-affinity GMR at 4°C. (E) GM-CSF association with high-affinity GMR/β at 4°C. (F) Analyses of association rate constants (see Eq. 1 in Materials and Methods) for low-affinity (□) and high-affinity (▪) GM-CSF receptors shown in (D) and (E); kon (GMR) = 8.44 × 10−7 pmol/L−1 min−1and kon (GMR/β) = 8.89 × 10−5pmol/L−1 min−1.
The high-affinity receptor has discrete rates of ligand dissociation.
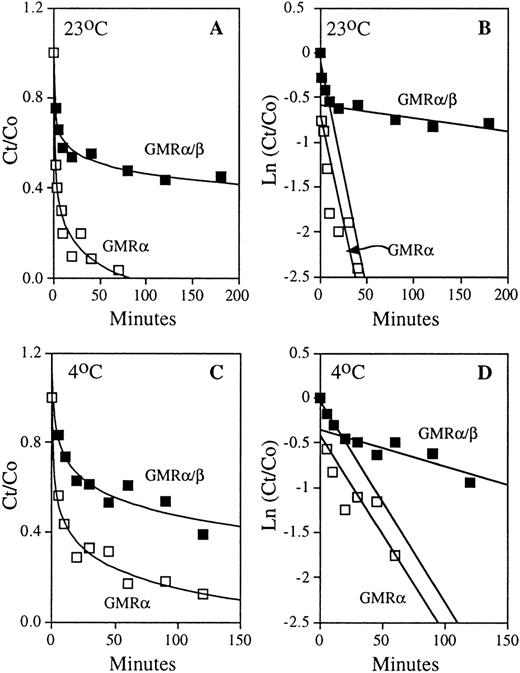
To ascertain the contribution of the β subunit in the dissociation component of the ligand-receptor interaction, HL-60 cells expressing only GMRα/β and COS cells transfected with GMRα were pre-equilibrated with 125I-labeled GM-CSF, and the time course of dissociation was measured directly by adding a high concentration of nonradioactive ligand to occupy free receptors (Fig 3A and C). Analysis of the dissociation curves (see Materials and Methods Eq. 4) yielded off-rate constants for the high- and low-affinity GM-CSF receptors (Fig 3B and D). In the case of high-affinity GM-CSF binding, there are two distinct dissociation rates indicating the presence of two classes of ligand-receptor complex with respect to dissociation: one that exhibits “tight” binding to the ligand and one with “loose” binding. At both 23°C and 4°C, the ratio of loose versus tight high-affinity binding complex is approximately 1:1 (Fig 3). The average dissociation rate constants obtained at both high and low temperatures for GMRα and GMRα/β are presented in Table 1. The koff of the tight complex is 8- to 22-fold lower than that of the loose complex. The average t1/2 values of ligand release from the loose complex are approximately 15 minutes at 23°C and 25 minutes at 4°C. For the tight complex, they are approximately 333 minutes at 23°C and 190 minutes at 4°C. GM-CSF dissociates from the low-affinity GMRα with average t1/2 values of approximately 12 minutes at 23°C and 18 minutes at 4°C. Thus, the koff of ligand release from the loose complex in high-affinity binding is similar to that observed in the low-affinity receptor. In contrast, the off-rate constant of the tight complex is much lower than that measured for GMRα. These results suggest a model in which the GMRβ subunit inhibits ligand release from the tight ligand-receptor complex in high-affinity GM-CSF binding, but may not play a role in ligand release from the loose complex. Therefore, the β chain of the GM-CSF receptor contributes to high-affinity binding by attenuation of ligand release in a distinct tight binding complex formed by the high-affinity receptor.
Dissociation kinetics of the low- and high-affinity GM-CSF receptors. COS cells expressing human low-affinity GMR and HL-60 promyelocytic cells expressing only high-affinity GMR/β were pre-equilibrated with 125I-labeled GM-CSF. The time courses of dissociation of radioactive ligand were determined by addition of 2 μmol/L of competing nonradioactive GM-CSF, as described in the experimental procedures. Representative results obtained at 23°C and 4°C are shown. (A) Time courses of GM-CSF dissociation from low-affinity GMR and high-affinity GMR/β at 23°C. (B) Analyses of dissociation rate constants (see Eq. 4 in Materials and Methods) of the low-affinity (□) and high-affinity (▪) GM-CSF receptors shown in (A); koff (GMR) = 4.74 × 10−2 min−1, loose complex koff (GMR/β) = 5.02 × 10−2min−1, and tight complex koff (GMR/β) = 1.41 × 10−3 min−1. (C) Time courses of GM-CSF dissociation from low-affinity GMR and high-affinity GMR/β at 4°C. (D) Analyses of dissociation rate constants (see Eq. 4 in Materials and Methods) of the low-affinity (□) and high-affinity (▪) GM-CSF receptors shown in (C); koff(GMR) = 2.22 × 10−2 min−1, loose complex koff (GMR/β) = 2.24 × 10−2min−1, and tight complex koff (GMR/β) = 4.09 × 10−3 min−1.
Dissociation kinetics of the low- and high-affinity GM-CSF receptors. COS cells expressing human low-affinity GMR and HL-60 promyelocytic cells expressing only high-affinity GMR/β were pre-equilibrated with 125I-labeled GM-CSF. The time courses of dissociation of radioactive ligand were determined by addition of 2 μmol/L of competing nonradioactive GM-CSF, as described in the experimental procedures. Representative results obtained at 23°C and 4°C are shown. (A) Time courses of GM-CSF dissociation from low-affinity GMR and high-affinity GMR/β at 23°C. (B) Analyses of dissociation rate constants (see Eq. 4 in Materials and Methods) of the low-affinity (□) and high-affinity (▪) GM-CSF receptors shown in (A); koff (GMR) = 4.74 × 10−2 min−1, loose complex koff (GMR/β) = 5.02 × 10−2min−1, and tight complex koff (GMR/β) = 1.41 × 10−3 min−1. (C) Time courses of GM-CSF dissociation from low-affinity GMR and high-affinity GMR/β at 4°C. (D) Analyses of dissociation rate constants (see Eq. 4 in Materials and Methods) of the low-affinity (□) and high-affinity (▪) GM-CSF receptors shown in (C); koff(GMR) = 2.22 × 10−2 min−1, loose complex koff (GMR/β) = 2.24 × 10−2min−1, and tight complex koff (GMR/β) = 4.09 × 10−3 min−1.
DISCUSSION
To illuminate the mechanism of interaction between GM-CSF and its receptor subunit components, we performed kinetic analyses of binding with HL-60 cells expressing the GMRα/β complex and COS cells transfected with low-affinity GMRα. We found that GMRβ both facilitates ligand association and retards ligand release in the high-affinity GM-CSF binding complex. The on rate constants in this study were determined by measurement of dynamic association time courses using a general second-order equation. This method of analysis offers several technical advantages that provide a more precise assessment of binding kinetics: (1) depletion of ligand and receptor as well as simultaneous ligand release during the course of the ligand-receptor association reaction are accounted for in the derivation of the second-order equation26; (2) unlike the initial rate method, in which the on rate constant is determined wholly by measurement of ligand binding at early time points, determination of kon by the second-order method uses all the time points over the entire course of the experiment; and (3) with the second-order equation, relatively low concentrations of ligand and receptor can be used to allow technically facile measurement of the association rate. In contrast, the commonly used pseudo-first-order method requires higher ligand concentrations to ensure that the free GM-CSF concentration does not change appreciably during binding. Using a second-order analytic model, we found that GM-CSF binds to the high-affinity receptor much more rapidly than the low-affinity receptor, indicating that GMRβ plays a crucial role in the initial association step of ligand-receptor interaction.
The finding of two discrete dissociation kinetics for the high-affinity receptor suggests that the ligand-receptor complex exists in “loose” and “tight” binding forms. In the loose complex, the koff of GM-CSF release from GMRα/β is similar to that of the low-affinity GMRα, implying that the β subunit contributes to high-affinity binding in the loose conformation only by facilitating GM-CSF association to the receptor. The koffmeasured in the “tight” form of the high-affinity receptor suggests that GMRβ is able to increase ligand-binding affinity by inhibition of ligand release as well as acceleration of GM-CSF association. The effect of GMRβ on ligand association is proportionally higher (60- to 69-fold) than its effect to slow ligand dissociation (10- to 25-fold).
The kon and koff define the equilibrium dissociation constant kd, which can be determined directly by measuring ligand binding at equilibrium.30 The calculated kd derived from the individual konand koff rate constants is in good agreement with experimentally derived equilibrium kd with differences that are less than threefold. With regard to high-affinity binding, the presence of heterogeneity in the ligand-dissociation step suggests that the measured kd value of the high-affinity receptor is the result of a homogeneous kon and a heterogeneous koff of loose and tight ligand-receptor complexes. GMRα/β exhibits a higher equilibrium dissociation constant at 4°C than at 23°C. Although a modest increase in the kon of GMRα/β at the higher temperature may explain some of the difference in the apparent kd values at the two temperatures, comparison of the individual dissociation constants of high-affinity binding at 4°C and 23°C suggests a small increase in the loose koff and a slight decrease in the tight koff for GMRα/β at the higher temperature (Table 1). Although the proportion of tight and loose complexes at 23°C and 4°C seem to be similar, a temperature effect on the ratio of tight and loose complexes cannot be excluded. Nonetheless, at both 4°C and 23°C, the β subunit causes enhanced GM-CSF association and the formation of loose and tight ligand-receptor complexes, which has an overall effect of retarding ligand release. The presence of two components of ligand release observed in the high-affinity binding complex may also contribute to the variability of dissociation constants observed in high-affinity GM-CSF binding.5-7
Although the β chain alone has no detectable ligand-binding activity, it plays a critical role in increasing receptor-binding affinity and in transmitting intracellular signals. Lopez et al31 found that amino acid substitutions at residue 21 of human GM-CSF substantially reduced high-affinity binding without affecting low-affinity binding, supporting the notion of a direct interaction between the common β subunit and ligand in the context of the high-affinity receptor complex. In this study, we found that the β chain functions actively in the initial acquisition of GM-CSF to the high-affinity receptor. The substantially increased association rate constant of the high-affinity receptor suggests that the β chain interacts with the α subunit in a manner that facilitates access of ligand to the binding pocket. Thus, the rate of ligand-receptor complex formation is accelerated in the high-affinity receptor. The slower koff displayed by the high-affinity receptors in the “tight” complex compared with GMRα alone indicates that the β subunit also inhibits GM-CSF release from the GMRα/β complex during ligand dissociation. The physical basis for the slower off rate is unknown. Heparan sulfate proteoglycans have been reported to influence ligand-receptor binding by interacting with ligands to create pockets of locally increased ligand concentration.32-35 We found that treatment of HL-60 promyelocytic cells with heparinase and heparitinase had no effect on high-affinity binding (data not shown), suggesting that it is unlikely that the extracellular matrix has a substantial role in high-affinity GM-CSF binding. Likewise, high-affinity binding in HL-60 cells is similar to what we previously found in COS cells transfected with the GMRα and GMRβ subunits.24 Thus, tissue and species-specific cellular backgrounds do not explain our findings. The possibility that the tight and loose complexes are caused by the effects of localized membrane microdomains, however, cannot be excluded. Several lines of evidence support the notion of a physical association between the α and β subunits in the absence of ligand.36 37 A preformed α/β complex may explain the faster on rate of the high affinity receptor and raises the possibility of distinct subclasses of GM-CSF receptors with different binding kinetics. Nonetheless, the differences in the off rate are likely a result of conformational change in the ligand-receptor complex after binding. The combined kinetic effects result in a GMRα/β complex that exhibits much higher equilibrium binding affinity than the α subunit alone, suggesting that the β chain increases binding energy in the ligand-receptor interaction, and that there are two distinct components of high-affinity binding.
The high-affinity receptors of GM-CSF, interleukin-3, and interleukin-5 share a common β subunit.5,38 Although the ligand binding determinants in the β chain for these cytokines may be different,37 39 it is possible that the β subunit has similar effects on ligand binding in these related receptors.
ACKNOWLEDGMENT
The authors thank Rong-Hua Zhang for excellent technical assistance, and Elizabeth P. Koers for expert editorial assistance.
Supported by Grants RO1 CA30388, RO1 HL42107, P30 CA08748 from the National Institutes of Health, The Leukemia Society of America, and The Schultz Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to David W. Golde, MD, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10021; e-mail: d-golde@ski.mskcc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal