Abstract
We used in vitro and in vivo approaches to examine whether tumor necrosis factor- (TNF-) and oncostatin M (OSM), cytokines that bind to distinct classes of receptors, differentially regulate expression of P- and E-selectin in murine and primate endothelial cells. In human umbilical vein endothelial cells, TNF- rapidly increased mRNA for E-selectin but not P-selectin. OSM elicited little or no change in mRNA for E-selectin, but induced a delayed and prolonged increase in P-selectin mRNA. TNF- and OSM did not cooperate to further enhance P- or E-selectin mRNA. Intravenous infusion of Escherichia coli, which markedly elevates plasma lipopolysaccharide and TNF-, increased mRNA for E-selectin but not P-selectin in baboons. In murine bEnd.3 endothelioma cells, TNF- and OSM individually and cooperatively increased mRNA and protein for both P- and E-selectin. Intravenous injection of these cytokines also individually and cooperatively increased mRNA for P- and E-selectin in mice. We conclude that the murine P- and E-selectin genes respond to both TNF- and OSM, whereas the primate P- and E-selectin genes have much more specialized responses. Such differences should be considered when extrapolating the functions of P- and E-selectin in murine models of inflammation to humans.
LEUKOCYTE EMIGRATION into sites of inflammation proceeds through a multistep series of adhesive and signaling events.1 Under shear flow, flowing leukocytes tether to and roll on the vascular surface, then adhere more firmly, and finally migrate between endothelial cells into the underlying tissues. A critical early event is the elaboration of inflammatory mediators that stimulate endothelial cells to express adhesion molecules, chemokines, and lipid autacoids.2 The adhesion molecules include P-selectin and E-selectin, which initiate the rolling of leukocytes through interactions with cell-surface glycoconjugates.3-5
The mediators tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), or lipopolysaccharide (LPS) induce endothelial cells to transcribe mRNA for E-selectin, and the translated protein is transported directly to the cell surface. Transient activation of the E-selectin gene involves the cooperative binding of nuclear factor κB (NF-κB), activating transcription factor-2 (ATF-2), and other transcription factors.6 Although the duration of transcriptional activation varies somewhat, TNF-α, IL-1β, or LPS clearly induce expression of E-selectin in both human and murine endothelial cells in vitro and in vivo.6
Thrombin, histamine, or other secretagogues redistribute P-selectin within minutes from the membranes of Weibel-Palade bodies to the surface of both human and murine endothelial cells.7-10Such early inducible expression does not require new protein synthesis because P-selectin is mobilized from an existing storage compartment. However, this pool of P-selectin is only transiently expressed on the cell surface, as it is proteolytically released into the circulation11,12 or it is rapidly endocytosed13and then recycled to Weibel-Palade bodies14 or degraded in lysosomes.15
Mechanisms for increasing synthesis of P-selectin are suggested by its presence on the apical surface of endothelial cells in human tissues with chronic or allergic inflammation.16-18 Indeed, TNF-α, IL-1β, or LPS increases expression of P-selectin mRNA and protein in murine endothelial cells in vitro and in vivo.10,19,20 Transient activation of the murine P-selectin gene requires the cooperative binding of NF-κB, ATF-2, and other transcription factors.21 However, the binding sites for NF-κB and ATF-2 found in the promoter of the murine P-selectin gene are not present in the promoter of the human P-selectin gene.22,23 Furthermore, TNF-α, IL-1β, or LPS does not increase P-selectin mRNA in human endothelial cells in vitro.24 25 It is unknown whether these mediators increase P-selectin mRNA in humans or in nonhuman primates in vivo, an important consideration because the regulation of gene expression in cultured cells may differ from that found in vivo. Confirmation of the inability of TNF-α, IL-1β, and LPS to increase P-selectin mRNA in primates in vivo would suggest that other cytokines must contribute to the inducible expression of P-selectin mRNA in humans.
Some members of the IL-6 family of cytokines induce proinflammatory responses in cultured human endothelial cells, including the expression of selectins. IL-6, oncostatin M (OSM), leukemia inhibitory factor (LIF), and other IL-6 family members bind to receptors that contain at least one of the signal-transducing subunit gp130.26 Human OSM binds to both low affinity and high affinity receptors on human endothelial cells.27 The high affinity receptor may be a heterodimer of gp130 and the OSM-specific receptor subunit, OSMRβ.28 Signaling through the high affinity receptor induces a marked increase in P-selectin mRNA.24 The OSM-induced increase in P-selectin mRNA requires new protein synthesis, is first observed 7 hours after addition of OSM, and persists for at least 72 hours.24 Signaling through the low affinity receptor increases E-selectin protein, but to a much lower level than that induced by TNF-α.29 IL-6 does not bind to human endothelial cells because they lack the IL-6–specific receptor subunit, IL-6Rα. However, soluble IL-6Rα complexed with IL-6 binds to gp130 on human endothelial cells, propagating signals that include the synthesis of E-selectin.30 31 It is not known whether IL-6–related cytokines affect selectin expression in murine endothelial cells in vitro or in vivo. This issue is important, because selectin genes in humans may have evolved to respond to different classes of cytokines.
Because of their small size and relatively low cost, mice are commonly used to study the functions of adhesion molecules, cytokines, and chemokines in models of inflammation, atherosclerosis, and vascular injury.32 Extrapolation of the results from mouse experiments to human biology, however, requires that the molecules of interest be regulated similarly in both species. The available data suggest that cytokine induction of selectin expression may differ in mice and humans to at least some extent. However, specific mediators have not always been tested in both mice and humans, and in other cases the comparisons have only been made in vitro. We have directly compared the effects of TNF-α and OSM in inducing expression of P- and E-selectin in cultured human and murine endothelial cells. To extend these results to in vivo settings, we have examined the effects of inflammatory mediators on selectin expression in nonhuman primates and in mice. Our results suggest that TNF-α and OSM function cooperatively to induce expression of P- and E-selectin in mice but diverge significantly in their effects on expression of P- and E-selectin in humans or nonhuman primates.
MATERIALS AND METHODS
Reagents and antibodies.
Recombinant human and murine OSM were purchased from R & D Systems, Inc (Minneapolis, MN). Recombinant human and murine TNF-α were obtained from Boehringer Mannheim Biochemicals (Indianapolis, IN). The murine anti-human P-selectin monoclonal antibody (MoAb) G1 and the murine anti-human E-selectin MoAb ES1 were prepared as described.33,34 The rat anti-murine P-selectin MoAb RB40.3435 and the rat anti-murine E-selectin MoAb 10E9.610 were kind gifts of Dr Dietmar Vestweber (University of Muenster, Muenster, Germany). Cycloheximide, actinomycin D, and LPS were from Sigma Chemical Co (St Louis, MO). Trizol reagent and the Superscript Preamplication System for first-strand cDNA synthesis were purchased from Life Technologies Inc (Grand Island, NY). The PCR Mimic construction kit was obtained from Clontech Laboratories, Inc (Palo Alto, CA). Hi-Lo DNA marker was obtained from Minnesota Molecular, Inc (Minneapolis, MN). All other reagents were obtained from Fisher Scientific (Pittsburgh, PA) unless noted otherwise.
Cell culture.
Human umbilical vein endothelial cells (HUVEC) and murine bEnd.3 endothelioma cells were cultured as described.24 Cytokines or pharmacologic agents, dissolved in fresh medium, were added to confluent cell monolayers for the time intervals and at the final concentrations indicated in the text. As a control, fresh medium lacking the cytokine or agent was added to other cells.
Northern blot analysis.
Western blots.
Equal numbers of HUVEC were lysed in sodium dodecyl sulfate (SDS) sample buffer in the absence of reducing agent and boiled for 5 minutes. The samples were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) in a 7.5% polyacrylamide gel, transferred to an Immobilon-P membrane (Amersham Corp, Arlington Heights, IL), and probed with 1 μg/mL anti–E-selectin MoAb ES1. Bound MoAb was detected using a horseradish peroxidase-conjugated goat anti-mouse antibody with enhanced chemiluminescence (Amersham).
Binding of 125I-labeled MoAbs to bEnd.3 cells.
Binding of 125I-labeled anti-murine P-selectin or E-selectin MoAbs to fixed, permeabilized bEnd.3 cell monolayers was performed exactly as described for binding of 125I-labeled anti-human P-selectin MoAb to HUVEC.24
Animal experiments.
Female 6- to 16-week-old Balb/C mice were purchased from Charles River Laboratories (Wilmington, MA). Each mouse received a tail-vein injection of 50 μL of buffer (phosphate-buffered saline, pH 7.4 plus 1% human serum albumin) as control, or 50 μL of buffer containing 25 ng murine OSM, 3000 U murine TNF-α, or both OSM and TNF-α. In other experiments mice were injected with buffer containing 25 ng human OSM. Mice were killed 1 hour, 4 hours, or 24 hours after the injection, and organs were then immediately collected for RNA isolation or immunohistochemistry.
Healthy adolescent baboons (Papio c. anubis) were purchased from a breeding colony maintained by the University of Oklahoma Health Sciences Center, Oklahoma City, OK. A dose of live Escherichia coli known to induce lethal septic shock was infused intravenously over a 2-hour period into a baboon as described.36-38 A control animal received no E colibut was otherwise treated identically. After 4 hours (2 hours after the end of the E coli infusion), the experimental and control animals were killed with a lethal intravenous infusion of sodium pentobarbitol, and the organs were then immediately collected for RNA isolation.
The protocols for mice and baboons were approved by the appropriate Animal Use Committees of the University of Oklahoma Health Sciences Center, the Veterans Administration Hospital, or the Oklahoma Medical Research Foundation, Oklahoma City, OK.
Quantitative reverse transcriptase-polymerase chain reaction (RT-PCR).
Total RNA was isolated from murine or baboon organs with the Trizol reagent. First-strand cDNA synthesis was performed with the Superscript Preamplification System as specified by the manufacturer. Briefly, RNA (5 μg) was incubated with 0.5 μg oligo-dT for 10 minutes at 70°C and then chilled on ice for 1 minute. A PCR buffer that included final concentrations of 0.2 mmol/L MgCl2, 1.0 mmol/L dNTP mix, and 0.01 mol/L DTT was added to each RNA/primer mix and incubated for 5 minutes at 42°C. Then 200 U of Superscript II reverse transcriptase was added to give a final volume of 20 μL, and the mixture was incubated for 50 minutes at 42°C, for 15 minutes at 70°C, and then chilled on ice. RNase H (1 μL containing 2 U) was added and incubated for 20 minutes at 37°C before PCR was conducted.
Quantitative PCR was performed with the PCR Mimic construction kit as specified by the manufacturer. Briefly, PCR mimics were constructed with 2 rounds of PCR amplification. Each composite primer consisted of target gene sequence (from P-selectin, E-selectin, or β-actin) attached to 1 of 2 20-nucleotide sequences designed to hybridize to opposite strands of a mimic DNA fragment (a 574-bp Bam HI/Eco RI fragment of the v-Erb gene). A dilution of the first PCR reaction was reamplified using only gene-specific primers. The PCR mimic was then purified on a ChromaSpin+TE-100 column. The purified mimic was adjusted to a concentration of 100 attomoles/μL. For quantitative PCR, 10-fold dilutions of mimic were added to a series of tubes containing a fixed concentration of first-strand cDNA and a pair of gene-specific primers. The PCR conditions were as follows: 25 cycles at 94°C for 1 minute, 60°C for 1 minute, and 72°C for 1.5 minutes. The amplified products were electrophoresed in a 1.5% agarose gel, and the gel was stained with ethidium bromide. The concentration of mRNA for P-selectin, E-selectin, or actin was estimated from the lane in which the quantity of the amplified target cDNA was similar to that of the mimic cDNA. For each such lane, the original concentration of mimic DNA (in attomoles/μL) was used to assign a semi-quantitative measurement to the concentration of mRNA as follows: − < 10−5, + = 10−4, ++ = 10−3, +++ = 10−2, ++++ = 10−1.
The gene-specific primers to amplify baboon P- and E-selectin were derived from the published sequences for the human cDNAs for P- and E-selectin.39,40 The size of the amplified products exactly matched the predicted size of the human orthologues. The identity of the amplified baboon cDNA fragments was confirmed by DNA sequencing; the nucleotide sequence of the baboon P-selectin cDNA fragment was 99% identical to that of the human P-selectin cDNA, and the nucleotide sequence of the baboon E-selectin cDNA was 97% identical to that of the human E-selectin cDNA. For the experiments shown in Fig 2 and Table1, the primers for P-selectin were derived from the experimentally determined baboon sequence. The baboon E-selectin product was amplified using the primers derived from the human E-selectin sequence. The gene-specific primers for murine P- and E-selectin were derived from the published sequences for the murine cDNAs for P- and E-selectin.19 The sizes of the amplified products exactly matched those predicted. The identity of the amplified fragments was further confirmed by restriction digestions with appropriate enzymes.
Human and murine β-actin mimic and primers were provided by the manufacturer. The P- and E-selectin primers were as follows: Murine P-selectin sense primer: 5′-CTATACCTGCTCCTGCTACCCAGGC-3′ (nt 549-573), antisense primer: 5′-TTCACTCCACTGACCAGAGCCAGTG-3′ (nt 951-937); murine E-selectin sense primer: 5′-CCTCTGACAGAGGAAGCTCAGAACT-3′ (nt 401-425), antisense primer: 5′-TCCACTCTCCAGAGGACGTACACCG-3′ (nt 830-806); baboon P-selectin sense primer: 5′-GACATGTCCTGCAGCAAACAAGGAGAGTGC-3′ (nt 531-560), antisense primer 5′-CCGGTCCAACTAATGCAAATCCCTCTTCAC-3′ (nt 942-913); human E-selectin sense primer: 5′-CAGCAAGAAGAAGCTTGCCCTATG-3′ (nt 506-530), antisense primer 5′-TTGTGTTCCATGGGAAGCTTCCAGG-3′ (nt 915-891).
Immunohistochemistry.
Frozen sections of murine brain, skin, heart, mediastinal large veins and elastic arteries, lungs, spleen, kidneys, liver, and esophagus were fixed in 4% buffered paraformaldehyde for 10 minutes at 4°C. Immunohistochemical staining was performed using a standardized streptavidin-biotin-peroxidase method. All incubations were at room temperature and were separated by washes with PBS. The sections were treated with 1.25% hydrogen peroxide to block endogenous peroxidase activity and with PBS containing 10% rabbit serum to reduce nonspecific antibody binding. The sections were next treated for 20 minutes with 10 μg/mL of anti–P-selectin MoAb RB40.34 or anti–E-selectin MoAb 10E9.6, or as a negative control, with buffer containing no primary antibody. The sections were then incubated with 10 μg/mL biotin-conjugated rabbit anti-rat IgG (Dako Corporation, Carpinteria, CA) for 20 minutes and finally with a streptavidin-peroxidase reagent (Dako) for 30 minutes. Diaminobenzidine (Sigma) was used as chromagen, and hematoxylin was used for nuclear counterstaining.
RESULTS
Stimulation of HUVEC with TNF-α plus OSM does not cooperatively increase mRNA for P- and E-selectin.
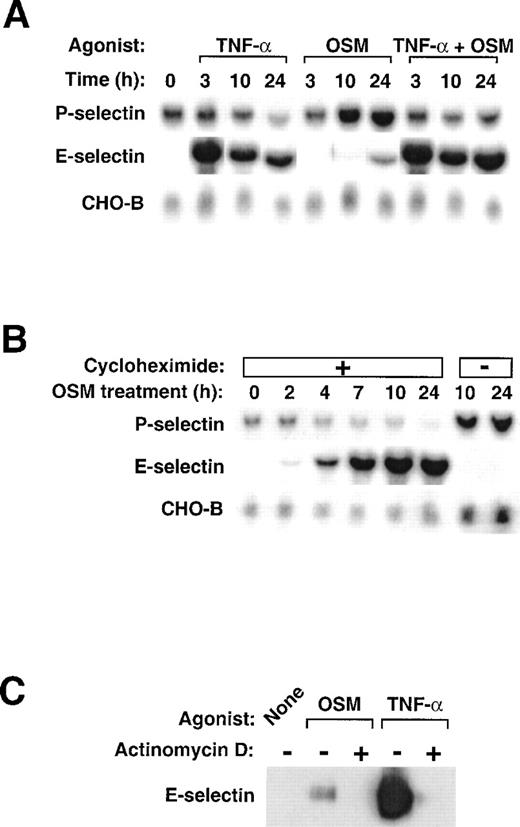
We used Northern blot analysis to examine the effects of human OSM and TNF-α on expression of P- and E-selectin in HUVEC. We also measured the mRNA levels of CHO-B, a control, ubiquitously expressed transcript that does not change its levels when cells are stimulated.41 As observed previously,24 OSM induced a delayed and sustained increase in P-selectin mRNA levels that reached maximum within 10 to 24 hours (Fig1A). In contrast, stimulation with TNF-α did not change or even slightly decreased P-selectin mRNA levels. Costimulation of HUVEC with TNF-α and OSM did not cooperatively increase P-selectin mRNA and, in fact, often blunted the OSM-induced increase in P-selectin mRNA levels. As documented previously,40 stimulation of HUVEC with TNF-α markedly increased E-selectin mRNA within 3 hours, followed by a gradual decline over 10 to 24 hours. Stimulation with OSM, in contrast, had little effect on E-selectin mRNA levels, although a small accumulation of transcripts was sometimes observed after 24 hours. Costimulation with TNF-α and OSM increased E-selectin mRNA to levels observed after TNF-α alone, although a slight further increase was sometimes observed after 24 hours. These data show that TNF-α and OSM do not cooperatively increase mRNA for P- or E-selectin in HUVEC. OSM is the major inducer of P-selectin mRNA, whereas TNF-α is the major inducer of E-selectin mRNA.
Stimulation of HUVEC with TNF- plus OSM does not cooperatively increase mRNA for P- and E-selectin. (A) Confluent monolayers of HUVEC were treated with 10 ng/mL human OSM, 100 U/mL human TNF-, or a combination of both cytokines. After the indicated time, total RNA was isolated, and 20 μg of RNA was electrophoresed and then transferred to a membrane for Northern blot analysis. The same membrane was sequentially hybridized with the indicated cDNA probes. The mobilities of the hybridized transcripts corresponded to published values. (B) HUVEC were treated with 10 ng/mL OSM in the presence or absence of 10 μg/mL cycloheximide. After the indicated time, total RNA was isolated and analyzed by Northern blotting. (C) HUVEC were treated with 1 μg/mL OSM or 100 U/mL TNF- in the presence or absence of 5 μg/mL actinomycin D. After 4 hours, the cells were lysed and analyzed by Western blotting with a MoAb to E-selectin.
Stimulation of HUVEC with TNF- plus OSM does not cooperatively increase mRNA for P- and E-selectin. (A) Confluent monolayers of HUVEC were treated with 10 ng/mL human OSM, 100 U/mL human TNF-, or a combination of both cytokines. After the indicated time, total RNA was isolated, and 20 μg of RNA was electrophoresed and then transferred to a membrane for Northern blot analysis. The same membrane was sequentially hybridized with the indicated cDNA probes. The mobilities of the hybridized transcripts corresponded to published values. (B) HUVEC were treated with 10 ng/mL OSM in the presence or absence of 10 μg/mL cycloheximide. After the indicated time, total RNA was isolated and analyzed by Northern blotting. (C) HUVEC were treated with 1 μg/mL OSM or 100 U/mL TNF- in the presence or absence of 5 μg/mL actinomycin D. After 4 hours, the cells were lysed and analyzed by Western blotting with a MoAb to E-selectin.
The lag period required for OSM to increase P-selectin mRNA differs from the ability of TNF-α to rapidly increase mRNA for E-selectin, a characteristic of immediate early genes. When pretreated with cycloheximide, HUVEC did not increase P-selectin mRNA levels in response to OSM (Fig 1B). In contrast, these cells expressed higher levels of E-selectin mRNA, presumably because they could not synthesize new IκB-α to retain heterodimeric NF-κB complexes in the cytoplasm.42 These results show that, to fully activate the P-selectin gene, OSM must first induce the synthesis of one or more proteins in HUVEC.
The HUVEC in Fig 1A and 1B were stimulated with OSM at 10 ng/mL (0.45 nmol/L), a concentration that saturates the high affinity receptors for OSM on HUVEC27 and causes maximal accumulation of P-selectin mRNA.24 OSM at a higher concentration (1 μg/mL; 45 nmol/L) binds to low affinity receptors and increases E-selectin protein, although to a much lower level than does TNF-α.29 Treatment of HUVEC with 45 nmol/L OSM only slightly increased E-selectin mRNA levels over that observed with 0.45 nmol/L OSM in Fig 1A (data not shown). As observed previously,29 stimulation of HUVEC with 45 nmol/L OSM for 4 hours did increase E-selectin protein as detected by immunoblotting, but the increase was only 10% of that observed after stimulation with TNF-α (Fig 1C). Pretreatment of HUVEC with actinomycin D blocked the increase in E-selectin protein, suggesting that OSM induced E-selectin expression at the transcriptional level. These data indicate that high concentrations of OSM do activate the E-selectin gene, but to a much lesser extent than does TNF-α.
Intravenous infusion of E coli increases mRNA for E-selectin but not P-selectin in baboon tissues.
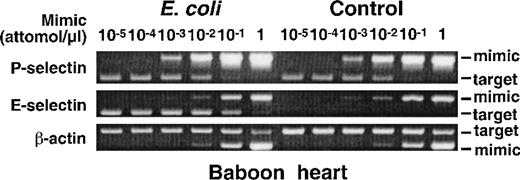
TNF-α, IL-1β, or LPS increases mRNA for E-selectin but not for P-selectin in HUVEC.6,24 (See also Fig 1A.) This strongly suggests that these mediators, which function by activating NF-κB heterodimers and ATF-2-containing dimers, do not activate the P-selectin gene in human endothelial cells. However, transcriptional regulation of genes in vitro and in vivo may differ, because the environment of cultured cells has been drastically altered from the normal environment. We therefore used a well-characterized, nonhuman primate model to test whether LPS increases mRNA for P- and E-selectin in vivo. A baboon was given an intravenous infusion of E coliat a dose that consistently induces death through septic shock within 12 to 36 hours.38 The injected E coli produce high circulating levels of LPS, which initiates signals that elevate plasma levels of TNF-α within 1 to 2 hours.36 After 4 hours the baboon was killed, and RNA was isolated from multiple organs; as a control, RNA was isolated from the organs of a baboon that did not receive E coli. The levels of mRNA for P-selectin, E-selectin, or β-actin were measured by a competitive RT-PCR method in which serial dilutions of a DNA mimic containing flanking sequences matching the target DNA were added to first-strand cDNA prepared from a fixed concentration of tissue RNA. The amount of mimic that allows amplification of equal quantitities of mimic and target DNA provides a measure of the level of target DNA, and thus mRNA, in the sample. Figure 2 shows representative agarose gel electrophoresis of RT-PCR products from RNA of the heart. The levels of P-selectin mRNA, like those of the control β-actin mRNA, were identical in the control baboon and in the baboon that received E coli. In contrast, mRNA levels for E-selectin were significantly higher in the baboon that received E coli than in the control baboon. Similar results were obtained when mRNA from other organs was measured (Table 1). The E coliinfusion markedly increased mRNA for E-selectin in all organs, whereas it did not change or even decreased mRNA for P-selectin. These data show that circulating LPS and TNF-α in concentrations that significantly increase E-selectin mRNA do not increase P-selectin mRNA in a nonhuman primate. Thus, the results obtained in vivo support the observations made in cultured human endothelial cells.
Intravenous infusion of E coli increases mRNA for E-selectin but not P-selectin in baboon tissues. A lethal dose of E coli was infused intravenously into a baboon. A control baboon received no E coli but was otherwise treated identically. After 4 hours, the animals were killed, and the organs were immediately collected for RNA isolation. The levels of mRNA for P-selectin, E-selectin, and β-actin were measured by a competitive RT-PCR method in which serial dilutions of a DNA mimic containing flanking sequences matching the target DNA were added to first-strand cDNA prepared from a fixed concentration of tissue RNA. The amount of mimic that allows amplification of equal quantitities of mimic and target DNA provides a measure of the level of target DNA, and thus mRNA, in the sample. Shown is representative agarose gel electrophoresis of the PCR products from baboon heart. In this example, mimic cDNA at a concentration of 10−3 attomoles/μL allowed equal amplification of mimic and P-selectin cDNA in both the control baboon and the baboon that received E coli. Mimic cDNA at a concentration of 10−1 attomoles/μL allowed equal amplification of mimic and E-selectin cDNA in the E coli baboon, whereas no E-selectin cDNA was amplified in the control baboon even at the lowest concentration of mimic (10−5 attomoles/μL). Mimic cDNA at a concentration of 10−1 attomoles/μL allowed equal amplification of mimic and β-actin cDNA in both the control baboon and the E coli baboon. Identical results were obtained with another pair of baboons, one of which received E coli.
Intravenous infusion of E coli increases mRNA for E-selectin but not P-selectin in baboon tissues. A lethal dose of E coli was infused intravenously into a baboon. A control baboon received no E coli but was otherwise treated identically. After 4 hours, the animals were killed, and the organs were immediately collected for RNA isolation. The levels of mRNA for P-selectin, E-selectin, and β-actin were measured by a competitive RT-PCR method in which serial dilutions of a DNA mimic containing flanking sequences matching the target DNA were added to first-strand cDNA prepared from a fixed concentration of tissue RNA. The amount of mimic that allows amplification of equal quantitities of mimic and target DNA provides a measure of the level of target DNA, and thus mRNA, in the sample. Shown is representative agarose gel electrophoresis of the PCR products from baboon heart. In this example, mimic cDNA at a concentration of 10−3 attomoles/μL allowed equal amplification of mimic and P-selectin cDNA in both the control baboon and the baboon that received E coli. Mimic cDNA at a concentration of 10−1 attomoles/μL allowed equal amplification of mimic and E-selectin cDNA in the E coli baboon, whereas no E-selectin cDNA was amplified in the control baboon even at the lowest concentration of mimic (10−5 attomoles/μL). Mimic cDNA at a concentration of 10−1 attomoles/μL allowed equal amplification of mimic and β-actin cDNA in both the control baboon and the E coli baboon. Identical results were obtained with another pair of baboons, one of which received E coli.
Effects of Infusion of E coli on mRNA Levels for P- and E-Selectin in Baboon Organs
| . | P-Selectin . | E-Selectin . | ||
|---|---|---|---|---|
| Control . | E coli . | Control . | E coli . | |
| Heart | ++ | ++ | − | ++++ |
| Lung | ++ | + | − | ++++ |
| Kidney | ++ | ++ | − | ++++ |
| Liver | ++ | ++ | − | ++++ |
| Spleen | ++ | + | − | ++++ |
| . | P-Selectin . | E-Selectin . | ||
|---|---|---|---|---|
| Control . | E coli . | Control . | E coli . | |
| Heart | ++ | ++ | − | ++++ |
| Lung | ++ | + | − | ++++ |
| Kidney | ++ | ++ | − | ++++ |
| Liver | ++ | ++ | − | ++++ |
| Spleen | ++ | + | − | ++++ |
The levels of mRNA were measured by competitive RT-PCR as described in Materials and Methods. The values represent the results from organs isolated from 2 control baboons and from 2 baboons that received E coli.
Stimulation of murine bEnd.3 cells with TNF-α plus OSM cooperatively increases mRNA for P- and E-selectin.
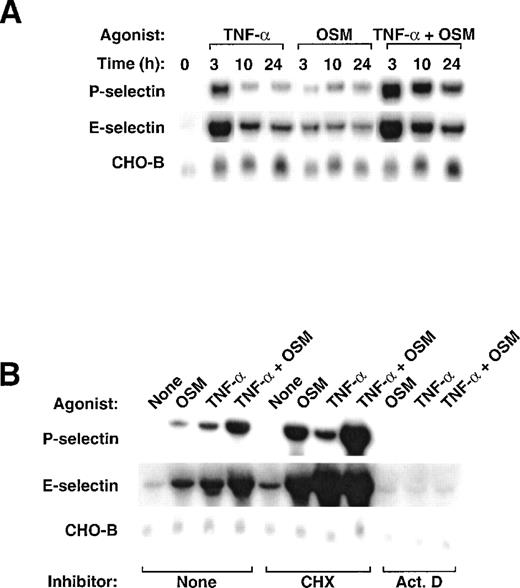
To determine whether OSM affected expression of P- or E-selectin in murine endothelial cells, we treated bEnd.3 cells with 25 ng/mL (1.125 nmol/L) murine OSM for various intervals. OSM markedly increased the levels of P-selectin mRNA (Fig 3A). Unlike the delayed accumulation of P-selectin mRNA in HUVEC after OSM stimulation, P-selectin mRNA in bEnd.3 cells increased rapidly, reaching maximum within 1 to 3 hours. Levels declined within 10 hours but remained elevated at least 48 hours after addition of OSM. Unlike the little or no change in E-selectin mRNA in HUVEC after OSM stimulation, E-selectin mRNA in bEnd.3 cells increased rapidly, reaching maximum within 1 hour. Levels then declined but remained slightly elevated at least 48 hours after addition of OSM. Quantification of selectin transcripts, normalized for the level of CHO-B mRNA, indicated that OSM increased P-selectin mRNA up to 8-fold (Fig 3B) and E-selectin mRNA up to 14-fold (Fig 3C). OSM increased P- and E-selectin mRNA in a concentration-dependent manner, with maximal effects achieved at 25 ng/mL (data not shown). The effect of murine OSM was not because of contamination with LPS because boiled OSM did not increase P- or E-selectin transcripts. In contrast, added LPS increased both P- and E-selectin mRNA (Fig 3A). Stimulation of bEnd.3 cells with 25 ng/mL human OSM increased P- and E-selectin mRNA exactly like that observed with murine OSM (data not shown).
Stimulation of murine bEnd.3 cells with OSM increases mRNA for both P- and E-selectin. (A) Confluent monolayers of bEnd.3 cells were incubated in the presence or absence of 25 ng/mL murine OSM. To ensure that the effects of OSM were not caused by contaminating LPS, some cells were treated with OSM that was boiled to inactive the cytokine, or with 10 ng/mL of exogenously added LPS. After the indicated time, total RNA was isolated and analyzed by Northern blotting. (B) and (C) The OSM-induced increase in P- and E-selectin mRNA in each lane was quantified by densitometric scanning. The level of P- and E-selectin mRNA in each lane was normalized according to the level of CHO-B mRNA, which was not affected by OSM. The data are representative of 4 independent experiments.
Stimulation of murine bEnd.3 cells with OSM increases mRNA for both P- and E-selectin. (A) Confluent monolayers of bEnd.3 cells were incubated in the presence or absence of 25 ng/mL murine OSM. To ensure that the effects of OSM were not caused by contaminating LPS, some cells were treated with OSM that was boiled to inactive the cytokine, or with 10 ng/mL of exogenously added LPS. After the indicated time, total RNA was isolated and analyzed by Northern blotting. (B) and (C) The OSM-induced increase in P- and E-selectin mRNA in each lane was quantified by densitometric scanning. The level of P- and E-selectin mRNA in each lane was normalized according to the level of CHO-B mRNA, which was not affected by OSM. The data are representative of 4 independent experiments.
Previous studies showed that TNF-α increases mRNA expression for P- and E-selectin in murine endothelioma cells.10 19 We treated bEnd.3 cells with murine TNF-α, OSM, or both cytokines. The combination of TNF-α and OSM increased mRNA for both selectins to much higher levels than those observed after treatment with either individual cytokine (Fig 4A). The increase was most prominent after 3 hours but was still observed at least 24 hours after addition of the mediators. These data show that, unlike their lack of cooperativity in HUVEC, TNF-α and OSM cooperatively increase mRNA for both P- and E-selectin in bEnd.3 cells. TNF-α and OSM also cooperatively increased protein for both P- and E-selectin in bEnd.3 cells (data not shown).
Stimulation of bEnd.3 cells with TNF- plus OSM cooperatively increases mRNA for P- and E-selectin. (A) bEnd.3 cells were treated with 25 ng/mL murine OSM, 100 U/mL murine TNF-, or a combination of both cytokines. After the indicated time, total RNA was isolated and analyzed by Northern blotting. (B) bEnd.3 cells were treated with fresh medium in the presence or absence of 25 ng/mL murine OSM, 100 U/mL murine TNF-, or a combination of both cytokines, in the presence or absence of 10 μg/mL cycloheximide or 5 μg/mL actinomycin D (Act. D). After 3 hours, total RNA was isolated and analyzed by Northern blotting.
Stimulation of bEnd.3 cells with TNF- plus OSM cooperatively increases mRNA for P- and E-selectin. (A) bEnd.3 cells were treated with 25 ng/mL murine OSM, 100 U/mL murine TNF-, or a combination of both cytokines. After the indicated time, total RNA was isolated and analyzed by Northern blotting. (B) bEnd.3 cells were treated with fresh medium in the presence or absence of 25 ng/mL murine OSM, 100 U/mL murine TNF-, or a combination of both cytokines, in the presence or absence of 10 μg/mL cycloheximide or 5 μg/mL actinomycin D (Act. D). After 3 hours, total RNA was isolated and analyzed by Northern blotting.
The rapid increase in mRNA for P- and E-selectin in response to TNF-α or OSM is characteristic of immediate early genes, which do not require new protein synthesis for their activation. Consistent with this notion, pretreatment of bEnd.3 cells with cycloheximide did not block the induction of P- and E-selectin mRNA by either or both cytokines (Fig 4B). In fact, cycloheximide potentiated the ability of TNF-α or OSM to increase selectin transcripts, perhaps because it prevented the synthesis of labile protein inhibitors of transcription. Pretreatment of bEnd.3 cells with actinomycin D blocked the increase in P- and E-selectin mRNA, suggesting that the cytokines acted at the level of mRNA transcription.
Intravenous injection of TNF-α and OSM cooperatively increases mRNA for P- and E-selectin in murine tissues.
The experiments with bEnd.3 cells showed that TNF-α and OSM independently increase mRNA for murine P- and E-selectin, and act cooperatively to further increase selectin mRNA expression. To determine whether these in vitro experiments predicted in vivo responses, mice were given an intravenous injection of 25 ng of murine OSM, 3,000 U of murine TNF-α, or a combination of both cytokines. Control mice received an injection of buffer alone. After 1 hour, the mice were killed, and RNA was isolated from multiple organs; the short 1-hour time interval was selected to maximize the probability that any observed changes in mRNA levels were because of direct actions of TNF-α or OSM on the endothelium rather than to induced expression of mediators in other cells that then stimulated the endothelium. The levels of mRNA for murine P-selectin, E-selectin, and β-actin were measured by the competitive RT-PCR method used to measure baboon mRNA levels. Figure 5 shows representative agarose gel electrophoresis of RT-PCR products from RNA of murine kidney. The cytokines did not increase mRNA for β-actin. In contrast, injection of either OSM (Fig 5A) or TNF-α (Fig 5B) significantly increased mRNA levels for both P- and E-selectin. Injection of both cytokines increased mRNA levels for P- and E-selectin to even higher levels (Fig 5C). Similar results were obtained in other murine organs (Table 2). Immunohistochemical analysis revealed that combined infusion of TNF-α and OSM increased expression of P- and E-selectin protein only in endothelial cells, primarily in small veins and postcapillary venules of multiple organs (data not shown). Injection of 25 ng human OSM for 1 hour increased P- and E-selectin mRNA to levels identical to those observed after injection of murine OSM (data not shown). Unlike the results obtained after 1 hour, no increase in P- or E-selectin mRNA was observed 24 hours after injection of murine OSM and TNF-α, either alone or in combination (data not shown). These results show that TNF-α and OSM cooperatively increase mRNA and protein for P- and E-selectin in murine tissues. Thus, the results obtained in vivo support the observations made in cultured murine endothelioma cells.
Intravenous injection of TNF- and OSM cooperatively increases mRNA for P- and E-selectin in murine tissues. Each mouse received an intravenous injection of buffer (Control), or of buffer containing 25 ng murine OSM, 3000 U murine TNF-, or a combination of both cytokines. After 1 hour, the mice were killed and the organs were immediately collected for RNA isolation. The levels of mRNA for P-selectin, E-selectin, and β-actin were measured by a competitive RT-PCR method as in Fig 2, except that the primers contained the respective murine cDNA sequences. Shown is representative agarose gel electrophoresis of the PCR products from mouse kidney. The data are representative of 4 independent experiments for control mice and mice receiving OSM, and 2 independent experiments for mice receiving TNF- or the combination of both cytokines.
Intravenous injection of TNF- and OSM cooperatively increases mRNA for P- and E-selectin in murine tissues. Each mouse received an intravenous injection of buffer (Control), or of buffer containing 25 ng murine OSM, 3000 U murine TNF-, or a combination of both cytokines. After 1 hour, the mice were killed and the organs were immediately collected for RNA isolation. The levels of mRNA for P-selectin, E-selectin, and β-actin were measured by a competitive RT-PCR method as in Fig 2, except that the primers contained the respective murine cDNA sequences. Shown is representative agarose gel electrophoresis of the PCR products from mouse kidney. The data are representative of 4 independent experiments for control mice and mice receiving OSM, and 2 independent experiments for mice receiving TNF- or the combination of both cytokines.
Effects of Infusion of OSM and/or TNF- on mRNA Levels for P- and E-Selectin in Mouse Organs
| . | P-Selectin . | E-Selectin . | ||||||
|---|---|---|---|---|---|---|---|---|
| Control . | OSM . | TNF-α . | OSM + TNF-α . | Control . | OSM . | TNF-α . | OSM + TNF-α . | |
| Heart | + | ++ | ++ | +++ | − | + | + | ++ |
| Lung | + | ++ | ++ | +++ | − | + | + | ++ |
| Kidney | + | ++ | ++ | +++ | − | + | + | + |
| Liver | + | ++ | ++ | +++ | − | − | + | + |
| Spleen | + | + | + | + | − | − | − | − |
| Brain | + | ++ | ++ | +++ | − | + | + | + |
| . | P-Selectin . | E-Selectin . | ||||||
|---|---|---|---|---|---|---|---|---|
| Control . | OSM . | TNF-α . | OSM + TNF-α . | Control . | OSM . | TNF-α . | OSM + TNF-α . | |
| Heart | + | ++ | ++ | +++ | − | + | + | ++ |
| Lung | + | ++ | ++ | +++ | − | + | + | ++ |
| Kidney | + | ++ | ++ | +++ | − | + | + | + |
| Liver | + | ++ | ++ | +++ | − | − | + | + |
| Spleen | + | + | + | + | − | − | − | − |
| Brain | + | ++ | ++ | +++ | − | + | + | + |
The levels of mRNA were measured by competitive RT-PCR as described in Materials and Methods. The values represent the results from organs isolated from 4 control mice; 2 mice treated with TNF-α, 4 mice treated with OSM, and 2 mice treated with both cytokines.
DISCUSSION
We used both in vitro and in vivo approaches to examine how TNF-α and OSM, cytokines that bind to distinct classes of receptors, affect the expression of P- and E-selectin in murine and primate endothelial cells. Our results indicate that the murine P- and E-selectin genes respond similarly to both cytokines. TNF-α or OSM markedly increased mRNA for both selectins, and a combination of both cytokines elicited additive or synergistic increases in P- and E-selectin mRNA and protein. In marked contrast, the human and nonhuman primate P- and E-selectin genes have diverged in their responsiveness to specific cytokines. OSM, but not TNF-α, increased P-selectin mRNA. TNF-α was the major inducer of E-selectin mRNA, whereas OSM even at high concentrations was a much weaker agonist. Notably, a combination of TNF-α and OSM did not cooperatively increase human P- or E-selectin mRNA.
TNF-α or OSM rapidly increased P- and E-selectin mRNA in murine bEnd.3 cells. The early induction and the lack of requirement for new protein synthesis are characteristic of the signal-mediated activation of immediate early genes. The relatively slow decline in transcript levels could reflect long mRNA half-lives and/or a biphasic response of the gene to differentially mobilized transcription factors. That cycloheximide potentiated the cytokine-mediated accumulation of mRNA suggests that short-lived proteins repress transcription of the genes for both P- and E-selectin. The ability of TNF-α and OSM to cooperatively increase selectin mRNA levels suggests that each cytokine activates gene expression through distinct signaling pathways that function additively or synergistically.
The ability of TNF-α and OSM to individually or cooperatively increase murine mRNA for P- and E-selectin in vivo supports the results obtained in vitro, and indicates that the signaling events observed in bEnd.3 cells are not unique to this cell line. The induction of P- and E-selectin mRNA only 1 hour after intravenous injection of TNF-α or OSM strongly suggests that each cytokine acted directly at the level of the endothelial cell. The immunohistochemical results indicate that inducible expression of P- and E-selectin protein was limited to endothelial cells. The lack of detectable increase in P- and E-selectin mRNA 24 hours after injection suggests that murine endothelial cells must be continuously exposed to cytokine, as performed in the in vitro experiments, to maintain elevated transcript levels. The dose of OSM injected, 25 ng, was low but was calculated to be sufficient to bind to high affinity receptors. In mice, murine OSM binds only to the OSM receptor, which consists of gp130 associated with the OSMRβ subunit, and human OSM binds only to the LIF receptor, which consists of gp130 associated with the LIFRβ subunit.43-45 Because murine or human OSM increased P- and E-selectin mRNA in bEnd.3 cells and in murine tissues in vivo, signaling through either the OSM receptor or the LIF receptor is sufficient to activate the murine P- and E-selectin genes. Signaling through the LIF receptor may account for the ability of subcutaneously injected human OSM to induce an acute inflammatory response in mice.29
The experiments in HUVEC suggest that the genes for P- and E-selectin in humans, unlike the corresponding genes in mice, have developed specialized responses to cytokine signals. TNF-α retained the ability to rapidly activate the human E-selectin gene but lost the ability to activate the human P-selectin gene, confirming previous studies.24,25 40 OSM had little or no ability to increase E-selectin mRNA levels, and did not cooperate with TNF-α to further increase E-selectin mRNA. In contrast, OSM markedly increased mRNA for P-selectin in a delayed and prolonged fashion that required new protein synthesis.
To extend the HUVEC studies to an in vivo setting, we examined the effects of infusion of a uniformly lethal dose of E coli on the expression of P- and E-selectin mRNA in baboons. The infused E coli produce high circulating levels of LPS, which, like TNF-α, augments E-selectin but not P-selectin mRNA in HUVEC.24Previous immunohistochemical analysis showed that E coliinduces expression of E-selectin protein in venular endothelium within 2 hours after injection into baboons.37 After 4 hours, we observed consistent induction of E-selectin mRNA in multiple baboon tissues, but found no increase in the constitutively expressed levels of P-selectin mRNA. The ability of LPS and TNF-α to increase mRNA for E-selectin, but not P-selectin, in a nonhuman primate supports the results obtained in vitro and indicates that the signaling events observed in HUVECs are not unique to these cultured human endothelial cells. We did not examine whether infusion of OSM altered selectin mRNA expression in baboons. High circulating levels of OSM have been reported in patients with septic shock.46 If OSM is generated after infusion of E coli in baboons, it might elevate P-selectin mRNA, but perhaps only after a lag period of 7 to 10 hours as observed in HUVEC.24 A similar lag might be required for signaling after binding of circulating IL-6/sIL-6Rα complexes to gp130 on endothelial cells.30 31
The different inducible responses of murine and human selectin genes extend to at least one other cytokine, IL-4. Like OSM, IL-4 increases P-selectin mRNA in both bEnd.3 cells and HUVEC.24 Also like OSM, IL-4 rapidly increases P-selectin mRNA in bEnd.3 cells, whereas it increases P-selectin mRNA in HUVEC in a delayed and prolonged fashion that requires new protein synthesis.24 Murine IL-4 rapidly increases E-selectin mRNA in bEnd.3 cells, and a combination of IL-4 and TNF-α further augments E-selectin mRNA (L.Y. and R.P.M., unpublished observations, October 1997). In contrast, human IL-4 does not activate E-selectin expression in HUVEC, and, in fact, inhibits the ability of TNF-α to increase E-selectin mRNA. IL-4 signals in part by activating Stat6.47,48 IL-4 prevents E-selectin expression in HUVEC by activating Stat6, which binds to a DNA element that overlaps a κB element in the human E-selectin promoter, thereby competitively inhibiting binding of NF-κB.49 Notably, the Stat6 recognition sequence is not conserved in the murine E-selectin promoter.50 There are 2 Stat6 elements in the human P-selectin promoter, which are in different locations than a single Stat6 element in the murine P-selectin promoter.22 Binding of activated Stat6 to the 2 elements in the human P-selectin gene contributes to IL-4–inducible expression.51
As in murine cells, TNF-α, IL-1β, or LPS rapidly increases mRNA for P- and E-selectin in rat,52 bovine,19,53 and canine54 endothelial cells. Thus, in the mammals studied, only the P-selectin gene in primates has lost its responsiveness to these mediators. It is not known whether the specialized responses of P- and E-selectin to OSM or IL-4 are limited to primates. What are the biological implications of these divergent responses across species? In mice, the ability of TNF-α and OSM to cooperatively stimulate both P- and E-selectin expression may ensure overlapping or redundant functions of these proteins during acute and chronic inflammatory responses. This may explain why targeted deletion of both the P- and E-selectin genes is required to significantly alter the phenotype in some murine models of inflammation or atherosclerosis.55-61 In humans, cytokines may have much more specific effects on expression of the P- or E-selectin gene. This specialized response to cytokines may allow P- and E-selectin to make more distinct contributions to leukocyte recruitment, depending on the nature of the inflammatory stimulus.
ACKNOWLEDGMENT
We thank Junliang Pan for helpful discussions, Gary Farrell and Michael McDaniel for valuable technical assistance, Kelsey Kennedy for assistance with the figures, Dietmar Vestweber for providing MoAbs, and James Morrissey and James Jarvis for critical reading of the manuscript.
Supported by Grant No. HL 54502 from the National Institutes of Health, Bethesda, MD. H.S. is the recipient of a postdoctoral fellowship award from the Oklahoma Affiliate of the American Heart Association.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Rodger P. McEver, MD, W.K. Warren Medical Research Institute, University of Oklahoma Health Sciences Center, 825 NE 13th St, Oklahoma City, OK 73104; e-mail: rodger-mcever@ouhsc.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal