Several features are characteristic for hairy cell leukemia (HCL). Among those are pancytopenia, bone marrow fibrosis, and the appearance of a defined tumor cell phenotype in peripheral blood (PB), bone marrow (BM), and spleen. Hairy cells (HC) coexpress antigens specific for B lymphocytes and monocytes/macrophages and thus the malignant cell does not seem to be restricted to a defined lineage. When serum or bone marrow aspirate was screened by enzyme-linked immunosorbent assay (ELISA) for basic fibroblast growth factor (bFGF), specimen derived from HCL (serum: mean value, 29 pg/mL; BM aspirate: mean value, 641 pg/mL) contained significantly higher levels than those from healthy subjects. To study whether peripheral blood mononuclear cells (PBMC) derived from patients suffering from HCL and healthy donors (HD) were capable of producing bFGF, culture supernatant (conditioned medium, [CM]) was tested for the presence of this cytokine. While bFGF was not detectable in cell cultures from HD, HCL-derived CM contained relatively high levels of bFGF. CM was successfully used for stimulation of mesenchymal cell proliferation, which could be inhibited by a neutralizing anti-bFGF antibody. Cellular activation by pokeweed mitogen (PWM) or the combination of 12-o-tetradecanoyl-phorbol-13-acetate (TPA) plus calcium ionophore (Ca-Ip) led to an enhanced mRNA expression. Results of Western blot experiments showed that HC synthesize at least three isoforms (approximately 18, 23, and 25 kD), but only the 23-kD isoform is exported. To assess the nature of the producer cell, double immunofluorescence analysis using a bFGF-specific and an anti-CD11c monoclonal antibody (MoAb) was undertaken. The majority of cells scoring positive for CD11c were also reactive with the anti-bFGF MoAb. Furthermore, enrichment of CD19/CD11c-positive cells correlated with enhanced bFGF levels, thereby supporting the argument for HC being the producer cells of bFGF. A biological function of bFGF in HCL might be mediation of chemoresistance, as 2-chlorodeoxyadenosine (2-CdA)–induced inhibition of cell proliferation can be reversed by bFGF. Endogenous bFGF production by HC is not affected by this purine analogue and 2-CdA–induced apoptosis is diminished in bFGF-producing HC as compared with normal PBMC. Therefore, bFGF expression by HC might be important for resistance to chemotherapy and survival of the malignant cells.
BASIC FIBROBLAST growth factor (bFGF), a pleiotropic cytokine, is important for embryogenesis, angiogenesis, wound healing, and mesodermal development. During these physiologic processes, its expression is tightly regulated.1,2 Due to its stimulatory capacity on the formation of new blood vessels, bFGF is suggested to play a major role for growth and dissemination of tumors. In fact, a high expression has been reported in various tumors.3-6
The producer cells of bFGF are mainly of mesenchymal origin. However, cells of the hematopoietic lineage have also been found to express and respond to this cytokine.7-9 Most of these studies have used permanent cell lines of myeloid origin.10,11 In primary cells derived from hematopoietic malignancies, bFGF, as well as its receptors (bFGF-R), seem to be preferentially produced by myeloid cells.12,13 More recently, however, intracellular bFGF has been detected in CD34+ cells,14 in B cells derived from chronic lymphocytic leukemia (CLL),15 and has been found in culture fluids of serum-starved, long-term T-cell cultures.16 17
Hairy cell leukemia (HCL) represents a distinct and rare form of chronic leukemias. Besides a characteristic morphology, the malignant cells coexpress both the B-cell–specific antigen, CD19, and the myeloid-specific antigen, CD11c.18 A functional feature invariably associated with HCL is bone marrow (BM) fibrosis.19 The mechanisms underlying this pathologic process are not completely understood. Because bFGF seems to be involved in fibrinogenic diseases such as liver fibrosis,20fibrosis in gastric carcinoma,21 or in systemic scleroderma,22 it is reasonable to speculate that it also plays a role in the development of BM fibrosis in HCL. Moreover, the production of bFGF by hairy cells (HC) would argue for a functional resemblance with myelomonocytic cells and would be compatible with the expression of CD11c on the surface of CD19-positive cells.
In the present study, we report on the capability of peripheral blood mononuclear cells (PBMC) derived from HCL to produce biologically active bFGF. Furthermore, we show that mRNA expression can be modulated by activators of protein kinase C (TPA+Ca-Ip), that bFGF is released by CD19/CD11c-positive cells as a high molecular weight isoform, and that bFGF mediates resistance to 2-chlorodeoxyadenosine (2-CdA)–induced apoptosis.
MATERIALS AND METHODS
Patients.
Seven patients suffering from HCL and seven healthy donors were investigated. HCL was diagnosed on the basis of cell morphology, double immunofluorescence staining using monoclonal antibodies (MoAb) against CD11c and CD19 and BM and splenic histology.
Cell culture.
NIH-3T3 mouse fibroblasts were obtained from American Type Culture Collection (ATCC; Rockville, MD). D15-7 (established in our own laboratory) are human skin fibroblasts from an 8-year-old healthy donor. The erythroleukemic cell line, K562, was kindly provided by Dr G. Klein (Karolinska Institute, Stockholm, Sweden).
PB was taken after informed consent from patients with HCL and healthy volunteers. PBMC were separated by density gradient centrifugation using Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). Cells banding at the interphase were washed twice in phosphate-buffered saline (PBS), resuspended in culture medium, and seeded in 6-well plates at 2 × 106 cells/mL. In certain experiments, T lymphocytes were depleted by a rosetting technique using neuraminidase-treated sheep red blood cells.
PBMC were grown in RPMI 1640 supplemented with 2 mmol L-glutamine, penicillin (100 U/mL), streptomycin (100 μg/mL), and 10% heat inactivated fetal calf serum (FCS) at 37°C in a humidified atmosphere containing 5% CO2. For certain experiments, cells were activated by pokeweed mitogen (PWM, 10 μg/mL) or 12-o-tetradecanoyl-phorbol-13-acetate (TPA, 10 ng/mL) plus calcium-ionophore A23187 (Ca-Ip, 10 ng/mL).
Production of conditioned medium (CM).
If not stated otherwise, culture supernatant was harvested from unstimulated cultures after 48 hours, centrifuged at 200g for 10 minutes, sterile filtered, and stored at −20°C until assayed.
Reagents and antibodies.
Cell culture media (RPMI 1640, MCDB 104), penicillin-streptomycin, L-glutamine, and FCS were obtained through GIBCO, Life Technologies (Paisley, UK). PWM, TPA, and Ca-Ip were purchased by Sigma Chemicals (St Louis, MO). 2-CdA was provided by Ortho Biotech (Raritan, NJ).
Monoclonal antibodies against CD3, CD5, CD11c, CD14, CD19, and the respective isotype controls, as well as ABComplex for Western blot analysis, were obtained through Dako (Copenhagen, Denmark), a Cy3-labeled anti-CD11c MoAb was purchased from Sigma Chemicals. A neutralizing mouse anti-bFGF MoAb and recombinant human bFGF (rhbFGF) were purchased from R&D Systems (Minneapolis, MN) and a biotin-labeled goat anti-mouse antibody was obtained through Accurate Chemicals (Westbury, NY). For Western blotting experiments, a mouse anti-bFGF MoAb (clone Ab-3, Oncogene Science, Uniondale, NY) was used.
Immunofluorescence and fluorescence-activated cell scanning (FACS) analysis.
Immunofluorescence analysis using a Cy3-labeled anti-CD11c and an anti-bFGF MoAb (R&D) was performed on aceton-fixed cells. Incubation steps with a biotinylated goat antimouse antibody and conjugates supplied in the TS amplification kit (Du-Pont-NEN, Boston, MA) were performed following instructions of the manufacturer.
MoAbs directed against CD3, CD5, CD11c, CD14, CD19 were used to analyze the cellular composition of the PBMC. For FACS analysis, 2 × 105 cells in PBS/bovine serum albumin (BSA) (1%) containing 0.1% sodium azide were stained with the fluorescein isothiocyanate (FITC)-labeled MoAb or isotype control for 30 minutes at 4°C. Samples were analyzed on a FACScan (Becton Dickinson, San Jose, CA). Gates for data analysis were set using the respective isotype staining as negative control. Quantitative analysis of apoptotic cells was performed using an Annexin V-FITC detection kit obtained from Pharmingen (San Diego, CA).
Detection of cytokines.
Sera, BM aspirates, cell lysates, and CM from cultures were tested for presence of bFGF by a commercially available ELISA-system (Bio-Trak; Amersham, Backinghamshire, UK) following the manufacturer’s protocol. Samples were tested in duplicates, optical density was measured, and bFGF values were calculated with a computer assisted plate reader (Dynatech, Guernsey Channel Islands, UK). The detection limit of the test is 1 pg/mL.
Western blot analysis.
Freshly isolated PBMC (107 cells) from a patient with HCL (66% CD19+/CD11c+ cells) and a healthy donor were cultured as described above. Supernatants from controls and PWM-stimulated cultures were passed through a 0.2-μm filter and incubated with 300 μL heparin-coated beads (AF-Hep 650M; Toso Haas, Tokyo, Japan) for 1 hour at room temperature (RT). rhbFGF was treated identically serving as positive control. Beads were washed twice in 1 mmol/L Tris/HCl, 0.2 mol NaCl, and bound proteins were eluted by boiling for 3 minutes in 300 μL electrophoresis buffer (0.125 mmol/L Tris/HCl; pH 6.8; 1% sodium dodecyl sulfate [SDS]). A total of 2 × 106 cells was resuspended in 1 mL electrophoresis buffer and lysed by boiling for 3 minutes. Unsoluble material was removed by centrifugation and aliquoted samples were stored at −20°C. Thirty-microliter samples were separated on homogenous gels under reducing conditions and transferred onto nitrocellulose by semidry blotting. Western blot analysis was performed using a mouse anti-bFGF MoAb followed by a biotin-streptavidin-horseradish-peroxidase incubation step. Positive reactions were visualized by enhanced chemiluminescence (Super Signal; Pierce, Rockford, IL) on ECL-Hyperfilm (Amersham, Backinghamshire, UK).
Proliferation assays.
NIH-3T3 fibroblasts and primary human dermal fibroblasts were cultured under serum-free conditions for 24 hours before addition of CM from PBMC of HCL patients or healthy donors. In a separate set of experiments, CM was preincubated for 1 hour at 37°C with a neutralizing anti-bFGF antibody (10 μg/mL) before addition to cultures. After 2 or 4 days, fibroblasts were pulsed with 1 μCi of3H-thymidine (3H-TdR; NEN) for 8 hours, harvested, and 3H-TdR uptake was determined using a Beta-Plate Scintillation Counter (Wallac-Pharmacia, Uppsala, Sweden). Cell cultures were performed in triplicates using 96-well plates.
To measure proliferation of PBMC, 2 × 105 cells/well were cultured in 96-well plates for 72 hours with and without 2-CdA and recombinant human bFGF, pulsed with 3H-TdR for 8 hours, and thymidine uptake was determined in a liquid scintillation counter.
mRNA-isolation and reverse transcription-polymerase chain reaction (RT-PCR).
Cells were harvested 48 hours after onset of cultures, washed twice in ice-cold PBS, and counted automatically (Coulter Counter, Coulter Electronics Ltd, Luton, UK). A total of 5 × 106 cells were subjected to RNA isolation by the guanidium-isothiocyanate-phenol-chloroform extraction procedure. cDNA was synthesized using 1 μg RNA as a template and a commercially available kit (Pharmacia). Polymerase chain reaction (PCR) was performed in a final volume of 50 μL in the presence of 0.25 mmol/L of each dNTP, 1 U Taq polymerase (Finnzyme, Helsinki, Finland), and 100 pmol/L of either bFGF- or β-actin–specific primers. For β-actin PCR (upstream primer: 5′-AGG CCG GCT TCG CGG GCG AC-3′; downstream primer: 5′-CTC GGG AGC CAC ACG CAG CTC-3′),23 30 cycles were performed at 94°C for 1 minute (denaturing), 60°C for 90 seconds (annealing), and 72°C for 90 seconds (extension). The final extension was performed at 72°C for 3 minutes. Each experiment was repeated at least two times and results showed no difference in β-actin expression, indicating equal amounts of mRNA. For bFGF-specific PCR (upstream primer: 5′-ACC ACG CTG CCC GCC TTG CCC-3′; downstream primer: 5′-CTT ATA GCC AGG TAA CGG TTA GCA-3′),12 40 cycles of amplification were performed (denaturation at 92°C/1 minute, annealing at 62°C/90 seconds, extension at 72°C/1 minute, final extension step at 72°C/5 minutes). Amplified DNA fragments were visualized by agarose gel electrophoresis. Southern blotting, hybridization to a digoxigenin (DIG)-labeled bFGF-specific cDNA probe (by courtesy of Dr N. Frazer, University of Oxford, Oxford, UK) and exposure to x-ray films was performed following standard procedures.
RESULTS
Detection of bFGF in serum and BM aspirate from patients with HCL.
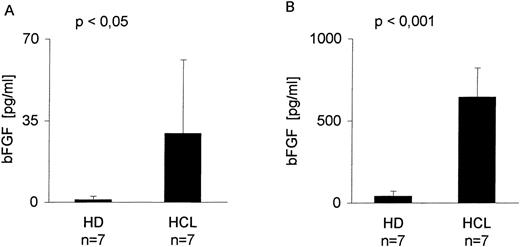
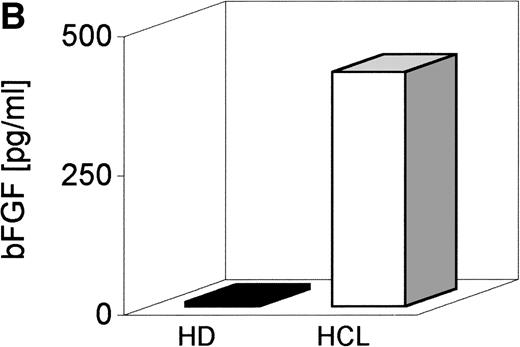
Serum derived from seven patients with HCL and seven healthy donors (HD) was screened by a bFGF-specific ELISA (detection limit, 1 pg/mL) (Table 1). Figure 1A indicates that in serum from all patients with HCL, bFGF was detectable, ranging from 1 to 90 pg/mL with a mean of 29 pg/mL. With the exception of one individual (4.6 pg/mL), no bFGF could be found in the serum from HD. Because BM is a primary site of HC appearance, BM aspirates were also tested. As indicated in Fig 1B, the mean concentration of bFGF in HCL-derived BM (641 pg/mL) was more than 16 times higher than in samples from HD (mean, 39 pg/mL).
Patient Characteristics
| Patient ID . | Sex/Age . | Treatment . | % CD19+/ CD11c+ Cells . | bFGF pg/mL† . |
|---|---|---|---|---|
| K.M. | F/81 | None | 1 | 1 |
| S.A. | M/59 | IFN-α | 1 | 6 |
| S.G. | M/55 | G-CSF | 2 | 12 |
| H.J. | M/61 | None | 9 | 27 |
| H.H. | M/67 | None | 49 | 53 |
| S.K. | M/46 | IFN-α | 80 | 16 |
| S.L. | F/84 | IFN-α | 84 | 90 |
| Mean values | 64 | 29.3 | ||
| HD (n = 7) | 5 M/2 F 39 | <1 | 0.8 |
| Patient ID . | Sex/Age . | Treatment . | % CD19+/ CD11c+ Cells . | bFGF pg/mL† . |
|---|---|---|---|---|
| K.M. | F/81 | None | 1 | 1 |
| S.A. | M/59 | IFN-α | 1 | 6 |
| S.G. | M/55 | G-CSF | 2 | 12 |
| H.J. | M/61 | None | 9 | 27 |
| H.H. | M/67 | None | 49 | 53 |
| S.K. | M/46 | IFN-α | 80 | 16 |
| S.L. | F/84 | IFN-α | 84 | 90 |
| Mean values | 64 | 29.3 | ||
| HD (n = 7) | 5 M/2 F 39 | <1 | 0.8 |
Abbreviations: G-CSF, granulocyte colony-stimulating factor; IFN, interferon.
Peripheral blood mononuclear cells.
bFGF levels in serum.
bFGF levels in sera and BM aspirates from healthy donors and HCL-patients. Sera from EDTA-drawn blood samples (n = 7) and BM aspirates (n = 7) were tested for bFGF contents by ELISA (limit of detection: 1 pg/mL). HCL-derived sera (range, 1 to 90 pg/mL; mean, 29 pg/mL) (A) and BM aspirates (range, 230 to 1,005 pg/mL; mean, 641 pg/mL) (B) contained significantly elevated amounts of bFGF. In HD, only one of seven serum samples was weakly positive (4.6 pg/mL) and BM samples showed a mean value of 39 pg/mL, range, 1 to 83 pg/mL bFGF.
bFGF levels in sera and BM aspirates from healthy donors and HCL-patients. Sera from EDTA-drawn blood samples (n = 7) and BM aspirates (n = 7) were tested for bFGF contents by ELISA (limit of detection: 1 pg/mL). HCL-derived sera (range, 1 to 90 pg/mL; mean, 29 pg/mL) (A) and BM aspirates (range, 230 to 1,005 pg/mL; mean, 641 pg/mL) (B) contained significantly elevated amounts of bFGF. In HD, only one of seven serum samples was weakly positive (4.6 pg/mL) and BM samples showed a mean value of 39 pg/mL, range, 1 to 83 pg/mL bFGF.
PBMC from patients with HCL release bFGF.
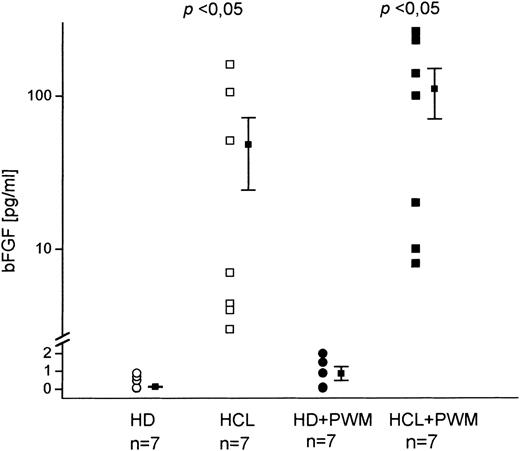
Because serum- or BM-derived bFGF might originate from nonhematopoietic cells or from cells that are disrupted during BM aspiration, we investigated whether PBMC from HD and HCL patients are capable of producing bFGF. Cells were cultured for 48 hours and culture supernatants were analyzed for bFGF by ELISA (Fig 2). While in cultures of unstimulated cells from HD no bFGF was detected, significant concentrations were found in HCL (mean value, 55 pg/mL). Stimulation with PWM led to a minute release of bFGF in only two of seven HD (2.4 pg/mL and 1.9 pg/mL). In HCL, however, an increase of up to 100% was seen (mean value, 110 pg/mL).
In vitro bFGF production by PBMC from patients with HCL and HD. A total of 2 × 106 cells were cultured in 12-well plates for 48 hours with and without PWM. Supernatants were assayed by a bFGF-specific ELISA. Unstimulated cultures from HD did not contain detectable amounts of bFGF in contrast to HCL-derived samples (mean, 55 pg/mL; range, 4 to 161 pg/mL). PWM stimulation led to an increased bFGF release in HCL (mean, 110 pg/mL; range, 8 to 262 pg/mL), while only two of seven HD showed weak positive reaction (2.4 pg/mL and 1.9 pg/mL).
In vitro bFGF production by PBMC from patients with HCL and HD. A total of 2 × 106 cells were cultured in 12-well plates for 48 hours with and without PWM. Supernatants were assayed by a bFGF-specific ELISA. Unstimulated cultures from HD did not contain detectable amounts of bFGF in contrast to HCL-derived samples (mean, 55 pg/mL; range, 4 to 161 pg/mL). PWM stimulation led to an increased bFGF release in HCL (mean, 110 pg/mL; range, 8 to 262 pg/mL), while only two of seven HD showed weak positive reaction (2.4 pg/mL and 1.9 pg/mL).
Stimulation of fibroblast proliferation by HCL-derived bFGF.
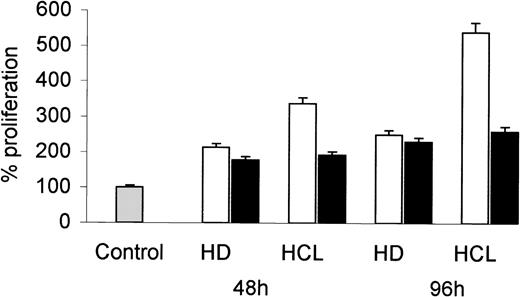
To examine if HCL-derived bFGF induces mesenchymal cell growth, human dermal fibroblasts were incubated with CM obtained from PBMC of an HCL patient and an HD. After 48 and 96 hours, cell proliferation was assessed by 3H-TdR uptake. As shown in Fig 3, CM from HCL induced a much higher increase in fibroblast proliferation than CM derived from HD. The addition of a neutralizing bFGF-specific MoAb markedly reduced the growth stimulatory effect of CM from HCL, but not that from HD.
Proliferative response of fibroblasts to CM from HD and HCL patients. Human dermal fibroblasts (D15-7) were cultured in the presence of 25% CM derived from PBMC of an HD and from an HCL patient (open bars). In a separate experiment, CM was preincubated (1 hour, 37°C) with a neutralizing anti-bFGF antibody (10 ng/mL) before addition to fibroblasts (solid bars). After 48 and 96 hours, proliferation was assessed by 3H-TdR incorporation. Proliferation of cells without CM was considered as 100%.
Proliferative response of fibroblasts to CM from HD and HCL patients. Human dermal fibroblasts (D15-7) were cultured in the presence of 25% CM derived from PBMC of an HD and from an HCL patient (open bars). In a separate experiment, CM was preincubated (1 hour, 37°C) with a neutralizing anti-bFGF antibody (10 ng/mL) before addition to fibroblasts (solid bars). After 48 and 96 hours, proliferation was assessed by 3H-TdR incorporation. Proliferation of cells without CM was considered as 100%.
Because binding to heparin is a feature of bFGF, CM from HCL patients was incubated with heparin beads for 30 minutes at RT. Heparin treatment of CM clearly reduced fibroblast proliferation triggered by HCL-derived CM or by 10 ng rhbFGF (data not shown).
Kinetics and regulation of bFGF mRNA expression in PBMC from HCL.
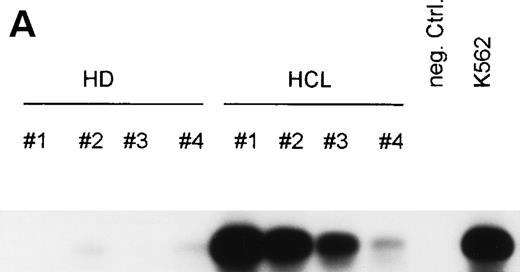
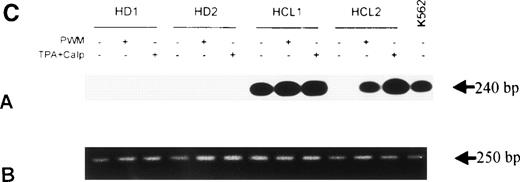
To test whether bFGF production results from constitutive expression or from in vitro activation, PBMC from HDs and patients with HCL were subjected to mRNA isolation immediately after blood donation and gradient centrifugation. Results of a RT-PCR are shown in Fig 4A. bFGF mRNA was highly expressed in HCL, while no or only minute mRNA expression could be detected in PBMC from HDs.
(A) Expression of bFGF-specific mRNA. PBMC from HDs (n = 4) and patients suffering from HCL (n = 4) were subjected to RNA isolation immediately after separation by gradient centrifugation. After RT-PCR amplification using bFGF-specific primers and Southern blotting, positive reactions were visualized by hybridization with a DIG-labeled bFGF cDNA probe followed by autoradiography. Negative control: no cDNA added; K562 mRNA served as positive control. In parallel experiments, β-actin expression was analyzed by RT-PCR to ensure that identical amounts of cDNA are used (not shown). (B) Synthesis of bFGF protein. PBMC derived from HCL patients (n = 3) and HD (n = 3) were analyzed for intracellular bFGF. A total of 2 × 106 cells were lysed and bFGF was determined by ELISA. Data are shown as mean values (HD, 7 pg/mL; HCL, 420 pg/mL). (C) Activation of bFGF-specific mRNA. PBMC from HDs (n = 2) and HCL patients (n = 2) were cultured for 48 hours in medium or stimulated with either PWM (10 μg/mL) or TPA (10 ng/mL) + Ca-Ip A23187 (10 ng/mL) (TPA+Ca-Ip). After RNA isolation, RT-PCR, Southern blotting, and hybridization with a DIG-labeled bFGF, cDNA-positive reactions were visualized by autoradiography (a). K562 mRNA served as positive control. (b) Shows results of β-actin–specific RT-PCR.
(A) Expression of bFGF-specific mRNA. PBMC from HDs (n = 4) and patients suffering from HCL (n = 4) were subjected to RNA isolation immediately after separation by gradient centrifugation. After RT-PCR amplification using bFGF-specific primers and Southern blotting, positive reactions were visualized by hybridization with a DIG-labeled bFGF cDNA probe followed by autoradiography. Negative control: no cDNA added; K562 mRNA served as positive control. In parallel experiments, β-actin expression was analyzed by RT-PCR to ensure that identical amounts of cDNA are used (not shown). (B) Synthesis of bFGF protein. PBMC derived from HCL patients (n = 3) and HD (n = 3) were analyzed for intracellular bFGF. A total of 2 × 106 cells were lysed and bFGF was determined by ELISA. Data are shown as mean values (HD, 7 pg/mL; HCL, 420 pg/mL). (C) Activation of bFGF-specific mRNA. PBMC from HDs (n = 2) and HCL patients (n = 2) were cultured for 48 hours in medium or stimulated with either PWM (10 μg/mL) or TPA (10 ng/mL) + Ca-Ip A23187 (10 ng/mL) (TPA+Ca-Ip). After RNA isolation, RT-PCR, Southern blotting, and hybridization with a DIG-labeled bFGF, cDNA-positive reactions were visualized by autoradiography (a). K562 mRNA served as positive control. (b) Shows results of β-actin–specific RT-PCR.
To examine if the expression of bFGF mRNA is paralleled by the synthesis of bFGF protein, lysates from freshly isolated cells from both HD and patients with HCL were subjected to bFGF-ELISA. As shown in Fig 4B, high amounts of intracellular bFGF were detectable in HCL-derived cells only.
Stimulation of PBMC for 48 hours by a mitogen (PWM) or activators of protein kinase-C (TPA+Ca-Ip) (Fig 4C) enhanced bFGF-specific mRNA expression. In HCL, the basic expression of bFGF mRNA was enhanced by both PWM and TPA+Ca-Ip. In HDs, treatment with either substances led to weak mRNA expression.
The concentration of extracellular bFGF protein in unstimulated HCL cultures did not change significantly over a period of 6 hours, but then gradually declined. In contrast, stimulation by TPA+Ca-Ip resulted in upregulation of bFGF after 24 hours, possibly indicating a correlation between cellular activation via protein kinase C and bFGF production (data not shown).
High-molecular-weight isoforms of bFGF are expressed and released by PBMC from HCL.
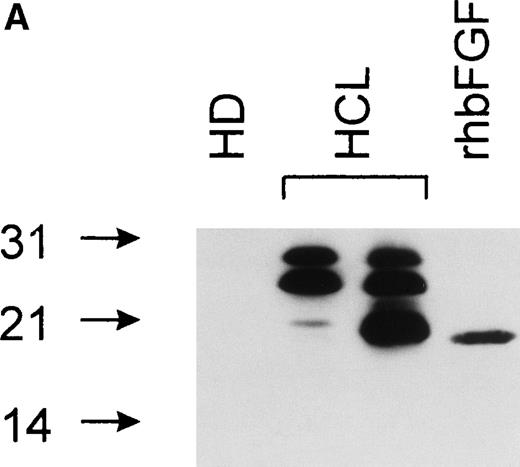
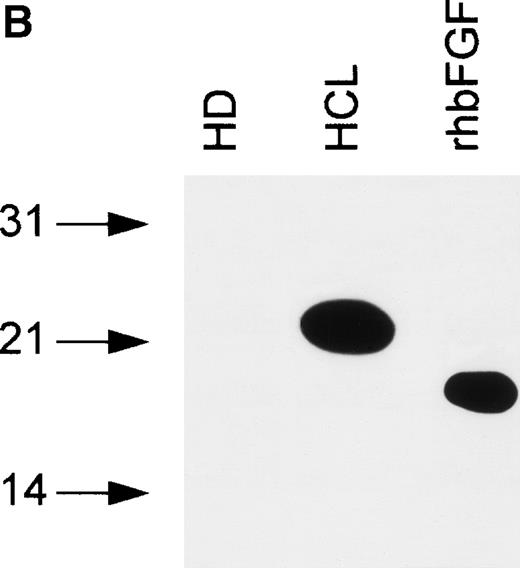
bFGF is expressed in several isoforms.24 To investigate if a particular isoform is predominantly produced in HCL, immunoblotting was performed. Samples were separated on SDS gels under reducing conditions (see Materials and Methods), blotted, and analyzed using an anti-bFGF MoAb. The pattern of bFGF protein expression detected in lysates derived from PBMC of HCL patients showed three distinct isoforms of approximately 18, 23, and 25 kD, while a lysate from an HD was negative (Fig 5A). In a second set of experiments, PBMC-derived cell-free CM from an HD and an HCL-patient were subjected to heparin chromatography. Bound proteins were eluted and analyzed as above. As demonstrated in Fig 5B, CM obtained from an HD again was essentially devoid of bFGF. In sharp contrast to lysates, the supernatant derived from a patient with HCL contained only the 23-kD high-molecular-weight isoform.
(A) Intracellular bFGF expression pattern of PBMC from HCL patients. A total of 30 μL of cell lysates (2 × 106cells/mL) were separated by SDS-PAGE, blotted, and analyzed by Western blotting. PBMC from HCL expressed three bFGF isoforms (approximately 18, 23, and 25 kD), while no bFGF could be detected in HD. rhbFGF was used as control. (B) Release of a 23-kD bFGF isoform. CM from HD and HCL cultures was harvested after 48 hours, subjected to heparin chromatography, and bulk eluted fractions were separated by SDS-PAGE. Proteins were transferred onto nitrocellulose and analyzed by Western blotting. rhbFGF served as a positive control.
(A) Intracellular bFGF expression pattern of PBMC from HCL patients. A total of 30 μL of cell lysates (2 × 106cells/mL) were separated by SDS-PAGE, blotted, and analyzed by Western blotting. PBMC from HCL expressed three bFGF isoforms (approximately 18, 23, and 25 kD), while no bFGF could be detected in HD. rhbFGF was used as control. (B) Release of a 23-kD bFGF isoform. CM from HD and HCL cultures was harvested after 48 hours, subjected to heparin chromatography, and bulk eluted fractions were separated by SDS-PAGE. Proteins were transferred onto nitrocellulose and analyzed by Western blotting. rhbFGF served as a positive control.
bFGF is produced by CD19+/CD11c+cells.
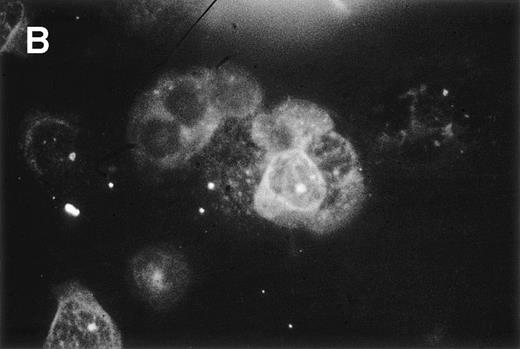
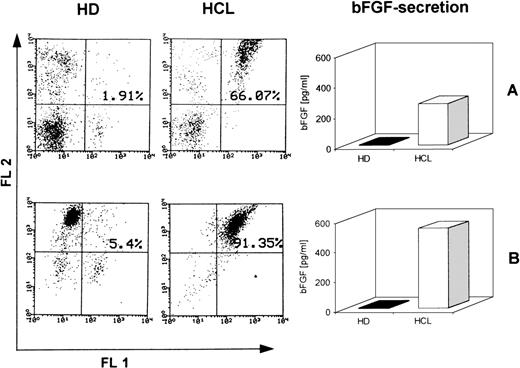
Double immunofluorescence analysis was performed to confirm whether HC are the producers of bFGF. PBMC from a patient with 66% CD19+/CD11c+ cells were examined. A typical reaction pattern of a bFGF- and a CD11c-specific MoAb is shown in Fig 6, showing that most of the CD11c-positive cells also express bFGF. In contrast, PBMC from an HD were negative for bFGF (data not shown). Because these results strongly suggested that CD19+/CD11c+ cells might be the producers of bFGF, we investigated the capacity of this particular cell population to produce bFGF. PBMC from the same patient and from an HD were investigated for coexpression of CD11c and CD19 by FACS analysis. While 66% of the PBMC from the patient expressed CD19, as well as CD11c, only 1.9% of the PBMC from the HD were double-positive for these antigens. When cells were cultured for 48 hours, only PBMC from the patient with HCL released bFGF (272 pg/mL; Fig 7A). In a parallel set of experiments, PBMC were separated from E-rosetting cells by gradient centrifugation. While in the case of HCL, this procedure yielded 91% CD19+/CD11c+ cells (<2% CD3 or CD5 and <1% CD14-positive cells), the non–T-cell population from the healthy donor contained 5% CD19+/CD11c+ cells only (<5% CD3, CD5, and CD14-positive cells, Fig 7B). Again, non-T cells from HD did not produce any detectable bFGF. In contrast, CM from PBMC enriched for CD19+/CD11c+ cells of the HCL patient contained high levels of bFGF (539 pg/mL). Therefore, enrichment for CD19+/CD11c+ cells leads to an increase of almost 100% (272 pg/mL to 539 pg/mL).
Double-immunofluorescence staining of PBMC from an HCL patient. Cell smears were fixed in acetone and incubated with a Cy3-labeled mouse anti-CD11c MoAb and a mouse anti-bFGF MoAb followed by the TS amplification system (see Materials and Methods). Green (A, anti-bFGF) and red (B, anti-CD11c) fluorescent signals from the same field were recorded sequentially. Experiments using isotype control antibodies or PBMC from an HD were negative (not shown).
Double-immunofluorescence staining of PBMC from an HCL patient. Cell smears were fixed in acetone and incubated with a Cy3-labeled mouse anti-CD11c MoAb and a mouse anti-bFGF MoAb followed by the TS amplification system (see Materials and Methods). Green (A, anti-bFGF) and red (B, anti-CD11c) fluorescent signals from the same field were recorded sequentially. Experiments using isotype control antibodies or PBMC from an HD were negative (not shown).
Production of bFGF by CD19+/CD11c+ cells. PBMC from HD and HCL were enriched for CD19+/CD11c+cells by T-cell depletion using a rosetting technique. FACS staining showed <3% CD3- and CD5-positive cells, respectively. Cells were cultured for 48 hours and supernatants were screened by ELISA. (A) Represents FACS profiles and bFGF production of unseparated cells, and (B) shows results of T-cell–depleted populations. Fluorescence-1 (FL1) and fluorescence-2 (FL2) represent CD19+ and CD11c+ populations, respectively.
Production of bFGF by CD19+/CD11c+ cells. PBMC from HD and HCL were enriched for CD19+/CD11c+cells by T-cell depletion using a rosetting technique. FACS staining showed <3% CD3- and CD5-positive cells, respectively. Cells were cultured for 48 hours and supernatants were screened by ELISA. (A) Represents FACS profiles and bFGF production of unseparated cells, and (B) shows results of T-cell–depleted populations. Fluorescence-1 (FL1) and fluorescence-2 (FL2) represent CD19+ and CD11c+ populations, respectively.
bFGF mediates resistance of PBMC to 2-CdA.
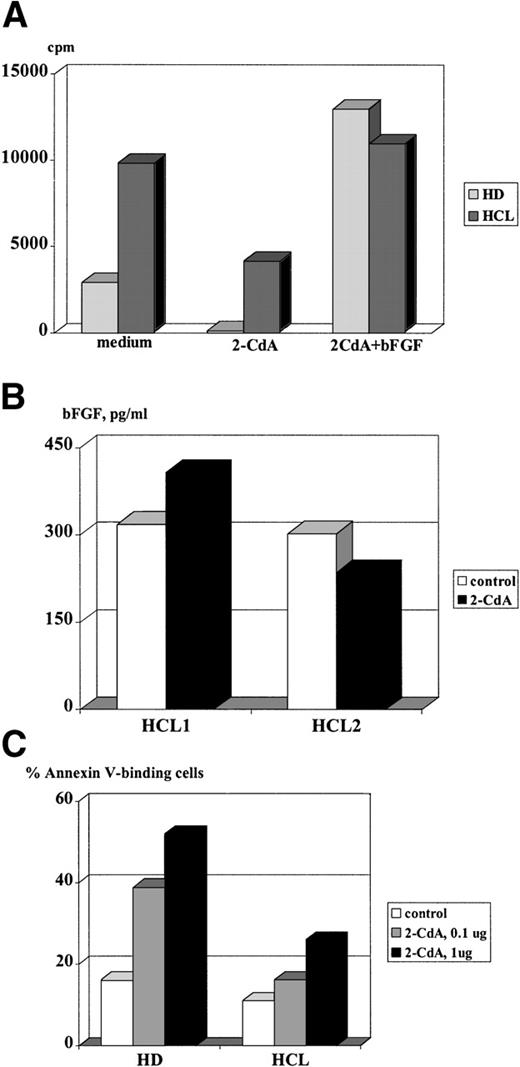
Endogenous bFGF production has a beneficial effect on cell proliferation and rescue from apoptotic cell death in various cell types, as has been reported earlier.25-28 To test whether bFGF exerts a protective effect on HC, cultures were set up in the presence of 2-CdA, a drug that induces apoptosis in HC29and is successfully used for treatment of HCL.30 As can be seen from Fig 8A, bFGF-producing cells from HCL could only be partially inhibited by the addition of 2-CdA. In contrast, proliferation of PBMC derived from an HD was completely abrogated. A possible explanation for the weak inhibition of HC might be that these cells express bFGF spontaneously and therefore are protected from the cytopathic effects of 2-CdA. This assumption is supported by results of experiments in which cultures treated with 2-CdA were supplemented with rhbFGF. Despite the presence of 2-CdA, exogenous rhbFGF restored the proliferative capacity of PBMC from a patient with HCL and an HD, suggesting that bFGF protects PBMC from the cytopathic action of 2-CdA. In vivo, such protection would require a constitutive expression of this cytokine even in the presence of 2-CdA. Thus, we examined if 2-CdA has an influence on bFGF release by HC. PBMC derived from two patients suffering from HCL were cultured for 48 hours and supernatants were screened for bFGF by ELISA. Indeed, PBMC from patient no. 1 produced high levels of bFGF, regardless of the presence of 2-CdA, while PBMC from patient no. 2 were inhibited by 22% only (Fig 8B). In this regard, it is interesting to note that patient no. 1 is resistant to therapy with 2-CdA.
Effect of 2-CdA on proliferation, bFGF release, and apoptosis of HC. (A) A total of 2 × 105 cells/well were cultured in medium or medium supplemented with bFGF (100 pg/mL) with or without 2-CdA (1 μg/mL). After 72 hours, cells were pulsed with 5 μCi/mL 3H-TdR and incorporation was measured by liquid scintillation counting. Mean values of triplicate cultures are shown. (B) A total of 2 × 106 PBMC from two patients with HCL were cultured in 6-well plates in the absence or presence of 2-CdA (1 μg/mL). After 48 hours, culture supernatants were screened by an ELISA specific for human bFGF. Results are expressed as mean values of triplicate cultures. (C) A total of 2 × 106 PBMC from a healthy donor and a patient with HCL were cultured in medium or medium containing 2-CdA (1 μg/mL, 0.1 μg/mL). Cells were harvested after 48 hours and assayed for FITC-labeled Annexin binding by FACS. Shown are results of a representative experiment.
Effect of 2-CdA on proliferation, bFGF release, and apoptosis of HC. (A) A total of 2 × 105 cells/well were cultured in medium or medium supplemented with bFGF (100 pg/mL) with or without 2-CdA (1 μg/mL). After 72 hours, cells were pulsed with 5 μCi/mL 3H-TdR and incorporation was measured by liquid scintillation counting. Mean values of triplicate cultures are shown. (B) A total of 2 × 106 PBMC from two patients with HCL were cultured in 6-well plates in the absence or presence of 2-CdA (1 μg/mL). After 48 hours, culture supernatants were screened by an ELISA specific for human bFGF. Results are expressed as mean values of triplicate cultures. (C) A total of 2 × 106 PBMC from a healthy donor and a patient with HCL were cultured in medium or medium containing 2-CdA (1 μg/mL, 0.1 μg/mL). Cells were harvested after 48 hours and assayed for FITC-labeled Annexin binding by FACS. Shown are results of a representative experiment.
In the next set of experiments, we tested whether bFGF expression in HC is paralleled by a reduced sensitivity for 2-CdA–mediated apoptosis. Cells were cultured for 48 hours and analyzed for Annexin-V binding, a feature of cells undergoing apoptosis. Figure 8C shows that 2-CdA induced Annexin-V binding in 52% of PBMC from a healthy donor, while only 26% of bFGF-producing HC bound Annexin-V. These results might indicate resistance to 2-CdA via bFGF.
DISCUSSION
The present study reports on the detection of elevated levels of bFGF in serum and BM from patients with HCL. This study also indicates that in HCL, bFGF is released in a biologically active form by the malignant cells, as CM derived from HC was shown to induce a proliferative response on fibroblasts, which could be diminished by treatment with a neutralizing anti-bFGF antibody. Stimulation of PBMC with PWM or TPA plus Ca-Ip led to an increase in bFGF mRNA expression in HCL, substantiating that bFGF expression can be upregulated by cellular activation. In cell lysates, three different isoforms can be detected by immunoblotting, but only a single bFGF isoform of approximately 23 kD is released by HC. Double-immunofluorescence and cell separation studies showed that the cytokine is produced and exported by CD19+/CD11c+ cells. Finally, bFGF expression is not blocked by 2-CdA and seems to mediate resistance to 2-CdA–mediated apoptosis.
Expression of bFGF and its receptors has been shown in few other hematologic malignancies, and the cytokine was implicated to play a potential pathological role in these diseases. Thus, in patients with myelofibrosis, an increased production of bFGF has been correlated with a possible involvement with the fibrotic process.14 In chronic lymphocytic leukemia, elevated bFGF levels were found to correlate with the stage of disease activity and resistance to fludarabine.15
HCL, a rare form of chronic leukemia, is characterized by several unique and unusual features (reviewed in Hess31). Some of them might be explained by abnormalities in cytokine production32 leading to BM insufficiency or deregulation of adhesion molecule expression.33
It is important to note that release of bFGF seems to be a feature of tumor cells, as transformed cells derived from breast carcinoma or fibrosarcomas export bFGF, while in nontransformed fibroblasts, bFGF remains cell-associated.34-36 In addition, acquisition of a signal peptide by molecular cloning converts bFGF to induce transformation of mouse fibroblasts, which are tumorigenic and metastatic in nude mice.37
The bFGF detected in culture supernatants from HCL patients was capable of inducing proliferation of primary human fibroblasts. This might implicate that the cytokine exerts important effects also on cells in the close proximity to HC, ie, on mesenchymal cells, or molecules deposited within the extracellular matrix, eg, heparan sulfate proteoglycans (HSPG). Complex formation of HSPG with bFGF facilitates binding to FGF receptors and thereby triggers cellular activation.38 39
In HCL, bFGF might also be operative through other growth factors. Among these transforming growth factor-β1 (TGF-β1) is a possible candidate. Interactions between bFGF and TGF-β1 have been described in several biological systems40-42 and may also be relevant for BM fibrosis seen in HCL. In this context, bFGF might contribute to BM fibrosis via activation of latent TGF-β1 in the BM microenvironment and consequently induction of extracellular matrix (ECM) synthesis and accumulation.43Indeed, both active and latent forms of TGF-β are highly elevated in HCL, particularly in BM (M. Shehata, in preparation).
One possible mechanism by which bFGF might exert a direct role in leukemogenesis could be through the prevention of apoptosis. There is evidence presented by Menzel et al15 who showed that bFGF accumulates intracellularly in malignant B cells from chronic lymphocytic leukemia (B-CLL) and delays fludarabine-induced cell death. Similarily, experiments described in this report showed that recombinant bFGF was capable of protecting HD-derived, as well as HCL-derived PBMCs, from the cytopathic effects of 2-CdA. Moreover, production and release of endogenous bFGF was not affected by 2-CdA. Therefore, overexpression and export of bFGF may mediate resistance to therapy and contribute to tumor progression.
Besides a possible direct effect on the survival and growth of CD19+/CD11c+ B cells, bFGF may also influence immune surveillance. In vitro and in vivo studies have shown that bFGF inhibits adhesion of natural killer (NK) cells to endothelial cells.33 Provided that bFGF functions in a similar way on HC, it may exert a protective mechanism for the malignant cells in the presence of NK cells. Evidence for a reduced NK cell activity in HCL has been provided earlier.44 45
In primary mesenchymal cells, bFGF is produced as an 18-kD glycoprotein without a signal sequence.46,47 In addition to the 18-kD form, at least three N-terminal extended bFGF isoforms of approximately 22, 23, and 25 kD molecular weight have been described. They originate from unusual translational initiation at CUG codons upstream of the original AUG initiation codon.48 While the 18-kD form of bFGF is present in the cytosol, these high molecular weight forms are predominantly associated with the nucleus due to a nuclear localization signal in the alternatively expressed N-terminal region.49Interestingly, bFGF derived from HCL displayed a molecular weight (MW) of 23 kD, an isoform that is characterized by its nuclear localization. It is feasible that high MW forms of bFGF also control gene expression because the presence of a high MW form within the nucleus may facilitate interaction with transcription factors. It has been shown by Kevil et al50 that the translation initiation factor eIF-4 preferentially increases translation of CUG-1 isoforms, resulting in release of large quantities of the 23-kD isoform. Whether or not transcription factor-mediated mechanisms are also responsible for the elevated bFGF expression seen in HCL remains to be elucidated.
Both production and distribution of a particular isoform may be instrumental for cell proliferation, as well as development and progression of tumors. Therefore, bFGF may play a role in the development of pathological conditions in HCL and hence be involved in myelofibrosis, prevention of apoptosis, and proliferation of HC.
Supported in part by Grants No. 9217 and 11210 from the Austrian Science Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal