We evaluated 18,014 patients who underwent allogeneic bone marrow transplantation (BMT) at 235 centers worldwide to examine the incidence of and risk factors for posttransplant lymphoproliferative disorders (PTLD). PTLD developed in 78 recipients, with 64 cases occurring less than 1 year after transplantation. The cumulative incidence of PTLD was 1.0% ± 0.3% at 10 years. Incidence was highest 1 to 5 months posttransplant (120 cases/10,000 patients/yr) followed by a steep decline to less than 5/10,000/yr among ≥1-year survivors. In multivariate analyses, risk of early-onset PTLD (<1 year) was strongly associated (P < .0001) with unrelated or human leukocyte antigen (HLA) mismatched related donor (relative risk [RR] = 4.1), T-cell depletion of donor marrow (RR = 12.7), and use of antithymocyte globulin (RR = 6.4) or anti-CD3 monoclonal antibody (RR = 43.2) for prophylaxis or treatment of acute graft-versus-host disease (GVHD). There was a weaker association with the occurrence of acute GVHD grades II to IV (RR = 1.9, P = .02) and with conditioning regimens that included radiation (RR = 2.9, P = .02). Methods of T-cell depletion that selectively targeted T cells or T plus natural killer (NK) cells were associated with markedly higher risks of PTLD than methods that removed both T and B cells, such as the CAMPATH-1 monoclonal antibody or elutriation (P = .009). The only risk factor identified for late-onset PTLD was extensive chronic GVHD (RR = 4.0, P = .01). Rates of PTLD among patients with 2 or ≥3 major risk factors were 8.0% ± 2.9% and 22% ± 17.9%, respectively. We conclude that factors associated with altered immunity and T-cell regulatory mechanisms are predictors of both early- and late-onset PTLD.
ALLOGENEIC BONE MARROW transplantation (BMT), an effective treatment for leukemia and other disorders, produces profound immune deficiency in the early period after transplantation. Posttransplant lymphoproliferative disorders (PTLD) are an uncommon, but frequently fatal, complication of this defective immune function.1-3 PTLD typically develop in the first 6 months posttransplant as clinically aggressive lymphomas of donor origin; most are related to Epstein-Barr virus (EBV).1,4 5Previous studies indicate that patients at highest risk of PTLD are those receiving unrelated donor or human leukocyte antigen (HLA) mismatched related donor transplants, T-cell depletion, or antithymocyte globulin. However, prior studies of PTLD generally included small numbers of cases treated in a single institution, and did not separately analyze lymphoproliferative disorders that occurred beyond the first posttransplant year. We evaluated the long-term incidence, risk factors, and outcome of lymphoproliferative disorders in a cohort of more than 18,000 allogeneic BMT recipients.
MATERIALS AND METHODS
Patients.
The study included 18,014 patients receiving allogeneic BMT at 234 transplant centers reporting to the International Bone Marrow Transplant Registry (IBMTR), Milwaukee, between 1964 and 1990, and at the Fred Hutchinson Cancer Research Center, Seattle, between 1969 and 1992. Because of the potential difficulty in distinguishing PTLD from recurrent non-Hodgkin’s lymphoma (NHL), we did not consider 729 patients transplanted for NHL. Also excluded were 577 patients transplanted for Fanconi anemia or a primary immunodeficiency disease who are known to have an increased susceptibility to cancer.6 7 Complete follow-up data through the study end date (December 31, 1991 and December 31, 1992 for the IBMTR and Seattle patients, respectively) were obtained for 91% of transplant recipients.
Table 1 lists subject- and transplant-related characteristics. Eighty-nine percent of patients received a transplant for leukemia or severe aplastic anemia; 81% of donors were HLA-identical siblings. Median age at transplant was 25 years (range, <1 to 72). Common conditioning regimens consisted of total body irradiation (TBI) combined with cyclophosphamide (64%) or other cytotoxic drugs (8%). Patients not given radiation typically received cyclophosphamide and busulfan (15%). In 14% of transplants, the graft was depleted of T cells, with the most frequent methods using monoclonal antibodies targeting T cells or T plus natural killer (NK) cells, CAMPATH-1 monoclonal antibodies, or elutriation. Most patients received posttransplant immune suppression to prevent graft-versus-host disease (GVHD), usually with cyclosporine, methotrexate, and/or corticosteroids. Acute GVHD was typically treated with corticosteroids, cyclosporine (usually continued from prophylaxis), antithymocyte (or antilymphocyte) globulin, or combinations of these drugs. Twenty-one patients from Seattle received an anti-CD3 monoclonal antibody (64.1) to treat acute GVHD.8 Data on drugs used to treat chronic GVHD were incomplete, and thus not further analyzed.
Characteristics of 18,014 Patients Undergoing Allogeneic BMT
| Characteristic . | No. of Patients . | Percent* . |
|---|---|---|
| Cohort | ||
| IBMTR, 1964-1990 | 14,041 | 77.9 |
| FHCRC, 1969-1992 | 3,973 | 22.1 |
| Female sex | 7,404 | 41.1 |
| Age at transplant (yr) | ||
| <10 | 2,623 | 14.6 |
| 10-19 | 3,929 | 21.8 |
| 20-29 | 4,654 | 25.8 |
| 30-39 | 4,191 | 23.3 |
| 40+ | 2,617 | 14.5 |
| Calendar year of transplant | ||
| 1964-1979 | 1,190 | 6.6 |
| 1980-1984 | 4,166 | 23.1 |
| 1985-1989 | 9,662 | 53.7 |
| 1990-1992 | 2,996 | 16.6 |
| Primary disease† | ||
| Acute nonlymphocytic leukemia | 5,065 | 28.1 |
| Chronic granulocytic leukemia | 4,770 | 26.5 |
| Acute lymphoblastic leukemia | 4,139 | 23.0 |
| Severe aplastic anemia | 2,114 | 11.7 |
| Other | 1,926 | 10.7 |
| Donor-recipient relationship and histocompatibility | ||
| HLA-identical sibling | 14,624 | 81.2 |
| 1 HLA-antigen mismatched sibling, relative | 1,356 | 7.5 |
| ≥2 HLA-antigen mismatched sibling, relative | 835 | 4.6 |
| Unrelated donor | 1,068 | 5.9 |
| Other, uncertain | 131 | 0.7 |
| Transplant conditioning regimen | ||
| TBI + Cy ± other drugs | 11,544 | 64.1 |
| TBI ± other drugs (no Cy) | 1,481 | 8.2 |
| LFI ± Cy ± other drugs | 655 | 3.6 |
| Busulfan + Cy ± other drugs | 2,775 | 15.4 |
| Cy ± other drugs | 1,391 | 7.7 |
| Other | 168 | 0.9 |
| T-cell depletion of marrow | ||
| No T-cell depletion | 15,518 | 86.1 |
| Anti-T or anti-T + NK MoAb | 1,255 | 7.0 |
| Sheep red blood cell rosetting | 166 | 0.1 |
| Lectins | 133 | 0.1 |
| CAMPATH-1 MoAb | 608 | 3.3 |
| Elutriation/density gradient centrifugation | 250 | 1.0 |
| Unclassified/other | 84 | 0.1 |
| Drugs given for GVHD prophylaxis | ||
| CsA + MTX (no ATG) | 6,659 | 37.0 |
| CsA (no MTX, no ATG) | 5,628 | 31.2 |
| MTX (no CsA, no ATG) | 3,871 | 21.5 |
| Any ATG | 525 | 2.9 |
| Other, none | 1,331 | 7.4 |
| Occurrence of acute GVHD II-IV | 7,063 | 39.2 |
| Treatment for acute GVHD II-IV | ||
| Steroids (No CsA, no ATG) | 3,285 | 18.2 |
| CsA + steroids (no ATG) | 1,776 | 9.9 |
| Any ATG | 1,101 | 6.1 |
| Other, none | 901 | 5.0 |
| Occurrence of extensive chronic GVHD‡ | 3,872 | 30.0 |
| Characteristic . | No. of Patients . | Percent* . |
|---|---|---|
| Cohort | ||
| IBMTR, 1964-1990 | 14,041 | 77.9 |
| FHCRC, 1969-1992 | 3,973 | 22.1 |
| Female sex | 7,404 | 41.1 |
| Age at transplant (yr) | ||
| <10 | 2,623 | 14.6 |
| 10-19 | 3,929 | 21.8 |
| 20-29 | 4,654 | 25.8 |
| 30-39 | 4,191 | 23.3 |
| 40+ | 2,617 | 14.5 |
| Calendar year of transplant | ||
| 1964-1979 | 1,190 | 6.6 |
| 1980-1984 | 4,166 | 23.1 |
| 1985-1989 | 9,662 | 53.7 |
| 1990-1992 | 2,996 | 16.6 |
| Primary disease† | ||
| Acute nonlymphocytic leukemia | 5,065 | 28.1 |
| Chronic granulocytic leukemia | 4,770 | 26.5 |
| Acute lymphoblastic leukemia | 4,139 | 23.0 |
| Severe aplastic anemia | 2,114 | 11.7 |
| Other | 1,926 | 10.7 |
| Donor-recipient relationship and histocompatibility | ||
| HLA-identical sibling | 14,624 | 81.2 |
| 1 HLA-antigen mismatched sibling, relative | 1,356 | 7.5 |
| ≥2 HLA-antigen mismatched sibling, relative | 835 | 4.6 |
| Unrelated donor | 1,068 | 5.9 |
| Other, uncertain | 131 | 0.7 |
| Transplant conditioning regimen | ||
| TBI + Cy ± other drugs | 11,544 | 64.1 |
| TBI ± other drugs (no Cy) | 1,481 | 8.2 |
| LFI ± Cy ± other drugs | 655 | 3.6 |
| Busulfan + Cy ± other drugs | 2,775 | 15.4 |
| Cy ± other drugs | 1,391 | 7.7 |
| Other | 168 | 0.9 |
| T-cell depletion of marrow | ||
| No T-cell depletion | 15,518 | 86.1 |
| Anti-T or anti-T + NK MoAb | 1,255 | 7.0 |
| Sheep red blood cell rosetting | 166 | 0.1 |
| Lectins | 133 | 0.1 |
| CAMPATH-1 MoAb | 608 | 3.3 |
| Elutriation/density gradient centrifugation | 250 | 1.0 |
| Unclassified/other | 84 | 0.1 |
| Drugs given for GVHD prophylaxis | ||
| CsA + MTX (no ATG) | 6,659 | 37.0 |
| CsA (no MTX, no ATG) | 5,628 | 31.2 |
| MTX (no CsA, no ATG) | 3,871 | 21.5 |
| Any ATG | 525 | 2.9 |
| Other, none | 1,331 | 7.4 |
| Occurrence of acute GVHD II-IV | 7,063 | 39.2 |
| Treatment for acute GVHD II-IV | ||
| Steroids (No CsA, no ATG) | 3,285 | 18.2 |
| CsA + steroids (no ATG) | 1,776 | 9.9 |
| Any ATG | 1,101 | 6.1 |
| Other, none | 901 | 5.0 |
| Occurrence of extensive chronic GVHD‡ | 3,872 | 30.0 |
Abbreviations: IBMTR, International Bone Marrow Transplant Registry; FHCRC, Fred Hutchinson Cancer Research Center; TBI, total-body irradiation; Cy, cyclophosphamide; LFI, limited-field irradiation; CsA, cyclosporine; MTX, methotrexate; GVHD, graft-versus-host disease; NK, natural killer cells; MoAb, monoclonal antibody; ATG, antithymocyte globulin.
Percents do not always add to 100% because of rounding.
Primary diseases excluded were non-Hodgkin’s lymphomas (n = 729), Fanconi’s anemia (n = 201), and immune deficiency diseases (n = 376). Other primary diseases included Hodgkin’s disease (n = 163), other malignancies (n = 347), myelodysplastic syndromes or myeloproliferative disorders (n = 632), and other smaller groups of primarily nonmalignant diseases (n = 784), including inherited disorders of metabolism (n = 158), and hemoglobinopathies (n = 315).
Occurrence of extensive chronic GVHD among 13,107 patients who survived ≥90 days posttransplant.
We identified 78 PTLD using well-established criteria9; some of these cases were described previously.2,3,10-15Fifty-three cases (68%) were confirmed by centralized histopathologic examination of archived tissue or slides (D.K., E.J.), 18% by review of clinical and pathology reports (P.B.), 9% by review of published case details,11,12,14 and 5% (4 cases) were evaluated using the transplant team report only. Sufficient tissue was available for 36 PTLD to perform in situ hybridization to detect expression of EBV-encoded RNA (EBER1) (methods described in Kingma et al16). Clonality and immunoglobulin gene rearrangement studies were not performed.
Statistical analyses.
For each transplant recipient, person-years at risk were compiled from the date of transplant until one of the following events: death, last known follow-up, diagnosis of new malignancy (including PTLD), or end of study, whichever occurred first. The change in PTLD risk over posttransplant intervals was initially evaluated by estimating crude incidence rates, defined as the number of PTLD events divided by the person-years at risk accrued in that interval. To allow comparisons with previously published estimates, the observed (O) number of PTLD was compared with the expected (E) number of NHL in the general population by applying age-, gender-, calendar year-, and region-specific population-based incidence rates to the appropriate person-years at risk.17 However, it should be recognized that lymphoproliferative disorders after BMT are a unique set of polyclonal and monoclonal tumors, and thus not directly comparable to NHL in the general population. Kaplan-Meier methods were used to calculate the cumulative probability of developing a PTLD.18
Poisson regression methods for grouped survival data19 and Cox proportional hazards regression techniques20 were used to compute estimates of relative risk (RR) of PTLD associated with various patient-, treatment-, and transplant-related variables. These 2 approaches gave nearly identical results and only Poisson analyses are presented. A forward step-wise selection procedure was used for variable selection. Poisson models included stratification on time since transplantation in 14 intervals to account for the sharp decline in the patients at risk during the first year post-transplant. Monthly cut-points were used during the first 9 months following transplantation, and at 1, 2.5, 5, 7.5, and 10 years thereafter. Models were also stratified by the underlying disease for which the transplant was performed using 5 categories: acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML), chronic myelogenous leukemia (CML), severe aplastic anemia, and other diseases. Patient-related variables evaluated in multivariate models included cohort (IBMTR vSeattle), geographic region, gender, age and calendar year of transplant, and primary disease. Transplant-related risk factors included degree of HLA match and donor relationship, conditioning regimen, use of antithymocyte globulin for conditioning, occurrence and method of T-cell depletion, prophylaxis, or treatment for acute GVHD with antithymocyte globulin or anti-CD3 monoclonal antibody 64.1 (Seattle only), all of which have been identified as risk factors for PTLD in 1 or more prior studies.1-3 The occurrence and treatment of acute GVHD (grades II to IV) and the occurrence of extensive chronic GVHD were entered into the model as time-dependent covariates. We also considered drugs commonly used to prevent or treat acute GVHD. Tests of statistical significance were 2-sided and 95% likelihood-based confidence intervals (CIs) were calculated.
To account for the nonconstant relative hazard for several risk factors over time since transplantation, we constructed a series of Poisson regression models, each of which included risk factors plus interaction terms. These interaction terms consisted of indicator variables (values 0, 1) that allowed for a step in the hazard function for a particular risk factor at prespecified follow-up times (ie, 4, 5, 6, 7, 8, 9 months, and 1 year posttransplant). Because the 1-year cut-point minimized the model deviance, these results will be presented in Table3.
RESULTS
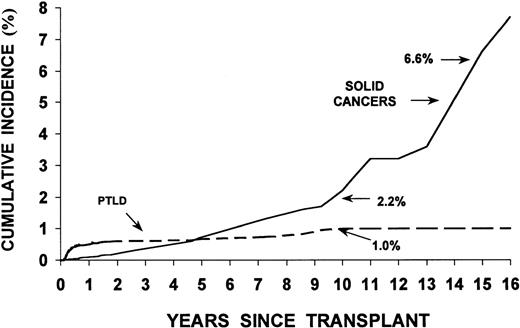
We identified 78 patients who developed PTLD, compared with 1.5 cases of NHL expected in the general population (O/E = 51.5; 95% CI, 40.7% to 64.3%). Sixty-four lymphoproliferative disorders occurred within the first year posttransplant (early-onset) while 14 arose 1 or more years after transplant (late-onset: range, 1 to 8.6 years posttransplant). Incidence was highest 1 to 5 months after transplant (120 cases/10,000 patients/yr) (Table2). The peak incidence (210 cases/10,000/yr) occurred during the third month posttransplant; thereafter, a gradual but steep decline in the PTLD rate was observed, with the lowest incidence occurring among ≥1-year survivors (<5 PTLD cases/10,000/yr). The risk of late-onset PTLD remained significantly higher than would be expected for de novo NHL in the general population (O = 14, O/E = 12.8; 95% CI, 7.0% to 21.4%). The overall cumulative incidence of PTLD was low, 1.0% ± 0.3% at 10 years. Although no PTLD cases occurred among the 625 patients who survived more than 10 years post-BMT (Fig 1), only a small percentage of these long-term survivors were recipients of family mismatched, unrelated, or T-cell depleted bone marrow. The cumulative incidence of PTLD exceeded the rate for invasive solid tumors in this multiinstitutional cohort for nearly 5 years posttransplant (data for solid cancers from Curtis et al21).
Incidence Rate of PTLD by Time Since Transplantation
| Time Since Transplantation . | No. of Patients . | Person-Years at Risk . | No. of PTLD . | Incidence Rate* . |
|---|---|---|---|---|
| 0-<1 mo | 18,014 | 1,339 | 0 | 0.0 |
| 1-<2 mo | 16,634 | 1,330 | 12 | 90.2 |
| 2-<3 mo | 14,732 | 1,138 | 24 | 210.9 |
| 3-<4 mo | 13,120 | 1,103 | 10 | 90.7 |
| 4-<5 mo | 12,148 | 998 | 9 | 90.2 |
| 5-<6 mo | 11,417 | 943 | 2 | 21.2 |
| 6-<12 mo | 10,826 | 4,845 | 7 | 14.4 |
| 1-<2.5 yr | 8,919 | 10,716 | 8 | 7.5 |
| 2.5-<10 yr | 5,771 | 18,456 | 6 | 3.3 |
| 10+ yr | 625 | 1,481 | 0 | 0.0 |
| Total years | 18,014 | 42,349 | 78 | 18.4 |
| Time Since Transplantation . | No. of Patients . | Person-Years at Risk . | No. of PTLD . | Incidence Rate* . |
|---|---|---|---|---|
| 0-<1 mo | 18,014 | 1,339 | 0 | 0.0 |
| 1-<2 mo | 16,634 | 1,330 | 12 | 90.2 |
| 2-<3 mo | 14,732 | 1,138 | 24 | 210.9 |
| 3-<4 mo | 13,120 | 1,103 | 10 | 90.7 |
| 4-<5 mo | 12,148 | 998 | 9 | 90.2 |
| 5-<6 mo | 11,417 | 943 | 2 | 21.2 |
| 6-<12 mo | 10,826 | 4,845 | 7 | 14.4 |
| 1-<2.5 yr | 8,919 | 10,716 | 8 | 7.5 |
| 2.5-<10 yr | 5,771 | 18,456 | 6 | 3.3 |
| 10+ yr | 625 | 1,481 | 0 | 0.0 |
| Total years | 18,014 | 42,349 | 78 | 18.4 |
Incidence rate is the observed number of PTLD cases divided by person-years at risk (×104).
Cumulative incidence (%) of PTLD (78 cases) and invasive solid cancers (80 cases) following an allogeneic BMT; multi-institutional cohort of 235 transplant centers. (Data for solid tumors taken from Curtis et al.21)
Cumulative incidence (%) of PTLD (78 cases) and invasive solid cancers (80 cases) following an allogeneic BMT; multi-institutional cohort of 235 transplant centers. (Data for solid tumors taken from Curtis et al.21)
Most of the 64 recipients with early-onset PTLD had a primary disease of leukemia, similar to the distribution in the entire cohort (16 ALL, 15 AML, 19 CML, 5 severe aplastic anemia, 5 myelodysplastic syndrome, 2 lysosomal storage disease, 1 Hodgkin’s disease, and 1 hemoglobinopathy). EBV-related sequences were detected by in situ hybridization in all 36 evaluable cases. Fifty-five (86%) patients with PTLD died during the survey period; in 51 cases, the PTLD was the primary (n = 39) or contributing (n = 12) cause of death. The diagnosis of PTLD was made premortem in 39 cases and after death in 16. Disease progression was rapid among the 39 patients diagnosed before death (median survival, 0.6 months; range, 0.03 to 15 months). Nine patients with early-onset PTLD are alive at a median follow-up duration of 88 months after PTLD diagnosis (range, 3 to 131 months).
Among 14 patients with late-onset PTLD, 13 had a diagnosis of leukemia (5 ALL, 4 AML, 4 CML) and 1 had severe aplastic anemia. None of the late-onset cases was evaluable for EBV status in the current study; however, published case details indicate that 2 B-cell PTLD were EBV-related,11,15 while 3 cases (1 B-cell and 2 T-cell lymphomas) had no evidence of EBV-related sequences.12 13Patients who developed late-onset PTLD had significantly longer survival after diagnosis than those with early-onset tumors (P= .03). Eleven of 14 patients (79%) with late-onset PTLD died; in 9 cases the lymphoma was either the primary (n = 8) or contributing (n = 1) cause of death. Two PTLD cases were diagnosed after death; median survival for the 9 patients diagnosed before death was 6 months (range, 1 to 22 months). Three patients are alive at 37, 46, and 112 months after developing PTLD.
Table 3 presents results from multivariate models evaluating risk factors for PTLD diagnosed less than 1 year and ≥1 year posttransplant and for all time periods combined. The risk of early-onset PTLD was strongly associated (P < .0001) with T-cell depletion of the graft (RR = 12.7), unrelated donor or ≥2 HLA-antigen mismatched related donor (RR = 4.1) and use of anti-CD3 monoclonal antibody 64.1 (RR = 43.2) or antithymocyte globulin (RR = 6.4) as prophylaxis or treatment of acute GVHD. A weaker association was observed with the occurrence of acute GVHD grades II-IV (RR = 1.9,P = .02). Recipients of transplants from 1 HLA-antigen mismatched related donors (RR = 1.9, P = .20) were not significantly different in PTLD risk from those with HLA-identical sibling grafts. Patients who received unrelated donor grafts (RR = 3.3) had risks similar to those with ≥2 HLA-antigen mismatched related transplants (RR = 4.8), and thus were grouped for analyses.
Risk Factors for PTLD, by Time Since Transplantation
| Variable . | Time Since Transplantation . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All Periods (78 PTLD, 42,349 PYR) . | <1 yr (64 PTLD, 11,696 PYR) . | ≥1 yr (14 PTLD, 30,653 PYR) . | |||||||
| No. of PTLD . | RR . | 95% CI . | No. of PTLD . | RR . | 95% CI . | No. of PTLD . | RR . | 95% CI . | |
| Conditioning radiation (TBI or LFI), yes v no | 74 | 2.93-150 | 1.1-10.1 | 61 | 2.8 | 0.9-11.8 | 13 | 2.4 | 0.4-49.6 |
| Unrelated or ≥2 HLA-Ag mismatched related donor v matched sibling or 1 HLA-Ag mismatched relative | 30 | 3.73-150 | 2.2-6.0 | 30 | 4.13-150 | 2.4-6.9 | 0 | 0.0 | 0.0-2.4 |
| T-cell depletion, yesv no | 42 | 9.13-150 | 5.5-15.1 | 40 | 12.73-150 | 7.2-23.0 | 2 | 1.7 | 0.3-6.5 |
| Acute GVHD: grade II-IV v grade I or no acute GVHD | 42 | 1.6 | 1.0-2.7 | 36 | 1.93-150 | 1.1-3.3 | 6 | 1.1 | 0.3-3.3 |
| ATG/ALG for acute GVHD, prophylaxis or therapy, yes v no | 25 | 5.53-150 | 3.2-9.1 | 23 | 6.43-150 | 3.6-11.0 | 2 | 2.1 | 0.3-8.2 |
| Anti-CD3 MoAb (64.1) for acute GVHD therapy yes v no | 3 | 35.93-150 | 8.4-107 | 3 | 43.23-150 | 9.8-134 | 0 | 0.0 | — |
| Chronic GVHD, extensive v none, limited | 16 | 1.93-150 | 1.0-3.6 | 7 | 1.2 | 0.5-2.7 | 9 | 4.03-150 | 1.3-13.5 |
| Variable . | Time Since Transplantation . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All Periods (78 PTLD, 42,349 PYR) . | <1 yr (64 PTLD, 11,696 PYR) . | ≥1 yr (14 PTLD, 30,653 PYR) . | |||||||
| No. of PTLD . | RR . | 95% CI . | No. of PTLD . | RR . | 95% CI . | No. of PTLD . | RR . | 95% CI . | |
| Conditioning radiation (TBI or LFI), yes v no | 74 | 2.93-150 | 1.1-10.1 | 61 | 2.8 | 0.9-11.8 | 13 | 2.4 | 0.4-49.6 |
| Unrelated or ≥2 HLA-Ag mismatched related donor v matched sibling or 1 HLA-Ag mismatched relative | 30 | 3.73-150 | 2.2-6.0 | 30 | 4.13-150 | 2.4-6.9 | 0 | 0.0 | 0.0-2.4 |
| T-cell depletion, yesv no | 42 | 9.13-150 | 5.5-15.1 | 40 | 12.73-150 | 7.2-23.0 | 2 | 1.7 | 0.3-6.5 |
| Acute GVHD: grade II-IV v grade I or no acute GVHD | 42 | 1.6 | 1.0-2.7 | 36 | 1.93-150 | 1.1-3.3 | 6 | 1.1 | 0.3-3.3 |
| ATG/ALG for acute GVHD, prophylaxis or therapy, yes v no | 25 | 5.53-150 | 3.2-9.1 | 23 | 6.43-150 | 3.6-11.0 | 2 | 2.1 | 0.3-8.2 |
| Anti-CD3 MoAb (64.1) for acute GVHD therapy yes v no | 3 | 35.93-150 | 8.4-107 | 3 | 43.23-150 | 9.8-134 | 0 | 0.0 | — |
| Chronic GVHD, extensive v none, limited | 16 | 1.93-150 | 1.0-3.6 | 7 | 1.2 | 0.5-2.7 | 9 | 4.03-150 | 1.3-13.5 |
Abbreviations: PYR, person-years at risk; CI, confidence interval; Ag, antigen; ATG/ALG, antithymocyte globulin or serum, antilymphocyte globulin or serum; RR, relative risk; TBI, total body irradiation; LFI, limited field irradiation.
Poisson regression models stratified by time since transplantation (14 groups) and primary disease group (5 groups).
Denotes relative risks that are statistically significant (P < .05).
Among patients who survived more than 1 year posttransplant, the relationship between PTLD risk and previously identified risk factors (T-cell depletion, HLA disparity, use of antithymocyte globulin or monoclonal antibody CD3 therapy) was greatly diminished (Table 3). The only risk factor identified for late-onset PTLD was extensive chronic GVHD (RR = 4.0, P = .01).
For all time intervals combined, conditioning regimens with radiation were associated with risk of PTLD (RR = 2.9, P = .02). However, only small numbers of PTLD patients (n = 4) did not receive radiation and risk was inconsistent across geographic regions. Risk appeared to vary by dose of fractionated TBI with 3.5- to 4.3-fold risks seen for doses ≥13 Gy.
We compared the risk of PTLD associated with 5 different methods of T-cell depletion of bone marrow (Table 4), while controlling for other major risk factors. Significant differences among T-cell depletion techniques were detected (test of homogeneity,P = .009). Particularly high risks were seen for methods using monoclonal antibodies specifically directed against T cells or T plus NK cells (RR = 12.3) and for sheep red blood cell E-rosetting techniques (RR = 15.6). Only 4 PTLD developed in recipients of grafts depleted of T cells using CAMPATH-1 monoclonal antibodies, elutriation, or lectins, all of which remove both T and B cells.
Relative Risk (RR) of PTLD by Method of T-Cell Depletion of the Bone Marrow
| Method of T-Cell Depletion . | No. of PTLD . | RR4-150 . | 95% CI . |
|---|---|---|---|
| No T-cell depletion | 36 | 1.0 | Reference |
| Anti-T or anti-T + NK MoAb4-151 | 31 | 12.3 | 7.1-21.3 |
| SRBC rosetting‡ | 5 | 15.6 | 5.3-37.5 |
| Lectins4-153 | 1 | 4.1 | 0.2-19.8 |
| CAMPATH-1 MoAb | 2 | 2.0 | 0.3-6.7 |
| Elutriation/density gradient centrifugation4-155 | 1 | 2.6 | 0.1-12.4 |
| Unclassified/unknown | 2 | 12.7 | 2.0-43.1 |
| Method of T-Cell Depletion . | No. of PTLD . | RR4-150 . | 95% CI . |
|---|---|---|---|
| No T-cell depletion | 36 | 1.0 | Reference |
| Anti-T or anti-T + NK MoAb4-151 | 31 | 12.3 | 7.1-21.3 |
| SRBC rosetting‡ | 5 | 15.6 | 5.3-37.5 |
| Lectins4-153 | 1 | 4.1 | 0.2-19.8 |
| CAMPATH-1 MoAb | 2 | 2.0 | 0.3-6.7 |
| Elutriation/density gradient centrifugation4-155 | 1 | 2.6 | 0.1-12.4 |
| Unclassified/unknown | 2 | 12.7 | 2.0-43.1 |
Relative risks were adjusted for HLA disparity, use of ATG or MoAb anti-CD3 A641 for acute GVHD, occurrence of acute GVHD II-IV, occurrence of extensive chronic GVHD, and radiation conditioning.
Anti-T methods include monoclonal antibodies directed against T-cell–specific epitopes (including single antibodies or combinations): CD3, CD3/CD7, CD4, CD4/CD8, CD4/CD5/CD8, CD5, CD5/CD8, CD5/ricin, T10 B9, CD6/CD7/CD8, CD6/CD7, CD6/CD8, CD8, CD7/CD8. Anti-T and anti-NK methods include monoclonal antibodies directed against T-cell– and NK-cell–specific epitopes (including single antibodies or combinations): CD2, CD2/CD3, CD2/CD8, CD2/CD4/CD8, CD2/CD6/CD8/CD28, CD2/CD7, CD2/CD5/CD7, CD2/CD5, CD8, CD2/CD3/CD8, CD2/CD8, ATG incubation.
SRBC (sheep red blood cell) rosetting methods includes SRBC rosetting + density gradient centrifugation.
Lectin methods include lectin or lectin and E-rosetting (n = 126), and lectin and MoAb CD5/CD8 (n = 7).
Elutriation methods include elutriation, density gradient centrifugation, and elutriation/density gradient centrifugation.
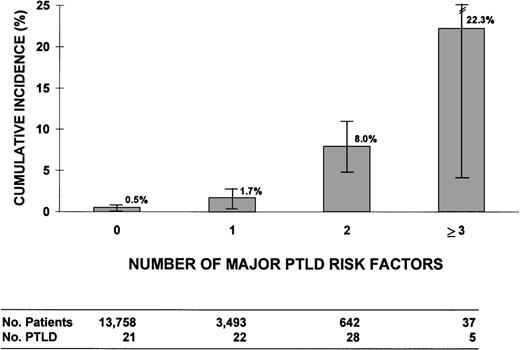
We assessed the risk of PTLD related to the number of risk factors for PTLD. Four major risk factors were defined: (1) T-cell depletion using monoclonal antibodies directed at T cells or T plus NK cells, or E-rosetting; (2) unrelated or ≥2 HLA-antigen mismatched related donor; (3) prophylaxis or therapy for acute GVHD with antithymocyte globulin; and (4) treatment of acute GVHD with anti-CD3 monoclonal antibody 64.1. Patients with unclassified or other methods of T-cell depletion were excluded (84 patients and 2 PTLD cases). Figure2 shows 10-year cumulative incidence rates of PTLD in patients with 0, 1, 2, or 3 to 4 major risk factors, with incidences ranging from 0.5% ± 0.3% in those with no major risk factors to high rates among recipients with 2 and ≥3 major risk factors (8.0% ± 2.9% and 22.3% ± 17.9%, respectively). We observed significant heterogeneity among patients with only 1 major risk factor (P = .0001). No PTLD developed among 1,240 patients (1,412 person-years) with unrelated or ≥2 HLA-antigen mismatched related donor grafts who did not have other major risk factors, and this group was not significantly different in PTLD risk (P = .08) from those with no major risk factors (21 cases, 6/10,000/yr). This result contrasted with the substantial increased risk seen among patients in other single-risk factor groups: T-cell–depleted graft alone (14 cases, 71/10,000/yr) or antithymocyte globulin therapy alone (7 cases, 27/10,000/yr). Of the 21 PTLD patients with no major risk factors, 20 received radiation for conditioning, 11 had acute or chronic GVHD (or both), and 3 received a graft that was T-cell–depleted with CAMPATH-1 monoclonal antibodies or lectin techniques.
Cumulative incidence (%) of PTLD for patients with 0, 1, 2, or 3 to 4 major risk factors for PTLD. The 4 risk factors were defined as: (1) T-cell depletion methods that selectively target T cells or T + NK cells or E-rosetting; (2) unrelated or ≥2 HLA antigen mismatched related donor; (3) antithymocyte globulin used as prophylaxis or therapy for acute GVHD; and (4) anti-CD3 monoclonal antibody 64.1 given as therapy for acute GVHD (Seattle only). Analysis excludes 2 PTLD and 84 patients with unclassified or other methods of T-cell depletion.
Cumulative incidence (%) of PTLD for patients with 0, 1, 2, or 3 to 4 major risk factors for PTLD. The 4 risk factors were defined as: (1) T-cell depletion methods that selectively target T cells or T + NK cells or E-rosetting; (2) unrelated or ≥2 HLA antigen mismatched related donor; (3) antithymocyte globulin used as prophylaxis or therapy for acute GVHD; and (4) anti-CD3 monoclonal antibody 64.1 given as therapy for acute GVHD (Seattle only). Analysis excludes 2 PTLD and 84 patients with unclassified or other methods of T-cell depletion.
Disease for which the transplant was performed and age at transplantation had no significant association with risk of PTLD in multivariate regression analyses. Drugs used for conditioning, evaluated singly and in combination, were also not significantly related to PTLD risk, and no increase in risk was observed for patients who received antithymocyte globulin for conditioning (RR = 0.8,P = .68), as previously reported.2 There were no significant associations with specific immunosuppressive drugs used to prevent or treat acute GVHD, other than those described above.
DISCUSSION
Lymphoproliferative disorders after bone marrow and organ transplantation and among patients infected with AIDS are believed to result from uncontrolled proliferation of EBV-transformed B-lymphocytes in the setting of immune dysfunction.1,22-28 Recent evidence suggests that most of the latent EBV proteins, including EBNA-1, -2, and -3, and LMP-1, are expressed on the malignant cells in EBV-induced PTLD1,4 and that LMP-1 functions as a signaling protein in these tumors.29 PTLD in the solid-organ transplant setting have been extensively studied over the last 3 decades. A wide range of tumors, from lymphoid hyperplasias that resolve after withdrawal of immunosuppression to aggressive monoclonal lymphomas with poor outcomes, are reported.9,30,31 In contrast, lymphoproliferative disorders after allogeneic BMT have been studied more recently.11,32 Most tumors after BMT appear to be rapidly fatal, monoclonal or oligoclonal B-cell lymphomas that occur early after transplant. Recent advances in the therapy of EBV-related lymphomas following BMT offer hope for improved survival following this complication, and efforts have intensified to identify and monitor patients at highest risk.33-35 Our current study evaluated 78 lymphoproliferative disorders occurring in a large cohort of 18,000 BMT recipients, and assessed incidence and risk factors for early-onset versus late-onset PTLD.
Our 1.0% cumulative incidence of PTLD at 10 years after BMT is comparable to rates of 0.5% to 1.8% reported from single centers.2,11,36 Data from our study also demonstrate that PTLD incidence varies markedly with time after transplantation, with particularly high rates occurring during the first 5 months, followed by a steep decline in incidence between 6 and 12 months posttransplant. A significantly increased risk of PTLD continues among longer term survivors, although the rate is greatly diminished. The high incidence of PTLD during the first few months after transplant is consistent with clinical investigations of the temporal pattern of immune reconstitution in marrow transplant recipients. Lucas et al reported that levels of anti-EBV cytotoxic T-lymphocyte precursors (CTLp) appear to return to normal by 6 months posttransplant in most patients, and that this interval of low CTLp frequency corresponds to the period of highest risk of EBV-related PTLD.37 Although T-cell immunity can be impaired in some patients for a prolonged interval, most transplant recipients without chronic GVHD have substantial recovery of T-cell function within 1 year.38 39 It is perhaps not surprising then that PTLD incidence declines during the first 12 months posttransplant from a high of 210 cases/10,000/yr to less than 5 cases/10,000/yr.
Previous investigations of risk factors for lymphomas after BMT were limited mostly to early-onset tumors.1-3,35,40 The 2 largest series evaluated 22 PTLD occurring among 2,150 BMT recipients at the University of Minnesota2 and 16 PTLD among 2,246 patients at Seattle.3 In those studies and the current study, factors associated with severe immune dysfunction and altered T-cell regulatory mechanisms were major predictors of early-onset lymphomas. Significantly increased risks were seen with T-cell depletion of the graft,2,3 unrelated donor or donor-recipient HLA disparity,2,3 anti-CD3 monoclonal antibody 64.1 therapy for acute GVHD,3 use of antithymocyte globulin,2,3 and transplantation for primary immunodeficiency.2 New findings in the current study indicate that the occurrence of acute GVHD of grades II to IV is significantly related to risk of early-appearing PTLD and that patients who receive 1 HLA-antigen mismatched related donor marrow are not significantly different in risk from recipients of an HLA-identical sibling graft. Radiation given as part of the conditioning regimen may be an additional risk factor for PTLD, although there was inconsistency in risk estimates across geographic areas. The mechanism by which pretransplant radiation might contribute to the development of PTLD is unclear. Higher TBI doses have been reported to increase GVHD severity,41 and exaggerated acute GVHD may trigger the need for prolonged immunosuppressive therapy, making it difficult to quantify the relative contributions of these effects to PTLD risk.
Our study is the first to evaluate risk factors for late-onset PTLD following allogeneic BMT, although case reports have described late-occurring lymphomas.3,12,13,15,42-44 Risk factors for early-onset PTLD in our study did not predict late-onset PTLD. However, chronic GVHD, an established correlate of immune dysregulation in long-term survivors, was identified as a strong risk factor. Patients with chronic GVHD may also require long-term treatment with immunosuppressive drugs, which is linked to an excess of PTLD following solid-organ transplants.45 Thus, it is likely that immune dysfunction and immunosuppressive mechanisms continue to play a role in the development of PTLD among long-term survivors of BMT, although different risk factors may be involved.
We found significant heterogeneity in PTLD risk among the nearly 2,500 patients who received T-cell–depleted grafts. Particularly high risks were observed among recipients of grafts T-cell–depleted with monoclonal antibodies or E-rosetting techniques that selectively target T (or T plus NK) cells, whereas lower rates were associated with methods that remove both T and B cells (CAMPATH-1 monoclonal antibodies, elutriation, lectins). Similarly, reports from single centers have described PTLD incidence rates as high as 25% in patients depleted with T-cell–specific monoclonal antibodies or E-rosetting.2,5,11,40,46-48 In a recent multicenter study of 2,401 recipients T-cell–depleted with CAMPATH-1M or 1G, Hale et al49 reported a low 1.1% cumulative risk of PTLD and hypothesized that depletion of B cells, as well as T cells, may reduce the viral load or virus target tissue in the interval before full recovery of the T-cell population. Low PTLD rates have been observed also after transplants using marrow that was T-cell–depleted with counterflow centrifugal elutriation50 or with lectin agglutination/E-rosette–depleted grafts without additional immunosuppression.4,35 Thus, the risk of PTLD appears much less with T-cell depletion techniques removing both T and B lymphocytes, possibly related to the reduced numbers of EBV-transformed B lymphocytes.4
Although PTLD incidence is reported to increase to high levels (up to 25%) among patients with multiple risk factors,4,35 few data are available to evaluate the individual and combined effects of each of the PTLD risk determinants. Our results indicate a steep increase in cumulative incidence with greater numbers of major risk factors, each of which are markers of altered immunity or T-cell function. In previous studies, patients receiving unrelated or HLA-mismatched related donor transplants were reported to be at higher risk of PTLD than those with HLA-identical sibling donors,2,3,32,37,46,47 although most patients in these investigations received a T-cell–depleted graft or other T-cell manipulation. Our study evaluated recipients of an unrelated or ≥2 HLA-antigen mismatched related graft who had no other major risk factors and found a low PTLD risk. This result contrasts with elevated PTLD rates among patients who received antithymocyte globulin alone or T-cell–depleted grafts alone (no other major risk factors). Although caution is indicated due to the high early mortality rate after HLA mismatched transplants and the strong correlation between HLA disparate donors and other risk factors, such as acute GVHD, these results suggest that HLA incompatibility by itself may be less important than other variables affecting PTLD risk. Along with the factors considered in the current study are other variables that appear to influence susceptibility to EBV-related lymphoproliferative disorders in solid-organ and marrow transplants, such as EBV viral burden, specific EBV strain (EBER A v EBER B), and deficiencies in cellular immunity to EBV.4,33,37 51 Even patients with no identifiable risk factors, except possibly radiation, may develop PTLD, although the risk is relatively low, reflecting variability in immune reconstitution not accounted for by known determinants.
Early-onset PTLD in our series were uniformly EBV-positive and most developed progressive disease that proved rapidly fatal. The 14 patients with late-onset PTLD had a significantly longer survival postdiagnosis than those with early-onset tumors. In addition, 3 of the late-onset PTLD cases in our series were described in published reports to be EBV-negative and 2 were T-cell PTLD.12,13 Recent evidence from solid-organ transplants suggests that EBV-negative PTLD are morphologically and clinically distinct from EBV-positive PTLD; EBV-negative PTLD after organ transplants tended to have later onset (median, 4.9 years), a higher prevalence of monomorphic lymphomas, and a greater proportion derived from T cells.52 These differences indicate the need for further research to fully characterize the clinicopathologic features of late-onset PTLD following BMT.
Most patients in our study developed PTLD during the 1980s and early 1990s, when available treatments (ie, antiviral and chemotherapeutic agents) were largely ineffective. Underreporting of PTLD during this period may be substantial due to difficulties in diagnosis, so that our estimates of PTLD incidence are likely to be conservative. In recent years, considerable progress has been made in the diagnosis, prevention, and treatment of PTLD among allogeneic BMT recipients. Immunovirologic assays are available to detect high levels of EBV DNA, which strongly correlates with increased risks of EBV-related PTLD.33,53,54 New approaches for prophylaxis and treatment have been reported, including successful therapy with infusions of donor leukocytes.35 Recent promising strategies include antiviral prophylaxis and treatment with gene-marked EBV-specific T lymphocytes,34,55 and the use of specific anti-CD21 and anti-CD24 murine monoclonal antibodies to control severe PTLD.56 These advances may improve the prognosis for patients at increased risk of PTLD.
We conclude from these analyses in a large cohort of BMT recipients that the risk of PTLD persists among patients surviving more than 1 year after transplant, although it is greatly decreased compared with the early posttransplant period. Moreover, our data indicate that risk factors associated with altered immunity and T-cell regulatory mechanisms are predictors of both early- and late-onset PTLD. Patients at highest risk of PTLD are candidates for trials of prophylactic or preemptive therapies.
ACKNOWLEDGMENT
We are indebted to all collaborating investigators and staff from the participating transplant centers who contributed data to this study. We wish to thank Muriel Siadek, K. Erne, and Gary Schoch from Fred Hutchinson Cancer Research Center, Seattle, and Diane Knutson and Sharon Nell from the IBMTR, Milwaukee, for support in data collection. We acknowledge Kathy Chimes, Elena Adrianza, Andy Singer, and Diane Fuchs from Westat, Inc for coordination of field studies, George Geise and Christel McCarty from Information Management Services for computing support, and Denise Duong and Rebecca Albert for manuscript preparation.
This research is supported by Contracts No. CP-51027 and CP-51028 from the National Cancer Institute. The International Bone Marrow Transplant Registry is supported by Public Health Service Grant no. PO1-CA-40053 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute of the US Department of Health and Human Services; and grants from Alpha Therapeutic Corp; Amgen, Inc; Anonymous; Baxter Healthcare Corporation; Bayer Corp; Berlex Laboratories; Blue Cross and Blue Shield Association; Lynde and Harry Bradley Foundation; Bristol-Myers Squibb Co; Cell Pro, Inc; Centeon; Center for Advanced Studies in Leukemia; Chimeric Therapies; Chiron Therapeutics; Charles E. Culpeper Foundation; Eleanor Naylor Dana Charitable Trust; Eppley Foundation for Research; Genentech, Inc; Glaxo Wellcome Co; ICN Pharmaceuticals; Immunex Corp; Kettering Family Foundation; Kirin Brewery Company; Robert J. Kleberg, Jr and Helen C. Kleberg Foundation; Herbert H. Kohl Charities, Inc; Nada and Herbert P. Mahler Charities; Milstein Family Foundation; Milwaukee Foundation /Elsa Schoeneich Research Fund; NeXstar Pharmaceuticals, Inc; Samuel Roberts Noble Foundation; Novartis Pharmaceuticals; Ortho Biotech, Inc; John Oster Family Foundation; Jane and Lloyd Pettit Foundation; Alirio Pfiffer Bone Marrow Transplant Support Association; Pfizer, Inc; Pharmacia and Upjohn; Principal Mutual Life Insurance Co; RGK Foundation; Rockwell Automation Allen Bradley Co; Roche Laboratories; SangStat Medical Corp; Schering-Plough Oncology; Searle; Stackner Family Foundation; Starr Foundation; Joan and Jack Stein Foundation; SyStemix; United Resource Networks; and Wyeth-Ayerst Laboratories. The Fred Hutchinson Cancer Research Center investigators are supported by Grants No. P01-CA 18029, P01-CA-18221 and P01-CA-15704 from the National Cancer Institute, and P01-HL-36444 from the National Heart, Lung, and Blood Institute.
See for grant support information.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
This is a US government work. There are no restrictions on its use.
REFERENCES
Author notes
Address reprint requests to Rochelle E. Curtis, MA, Executive Plaza South, Room 7042, National Cancer Institute, Bethesda, MD 20892; e-mail: curtisr@epndce.nci.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal