Abstract
The actin regulatory protein gelsolin cleaves actin filaments in a calcium- and polyphosphoinositide-dependent manner. Gelsolin has recently been described as a novel substrate of the cysteinyl protease caspase-3, an effector protease activated during apoptosis. Cleavage by caspase-3 generates an amino-terminal fragment of gelsolin that can sever actin filaments independently of calcium regulation. The disruption of the actin cytoskeleton by cleaved gelsolin is hypothesized to mediate many of the downstream morphological changes associated with apoptosis. In contrast, overexpression of full-length gelsolin has also been reported to inhibit apoptotic cell death upstream of the activation of caspase-3, suggesting that gelsolin may also act prior to commitment to cell death. The authors previously observed that actin stabilization by the cell permeant agent jasplakinolide enhanced cell death upon interleukin (IL)-2 or IL-3 withdrawal from growth-factor–dependent lymphocyte cell lines, and hypothesized that actin polymerization could alter the activity of gelsolin, thus enhancing apoptosis. Here the authors show that constitutive overexpression of gelsolin did not, however, inhibit or dramatically enhance apoptotic cell death upon growth-factor withdrawal, nor did it modify sensitivity to jasplakinolide. In contrast to previous reports, overexpression of gelsolin in Jurkat T cells did not prevent or delay apoptosis induced by Fas ligation or ceramide treatment. Overexpressed gelsolin protein was cleaved during apoptosis, as seen previously in this and other cell types. In these model systems, therefore, the level of gelsolin expression was not a rate-limiting determinant in commitment to or time to the morphological changes of apoptosis.
Apoptosis, a process of programmed cell death characterized by defined biochemical and morphological changes, is often divided into 2 phases, an initiation/commitment phase and a downstream effector phase.1 A number of stimuli can trigger different signaling pathways that culminate in cellular commitment to apoptosis. The precise events that constitute irreversible commitment are as yet unknown, but upon activation of a family of cysteinyl proteases (caspases), commitment has occurred and cell death is irreversible. In this regard, caspase-3 appears to play a critical role as an executioner of the effector phase of apoptosis, since its activation is often required for the downstream morphological changes that define apoptosis, including membrane blebbing, DNA degradation, and nuclear condensation.1 The interrelationships between commitment to apoptosis, activation of caspase-3, and the resulting morphological changes are not fully understood.
One substrate of caspase-3 that has been recently identified is gelsolin, an 80-kd actin-binding protein.2Gelsolin consists of 6 repeating domains, with separate, distinct sites for binding actin monomers and polymers.3 Through the sequestration of actin monomers and the severing and capping of actin filaments, gelsolin regulates the rates of both actin polymerization and depolymerization. The activity of gelsolin itself is regulated by calcium and polyphosphoinositide binding.4,5 Gelsolin contains a consensus caspase cleavage site (DQTD352G) between the third and fourth repeating domains. Cleavage at this site by caspase-3 generates an amino-terminal fragment that binds and severs actin filaments independently of calcium regulation.2Overexpression of this fragment generates many of the characteristic morphological changes of apoptosis, including DNA fragmentation, without activating the caspase cascade, and it has been suggested that the cleavage of gelsolin is a critical element in the progression of the “effector” phase of apoptosis.2 Interestingly, it has also been reported that the overexpression of gelsolin prevented apoptosis induced by Fas ligation, by ceramide, and by dexamethasone in Jurkat T cells by preventing the release of cytochrome c from the mitochondrial membrane and the subsequent activation of caspase-3.6 7 These results provide support for an additional role for gelsolin in the upstream, commitment phase of apoptosis or for the possibility that the interaction between caspase-3 and gelsolin is more complex than was initially described.
We have recently demonstrated that pharmacological stabilization of the actin cytoskeleton by the compound jasplakinolide enhanced apoptosis of the interleukin (IL)-2–dependent T-cell line CTLL-20 induced by cytokine deprivation.8 Incubation of the cells with jasplakinolide after IL-2 deprivation decreased the time to commitment to the apoptotic pathway. The enhancement of apoptosis occurred upstream of the caspase cascade and could be inhibited by the overexpression of the anti-apoptotic protein Bcl-xL. CTLL-20 cells treated with jasplakinolide did not undergo apoptosis when incubated in the presence of exogenous IL-2, arguing that this compound was not toxic to the cells. These data suggested a specific role for the actin cytoskeleton in the upstream “commitment” phase of apoptosis. On the basis of the reports of the involvement of gelsolin in apoptosis, we hypothesized that jasplakinolide-mediated actin stabilization altered gelsolin activity and that the modulation of gelsolin in turn altered the apoptotic process. Here we tested this hypothesis by overexpressing gelsolin in CTLL-20 cells, in the murine IL-3 dependent pre-B cell line Ba/F3, and in the human T-cell line Jurkat. As expected, gelsolin was cleaved during apoptosis; surprisingly, however, its overexpression did not inhibit apoptosis induced by cytokine deprivation, ceramide treatment, or Fas ligation. Furthermore, overexpression of gelsolin did not prevent the jasplakinolide-mediated enhancement of apoptosis induced by cytokine deprivation.
Materials and methods
Reagents
The actin-binding compound jasplakinolide was provided by the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment, National Cancer Institute, National Institutes of Health (Bethesda, MD). Jasplakinolide was stored in Me2SO at −20°C or −80°C and diluted into medium immediately prior to use. The human gelsolin expression vector LKCG and the control vector LK444 have been previously described.9 LKCG contains the complementary DNA for human gelsolin driven by the β-actin promoter and contains a neomycin resistance gene. The plasmids were linearized with ScaI (New England Biolabs, Beverly, MA) and purified prior to transfection.
The murine antihuman gelsolin monoclonal antibody (mAb) GS-2C4 was purchased from Sigma (St Louis, MO). C2-ceramide (D-erythro-sphingosine, N-Acetyl-) was purchased from Calbiochem (La Jolla, CA) and stored in Me2SO at −20°C. Antihuman Fas mAb 7C11 was kindly provided by Jerome Ritz (Dana-Farber Cancer Institute, Boston, MA). Antihuman Fas mAb CH11 was purchased from Upstate Biotechnologies (Lake Placid, NY). Antiactin mAb N350 was purchased from Amersham (Arlington Heights, IL). Anti–caspase-3 mAb was purchased from Transduction Laboratories (Lexington, KY).
Cell culture and transfection
The human leukemic T-cell line Jurkat was cultured in RPMI 1640 (MediaTech, Herndon, VA) supplemented with 10% heat-inactivated fetal calf serum (Life Technologies, Gaithersburg, MD); 2 mmol/L L-glutamine, 10 mmol/L Hepes, 100 U/mL penicillin, and 100 μg/mL streptomycin (MediaTech); and 50 μmol/L 2-mercaptoethanol (Sigma) (termed complete media, or cRPMI-10%). The IL-2–dependent murine T-cell line CTLL-20 was cultured in cRPMI-10% supplemented with 3% IL-2–containing supernatant derived from conconavalin A–stimulated rat splenocytes (T-Stim) (Collaborative Biomedical Products, Bedford, MA). The IL-3–dependent murine pre-B cell line Ba/F3 was cultured in cRPMI-10% supplemented with 5% IL-3–containing Wehi culture supernatant. All cells were free of mycoplasma as determined by routine polymerase chain reaction analysis (Mycoplasma PCR Primers; Stratagene, La Jolla, CA).
For transfection, 5 × 106 cells were resuspended in 500 μL RPMI plus 10% fetal calf serum and incubated in the electroporation chamber for 15 minutes with 30 μg linearized plasmid DNA at room temperature. Cells were electroporated at low resistance, 800 microfarads (μF), and 250 V (Cell-Porator) (Life Technologies) and resuspended in cRPMI-10%. After 48 hours, cells were plated at 1 × 104 cells/mL in 24-well plates in selection medium consisting of cRPMI-10% plus 1 mg/mL G418 (Geneticin) (Life Technologies) for Jurkat cells, cRPMI-10% plus 1 mg/mL G418 plus 5% Wehi-derived supernatant for Ba/F3 cells, and cRPMI-10% plus 0.3 mg/mL G418 plus 100 U/mL recombinant human IL-2 (kindly provided by Hoffman-LaRoche, Nutley, NJ) for CTLL-20 cells. G418-resistant colonies were screened for expression of gelsolin by Western blot, and stable transfectants were maintained in selection medium.
Apoptosis assays
Washed cells were resuspended at a density of 2 × 105 cells/mL for the induction of apoptosis. Apoptosis was induced by cytokine withdrawal, treatment with ceramide (50 or 100 μmol/L), or treatment with anti-Fas mAb (100 ng/mL CH11 or a 1:500 dilution of 7C11 ascites) for the indicated periods of time. For cytokine withdrawal, stably transfected cytokine-dependent cell lines CTLL-20 or Ba/F3 were washed 3 times in cRPMI-10% and then resuspended in cytokine-free cRPMI-10%. For the induction of apoptosis by ceramide treatment, cells were incubated in AIM-V serum free lymphocyte medium (Life Technologies). AIM-V medium was supplemented with 50 U/mL recombinant human IL-2 for experiments using CTLL-20 cells to prevent cytokine-withdrawal–induced death.
Cell death was assayed by trypan blue exclusion, nuclear morphology, or Annexin V immunofluorescent staining. For the assessment of nuclear morphology, cells induced to undergo apoptosis were fixed in an excess volume of 3:1 (vol/vol) methanol/acetic acid, dried onto glass slides, and stained with 50 μg/mL propidium iodide (Sigma) blocked with dried milk, and incubated with 0.5 μg/mL RNase (Boehringer Mannheim). The percentage of cells undergoing apoptosis was determined by quantifying the number of cells with and without condensed nuclei by fluorescence microscopy (Olympus Bmax BX50) (Olympus America, Melville, NY). More than 300 cells were counted for each sample. The 95% confidence interval for each sample within each experiment was determined according to the statistical definition of the variance of a proportion in a binomial experiment. The variance was computed as pq/n, where p = proportion apoptotic,q = 1−p, and n = the number of cells counted.
For Annexin-V immunofluorescence, cells were resuspended in 200 μL binding buffer (ApoAlert AnnexinV-FITC kit) (ClonTech, Palo Alto, CA) and then stained with 5 μL Annexin V conjugated to fluorescein isothiocyanate (FITC) and 20 μL Via-Probe (7-amino-actinomycin D [7-AAD]) (PharMingen, San Diego, CA) for a minimum of 15 minutes at room temperature. Staining was assayed by flow cytometry (Epics XL, Coulter, equipped with an argon laser emitting at 488 nm), measuring Annexin V-FITC staining on the FL1 channel (emission wavelength 535 nm) and 7-AAD fluorescence on the FL3 channel (emission wavelength 620 nm).
Immunoprecipitations and immunoblotting
For immunoprecipitation, 2 × 107 cells were lysed in 1 mL lysis buffer (1% Nonidet P-40 [Calbiochem], 150 mmol/L NaCl, 25 mmol/L Tris pH 7.5, 1 mmol/L EDTA, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF) (Boehringer Mannheim), and 1 mmol/L sodium orthovanadate). Postnuclear lysates were rotated for 2 hours at 4°C with 2 μL anti-gelsolin mAb and 20 μL Sepharose Protein G (Santa Cruz Biotechnology, Santa Cruz, CA). Beads were washed 3 times with wash buffer (0.1% Nonidet P-40, 150 mmol/L NaCl, 25 mmol/L Tris pH 7.5, 1 mmol/L EDTA, 1 mmol/L sodium orthovanadate), and proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).
Western blot assays were performed essentially as described.10 In brief, whole-cell lysates were generated by resuspending washed cells in SDS sample buffer and boiling for 10 minutes. Postnuclear lysates were generated by lysing washed cells in lysis buffer (1% Nonidet P-40, 150 mmol/L NaCl, 25 mmol/L Hepes, 1 mmol/L EDTA pH 7.4, 1 mmol/L sodium orthovanadate, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 10 μg/mL soybean trypsin inhibitor), incubating on ice for 20 minutes, and removing cell debris by centrifugation at 15 300 rpm for 10 minutes. Supernatants were mixed with 2 × SDS sample buffer and boiled for 10 minutes. Lysates or immunoprecipitates were separated by SDS-PAGE (10% or 12% acrylamide; Protogel) (National Diagnostics, Atlanta, GA). Proteins were transferred to polyvinylidene membranes (Millipore, Bedford, MA), which were blocked with a solution of 4% bovine serum albumin and then incubated with the indicated antibody. Bound antibodies were detected with the enhanced chemiluminescence system (Amersham Pharmacia Biotech) according to the manufacturer's instructions.
Results
Overexpression of gelsolin in CTLL-20 or Ba/F3 cells did not delay apoptosis in response to cytokine deprivation, nor did it prevent the enhancement of apoptosis by jasplakinolide
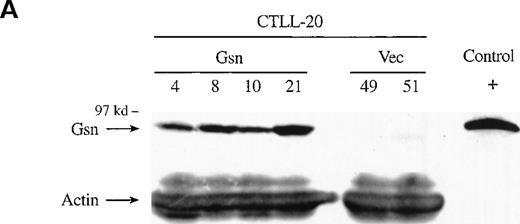
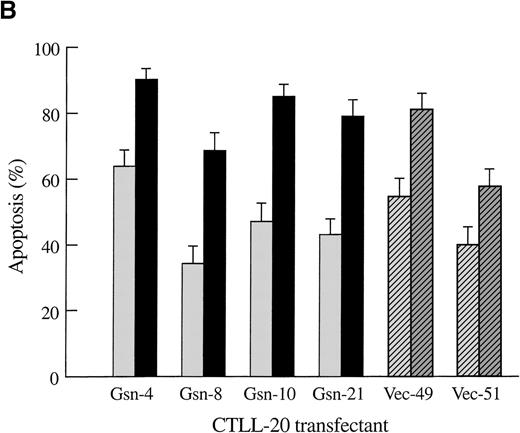
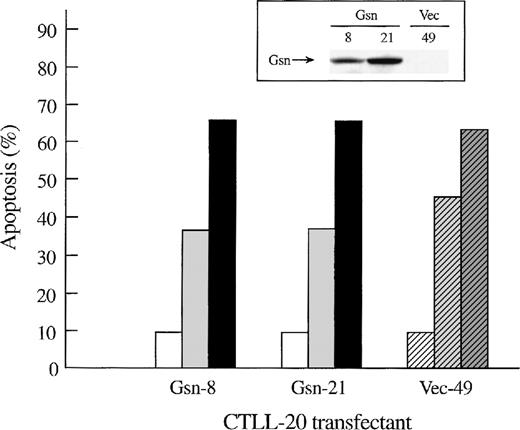
To determine whether overexpression of gelsolin delayed or inhibited apoptosis induced by cytokine deprivation, and to determine whether overexpression of gelsolin prevented the enhancement of apoptosis induced by actin polymerization, CTLL-20 cells were transfected with the gelsolin expression or control vector by electroporation. Stably transfected cell lines were selected by culture in G418 and screened by Western blot analysis (Figure1A). Endogenous gelsolin was expressed at very low levels in CTLL-20 cells (data not shown). Transfected cells were deprived of IL-2 and incubated for 15 hours in the presence of either 100 nmol/L jasplakinolide or the vehicle, 0.02% Me2SO. The percentage of apoptotic cells was determined by nuclear morphology. Overexpression of human gelsolin did not protect IL-2–deprived CTLL-20 cells from apoptosis, nor did it prevent the enhancement of apoptosis observed when actin was stabilized by the compound jasplakinolide (Figure 1B). Furthermore, the time of commitment to cell death and the final percentage of cells that underwent apoptosis were not changed by gelsolin overexpression (data not shown).
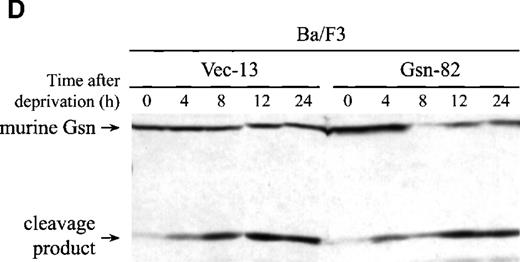
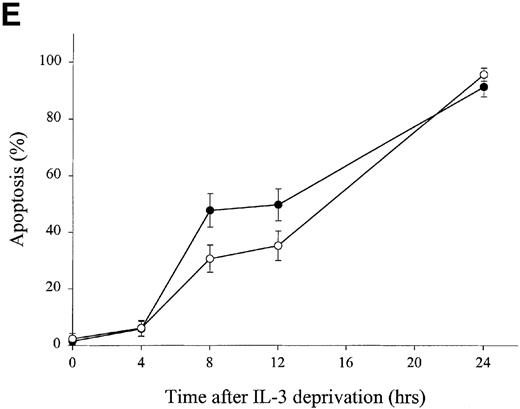
Effect of overexpression of gelsolin on timing of apoptosis of growth factor-dependent cells induced by cytokine deprivation.
Overexpression of gelsolin did not delay apoptosis of growth factor-dependent cells induced by cytokine deprivation, or prevent its enhancement by jasplakinolide, despite appropriate cleavage. (A) Whole cell lysates (1.25 × 106 cell equivalents) of transfected CTLL-20 cells were separated by SDS-PAGE and immunoblotted for gelsolin and for actin. Serum was used as a positive control. (B) Transfected CTLL-20 cells were incubated for 15 hours in the absence of IL-2 and in the presence of 100 nmol/L jasplakinolide (closed bars) or its vehicle, 0.02% Me2SO (open bars). After incubation, cells were fixed and stained with propidium iodide for quantification of apoptosis by nuclear morphology. Results are representative of 2 independent experiments. (C, D) Whole cell lysates (2 × 106 cell equivalents) of transfected Ba/F3 cells deprived of IL-3 for the indicated periods of time were separated by SDS-PAGE and immunoblotted for human (C) or murine (D) gelsolin. The time-dependent appearance of the 46 kd gelsolin cleavage product is shown. In (D) there was a slight loss of the cell sample at 8 hours. Results are representative of 2 independent experiments. (E) Parallel samples of transfected Ba/F3 cells deprived of IL-3 for the indicated periods of time were fixed and stained with propidium iodide for quantification of apoptosis by nuclear morphology. Closed circles represent Ba/F3 cells that overexpress gelsolin, while open circles represent the vector control. SDs were calculated as described in “Materials and methods.” The slight difference between the 2 cell lines at 8 and 12 hours was not evident by trypan blue exclusion.
Effect of overexpression of gelsolin on timing of apoptosis of growth factor-dependent cells induced by cytokine deprivation.
Overexpression of gelsolin did not delay apoptosis of growth factor-dependent cells induced by cytokine deprivation, or prevent its enhancement by jasplakinolide, despite appropriate cleavage. (A) Whole cell lysates (1.25 × 106 cell equivalents) of transfected CTLL-20 cells were separated by SDS-PAGE and immunoblotted for gelsolin and for actin. Serum was used as a positive control. (B) Transfected CTLL-20 cells were incubated for 15 hours in the absence of IL-2 and in the presence of 100 nmol/L jasplakinolide (closed bars) or its vehicle, 0.02% Me2SO (open bars). After incubation, cells were fixed and stained with propidium iodide for quantification of apoptosis by nuclear morphology. Results are representative of 2 independent experiments. (C, D) Whole cell lysates (2 × 106 cell equivalents) of transfected Ba/F3 cells deprived of IL-3 for the indicated periods of time were separated by SDS-PAGE and immunoblotted for human (C) or murine (D) gelsolin. The time-dependent appearance of the 46 kd gelsolin cleavage product is shown. In (D) there was a slight loss of the cell sample at 8 hours. Results are representative of 2 independent experiments. (E) Parallel samples of transfected Ba/F3 cells deprived of IL-3 for the indicated periods of time were fixed and stained with propidium iodide for quantification of apoptosis by nuclear morphology. Closed circles represent Ba/F3 cells that overexpress gelsolin, while open circles represent the vector control. SDs were calculated as described in “Materials and methods.” The slight difference between the 2 cell lines at 8 and 12 hours was not evident by trypan blue exclusion.
Stable transfectants of the IL-3–dependent pre-B cell line Ba/F3 were also generated (Figure 1C). Transfected Ba/F3 cells overexpressing human gelsolin were deprived of IL-3 for the indicated period of time; parallel samples were assayed for apoptosis and for cleavage of gelsolin. Human gelsolin (Figure 1C) was cleaved at the same rate as endogenous murine gelsolin (Figure 1D), confirming that human gelsolin remained a substrate for murine caspases in a murine environment. Consistent with the results observed in CTLL-20 cells, overexpression of gelsolin did not inhibit nuclear condensation indicative of apoptosis as measured by staining with propidium iodide (Figure 1E). There was a modest time-dependent increase in apoptotic death in cells overexpressing gelsolin following IL-3 deprivation; this increase may reflect a modest acceleration in the effector phase of apoptosis, consistent with an increase in levels of the amino-terminal fragment of gelsolin following cleavage by caspases.2 However, this difference was not reflected in cell death as measured by trypan blue exclusion at these or later time points (data not shown). Thus, the failure of gelsolin overexpression to inhibit apoptosis was not specific to a cell line or to dependence on a particular cytokine. Importantly, however, human gelsolin was able to interact with the murine apoptotic machinery, as the exogenous human gelsolin was cleaved at the same rate as the endogenous murine gelsolin (Figure 1C and 1D). In addition, the caspase machinery was not rate-limiting, as the endogenous gelsolin was cleaved at the same rate in Ba/F3 cell lines that either did or did not overexpress gelsolin (Figure 1D).
Overexpression of gelsolin did not prevent apoptosis in response to ceramide treatment or Fas ligation
While the role of gelsolin in apoptosis induced by cytokine withdrawal has not been previously examined, its overexpression has been reported to prevent ceramide-induced apoptosis in Jurkat T cells.6 We therefore treated transfected CTLL-20 cells with vehicle (0.05% Me2SO) and 50 μmol/L or 100 μmol/L C2-ceramide while maintaining the cells in the presence of 50 U/mL rhIL-2 to ensure growth-factor sufficiency. The percentage of apoptotic cells was determined by Annexin V-FITC staining (Figure 2), nuclear morphology, and/or trypan blue exclusion (data not shown). Overexpression of gelsolin did not delay apoptosis in response to ceramide treatment in CTLL-20 cells (Figure 2).
Effect of overexpression of gelsolin on apoptosis of CTLL-20 cells induced by C2-ceramide.
Overexpression of gelsolin did not prevent apoptosis of CTLL-20 cells induced by C2-ceramide. Transfected CTLL-20 cells overexpressing gelsolin (solid bars) or vector alone (hatched bars) were incubated for 12.5 hours in the presence of 50 U/mL recombinant human IL-2 and in the presence of 100 μmol/L C2-ceramide (closed or dark gray bars), 50 μmol/L C2-ceramide (gray bars), or its vehicle, 0.05% Me2SO (open bars). The percentage of apoptosis was determined by Annexin V-FITC staining as described in “Materials and methods.” Comparable results were obtained by trypan blue exclusion (data not shown). These results are representative of 3 independent experiments. Immunoblotting for gelsolin (inset) demonstrated that the gelsolin CTLL-20 transfectants were overexpressing gelsolin protein at the time of the experiment.
Effect of overexpression of gelsolin on apoptosis of CTLL-20 cells induced by C2-ceramide.
Overexpression of gelsolin did not prevent apoptosis of CTLL-20 cells induced by C2-ceramide. Transfected CTLL-20 cells overexpressing gelsolin (solid bars) or vector alone (hatched bars) were incubated for 12.5 hours in the presence of 50 U/mL recombinant human IL-2 and in the presence of 100 μmol/L C2-ceramide (closed or dark gray bars), 50 μmol/L C2-ceramide (gray bars), or its vehicle, 0.05% Me2SO (open bars). The percentage of apoptosis was determined by Annexin V-FITC staining as described in “Materials and methods.” Comparable results were obtained by trypan blue exclusion (data not shown). These results are representative of 3 independent experiments. Immunoblotting for gelsolin (inset) demonstrated that the gelsolin CTLL-20 transfectants were overexpressing gelsolin protein at the time of the experiment.
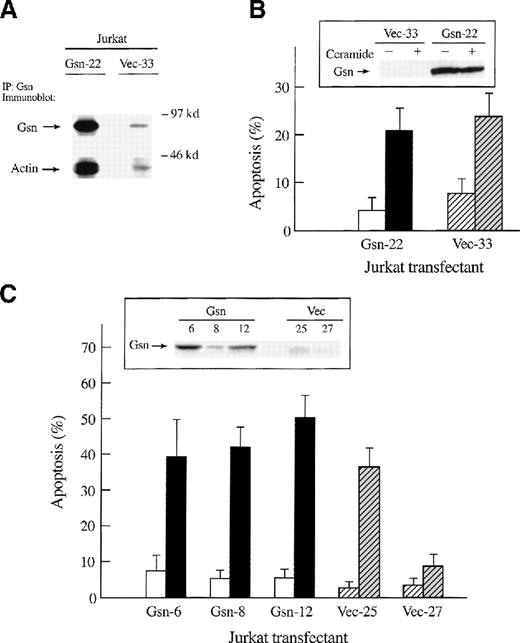
Our results were in striking contrast to the results reported for Jurkat cells.6 Therefore, by electroporation and selection in G418, stable transfectants of Jurkat T cells that overexpressed human gelsolin were generated. Expression of human gelsolin driven by the heterologous promoter was higher than that of endogenous protein (Figure 3A, 3B, 3C [insets]). As expected, endogenous gelsolin co-immunoprecipitated with actin; the amount of actin was dramatically increased in immunoprecipitates of Jurkat cells transfected with and overexpressing gelsolin (Figure 3A).
Effect of overexpression of gelsolin on apoptosis of Jurkat T cells in response to ceramide treatment or to Fas ligation.
Overexpression of gelsolin did not prevent apoptosis of Jurkat T cells in response to ceramide treatment or to Fas ligation, although gelsolin was cleaved during apoptosis. (A) Both endogenous and overexpressed gelsolin bound actin. Human gelsolin was immunoprecipitated from lysates of transfected Jurkat cells (2 × 107cells/sample). Precipitated proteins were separated by 10% SDS-PAGE on a 10% gel, transferred to PVDF membrane, and immunoblotted for gelsolin and actin as described. These results are representative of 2 independent experiments. (B) Transfected Jurkat T cells (solid bars represent those that overexpress gelsolin, hatched bars represent the vector control) were incubated with 50 μmol/L ceramide (closed or gray bar) or its vehicle, 0.025% Me2SO (open bars), for 12 hours. The percentage of apoptosis was determined by trypan blue exclusion. Level of expression of gelsolin during this experiment was determined by immunoblot (inset). (C) Transfected Jurkat cells (solid bars represent those that overexpress gelsolin, hatched bars represent the vector control) were incubated for 4 hours with anti-Fas mAb (1:500 dilution of 7C11) (closed or gray bars) or with medium alone (open bars), fixed, and stained with propidium iodide for quantification of apoptosis by nuclear morphology. Immunoblot confirmed the overexpression of gelsolin at the time of the experiment (inset). Results are representative of 4 independent experiments.
Effect of overexpression of gelsolin on apoptosis of Jurkat T cells in response to ceramide treatment or to Fas ligation.
Overexpression of gelsolin did not prevent apoptosis of Jurkat T cells in response to ceramide treatment or to Fas ligation, although gelsolin was cleaved during apoptosis. (A) Both endogenous and overexpressed gelsolin bound actin. Human gelsolin was immunoprecipitated from lysates of transfected Jurkat cells (2 × 107cells/sample). Precipitated proteins were separated by 10% SDS-PAGE on a 10% gel, transferred to PVDF membrane, and immunoblotted for gelsolin and actin as described. These results are representative of 2 independent experiments. (B) Transfected Jurkat T cells (solid bars represent those that overexpress gelsolin, hatched bars represent the vector control) were incubated with 50 μmol/L ceramide (closed or gray bar) or its vehicle, 0.025% Me2SO (open bars), for 12 hours. The percentage of apoptosis was determined by trypan blue exclusion. Level of expression of gelsolin during this experiment was determined by immunoblot (inset). (C) Transfected Jurkat cells (solid bars represent those that overexpress gelsolin, hatched bars represent the vector control) were incubated for 4 hours with anti-Fas mAb (1:500 dilution of 7C11) (closed or gray bars) or with medium alone (open bars), fixed, and stained with propidium iodide for quantification of apoptosis by nuclear morphology. Immunoblot confirmed the overexpression of gelsolin at the time of the experiment (inset). Results are representative of 4 independent experiments.
Transfected Jurkat cell lines were treated with 50 μmol/L C2-ceramide or vehicle control (0.025% Me2SO) for 12 hours, and cell death was assayed by trypan blue exclusion. Overexpression of gelsolin did not inhibit ceramide-induced apoptosis in Jurkat T cells (Figure 3B). Transfected Jurkat T-cell lines were treated with anti-Fas mAb and assayed for apoptosis by nuclear morphology (Figure 3C), trypan blue exclusion, and Annexin V-FITC staining (data not shown). Overexpression of gelsolin in Jurkat T cells did not inhibit Fas-dependent apoptosis, as measured by 3 different methods, again suggesting that gelsolin is not rate-limiting in the progression of apoptosis.
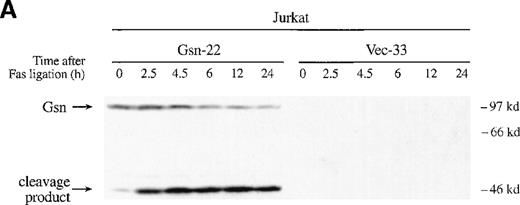
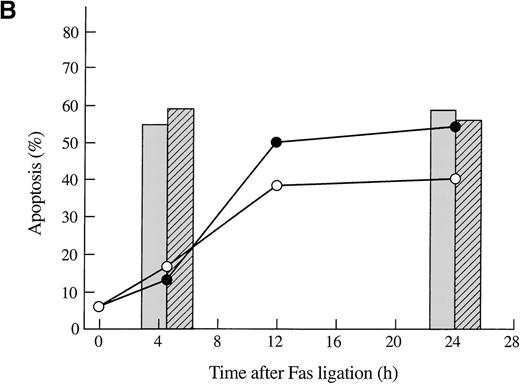
To confirm that the exogenous gelsolin was cleaved during apoptosis, we conducted a time-course experiment in which transfected Jurkat T cells were incubated with anti-Fas mAb for the indicated amount of time and then assayed for both apoptosis and cleavage of gelsolin. Exogenous gelsolin was cleaved during the apoptotic process (Figure 4A), but the rate and extent of cell death were not decreased by the overexpression of this caspase-3 substrate (Figure 4B). A slight increase in cell death of the Jurkat T-cell line that overexpressed gelsolin was evident by trypan blue exclusion, a finding that would be consistent with an acceleration of the effector phase of apoptosis mediated by an increase in the levels of the amino-terminal gelsolin fragment. However, this increase was not measurable by Annexin-V FITC staining, and no consistent, significant increase was seen in other experiments (Figure3C and data not shown).
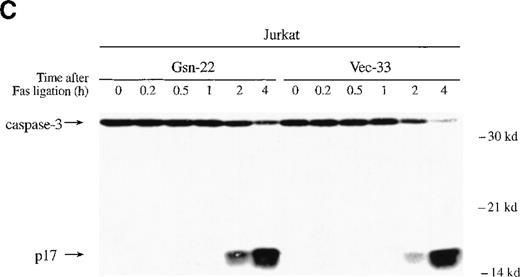
Gelsolin and caspase-3 were cleaved during apoptosis induced by Fas ligation.
(A) Transfected Jurkat cells were incubated for the indicated period of time with 100 ng/mL CH11 anti-Fas mAb. Immunoblot analysis of whole-cell lysates (1 × 106 cell equivalents) demonstrated overexpression of gelsolin and its cleavage during apoptosis. The gelsolin cleavage product is evident at approximately 46 kd. Results are representative of 4 independent experiments. (B) Parallel samples of transfected Jurkat cells incubated with 100 ng/mL CH11 anti-Fas mAb were assayed for the percentage of cell deaths by trypan blue exclusion (closed circles, cells overexpressing gelsolin, JK-Gsn-22; open circles, vector controls, JK-Vec-33). The percentage of cell deaths of parallel samples was confirmed by staining with Annexin V-FITC (solid bars, JK-Gsn-22; hatched bars, JK-Vec-33). (C) Transfected Jurkat cells were treated with anti-Fas mAb 100 ng/mL CH11 for the indicated periods of time. Immunoblot analysis of postnuclear lysates (1 × 106 cell equivalents) demonstrated cleavage of caspase-3; one of the fragments of caspase-3 generated during apoptosis is evident at approximately 17 kd. Results are representative of 3 independent experiments.
Gelsolin and caspase-3 were cleaved during apoptosis induced by Fas ligation.
(A) Transfected Jurkat cells were incubated for the indicated period of time with 100 ng/mL CH11 anti-Fas mAb. Immunoblot analysis of whole-cell lysates (1 × 106 cell equivalents) demonstrated overexpression of gelsolin and its cleavage during apoptosis. The gelsolin cleavage product is evident at approximately 46 kd. Results are representative of 4 independent experiments. (B) Parallel samples of transfected Jurkat cells incubated with 100 ng/mL CH11 anti-Fas mAb were assayed for the percentage of cell deaths by trypan blue exclusion (closed circles, cells overexpressing gelsolin, JK-Gsn-22; open circles, vector controls, JK-Vec-33). The percentage of cell deaths of parallel samples was confirmed by staining with Annexin V-FITC (solid bars, JK-Gsn-22; hatched bars, JK-Vec-33). (C) Transfected Jurkat cells were treated with anti-Fas mAb 100 ng/mL CH11 for the indicated periods of time. Immunoblot analysis of postnuclear lysates (1 × 106 cell equivalents) demonstrated cleavage of caspase-3; one of the fragments of caspase-3 generated during apoptosis is evident at approximately 17 kd. Results are representative of 3 independent experiments.
Overexpression of gelsolin had been reported to prevent the cleavage of caspase-3 in Jurkat T cells during Fas-induced apoptosis.6,7 In contrast to these results,6 7no delay in caspase-3 cleavage was evident in the Jurkat T cells that overexpressed gelsolin used here (Figure 4C). The cleavage product of caspase-3 (p17) appeared 2 hours after Fas ligation in both gelsolin and control Jurkat transfectants. Near-complete cleavage of caspase-3 was not apparent until 4 hours after Fas ligation (Figure 4C). Cleavage of both the endogenous and exogenous gelsolin occurred concurrently with caspase-3 cleavage, as did the appearance of dead cells assayed by trypan blue exclusion (data not shown).
Discussion
We have shown that overexpression of gelsolin in 3 different lymphocyte cell lines (CTLL-20, Ba/F3, and Jurkat) did not prevent or delay cell death in response to cytokine deprivation, ceramide treatment, or Fas ligation. A slight increase in death in 2 experiments (Figures 1E and 4B) could reflect an acceleration of the apoptotic “effector” phase by the increased levels of the amino-terminal fragment of gelsolin, which would be suggested by the results of Kothakota et al.2 However, as this increase was not seen in all experiments or by all methods of assaying cell death, we are unable to conclude that this is a consistent and biologically relevant result. Our results differ from those of Ohtsu et al,6 who reported that overexpression of gelsolin in Jurkat T cells delayed cell death in response to treatment with ceramide, dexamethasone, and anti-Fas antibody. Also in contrast to previous reports, we demonstrated that overexpression of gelsolin did not affect the rate of activation of caspase-3 in Jurkat T cells induced to undergo apoptosis by Fas ligation.6,7 Furthermore, overexpression of gelsolin did not modify the jasplakinolide-mediated enhancement of apoptosis of CTLL-20 cells induced by cytokine deprivation, suggesting that the enhancement of apoptosis by actin stabilization previously described8 was not affected by an increase in the molar content or actin-severing activity of gelsolin.
The observations of Ohtsu et al6 and Kamada et al7 regarding the ability of gelsolin to inhibit Fas-induced death may have differed from our observations because of differences in the pathways of Fas signaling in the 2 different populations of Jurkat cells under study. Scaffidi and coworkers11 recently reported that the Fas apoptotic pathway can proceed via 2 distinct pathways; in a given cell type, 1 of these 2 pathways is predominant. Cells designated type I were shown to cleave caspase-8 within 10 minutes of Fas ligation, while caspase-3 cleavage and subsequent activation were detected within 30 minutes. In these cells, caspase-3 was activated independently of mitochondrial permeability changes, and cell death was not inhibited by overexpression of the anti-apoptotic proteins Bcl-2 or Bcl-xL. In cells designated type II, however, caspase-8 cleavage was reported to appear only after 1 hour of anti-Fas stimulation, and caspase-3 cleavage appeared weakly after 2 hours. Activation of caspase-3 was dependent upon mitochondrial changes in type II cells, and apoptosis was inhibited by Bcl-2 or Bcl-xL. Caspase-8 overexpression converted a type II cell line into a type I.11 Kamada and coworkers7demonstrated that gelsolin overexpression inhibited cytochrome c release upstream of caspase activation, suggesting that their cells displayed a type II phenotype. Our Jurkat T cells also displayed type II kinetics of caspase-3 activation (Figure 4C), as the cleavage product of caspase-3 (p17) appeared after 2 hours of incubation with an anti-Fas antibody, similar to those seen by Kamada et al.7We do note that the kinetics of caspase-3 activation in their Jurkat T cells were different from those seen here: in their vector-transfected cell lines, caspase-3 cleavage was apparent within 1 hour after Fas ligation and appeared to be largely complete after 2 hours.7 The different rates of caspase-3 cleavage in these 2 populations of Jurkat T cells could be due to the higher concentrations of anti-Fas mAb used by Kamada et al7 (1 μg/mL in Kamada et al; 0.1 μg/mL here), or to clonal variation in the 2 Jurkat cell populations studied.
It is important to note that the demonstration of inhibition of apoptotic death by a particular protein may depend upon the method used to assay cell death. Following Fas ligation, caspase-3 is required for blebbing, DNA degradation, and nuclear condensation but not for the phosphatidylserine “flip” or for loss of membrane integrity as measured by uptake of propidium iodide.12 Thus, an inhibitor of caspase-3 activation, as gelsolin was proposed to be,6 would appear to inhibit cell death if cell death were assayed by membrane blebbing, but not if death were assayed by Annexin V staining. We used methods similar to those of Ohtsu et al6 to detect apoptosis; both methods were sensitive to early morphologic changes. It remains possible that the discrepancy between previously published results and those presented here may be due to different levels of (basal or transfected) expression of gelsolin, or that the gelsolin overexpressed in Jurkat T cells by other investigators was not cleaved during Fas-induced apoptosis. In their system, it is possible that uncleaved, full-length gelsolin was able to compete for substrates of the cleaved gelsolin that are required for progression of apoptosis, thus acting as a “dominant interfering” molecule. This competition could theoretically inhibit apoptosis. The discrepancy could also be due to other, as yet undefined, differences in apoptotic signaling following Fas ligation. That the regulation of induction and progression of apoptosis is complex has been shown in many systems, including Fas-induced death of Jurkat T lymphocytes.11 13-15
Different roles for gelsolin have previously been attributed to differences in the cellular environment and the apoptotic stimulus. Its overexpression has been reported to prevent Fas- and ceramide-induced death in Jurkat T cells,6 and its absence has been reported to protect neutrophils from apoptosis induced by TNFα.2Additionally, the absence of gelsolin sensitized hippocampal neurons to apoptosis induced by glutamate.16 We have confirmed that gelsolin is cleaved during lymphocyte apoptosis, but we failed to show that the quantitative increase in the generation of the active amino-terminal fragment of gelsolin dramatically increased the rate of the appearance of nuclear condensation or of cell death in lymphocytes as might have been predicted from the results of Kothakota et al.2 There did appear to be a variable, time-dependent increase in cell death following cytokine deprivation (Figure 1E) and after Fas ligation (Figure 4B), but this increase was not consistent at all time points (Figure 1E, 4-hour time point) or in all experiments (data not shown). In these lymphocyte cell lines, therefore, gelsolin and the generation of the amino-terminal fragment of gelsolin did not seem to be a significant or rate-limiting factor for apoptotic cell death.
S.C.P. was supported by a pre-Intramural Research Training Award from the National Heart, Lung and Blood Institute of the National Institutes of Health, Bethesda, MD. M.P.M. was supported by a fellowship from Fondazione, “Istituto Pasteur—Fondazione Cenci-Bolognetti,” University of Rome “La Sapienza,” Italy, and by a supplemental fellowship from the National Institutes of Health, Bethesda, MD. D.J.K. was supported by grant HL54188 from the National Institutes of Health, Bethesda, MD.
Reprints:Barbara E. Bierer, National Heart, Lung and Blood Institute, Bldg 10, Rm 5D49, 10 Center Dr, Bethesda, MD 20892; e-mail:biererb@nih.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal