Abstract
In a previous study, it was found that a truncated erythropoietin receptor transgene (tEpoR tg) enables multilineage hematopoietic progenitor amplification after treatment with erythropoietin (epo) in vitro and in vivo. This study used competitive bone marrow (BM) repopulation to show that tEpoR tg facilitates transplantation by hematopoietic stem cells (HSC). Individual multilineage colonies, committed myeloid progenitor colonies, and lymphoid colonies (pre-B colony-forming units) were grown from the marrow of animals 6 months after they received a 50/50 mixture of transgene and wild-type BM cells. In epo-treated recipients, the transgene-bearing cells significantly outcompeted the wild-type cells (84%-100% versus 16%-0%, respectively). In recipients treated with phosphate-buffered saline, the repopulation was minimally different from the donor mixture (49%-64% transgene versus 51%-36% wild-type). The epo-induced repopulation advantage is maintained in secondary transplants. In addition, neither accelerated HSC depletion nor uncontrollable proliferation occurred during epo-stimulated serial transplants of transgene-containing BM. Thus, the tEpoR tg functions in a benign fashion in HSC and allows for a significant and controllable repopulation advantage in vivo without excessive HSC depletion relative to wild-type BM.
Bone marrow transplantation (BMT) is often limited by low donor availability and the toxicity of the procedure. One approach to partially ameliorate these limitations is by controlling the survival or proliferation of hematopoietic stem cells (HSC). This has been attempted by a number of investigators, both in vivo with cells containing transgenes allowing for a selection advantage1-4and in vitro through cytokine-mediated expansion.5-9 The former approach has to date been implemented by selecting progenitors or HSC resistant to toxic substances; consequently, it does not enhance proliferation and therefore would not help with low numbers of HSC, for example, with umbilical cord blood transplants given to adult patients. A problem with the latter approach has been the loss of long-term repopulating ability with the induced expansion, thought to be due to HSC exhaustion/depletion.10-12 Our efforts have centered on using a benign transgene as a means of giving donor bone marrow (BM) cells a controllable transplantation advantage over untreated endogenous BM cells. Toward this end, we previously generated mice expressing a truncated erythropoietin receptor transgene (tEpoR tg).13 We now use BM from these animals in competitive transplants to test whether short-term treatment with erythropoietin (epo) at the time of transplant can give the transgene-containing HSC an advantage over wild-type HSC in repopulating lethally irradiated animals. The results of the competitive transplantation show that this does occur, that the effect is sustainable in secondary transplants and in older animals, and that in repeated epo-stimulated serial transplants, the repopulating ability of the HSC is not significantly exhausted by the presence of the tEpoR tg.
Materials and methods
Animals
Transgenic mice expressing tEpoR were generated as previously described.13 These animals contain a functional hypoxanthine phosphoribosyltransferase(HPRT) transgene and 2 copies of a tEpoR tg driven by a human β-actin promoter inserted into the X-linked HPRT locus. All animals were maintained in microisolator cages with autoclaved food. Male transgenic animals used for repopulation experiments had been back-crossed more than 6 times to C57BL/6 inbred animals. Competitor animals were inbred C57BL/6 males that carry, as a distinguishing marker, an inactivating deletion of the HPRT locus, which does not affect their hematopoiesis. Female inbred C57BL/6 animals of at least 8 weeks of age were used as recipients. For serial transplantation experiments, female B6.SJL-PtprcaPep3b/ByJ (Ly5.1) mice (Jackson Laboratory, Bar Harbor, ME), which express the Ly 5.1 antigen on their hematopoietic cells, were used as recipients; the corresponding wild-type and transgene C57BL/6 donors express the Ly5.2 antigen.
Competitive repopulation assays
Lethally irradiated mice (9.5 Gy total body irradiation from a137Cs source) were anesthetized with tribromoethanol (Avertin) before receiving by retro-orbital injection a 50/50 mixture of BM cells from the donor transgene and the wild-type competitor (2 × 106 cells total). Starting on the day of transplant, the recipient animals were treated by intraperitoneal injection for 2 weeks with either epo (10 U/mouse, about 400 U/kg, given 3 times per week) or a similar volume (0.1 mL) of sterile phosphate-buffered saline (PBS). The transplanted animals were maintained in microisolator cages with autoclaved food and acidified sterile water supplemented with neomycin sulfate for the first 2 weeks after transplant. In one experiment, wild-type littermates expressing a functional HPRT gene were used as the competitor with no significant repopulation difference noted between this experiment and those when the competitor lacks a functional HPRT gene. Secondary transplants were performed in a similar manner except with the donor BM coming from animals having previously received a 50/50 mixture of wild-type and transgene BM and PBS treatment at the time of the original transplant. Secondarily transplanted animals were treated with the same dose and schedule of either epo or PBS as the primary recipients.
Clonogenic assays
Anesthetized animals that were 6 months or more after transplant were killed and BM was harvested from their femora into RPMI media containing 10% fetal calf serum (FCS) and penicillin/streptomycin. Clonogenic assays for committed myeloid progenitor colonies (CFC), colony-forming units–granylocyte, erythrocyte, macrophage, megakaryocyte (CFU-GEMM), and pre-B colony-forming units (pre-B CFU) were established in methylcellulose media (Media 3434 and 3630; Stem Cell Technologies, Vancouver, BC, Canada) as previously described.13 Pre-B colonies and CFC were counted at day 7. CFU-GEMM colonies were counted at day 14. Individual colonies were picked in a sterile fashion with a glass micropipette for analysis by polymerase chain reaction (PCR).
Hematologic analysis
Peripheral blood was collected into EDTA by retro-orbital puncture after anesthesia. Complete blood counts and differential counts were performed on a Roche Hematology Analyzer equipped with software for murine blood analyses.
PCR analyses
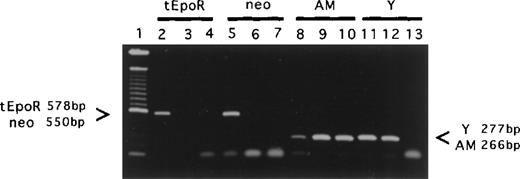
Individual colonies picked from cultures were washed with PBS to remove excess methylcellulose, centrifuged, and then resuspended in 50 μL of sterile water in Eppendorf tubes. Samples were heated to 95°C for 10 minutes and cooled briefly on ice. Proteinase K (PK; 40 μg/sample) was added and digestion was carried out at 55°C for 4 hours. PK was inactivated by heating again at 95°C for 10 minutes. Two microliters of the digested samples were used for PCR analysis. PCR reactions were carried out in a Perkin-Elmer 9700 PCR machine. Amplification was performed (after an initial 1 minute at 95°C) for 35 cycles of 1 minute at 95°C, 1 minute at the indicated annealing temperature, and 2 minutes at 72°C. Annealing temperatures were 68°C for the Y chromosome marker (to identify male donor-origin colonies), 66°C for the adrenomedullin(am) gene (a genotype-independent positive control), 63°C for tEpoR tg (to identify tEpoR donor-origin colonies), and 64°C for the Neo gene (to confirm tEpoR donor-origin colonies). Primers for the Y chromosome 5′ AGACAAGTTTTGGACTGGTGAC3′ and 5′AGCCCTCCGATGAGGCTGATA3′ amplify a 277 bp product;am primers 5′CCCACGACTTAGCGCCCACTTATT-CCAC3′ and 5′ATGAAGCTGGTTTCCATCGCC3′ amplify a 266 bp product; tEpoR primers 5′CCTCTGTCTCCTACTTGCTGG3′ and 5′CTCAGAACACACTCAGTG-CGG3′ amplify a 578 bp product; Neo primers 5′GTGTTCCGGCTGTCAGCGCA3′ and 5′GTCCTGATAGCGGTCCGCCA3′ amplify a 550 bp product. PCR reaction products were separated on 1.0% agarose gels and visualized after ethidium bromide staining.
Serial transplantation assays
The Ly5.1 primary recipient animals were lethally irradiated (9.5-Gy total body irradiation) and transplanted with either 2 or 4 × 106 BM cells from either wild-type Ly5.2 or transgene Ly5.2 donors. After 6 to 8 weeks of engraftment, BM was harvested from the femora of these recipients and analyzed by flow cytometry. BM from some of the recipient animals was transplanted into secondary, lethally irradiated Ly5.1 recipients. Tertiary transplants were performed in a similar fashion using BM harvested after 6 to 8 weeks of engraftment in the secondary recipients. The primary, secondary, and tertiary serial transplant recipients all received epo in the standard 2-week protocol.
Flow cytometry analysis
Bone marrow was harvested from the femora and tibiae of the serially transplanted recipients (primary, secondary, tertiary). After lysis of red blood cells with ACK solution, nucleated cells were stained with the following fluorochrome-labeled monoclonal antibody combinations: anti-Ly5.2-fluorescein isothiocyanate (FITC) and anti-CD3-phycoerythrin (PE) (Pharmingen). Cells were stained for 30 minutes at 4°C (1 μg antibody/106 cells) and then washed 3 times before analyses (FACScan; Becton Dickinson Biosciences, San Jose, CA) with Cyclops software (Cytomation, Ft Collins, CO).
Statistical analysis
Two-tailed Student t tests were performed for comparisons of complete blood counts and clonogenic cell contents of posttransplant BM from epo-treated compared to PBS-treated transplanted animals and for comparison of percentage donor engraftment between wild-type and transgene donors in the serial transplantation experiments. The ztest was used to compare the observed versus input proportions of transgene repopulation in the primary transplant recipients. Sigmastat software (for Windows 98) was used for statistical calculations.
Results
Competitive repopulation assay
Mice expressing tEpoR tg were created as previously described.13 BM was harvested from male tEpoR tg-bearing and wild-type mice, and mixtures of BM cells were prepared to contain 50% tEpoR tg cells and 50% wild-type competitor cells. This mixture of cells (2 × 106 nucleated cells/animal) was retro-orbitally injected into lethally irradiated female recipient mice. Starting at the time of transplant, the recipient animals were treated with injections of either PBS or epo (10 U/mouse) 3 times per week for 2 weeks. No further injections were given after the initial 2 weeks, although endogenous epo is naturally present. Animals were then maintained for up to 12 months after transplant, with most analyses being performed at 6 to 8 months. After the chosen time, BM was harvested from the femora of transplanted animals for clonal assays to assess the total clonogenic cell content and the source (transgene donor, wild-type donor, or recipient) of the clonogenic cells.
Hematologic analyses after competitive repopulation
To determine that adequate BM engraftment had occurred, peripheral blood and marrow specimens from the primary 50/50 transplant recipients were examined histologically and complete blood counts were performed with a Roche hematology analyzer. At the time of BM harvest (6 months after transplant), there were no significant differences in the white blood cell count, platelet counts, hemoglobin concentrations, or leukocyte differential counts between untreated mice and the primary BM recipients treated with either epo or with PBS (data not shown). Thus functional engraftment following the 50/50 transplant was complete.
Recovery of clonogenic cells after competitive repopulation
To look for changes in the recipient BMs in the animals receiving the 50/50 mix and treated with epo or with PBS, we performed a series of clonogenic assays. The upper portion of Table1 presents the resulting data from animals 6 months after the primary 50/50 transplantation. There were significant modest increases (range, 131%-159%) in the total recovery of myeloid CFU-GEMM (P = .013) and CFC (P < .001) in the epo-treated compared to the PBS-treated primary transplanted animals. The epo treatment had no significant effect on the total recovery of lymphoid pre-B CFU. There were also modest increases (116%-139%) in all transgenic CFU with epo treatment after secondary transplant. There was no significant increase (data not shown) in the total nucleated cell content of the BM in response to the epo treatment (based on 4 bones harvested). Thus, the presence of the transgene in the donor cells caused a modest increase in recovery of clonogenic cells when the recipient was treated with exogenous epo, but the repopulated BM from untreated animals showed no indications of excessive proliferation of transgenic compared to wild-type clonogenic cells.
Bone marrow clonogenic cells recovered from competitively transplanted animals
| Treatment . | CFU-GEMM . | CFC . | Pre-B CFU . | |||
|---|---|---|---|---|---|---|
| PBS . | Epo . | PBS . | Epo . | PBS . | Epo . | |
| Primary recipients | 20.7 ± 1.8 | 32.9 ± 4.4 | 284 ± 10.2 | 373 ± 11.5 | 10.2 ± 1.2 | 9.4 ± .91 |
| (159% ± 21%) | (131% ± 4%) | (92% ± 9%) | ||||
| P = .013 | P < .001 | P = .189 | ||||
| Secondary recipients | 25.8 ± 4.8 | 35.8 ± 4.0 | 291 ± 13.7 | 337 ± 18.1 | 3.2 ± 0.28 | 4.1 ± 1.1 |
| (139% ± 16%) | (116% ± 6%) | (128% ± 34%) | ||||
| P = .125 | P = .056 | P = .665 | ||||
| Treatment . | CFU-GEMM . | CFC . | Pre-B CFU . | |||
|---|---|---|---|---|---|---|
| PBS . | Epo . | PBS . | Epo . | PBS . | Epo . | |
| Primary recipients | 20.7 ± 1.8 | 32.9 ± 4.4 | 284 ± 10.2 | 373 ± 11.5 | 10.2 ± 1.2 | 9.4 ± .91 |
| (159% ± 21%) | (131% ± 4%) | (92% ± 9%) | ||||
| P = .013 | P < .001 | P = .189 | ||||
| Secondary recipients | 25.8 ± 4.8 | 35.8 ± 4.0 | 291 ± 13.7 | 337 ± 18.1 | 3.2 ± 0.28 | 4.1 ± 1.1 |
| (139% ± 16%) | (116% ± 6%) | (128% ± 34%) | ||||
| P = .125 | P = .056 | P = .665 | ||||
Numbers represent mean SEM of CFU/106 cells plated. Percentages (in parentheses) represent the numbers with epo treatment relative to the numbers with PBS treatment. P values are for comparisons of the mean CFU between the epo-treated and PBS-treated recipients.
CFC indicates committed myeloid progenitor colonies; CFU, colony-forming units; CFU-GEMM, colony-forming units–granulocyte, erythrocyte, macrophage, megakaryocyte; Epo, erythropoietin; PBS, phosphate-buffered saline.
Epo-inducible competitive repopulation by the donor transgenic HSC
To determine the source of the clonogenic cells recovered from recipients 6 months or more after the primary 50/50 transplantation, we performed PCR analyses of individual colonies (Figure1). The upper portion of Table2 presents the percentages of clonogenic cells derived from the transgene-bearing donor HSC in the BM of the primary recipients after PBS or epo treatment at the time of transplant. No colonies of the recipient genotype were recovered (data not shown), indicating that the irradiation dose used pretransplantation was adequate to suppress endogenous hematopoiesis. The PBS-treated recipients of the primary 50/50 mix of transgene and wild-type BM cells showed repopulation of all lineages with transgene cells at close to the input 50% level (range 49%-64%). Of the clonogenic precursors analyzed in these primary recipients, only the proportion of transgene CFU-GEMM (64%) differed significantly (P = .04) from the expected 50% proportion. This suggests that a slight increase in the transgene-bearing donor population of progenitors occurred even in the absence of exogenous epo, probably under the influence of the increased endogenous epo levels present after irradiation.14-17 In the epo-treated recipients, in contrast, all categories of CFU were nearly completely repopulated with progeny from the tEpoR tg HSC (range 84%-100%; P versus PBS-treated ≤ .05 and versus the input 50/50 ratio < .001). Thus, the brief epo treatment at the time of transplantation gives the tEpoR tg HSC a considerable repopulation advantage over wild-type cells. The transgene donors in these experiments were back-crossed 6 to 9 times. No differences from these experiments have been seen in more recent experiments with animals back-crossed more than 10 times (data not shown).
PCR analyses of individual colonies.
DNA was processed from individually picked colonies (CFU-GEMM, CFC, and pre-B CFU) and analyzed by PCR for (1) quality of DNA preparation using the am (an autosomal gene) primers, (2) donor rather than recipient origin using Y chromosome-specific primers, and (3) transgene donor origin using both tEpoR and Neo primers. Shown is a representative sample of the PCR products from a male tEpoR tg donor (lanes 2, 5, 8, 11), male wild-type donor (lanes 3, 6, 9, 12), and female wild-type recipient (lanes 4, 7, 10, 13). Lane 1 contains Gibco 100-bp marker fragments. The primers used are indicated above the lane numbers. The bands corresponding to the primers are marked by arrows. Note, for example, the presence of the transgene-specific and Neo-specific bands in lanes 2 and 5, indicative of male-derived transgene-bearing colonies, and the absence of the Y-specific band in lane 13, indicative of a female-derived colony.
PCR analyses of individual colonies.
DNA was processed from individually picked colonies (CFU-GEMM, CFC, and pre-B CFU) and analyzed by PCR for (1) quality of DNA preparation using the am (an autosomal gene) primers, (2) donor rather than recipient origin using Y chromosome-specific primers, and (3) transgene donor origin using both tEpoR and Neo primers. Shown is a representative sample of the PCR products from a male tEpoR tg donor (lanes 2, 5, 8, 11), male wild-type donor (lanes 3, 6, 9, 12), and female wild-type recipient (lanes 4, 7, 10, 13). Lane 1 contains Gibco 100-bp marker fragments. The primers used are indicated above the lane numbers. The bands corresponding to the primers are marked by arrows. Note, for example, the presence of the transgene-specific and Neo-specific bands in lanes 2 and 5, indicative of male-derived transgene-bearing colonies, and the absence of the Y-specific band in lane 13, indicative of a female-derived colony.
Competitive repopulation in primary and secondary transplants
| Treatment . | CFU-GEMM . | CFC . | Pre-B CFU . | |||
|---|---|---|---|---|---|---|
| PBS . | Epo . | PBS . | Epo . | PBS . | Epo . | |
| Primary recipients | 64 ± 9.5% (119) | 100 ± 0% (89) | 49 ± 7.3% (72) | 100 ± 0% (109) | 52 ± 9.6% (204) | 84 ± 8.3% (153) |
| Secondary recipients | 71 ± 14% (28) | 96 ± 3.5% (25) | 70 ± 10% (35) | 86 ± 4.0% (20) | 71 ± 5.0% (24) | 86 ± 4.0% (20) |
| Treatment . | CFU-GEMM . | CFC . | Pre-B CFU . | |||
|---|---|---|---|---|---|---|
| PBS . | Epo . | PBS . | Epo . | PBS . | Epo . | |
| Primary recipients | 64 ± 9.5% (119) | 100 ± 0% (89) | 49 ± 7.3% (72) | 100 ± 0% (109) | 52 ± 9.6% (204) | 84 ± 8.3% (153) |
| Secondary recipients | 71 ± 14% (28) | 96 ± 3.5% (25) | 70 ± 10% (35) | 86 ± 4.0% (20) | 71 ± 5.0% (24) | 86 ± 4.0% (20) |
Percentages are the mean % ± SEM of transgene-bearing colonies detected in BM harvested from animals 6 months after primary and secondary transplants. Numbers in parentheses represent total colonies of each class analyzed. There were a total of 11 to 15 different animals for each group (PBS and epo-treated) in the primary transplants and 2 animals for each group in the secondary transplants. P ≤ .05 for each type of CFU when percentage of cells of transgene origin in epo-treated primary recipients is compared to the percentage in PBS-treated animals, and <.001 when compared to the input ratio (50/50). P values for percent transgene-bearing CFU in the secondary transplant epo-treated animals when compared to secondary PBS-treated animals were .15, .038, and .18 (GEMM, CFC, pre-B) and .018, .28, .061 when compared to the percent transgene-bearing CFU in the primary PBS-treated donor animals.
Abbreviations are as in Table 1.
The tEpoR tg can provide an advantage in secondary transplants
To determine whether the transgene-bearing HSC are still present after a primary transplant, we performed secondary transplants 6 months after the 50/50 initial transplant. The secondary recipients received 2 × 106 nucleated BM cells from primary recipients that had been treated with PBS. The secondary recipients were treated with PBS or with epo, and their BM was harvested and analyzed in the same way as in the primary transplanted animals. The total clonogenic CFU-GEMM and CFC recoveries in the secondarily transplanted recipients, PBS or epo-treated, were not significantly different from those recovered in the primary recipients (Table 1, lower part compared with upper part). There was a decrease in pre-B CFU in both PBS and epo-treated secondary recipients compared with the primary transplant animals. This was not unexpected because several studies have shown that lymphoid populations decrease with aging and after serial transplantation in mice.18-20
The source of the recovered clonogenic cells after the secondary transplant was determined in the same manner as was used in the primary transplant. The resulting data (Table 2, lower part) show that the proportion of all categories of transgene-bearing cells is now significantly greater than the input 50/50 mix (P < .001 versus 50/50) even in the PBS-treated recipients; this was again expected because the HSC have been twice exposed to increased endogenous epo. Nevertheless the secondary recipients still show a further increase in transgene-bearing cells of all categories when treated with exogenous epo. These data demonstrate that transgene-bearing HSC can be easily detected during a secondary transplant 6 months after the primary transplantation and that epo can enhance their effectiveness in repopulation.
The tEpoR tg does not accelerate HSC exhaustion
To determine whether epo stimulation of transgene-bearing HSC results in their exhaustion, we performed serial transplants of un-fractionated BM cells from wild-type or transgene donors carrying the Ly5.2 antigenic marker into recipients carrying the Ly5.1 marker, with the recipients receiving 2 weeks of epo supplementation at the time of each serial transplant. After 6 to 8 weeks of engraftment, BM was harvested from the femora of recipients and analyzed by flow cytometry to determine the percent donor versus recipient contribution to myeloid and lymphoid cells. Representative analyses are shown in Figure 2. The overall data are presented in Table 3 and show that the trangsene and wild-type donor BM are not significantly different in their engraftment capabilities during the successive transplants, although both the transgene and wild-type donors show some HSC loss with each serial transplant. We conclude that the transgene donor HSC following serial transplantation (accompanied by epo stimulation at each transplant) are not depleted relative to wild-type HSC. It is also notable that this repeated exposure to pharmacologic doses of epo did not lead to any uncontrollable expansion in the transgene donor BM cells as judged by the normality of their BM and peripheral blood cell counts. Nor were any quantitative abnormalities seen in the lymphoid populations (data not shown). Lastly, in some of these animals both peripheral blood and BM were harvested, with no significant differences seen in the relative repopulation of each compartment by either transgene or wild-type donors (data not shown). In addition, in more recent sublethal irradiation experiments BM, peripheral blood, and spleen hematopoietic cells were harvested simultaneously and analyzed by flow cytometry. The percentage repopulation with Ly5.2+ donor cells did not differ significantly in these compartments (unpublished data).
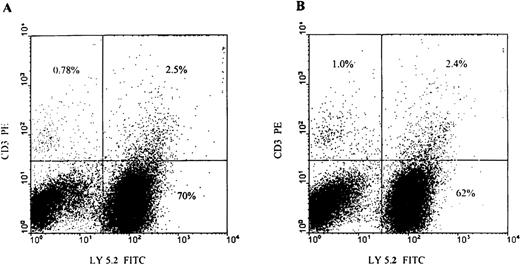
Flow cytometry analyses of BM cells after serial transplantation.
Lethally irradiated Ly5.1 primary recipient animals received either wild-type or transgene Ly5.2 donor cells (2-4 × 106unfractionated BM cells). After 6 to 8 weeks, BM was harvested from the recipient animals, stained with fluorochrome-labeled antibodies as shown, and analyzed by flow cytometry to determine their origin (donor or endogenous). Secondary recipients received BM from several of these primary recipient animals. Tertiary transplants were performed in a similar fashion after 6 to 8 weeks of engraftment in secondary recipients. All recipient animals received epo supplementation for a period of 2 weeks after BM transplant. Shown is a representative sample of the BM from wild-type (A) and transgene donor (B) transplanted secondary recipients analyzed by flow cytometry. Note the presence in the recipient animals of both donor (right upper quadrant) and recipient (left upper quadrant) T cells and donor and recipient myeloid/B cells (right lower quadrant and left lower quadrant, respectively) present in the recipient animals.
Flow cytometry analyses of BM cells after serial transplantation.
Lethally irradiated Ly5.1 primary recipient animals received either wild-type or transgene Ly5.2 donor cells (2-4 × 106unfractionated BM cells). After 6 to 8 weeks, BM was harvested from the recipient animals, stained with fluorochrome-labeled antibodies as shown, and analyzed by flow cytometry to determine their origin (donor or endogenous). Secondary recipients received BM from several of these primary recipient animals. Tertiary transplants were performed in a similar fashion after 6 to 8 weeks of engraftment in secondary recipients. All recipient animals received epo supplementation for a period of 2 weeks after BM transplant. Shown is a representative sample of the BM from wild-type (A) and transgene donor (B) transplanted secondary recipients analyzed by flow cytometry. Note the presence in the recipient animals of both donor (right upper quadrant) and recipient (left upper quadrant) T cells and donor and recipient myeloid/B cells (right lower quadrant and left lower quadrant, respectively) present in the recipient animals.
Percent donor composition of BM after serial transplantation
| Genotype . | Primary . | Secondary . | Tertiary . |
|---|---|---|---|
| Wild-type | 94.0 ± 0.7 | 87.4 ± 7.2 | 59.3 ± 13.4 |
| Transgene | 92.2 ± 0.6 | 75.9 ± 8.5 | 63.5 ± 14.8 |
| P value | (.60) | (.34) | (.84) |
| Genotype . | Primary . | Secondary . | Tertiary . |
|---|---|---|---|
| Wild-type | 94.0 ± 0.7 | 87.4 ± 7.2 | 59.3 ± 13.4 |
| Transgene | 92.2 ± 0.6 | 75.9 ± 8.5 | 63.5 ± 14.8 |
| P value | (.60) | (.34) | (.84) |
Mean (±SEM) percent Ly5.2 cells recovered, normalized to equal numbers of donor cells transferred. Numbers of animals of each genotype per group were 5-7. P values are for differences between wild-type and transgene donor engraftment, as represented by percent Ly5.2 cells.
Transgene-bearing HSC function is maintained with aging
Any potential agent for enhancing BM transplantation should not lead to any undesirable loss of BM function or to malignant transformation later in life. To test for these possibilities we examined untreated, nontransplanted wild-type and transgene animals at 19 to 22 months of age. Table 4 shows the recovery of clonogenic cells from these older animals. There was a slight deficit in transgene-bearing CFU-GEMM (21%; P = .40) and CFC (28%;P = .012) and a slight excess in transgene-bearing pre-B CFU (119%; P = .30), but none of these changes are sufficient to signal biologically significant expansion or exhaustion of clonogenic precursors. Hematologic analyses of the primarily transplanted animals (both PBS and epo treated) performed 1 year after their initial transplant showed no differences in peripheral blood counts and differential counts compared to the counts at 6 months (data not shown), indicating that the donor cells were neither increased nor lost over time after the initial expansion. Furthermore, no signs of any myeloproliferative disorders, leukemia, or lymphoma have developed in any of our animals with the tEpoR tg even when (data not shown) treated with epo for as long as 8 weeks. We conclude from these tests that the EpoR tg is completely benign within the limits of the current experiments.
CFU from aged animals
| Genotype . | CFU-GEMM . | CFC . | Pre-B CFU . |
|---|---|---|---|
| Wild-type | 85.0 ± 10.6 | 268 ± 9.1 | 17.1 ± 8.5 |
| Transgene | 67.3 ± 12.0 | 193 ± 16.7 | 20.3 ± 2.2 |
| P value | (.40) | (.012) | (.030) |
| Genotype . | CFU-GEMM . | CFC . | Pre-B CFU . |
|---|---|---|---|
| Wild-type | 85.0 ± 10.6 | 268 ± 9.1 | 17.1 ± 8.5 |
| Transgene | 67.3 ± 12.0 | 193 ± 16.7 | 20.3 ± 2.2 |
| P value | (.40) | (.012) | (.030) |
Mean (±SEM) CFU/106 cells plated are shown for 3-6 male animals of each genotype. Average age of animals was 20 months.P values are for differences between wild-type and transgene CFU.
Abbreviations are as in Table 1.
Discussion
Bone marrow transplantation is a powerful therapeutic modality, which could be more widely applicable if the procedure were less toxic, if HSC could be expanded in vitro or in vivo, or if the HSC could be given a repopulation advantage over endogenous cells during transplantation. Expansion of HSC in vitro has been attempted by improving the environment for culturing the BM cells, by using new types of stroma,21-25 or by adding exogenous growth factors.5-9 Although expansion of progenitor cells has been fairly easily obtained in this manner, there has not yet been proven expansion of long-term repopulating cells. Indeed, there have been several reports of the loss of long-term repopulating function during attempts to achieve in vitro expansion.10-12 These difficulties motivated our interest in generating an in vivo repopulation advantage. Generating a selection advantage for transplanted cells has been successful by transduction with genes conferring resistance to chemotherapeutic agents,1-4 but the selective agents used are themselves toxic. Our aim, therefore, has been to develop a suitable transgene that will enable a benign repopulation advantage to be conferred on HSC during transplantation without leading to their subsequent depletion or aberrant proliferation. We have chosen to test this concept by using a tEpoR tg targeted to a known locus so that in the future we can easily compare other transgenes. We previously showed that the tEpoR tg enables expansion of multilineage clonogenic progenitors under the stimulation of exogenous epo, both in vivo and in vitro. We also showed that even after 5 months of exposure to epo in vitro, the cultured BM cells from the transgene animals never transformed into malignant progeny or became factor independent.
This present study demonstrated that this transgene is effective in long-term repopulating HSC by showing that BM containing the transgene outcompetes wild-type BM cells during the repopulation of lethally irradiated recipients. We have further demonstrated that this advantage can be controlled during the transplantation by the administration of exogenous epo. Thus, a very short-term exposure to doses of epo in the therapeutic range was sufficient to produce considerably enhanced repopulation by transgene HSC relative to coinjected wild-type HSC. The recapitulation of this effect in secondary transplants, along with the maintenance of normal hematopoiesis in aged transgene animals (transplanted and nontransplanted), further showed that the presence of the transgene did not adversely affect overall HSC function several months after both primary and secondary transplants. In addition, serial transplants using repeated stimulation with pharmacologic doses of epo led neither to accelerated HSC exhaustion nor to uncontrollable proliferation from the transgene compared to wild-type donors. Furthermore, none of the transplanted animals, nor any of the untreated or epo-treated transgene animals, developed any malignant process or abnormal proliferation and differentiation of any hematopoietic lineage. The mechanisms of the tEpoR-induced repopulation advantage via HSC expansion, prevention of apoptosis, and interactions with facilitating cells remain to be determined.
Much needs to be done to optimize the constructs based on tEpoR and to determine whether the effect can be obtained (and remain benign) when the transgene is introduced into the genome at other sites than the current one, or by other means of gene transfer. For example, it is feasible that with the current system the transduced HSC or their progeny (or both) could abnormally proliferate in special clinical settings with prolonged exogenous epo supplementation, such as that seen with renal failure. There have been a number of recent reports of EpoR tgs introduced by retroviral vectors affecting multipotent or committed progenitors, but none of the transfected cells has been shown to yield any advantage during long-term repopulation.26-30The work we have presented here proves that a cytokine receptor transgene can confer on BM cells, including HSC, a benign and controllable advantage over unmodified cells during transplantation.
Acknowledgments
We thank Chad Cecil for technical assistance in this work and Jon Serody, Chris Walsh, and Beverly Mitchell for valuable comments regarding our manuscript.
Supported in part by National Institutes of Health grant number HL37001 (O.S.) S.K. was supported by the Leukemia Society of America Special Fellow Award and the Burroughs-Wellcome Fund Career Development Award.
Reprints:Suzanne Kirby, Division of Hematology/Oncology, CB #7295, LCCC, University of North Carolina, Chapel Hill, NC 27599-7295; e-mail: skirb@med.unc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal