Abstract
Lymphopenia and immune deficiency are significant problems following allogeneic hematopoietic cell transplantation (HCT). It is largely assumed that delayed immune reconstruction is due to a profound decrease in thymus-dependent lymphopoiesis, especially in older patients, but apoptosis is also known to play a significant role in lymphocyte homeostasis. Peripheral T cells from patients who received HCT were studied for evidence of increased cell death. Spontaneous apoptosis was measured in CD3+ T cells following a 24-hour incubation using 7-amino-actinomycin D in conjunction with the dual staining of cell surface antigens. Apoptosis was significantly greater among CD3+ T cells taken from patients 19-23 days after transplantation (30.4% ± 12.5%,P < .05), and 1 year after transplantation (9.7% ± 2.8%, P < .05) compared with healthy controls (4.0% ± 1.5%). Increased apoptosis occurred preferentially in HLA (human leukocyte antigen)-DR positive cells and in both CD3+/CD4+ and CD3+/CD8+ T-cell subsets, while CD56+/CD3− natural killer cells were relatively resistant to apoptosis. The extent of CD4+T-cell apoptosis was greater in patients with grade II-IV acute graft-versus-host disease (GVHD) (33.9% ± 11.3%) compared with grade 0-I GVHD (14.6 ± 6.5%, P < .05). T-cell apoptosis was also greater in patients who received transplantations from HLA-mismatched donors (39.5% ± 10.4%,P < .05) or HLA-matched unrelated donors (32.1% ± 11.4%, P < .05) compared with patients who received transplantations from HLA-identical siblings (19.6% ± 6.7%). The intensity of apoptosis among CD4+ T cells was significantly correlated with a lower CD4+ T-cell count. Together, these observations suggest that activation of T cells in vivo, presumably by alloantigens, predisposes the cells to spontaneous apoptosis, and this phenomenon is associated with lymphopenia. Activation-induced T-cell apoptosis may contribute to delayed immune reconstitution following HCT.
Programmed cell death or apoptosis is an important physiological pathway in embryogenesis and tissue renewal.1,2 Within the thymus, activation-induced apoptosis is triggered by the recognition of “self” antigens, a critical mechanism for the elimination of autoreactive T cells.3,4 A large fraction of mature T cells may undergo activation-induced cell death following antigen stimulation as a means for maintaining homeostasis of the peripheral lymphoid system.4-6 During allogeneic hematopoietic cell transplantation (HCT), donor T cells reacting to recipient alloantigens cause graft-versus-host disease (GVHD), a major complication associated with morbidity and mortality. Prolonged immune deficiency characterized by persistent lymphopenia and susceptibility to infection is a common problem in patients, especially patients with GVHD, who receive intensive chemotherapy following allogeneic HCT.7-10 Prolonged lymphopenia in adult patients is mainly characterized by a delay in the recovery of naive CD4+ T cells, presumably due to age-related loss of thymus function.11-13
The study reported here was undertaken to further characterize T-cell apoptosis that occurs following HCT and to determine if it might contribute to lymphopenia. Spontaneous apoptosis in vitro was detected using terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate (dUTP) nick end-labeling (TUNEL) assay and 7-amino-actinomycin D (7AAD) in conjunction with dual staining with monoclonal antibodies (mAbs) defining T-cell subsets.14 15 The results show that apoptosis is prevalent among peripheral blood T cells early after transplantation, especially in the presence of human leukocyte antigen (HLA) disparity and acute GVHD. Apoptosis in these patients occurred preferentially among activated T cells. The intensity of apoptosis was correlated with a deficiency of CD4+ helper T cells.
Patients and methods
Patients
Blood samples were collected from 51 patients 19-23 days following allogeneic HCT. The clinical characteristics of these patients are summarized in Table 1. All patients studied received methotrexate for GVHD prophylaxis per protocol on days 1, 3, 6, and 11 after transplantation. In preliminary studies, T-cell apoptosis was studied on days 14, 21, 35, 49, and 80 after transplantation. Subsequent studies were performed 19-23 days following transplantation because of earlier profound lymphopenia. Blood samples were also obtained from healthy controls. HLA matching was based on serotyping for HLA-A, B, and C antigens and genotyping for theHLA-DRB1 allele and the DQB1 allele by DNA hybridization with sequence-specific oligonucleotide probes, as previously described.16 All patients received T-cell replete grafts and cyclosporine and methotrexate for GVHD prophylaxis.17 The diagnosis of acute GVHD was based on clinical findings and biopsy of skin and/or stomach.18Glucocorticosteroid was given as first-line therapy for all patients with clinically significant acute GVHD, according to standard practice. Institution-approved informed consents were obtained for all patients and controls.
Clinical characteristics of patients studied 19-23 days after transplantation
| . | Donors . | Total, n = 51 . | ||
|---|---|---|---|---|
| HLA-identical sibling, n = 16 . | HLA-matched unrelated, n = 20* . | HLA-mismatched unrelated, n = 15 . | ||
| BMT/PBSCT recipients | 12/4 | 19/1 | 15/0 | 46/5 |
| Age, years | 21-59 | 26-55 | 19-49 | 19-59 |
| Median years | 46 | 38 | 36 | 39 |
| Maximum acute GVHD | ||||
| Grade 0/I | 5/1 | 2/0 | 0/0 | 7/1 |
| Grade II-IV† | 10 | 8 | 12 | 30 |
| Patients treated for acute GVHD prior to analysis of apoptosis‡ | 0 | 10 | 3 | 13 |
| . | Donors . | Total, n = 51 . | ||
|---|---|---|---|---|
| HLA-identical sibling, n = 16 . | HLA-matched unrelated, n = 20* . | HLA-mismatched unrelated, n = 15 . | ||
| BMT/PBSCT recipients | 12/4 | 19/1 | 15/0 | 46/5 |
| Age, years | 21-59 | 26-55 | 19-49 | 19-59 |
| Median years | 46 | 38 | 36 | 39 |
| Maximum acute GVHD | ||||
| Grade 0/I | 5/1 | 2/0 | 0/0 | 7/1 |
| Grade II-IV† | 10 | 8 | 12 | 30 |
| Patients treated for acute GVHD prior to analysis of apoptosis‡ | 0 | 10 | 3 | 13 |
Statistics are given for recipients of bone marrow transplantation/peripheral blood stem cell transplantation (BMT/PBSCT). The median day of study after transplantation was day 21 for all donor types.
HLA-matched unrelated donors were studied 20-23 days after transplantation.
At the time of study, 18 patients had grade II-IV clinical acute GVHD, and 12 patients developed acute GVHD within 24-72 days following transplantation (median, 30 days). All patients in this group were studied prior to receiving systemic glucocorticosteroids.
A total of 13 patients received systemic glucocorticosteroids for treatment of acute GVHD prior to analysis of apoptosis.
HLA-DR expression by CD4+ and CD8+ peripheral blood T cells
Aliquots of 50-100 μL whole blood were incubated with the following monoclonal antibodies (brand names noted in parentheses: fluorescein isothiocyanate–labeled (FITC-labeled) anti–HLA-DR (immunglobulin G2a [IgG2a], clone G46-6, PharMingen); phycoerythrin-labeled (PE-labeled) anti-CD4 (IgG1, clone RPA-T4, PharMingen); and cychrome-labeled anti-CD8 (IgG1, clone RPA-T8, PharMingen). In selected experiments, aliquots of whole blood were incubated with FITC-labeled anti–HLA-DR, PE-labeled anti-CD3 (IgG1, clone UCHT1; PharMingen), and cychrome-labeled anti-CD4 mAbs. Mouse IgG1 (clone X40; Becton Dickinson, San Jose, CA; clone MOPC-21, PharMingen) and IgG2a (clone G155-178, PharMingen) isotype mAbs were used as negative controls. After incubation on ice for 30 minutes, red cells were lysed by fluorescence activated cell sorter (FACS) lysis buffer (Becton Dickinson).
The remaining cells were washed twice with phosphate-buffered saline (PBS) supplemented with 0.05% NaN3 and 1% fetal calf serum (FCS) (HyClone, Logan, UT), fixed in 2% neutral buffered formalin or 1% paraformaldehyde, and then analyzed by flow cytometry (FACScan and CELLQuest software, Becton Dickinson). The lymphocyte gate was determined by forward light scatter criteria plus side scatter criteria (FSC/SSC) using FITC-labeled anti-CD45 mAb (IgG1, clone HI30; PharMingen) and PE-labeled anti-CD14 mAb (IgG2b, clone MφP9; Becton Dickinson) staining. When staining with anti–HLA-DR/CD4/CD8 mAbs, CD8+ T cells and CD8+ natural killer (NK) cells were distinguished according to bright versus weak staining with anti-CD8 mAb. The CD3+CD4− population was used to represent CD8+ T cells when staining with anti–HLA-DR/CD3/CD4 mAbs.
Absolute peripheral T-cell count
Whole blood was stained with FITC-labeled anti-CD45 and PE-labeled anti-CD3 mAbs. The absolute number of CD3+ T cells was calculated from the peripheral white blood cell count times the percent of CD3+ cells. T-cell subsets were identified by staining with FITC-labeled anti-CD8, PE-labeled anti-CD3, and cychrome-labeled anti-CD4 mAbs. The absolute number of CD4+ T cells was calculated by multiplying the CD3+ T-cell count times the percent of CD4+CD8− cells. The absolute number of CD8+ T cells was calculated by multiplying the CD3+ T-cell count times the percent of CD4−CD8+ cells.
Short-term culture
Peripheral blood mononuclear cells (PBMCs) were isolated from blood by Ficoll-Hypaque (FH) density gradient centrifugation within 6 hours of venipuncture and resuspended to a final concentration of 5 × 105 cells per mL in RPMI 1640 supplemented with 10% heat-inactivated FCS, 10 IU/mL penicillin, 10 μg/mL streptomycin, 2 mmol/L glutamine, and 1 mmol/L sodium pyruvate. We dispensed 100 μL aliquots (50 × 103 cells per well) into 96-well round-bottomed microtitre plates (Rainin, Woburn, MA) and incubated them in a 5% carbon dioxide–humidified atmosphere at 37°C. In selected experiments, PBMCs were also cultured for 24 hours in medium alone or stimulated with 1 μg/mL phytohemagglutinin (PHA) (Sigma, St Louis, MO) or 1 μg/mL ionomycin (Sigma).
Detection of T-cell apoptosis by 7AAD
Apoptotic T cells in freshly isolated blood were identified by incubating 100-200 μL whole blood aliquots with PE-labeled anti-CD3 mAb within 6 hours of venipuncture. After incubation on ice for 30 minutes, 3 mL red cell lysing buffer, comprising 160 mmol/L NH4Cl, 0.1 mmol/L EDTA (ethylenediamine tetraacetic acid), and 12 mmol/L NaHCO3, was added for 10 minutes. The cells were washed and then stained with 7AAD as described below. T-cell apoptosis was also identified in freshly isolated PBMCs or PBMCs cultured for 24 hours. PBMCs were washed with PBS containing 1% FCS and 0.05% NaN3. Aliquots containing 1-2 × 105 cells were stained with PE-labeled anti-CD3 and FITC-labeled anti-CD4 or FITC-labeled anti–HLA-DR mAbs or stained with PE-labeled anti-CD56 (IgG2b, clone NCAM16.2; Becton Dickinson) and FITC-labeled anti-CD3 mAbs for 30 minutes on ice. The cells were washed once, incubated with PBS containing 20 μg/mL actinomycin D (Sigma) on ice for 20 minutes, washed twice, and then fixed in 2% neutral buffered formalin or 1% paraformaldehyde in the presence of 20 μg/mL actinomycin D (Sigma). All samples were analyzed by flow cytometry within 24 hours. For analysis of PBMCs, data were collected on a minimum of 5000 CD3+ cells. The fluorescence of 7AAD was detected by red channel FL-3 (wavelength between 650 and 850 nm) of FACScan (Becton Dickinson).
TUNEL assay
After a 24-hour culture, a PBMC aliquot containing approximately 1-2 × 106 cells was stained with PE-labeled anti-CD3 mAb, washed, and then resuspended in PBS containing 20 μg/mL 7AAD for 20 minutes before sorting. Using an FACS Vantage cell sorter (Becton Dickinson), 7AAD+/CD3+ and 7AAD−/CD3+ cells were sorted for greater than 95% purity. The TUNEL assay (Oncor, Gaithersburg, MD) was performed according to the manufacturer's instructions. Briefly, 7AAD+/CD3+ or 7AAD−/CD3+ cells were mounted on slides by cytospin and fixed in 10% neutral buffered formalin. Cells were washed and then incubated with buffer containing digoxigenin-dUTP and terminal deoxynucleotidyl transferase at 37°C for 60 minutes. After the reaction was stopped, the cells were washed, incubated with FITC-labeled antidigoxigenin at room temperature for 30 minutes, and washed again before counterstaining with propidium iodide. Nuclear incorporation of FITC-dUTP was detected by examination on a fluorescent microscope.
Statistical analysis
Statistical significance was assessed using the Wilcoxon rank sum test for nonpaired samples and the signed rank test for paired samples. No adjustments were made for multiple comparisons, and all reportedP values are 2-sided. Linear regression was used to assess the correlation between the intensity of T-cell apoptosis and the expression of HLA-DR or the absolute number of peripheral blood T cells.
Results
T-cell apoptosis in whole blood and freshly isolated PBMCs
To determine if apoptosis could be detected among peripheral blood T cells, heparinized whole blood from 23 patients (20-28 days after transplantation) and 12 controls was stained with anti-CD3 mAb and 7AAD. There was a significantly higher incidence of apoptosis among T cells from patients (2.9% ± 1.5%), compared with controls (1.7% ± 0.8%, P < .05) (Table2). To determine if cell processing in vitro altered the level of detectable apoptosis, an aliquot of blood from patients and from controls was separated over FH density gradients, and the isolated PBMCs were stained with PE-labeled anti-CD3 mAb and 7AAD. There was a significantly higher incidence of apoptosis among FH-separated T cells from patients (4.0% ± 2.3%) compared with controls (1.5% ± 0.8%, P < .05). There was also a significant increase in apoptosis detected in T cells from patients immediately following FH separation (4.0% ± 2.3%) compared with T cells stained in whole blood (2.9% ± 1.5%,P < .05). Separation using FH density gradient, however, did not significantly change the degree of apoptosis detected in T cells from controls (P > .05).
T-cell apoptosis in freshly isolated whole blood and following separation of mononuclear cells
| . | Apoptosis among CD3+T cells, % . | |
|---|---|---|
| Whole blood . | PBMCs . | |
| Patients, n = 23 | 2.9 ± 1.5* | 4.0 ± 2.3*,† |
| (0.6-5.7) | (1.5-10.3) | |
| Controls, n = 12 | 1.7 ± 0.8 | 1.5 ± 0.8 |
| (0.7-2.9) | (0.7-2.6) | |
| . | Apoptosis among CD3+T cells, % . | |
|---|---|---|
| Whole blood . | PBMCs . | |
| Patients, n = 23 | 2.9 ± 1.5* | 4.0 ± 2.3*,† |
| (0.6-5.7) | (1.5-10.3) | |
| Controls, n = 12 | 1.7 ± 0.8 | 1.5 ± 0.8 |
| (0.7-2.9) | (0.7-2.6) | |
We studied 23 patients 20-28 days after transplantation. The percent of apoptosis plus or minus SD was measured within 6 hours of phlebotomy. The range is indicated in parentheses.
Indicates that P < .05 compared with controls.
Indicates that P < .05 compared with whole blood.
Apoptosis of T cells after incubation of whole blood and PBMCs
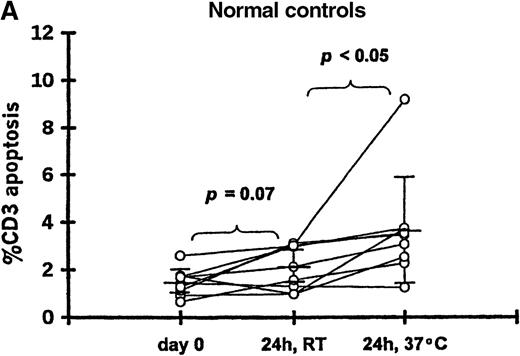
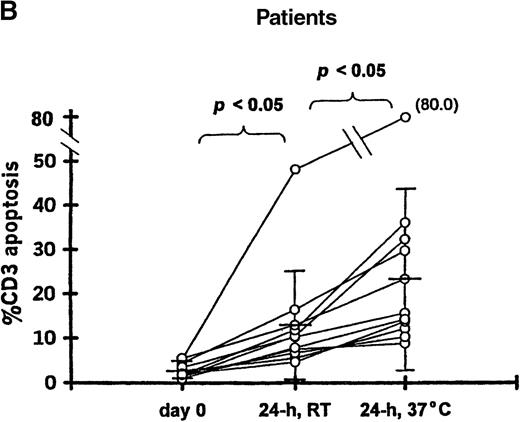
To determine if in vitro incubation affected the degree of T-cell apoptosis, whole blood from 11 patients (20-102 days after transplantation) and 9 controls was separated into aliquots in 12 × 75-mm Falcon tubes (Becton Dickinson, Lincoln Park, NJ). Aliquots were analyzed either immediately, after 24-hour incubation at room temperature, or after 24-hour incubation at 37°C. The frequency of 7AAD+/CD3+ cells was significantly increased when whole blood from patients was incubated 24 hours at room temperature (12.9% ± 12.2%) or 37°C (25.1% ± 20.5%) compared with the preincubation analysis (2.9% ± 1.6%;P < .05 for both incubation temperatures) (Figure 1B). Incubation for 24 hours resulted in a smaller increase in apoptosis among T cells from controls at room temperature (2.1% ± 0.9%) or 37°C (3.6% ± 2.2%) compared with the preincubation samples (1.5% ± 0.6%; P = .07 and P < .05, respectively) (Figure 1A).
T-cell apoptosis before and after incubation of whole blood.
Whole blood was incubated 24 hours at room temperature (24h, RT) or at 37°C (24h, 37°C); prior to incubation is noted as day 0. Samples were obtained from (A) 9 controls and (B) 11 patients studied 20-102 days after transplantation.
T-cell apoptosis before and after incubation of whole blood.
Whole blood was incubated 24 hours at room temperature (24h, RT) or at 37°C (24h, 37°C); prior to incubation is noted as day 0. Samples were obtained from (A) 9 controls and (B) 11 patients studied 20-102 days after transplantation.
Similarly, apoptosis increased when PBMCs from patients were cultured in vitro. After a 24-hour incubation at 37°C, there was a trend toward a higher rate of apoptosis among FH-separated T cells (31.0% ± 12.8%) compared with whole blood T cells (25.1% ± 20.5%, P = .15). To determine if cell death was temperature-dependent, PBMCs from 13 patients were analyzed immediately and after a 24-hour incubation at 4°C or 37°C. The intensity of apoptosis among T cells incubated 24 hours at 4°C (10.4% ± 6.7%) was significantly higher than in T cells analyzed before culture (2.7% ± 2.0%, P < .05). However, the highest level of apoptosis was observed in T cells incubated for 24 hours at 37°C (23.6% ± 11.3%,P < .05).
Apotosis of T cells after cryopreservation
T-cell apoptosis was measured in 12 patients before and after cryopreservation. The frequency of 7AAD+/CD3+cells was 3.4% ± 3.0% for fresh PBMCs and 15.2% ± 6.2% immediately following thawing of frozen cells. T-cell apoptosis was significantly greater among frozen PBMCs (57.5% ± 14.0%) compared with freshly isolated PBMCs (22.7% ± 9.1%) after a 24-hour incubation at 37°C (P < .05). Cryopreservation had a more profound effect on CD8+ T cells (66.0% ± 13.5% for frozen PBMCs and 22.1% ± 10.0% for freshly isolated PBMCs, respectively) compared with CD4+ T cells (39.4% ± 10.2% for frozen PBMCs and 24.6% ± 16.3% for freshly isolated PBMCs, respectively). To avoid the cryopreservation effect, subsequent experiments were completed exclusively with fresh PBMCs.
Phenotyping of 7AAD+ cells
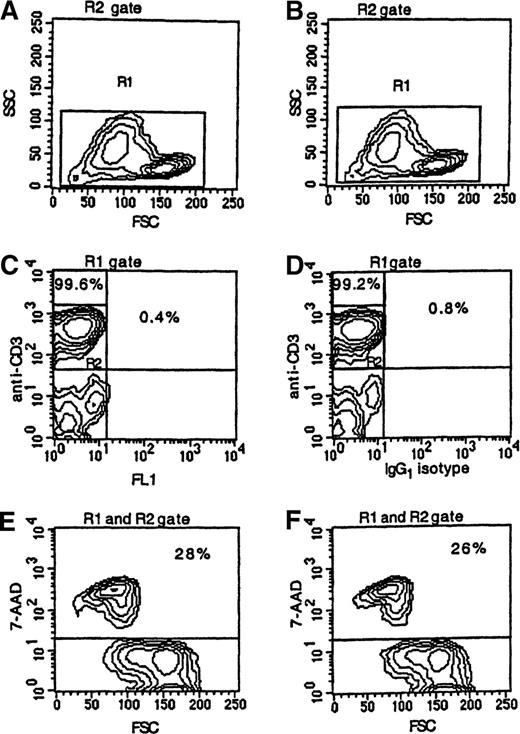
To determine if apoptotic T cells might label nonspecifically with mAbs, cultured PBMCs from 17 patients and 6 controls were dual-stained with FITC-labeled IgG1 isotype control antibody and PE-labeled anti-CD3 antibody. A low level of nonspecific staining of CD3+ cells was detected in both patients (2.3% ± 2.8%) and controls (0.7% ± 0.4%). The flow cytometric analysis of cultured PBMCs from a representative patient is illustrated in Figure 2. In this case, 28% of CD3+ cells were 7AAD+ (Figure 2E). When the cultured cells were stained with PE-labeled anti-CD3 antibody, FITC-labeled IgG1 isotype control antibody, and 7AAD, 26% of the FITC−/CD3+ cells were scored as 7AAD+ (Figure 2F), which demonstrates that there was no significant nonspecific binding of fluorescence-labeled antibody to apoptotic cells.
Apoptosis among CD3+ T cells.
PBMCs were obtained from a patient 21 days following transplantation, cultured for 24 hours, and stained with PE-labeled anti-CD3 mAb (panels A, C, and E) or with PE-labeled anti-CD3 mAb and FITC-labeled IgG1 isotype control antibody (panels B, D, and F) followed by 7AAD. Less than 1% of the T cells were stained with FITC-labeled IgG1 isotype control antibody. T-cell apoptosis was measured by using FSC and the intensity of 7AAD fluorescence (panel E or F) of cells noted in the R1 gate (panel A or B) and R2 gate (panel C or D).
Apoptosis among CD3+ T cells.
PBMCs were obtained from a patient 21 days following transplantation, cultured for 24 hours, and stained with PE-labeled anti-CD3 mAb (panels A, C, and E) or with PE-labeled anti-CD3 mAb and FITC-labeled IgG1 isotype control antibody (panels B, D, and F) followed by 7AAD. Less than 1% of the T cells were stained with FITC-labeled IgG1 isotype control antibody. T-cell apoptosis was measured by using FSC and the intensity of 7AAD fluorescence (panel E or F) of cells noted in the R1 gate (panel A or B) and R2 gate (panel C or D).
Morphology and terminal transferase labeling of 7AAD+T cells
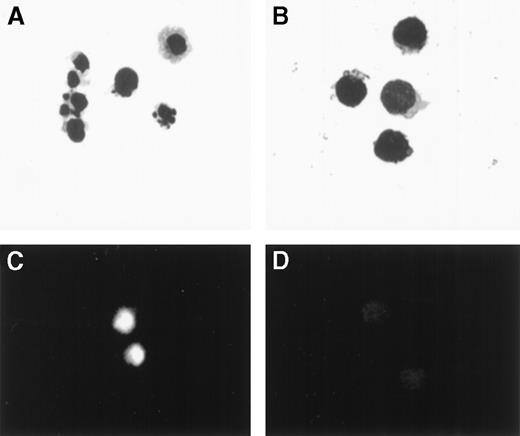
PBMCs from 5 patients were cultured for 24 hours and then sorted into 7AAD+/CD3+ and 7AAD−/CD3+ cells. Cells were mounted on slides, stained with FITC-dUTP, and examined by phase contrast and fluorescent microscopy. Results of a representative experiment are illustrated in Figure 3. In this case, 27.4% of CD3+ cells were 7AAD+. By morphological examination, cells in the 7AAD+/CD3+ fraction showed a marked reduction of cell volume, blebbing of plasma membranes, chromatin condensation, and nuclear fragmentation (Figure 3A) compared with cells in the 7AAD−/CD3+ fraction (Figure 3B). There was a significant incorporation of FITC-dUTP in the nuclei of 7AAD+/CD3+ cells but no incorporation in the nuclei of the 7AAD−/CD3+ cells (Figure3C, D).
Wright-Giemsa stain and TUNEL assay of PBMCs obtained from a patient 21 days following transplantation, incubated for 24 hours, and separated by sorting into CD3+/7AAD+and CD3+/7AAD− cells.
(A) Cells in the CD3+/7AAD+ fraction showed reduction in cell volume, blebbing of plasma membrane, chromatin condensation, and nuclear fragmentation (original magnification × 1000), (B) while cells in the CD3+/7AAD− fraction showed a typical morphological feature of lymphocytes. (C) Cells in the CD3+/7AAD+ fraction were brightly fluorescent, indicating incorporation of fluorescein-dUTP into fragmented DNA (original magnification × 400), (D) while cells in the CD3+/7AAD− fraction showed no significant fluorescence.
Wright-Giemsa stain and TUNEL assay of PBMCs obtained from a patient 21 days following transplantation, incubated for 24 hours, and separated by sorting into CD3+/7AAD+and CD3+/7AAD− cells.
(A) Cells in the CD3+/7AAD+ fraction showed reduction in cell volume, blebbing of plasma membrane, chromatin condensation, and nuclear fragmentation (original magnification × 1000), (B) while cells in the CD3+/7AAD− fraction showed a typical morphological feature of lymphocytes. (C) Cells in the CD3+/7AAD+ fraction were brightly fluorescent, indicating incorporation of fluorescein-dUTP into fragmented DNA (original magnification × 400), (D) while cells in the CD3+/7AAD− fraction showed no significant fluorescence.
Apoptosis occurs preferentially among CD4+ T cells
To determine if CD4+ and CD8+ cells were equally likely to undergo apoptosis, PBMCs cultured for 24 hours were stained with anti-CD3 mAb, anti-CD4 mAb, and 7AAD. In preliminary experiments we compared the relative number of CD4+ and CD8+ T cells by 2-color staining with CD3+/CD4+ antibodies or CD3+/CD8+ antibodies, and we found that the relative number of CD8+ T cells could be reliably determined by enumerating CD3+/CD4− cells (data not shown). For convenience and economy of cell supply, we chose to use the CD3+/CD4− parameter to estimate the number of CD8+ T cells. The degree of apoptosis was greater in both the CD4+ T cells (33.3% ± 14.6%) and the CD8+ T cells (26.0% ± 13.1%) from patients studied 19-23 days following transplantation compared with controls (4.2% ± 1.6% and 3.8% ± 2.0%, respectively; P < .05 for both types of cells) (Table 3). The degree of apoptosis was significantly greater in CD4+ T cells compared with CD8+ T cells (P < .05). The results were similar when we analyzed the 38 patients who did not receive systemic glucocorticosteroids before the study: 30.0% ± 13.0% for CD4+ T cells and 23.8% ± 13.5% for CD8+ T cells (P < .05). Among the 9 patients studied between 10 and 14 months following transplantation, the degree of apoptosis was substantially less in both the CD4+ T cells (11.8% ± 4.2%) and CD8+ T cells (8.8% ± 2.5%). However, the degree of apoptosis in these cells remained significantly higher than in the controls (P < .05 for both types of cells).
Apoptosis among CD4+, CD8+, and CD56+ cells following allogeneic HCT
| . | CD3+ . | Apoptosis of PBMCs, % . | ||
|---|---|---|---|---|
| CD4+ . | CD8+ . | CD3−/CD56+ . | ||
| Median days after transplantation and total patients, | ||||
| 19-23 days, n = 51 | 30.4 ± 12.53-150 | 33.3 ± 14.63-150,3-151 | 26.0 ± 13.13-150 | 2.2 ± 1.2 |
| (5.1-60.0) | (4.3-68.6) | (5.9 ± 59.7) | (1.1-4.3) | |
| 10-14 months, n = 9 | 9.7 ± 2.83-150 | 11.8 ± 4.23-150 | 8.8 ± 2.53-150 | 2.6 ± 1.5 |
| (5.4-12.2) | (6.2-18.1) | (5.3-13.5) | (1.0-4.7) | |
| Controls, n = 17 | 4.0 ± 1.5 | 4.2 ± 1.6 | 3.8 ± 2.0 | 2.2 ± 1.5 |
| (1.9-6.9) | (1.8-6.9) | (1.4-8.3) | (0.6-4.2) | |
| . | CD3+ . | Apoptosis of PBMCs, % . | ||
|---|---|---|---|---|
| CD4+ . | CD8+ . | CD3−/CD56+ . | ||
| Median days after transplantation and total patients, | ||||
| 19-23 days, n = 51 | 30.4 ± 12.53-150 | 33.3 ± 14.63-150,3-151 | 26.0 ± 13.13-150 | 2.2 ± 1.2 |
| (5.1-60.0) | (4.3-68.6) | (5.9 ± 59.7) | (1.1-4.3) | |
| 10-14 months, n = 9 | 9.7 ± 2.83-150 | 11.8 ± 4.23-150 | 8.8 ± 2.53-150 | 2.6 ± 1.5 |
| (5.4-12.2) | (6.2-18.1) | (5.3-13.5) | (1.0-4.7) | |
| Controls, n = 17 | 4.0 ± 1.5 | 4.2 ± 1.6 | 3.8 ± 2.0 | 2.2 ± 1.5 |
| (1.9-6.9) | (1.8-6.9) | (1.4-8.3) | (0.6-4.2) | |
The percent of apoptosis plus or minus SD after a 24-hour culture of PBMCs. The range is indicated in parentheses. Patients were studied at 19-23 days or 10-14 months following transplantation. Four patients were studied at both 20-21 days and 12-14 months following transplantation. Of the 9 patients studied 10-14 months after transplantation, 7 were still receiving cyclosporine alone or cyclosporine and glucocorticosteroids. The median age (range) of the 17 controls was 34 years (18-50 years).
P < .05 compared with controls.
P < .05 compared with CD8+ T cells.
Apoptosis among NK cells
There was no significant increase in apoptosis among CD56+/CD3− NK cells from patients at either 19-23 days (2.2% ± 1.2%) or 1 year (2.6% ± 1.5%) after transplantation compared with controls (2.2% ± 1.5%) (Table 3).
Apoptosis occurs preferentially among HLA-DR+ T cells
HLA-DR expression was significantly increased in both CD4+ T cells (37% ± 17%) and CD8+ T cells (48% ± 22%) from 41 patients (19-23 days after transplantation) compared with CD4+ T cells (5% ± 3%) and CD8+ T cells (11% ± 8%,P < .05 for both types of cells) from 17 controls. The expression of HLA-DR by CD3+ T cells was significantly higher in 10 patients who received transplantations from HLA-mismatched unrelated donors (59% ± 17%) compared with 15 patients who received transplantations from HLA-matched unrelated donors (43% ± 18%, P < .05) or 11 patients who received transplantations from HLA-identical siblings (31% ± 13%,P < .05). The intensity of T-cell apoptosis following a 24-hour culture of PBMCs was correlated with higher HLA-DR expression before culture (correlation efficient (R2) equal to 42.8%, P < .001) (Figure 4). Among 21 patients studied 19-23 days after transplantation, apoptosis was more frequent in DR+ T cells (37.9% ± 9.4%) compared with DR− T cells (18.3% ± 10.9%,P < .05). In 8 patients 10-14 months after transplantation, the expression of DR remained significantly increased in both CD4+ T cells (28% ± 13%) and CD8+ T cells (46% ± 22%, P < 0.5 for both types of cells).
Correlation between T-cell apoptosis following 24-hour culture and HLA-DR expression in T cells before culture in 36 patients studied 19-23 days after transplantation.
Linear regression curve is shown (R2 = 42.8%,P < .001).
Correlation between T-cell apoptosis following 24-hour culture and HLA-DR expression in T cells before culture in 36 patients studied 19-23 days after transplantation.
Linear regression curve is shown (R2 = 42.8%,P < .001).
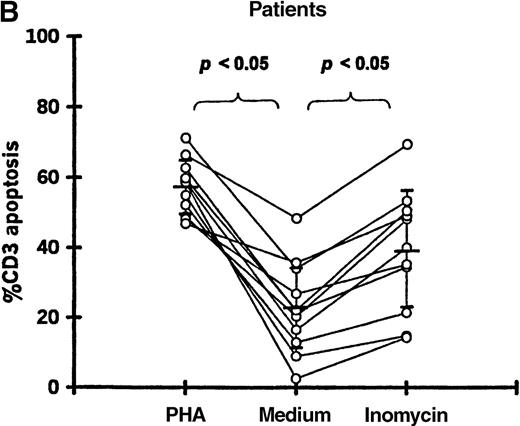
Enhancement of T-cell apoptosis by stimulation with PHA and ionomycin
PBMCs from 11 patients (19-87 days after transplantation) and 11 controls were incubated in medium alone, with PHA, or with ionomycin. Both PHA and ionomycin significantly increased T-cell apoptosis in patients and controls (Figure5). PHA- and ionomycin-induced T-cell apoptosis was significantly higher (P < .05) in patients (57.2% ± 7.9% and 39.2% ± 17.3%, respectively) compared with controls (23.2% ± 6.0% and 6.4% ± 2.1%, respectively).
T-cell apoptosis following stimulation by PHA or ionomycin.
Apoptosis was measured following 24-hour culture of PBMCs in (A) 11 controls and (B) 11 patients studied 19-87 days after transplantation.
T-cell apoptosis following stimulation by PHA or ionomycin.
Apoptosis was measured following 24-hour culture of PBMCs in (A) 11 controls and (B) 11 patients studied 19-87 days after transplantation.
Effect of HLA mismatch on T-cell apoptosis
Among the 51 patients studied 19-23 days after transplantation, the degree of T-cell apoptosis was significantly higher in the 15 patients who received transplantations from HLA-mismatched unrelated donors (39.5% ± 10.4%, P < .05) or in the 20 patients who received transplantations from HLA-matched unrelated donors (32.1% ± 11.4%, P < .05) compared with the 16 patients who received transplantations from HLA-identical siblings (19.6% ± 6.7%). To exclude the potential effect of treatment for GVHD, only the data for the 38 patients who did not receive systemic glucocorticosteroids were analyzed. The results showed a significantly higher intensity of T-cell apoptosis in transplantations from HLA-mismatched unrelated donors (37.9% ± 10.6%, P < .05) or HLA-matched unrelated donors (27.3% ± 9.6%, P < .05) compared with transplantations from HLA-identical sibling donors (19.6% ± 6.7%). No significant correlation was found between the degree of T-cell apoptosis and the day of transplantation (R2 = 0.4%, P > .05) or patient age (R2 = 3.9%, P > .05).
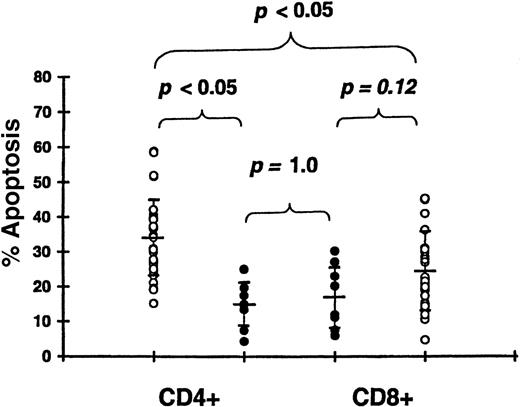
Effect of acute GVHD on T-cell apoptosis
We studied 30 patients 19-23 days (median, 21 days) after transplantation and prior to receiving glucocorticosteroids (Table 1). Apoptosis was significantly increased in CD4+ T cells of patients with grade II-IV GVHD (33.9% ± 11.3%) compared with patients with grade 0-I GVHD (14.6% ± 6.5%,P < .05) (Figure 6). Apoptosis in CD8+ T cells tended to be higher in patients with grade II-IV GVHD (24.8% ± 11.9%) compared with grade 0-I GVHD (17.2% ± 9.2%), but this difference was not significant (P = .12). There was no difference in apoptosis between CD4+ T cells (14.6% ± 6.5%) and CD8+ T cells (17.2% ± 9.2%) among patients with grade 0-I acute GVHD (P = 1.0). However, in patients with clinically significant acute GVHD, the intensity of apoptosis was higher in CD4+ T cells (33.9% ± 11.3%) compared with CD8+ T cells (24.8% ± 11.9%,P < .05). Apoptosis of both CD4+ and CD8+ T cells was higher in patients with grade 0-I GVHD compared with controls (P < .05 for both types of cells). In transplantations using HLA-identical sibling donors, apoptosis was significantly increased in CD4+ T cells of 10 patients with grade II-IV GVHD (24.7% ± 6.0%) compared with 6 patients with grade 0-I GVHD (15.3% ± 6.8%, P < .05).
Apoptosis of CD4+ and CD8+ T cells following 24-hour culture.
We studied 30 patients with grade II-IV acute GVHD 19-23 days after transplantation (open circle) and 8 patients with grade 0-I GVHD 20-22 days after transplantation (closed circle).
Apoptosis of CD4+ and CD8+ T cells following 24-hour culture.
We studied 30 patients with grade II-IV acute GVHD 19-23 days after transplantation (open circle) and 8 patients with grade 0-I GVHD 20-22 days after transplantation (closed circle).
CD4+ T-cell apoptosis correlates with a lower absolute number of CD4+ T cells
To determine if T-cell apoptosis observed after HCT may contribute to lymphopenia, T-cell apoptosis at 19-23 days after transplantation was correlated with the absolute number of CD4+ and CD8+ T cells. The number of CD8+ T cells was found to increase significantly from days 19-23 (28.4 ± 21.1 cells per μL) to days 75-91 (123.8 ± 179.2 cells per μL,P < .05). However, the CD4+ T cells did not increase significantly from days 19-23 (52.1 ± 40.0 cells per μL) to days 75-91 (63.0 ± 59.8 cells per μL,P > .05). The intensity of CD4+ T-cell apoptosis at days 19-23 was significantly correlated with a lower CD4+ T-cell count at days 19-23 (R2 = 19.6%, P < .05) and a lower CD4+ T-cell count at days 75-91 (R2 = 25.7%, P < .05) (Figure7A). In contrast, there was no significant correlation between the intensity of CD8+ T-cell apoptosis and the CD8+ T-cell count at days 19-23 (R2 = 0.7%, P > .05) or days 75-91 (R2 = 0.7%, P > .05) (Figure 7B). At days 75-91, the intensity of CD4+ T-cell apoptosis was significantly higher in 15 patients with a CD4/CD8 ratio of less than 1 (31.6% ± 22.8%) compared with 13 patients with a CD4/CD8 ratio of at least 1 (13.2% ± 5.6%,P < .05).
Intensity of spontaneous apoptosis measured 19-23 days after transplantation following 24-hour culture and the absolute number of peripheral blood T cells at days 75-91 among 28 patients.
(A) Apoptosis among CD4+ cells and absolute CD4+ T-cell count (R2 = 25.7%,P = .006). (B) Apoptosis among CD8+ cells and absolute CD8+ T-cell count (R2 = 0.7%,P = .671).
Intensity of spontaneous apoptosis measured 19-23 days after transplantation following 24-hour culture and the absolute number of peripheral blood T cells at days 75-91 among 28 patients.
(A) Apoptosis among CD4+ cells and absolute CD4+ T-cell count (R2 = 25.7%,P = .006). (B) Apoptosis among CD8+ cells and absolute CD8+ T-cell count (R2 = 0.7%,P = .671).
Discussion
This study demonstrates an increase in spontaneous apoptosis among peripheral blood T cells of patients following allogeneic HCT. Soon after transplantation, there was a significant increase in apoptotic T cells, which were detected in patients by direct staining of freshly isolated whole blood or PBMCs. There was also a significant progression of apoptosis following T-cell incubation in medium for 24 hours at 37°C. The intensity of apoptosis was further amplified by stimulation with PHA or ionomycin. It is unclear if the in vitro T-cell death observed in these patients represents an increased sensitivity to the stress of in vitro manipulation, or alternatively, if the cells undergoing apoptosis are committed to the cell death pathway prior to phlebotomy. The finding of apoptotic T cells in freshly isolated blood suggests that apoptosis is occurring in vivo in these patients. Detection of apoptosis in vivo may be limited, and the estimated frequency may be relatively low if there are very efficient mechanisms for removing cells from the circulation early in the apoptotic process.19,20 Rapid clearance of apoptotic T cells within the thymus has been described.21
Apoptosis in this study was more prevalent between 19-23 days and 75-91 days after transplantation compared with 10-14 months after transplantation. Soon after transplantation, HLA-DR+ and CD4+ T cells were more susceptible to apoptosis than DR− and CD8+ T cells. NK cells were resistant to apoptosis. T-cell apoptosis was greater in patients who received transplantations from HLA-mismatched unrelated donors and was more frequent in patients with clinically significant acute GVHD. The relevance of this finding is demonstrated by the correlation between the intensity of CD4+ T-cell apoptosis at days 19-23 and CD4+ T-cell lymphopenia at days 19-23 and days 75-91. These observations indicate that T-cell activation induced by alloantigen and associated with GVHD induces sufficient CD4+ T-cell death to significantly retard CD4+ T-cell reconstitution following transplantation.
The analysis of T-cell subsets among apoptotic cells may be confounded by nonspecific binding of mAbs, especially following 24-hour in vitro culture. Experiments with an isotype control antibody, however, indicated that specific staining of apoptotic T cells for CD3 could be achieved. In addition, the discrimination of CD4+ T cells from monocytes early after transplantation can be problematic. Monocytes weakly express CD4. In certain patients there can be a prominent overlap in FSC/SSC profiles, making it impossible to separate the cells simply by cell size and refractile properties. Furthermore, the expression of CD4 was found to diminish during apoptosis. It was possible to circumvent these problems by 3-color staining for CD3, CD4, and 7AAD. There was also a significant loss of CD8 expression during apoptosis, and it was not possible to reliably distinguish CD8+ T cells from CD8+/CD56+ NK cells after short-term culture by single-color CD8 staining. This problem was also minimized by 3-color staining for CD3, CD8, and 7AAD.
Donnenberg et al22 and Hebib et al23 have also reported increased T-cell apoptosis following marrow transplantation. Donnenberg et al studied allotransplantation and autotransplantation recipients between 7 and 175 days after transplantation using propidium iodide to identify apoptotic nuclei and single-color staining to define CD3, CD4, and CD8 cells following an 18-hour incubation. They reported 20% apoptosis in controls and 50% to 60% apoptosis in patients. Other investigators24-26 have reported control results similar to ours, with less than 5% CD3+ T-cell apoptosis following 24-hour culture or less than 10% CD4+ T-cell apoptosis following 18-48 hours of culture using 7AAD or Hoechst 33342 staining. Hebib et al23 studied cryopreserved PBMCs from 26 recipients of transplantations from HLA-identical siblings and measured apoptosis after an 18-hour culture using DiOC6 (3.3′-diethyloxacarbocyanine) fluorescent dye to stain mitochondrial membranes. They reported 9% CD4+ and 14% CD8+ T-cell apoptosis in controls compared with 36% CD4+ and 51% CD8+ T-cell apoptosis in patients 45-90 days after transplantation. Hebib et al23 also demonstrated an increased expression of CD95, the Fas receptor, and an increase in anti-Fas IgM-induced apoptosis in patients compared with controls.
The finding by Hebib et al23 that CD8+ T cells are more sensitive to in vitro apoptosis than CD4+ T cells appears to contradict the results of our study. This discrepancy may be explained in part by differences in the timing of the study, patient populations, severity of GVHD, and possibly also by the use of cryopreserved cells. All patients in the study reported by Hebib et al received transplantations from HLA-identical siblings, whereas 35 of 51 patients in our study received transplantations from unrelated donors. Thus the frequency and intensity of GVHD were surely greater in our study patients. We found that the degree of apoptosis was greater in CD4+ T cells compared with CD8+ T cells in the presence of clinically significant GVHD but not in the absence of GVHD (Figure 6). Patients in our study were initially studied at days 19-23 after transplantation, and 13 of 51 patients had already received systemic glucocorticosteroids for treatment of acute GVHD. Of the remaining patients, 8 remained free of clinically significant GVHD, 18 had grade II-IV acute GVHD at the time of study, and 12 subsequently developed grade II-IV acute GVHD. Patients with grade II-IV acute GVHD received additional immune suppression therapy, usually glucocorticosteroids. In preliminary studies we observed a significant decrease in apoptosis and CD4+ T-cell counts following initiation of GVHD treatment (data not shown). The results of Hebib et al may also have been significantly affected by the use of cryopreserved PBMCs for the evaluation of apoptosis. We found that freezing and thawing PBMCs from patients caused a significantly higher level of spontaneous T-cell apoptosis before and after a 24-hour culture compared with freshly isolated PBMCs. CD8+ T cells appeared to be more sensitive to cryopreservation than CD4+T cells.
Prolonged lymphopenia also occurs following nonmyeloablative chemotherapy, especially among naive CD4+ T cells in adult patients. Lymphopenia is likely due, at least in part, to age-related loss of thymic function.13 Apoptosis of CD4+ T cells following chemotherapy has also been described. It has been hypothesized that increased susceptibility to apoptosis in this situation may be a regulatory response secondary to an increased level of activation in the expanding CD4+population.26 Increased spontaneous T-cell apoptosis has also been described in patients who tested positive for human immunodeficiency virus (HIV), and apoptosis is likely to be a major cause of the progressive lymphopenia and immunodeficiency that occurs in these patients. Although HIV can be cytopathic to infected cells, apoptosis is observed in both HIV-infected and noninfected T cells, which suggests that other mechanisms induce cell death in bystander T cells.27-31
Profound lymphopenia and delayed immune reconstitution are significant problems in marrow allograft recipients.8-11 The data presented here suggest that stimulation by donor alloantigen and T-cell activation associated with the GVH reaction contributes to T-cell apoptosis, especially in CD4+ T cells early after transplantation. The degree of CD4+ T-cell apoptosis correlates with clinically significant GHVD and CD4 lymphopenia. We have no direct evidence, however, that T cells undergoing apoptosis are specific for host alloantigen. Massive activation-induced cell death following an initial brisk proliferation of donor T cells has recently been reported in a murine model of GVHD.32 A significant nonspecific bystander effect was seen with the concurrent death of T cells incapable of reacting to a host, a reaction shown to be mediated by the Fas/FasL pathway.
In summary, these findings demonstrate that T-cell apoptosis is prevalent early following allogeneic HCT, especially among activated CD4+ T cells. We postulate that stimulation by host alloantigen may drive a significant number of donor T cells into the activation-induced cell death pathway. A strong antihost allograft reaction mediated by mature donor T cells may thus contribute directly to lymphopenia and delayed immune reconstitution. Apoptosis may be an essential mechanism for eliminating T-cell clones capable of causing GVHD, but the killing of bystander cells through Fas and other pathways may lead to a profound global immune deficiency. Future studies aimed at understanding the mechanisms for regulating alloantigen-induced T-cell activation and posttransplant lymphopoiesis may provide the basis for novel strategies to facilitate tolerance and promote immune reconstitution.
Acknowledgments
The authors thank Jennie Lorenz and Sell Alison for assistance and help in preparing the manuscript and Dr Claudio Anasetti for helpful discussion.
Supported by grants AI33484, CA18029, CA15704, and CA18221 from the National Institutes of Health, Bethesda, MD.
Reprints:John A. Hansen, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D2-100, Seattle, WA 98109-1024; e-mail:Jhansen@fhcrc.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal