Abstract
Fes is a nonreceptor tyrosine kinase expressed at the highest level in macrophages. We previously showed that the overexpression of c-fes in murine macrophages of the BAC-1.2F5 cell line renders these cells independent of macrophage colony-stimulating factor (MCSF) for survival and proliferation, although no direct relationship could be established between tyrosine-phosphorylated substrates of Fes- and MCSF receptor–dependent signaling and mitogenesis. In this study, we investigated whether the mitogen-activated protein kinase (MAPK) pathway is involved in the growth factor–independent growth of v-fes–overexpressing macrophages. We found a constitutively increased phosphorylation of extracellularly regulated kinase (ERK) in v-fes–overexpressing macrophages as compared with mock-infected cells. This finding was associated with activation of mitogen/extracellular signal–regulated kinase (MEK) and with constitutive localization of ERK in the nucleus. Treatment of v-fes–overexpressing cells with the MEK-specific inhibitor PD98059 markedly reduced cell growth, hyperphosphorylation, and nuclear localization of ERK, indicating that the MAPK pathway mediates the mitogenic effect of v-fes.
The c-fes/fps proto-oncogene encodes a 92-kd nonreceptor protein tyrosine kinase (p92c-fes) expressed in myeloid and endothelial cells. The biological and biochemical effects of Fes in macrophages, a cell type in which this kinase is physiologically expressed at the highest levels, are still to be defined. The overexpression of c-fes in BAC-1.2F5 murine macrophages, which depend on macrophage colony-stimulating factor (MCSF) for survival and growth,1 resulted in the acquisition of MCSF-independent growth.2 However, the proteins so far shown to exhibit increased tyrosine phosphorylation in BAC-1.2F5 cells overexpressing c-fes, namely the Cas protein and an unidentified 75-kd protein, are involved in cell adhesion and cell-cell signaling2 3 but not mitogenesis.
In murine fibroblasts, where fes is not physiologically expressed, ectopic expression of this gene at high levels causes tumorigenic transformation involving the mitogen-activated protein kinase (MAPK) pathway.4 The best-characterized MAPK cascade involves the sequential activation of Ras, Raf-1, mitogen/extracellular signal–regulated kinase (MEK), and extracellularly regulated kinase (ERK). Active Raf-1 phosphorylates and activates the dual-specificity kinases MEK1 and MEK2, which, in turn, activate ERK1 and ERK2, respectively. In unstimulated cells, ERK1 and ERK2 are mostly located in the cytosol; upon phosphorylation, both proteins translocate to the nucleus5,6 to phosphorylate a number of transcription factors, Elk-1 being one of the best-characterized examples.7 8
In this study, we addressed the question of whether the MAPK pathway is involved in conveying Fes-dependent mitogenic signals in macrophages, where fes is physiologically expressed. In clones of BAC-1.2F5 macrophages overexpressing v-fes, we found a constitutive activation of ERK and MEK and demonstrated their involvement in growth factor–independent mitogenesis.
Materials and methods
Cells and cell culture
Murine macrophages of the BAC-1.2F5 cell line were infected with a helper virus (amphotropic-MuLV) alone (control) or carrying a pHFeSV plasmid containing v-fps/fes (VFB clones) encoding for the p108 protein,9 as already described.2 10 After v-fes infection, bulk cell population was washed twice in MCSF-free medium, resuspended in the same medium, and plated into 96-well multiculture plates at 0.3 cells in 0.2 mL per well. After 3 to 4 weeks of incubation, clones were transferred into 24-well multiculture plates and incubated in the same medium (1 mL per well). After reaching the confluence, clones were transferred to culture plates and expanded in the presence of MCSF (as indicated below). Following v-fes infection, frequency of appearence of MCSF-independent clones was 26.4%. No clone developed after limiting dilution of mock-infected, control cells, whose bulk cultures were therefore used (KB cells). Twenty-three v-fes–expressing MCSF-independent clones were isolated and expanded. The level of v-fes expression was homogeneous in all clones tested. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; EuroClone, Celbio Biotecnologie, Pero, MI, Italy) supplemented with 4-mmol/L glutamine (EuroClone), 10% fetal bovine serum (FBS; EuroClone), and 6-ng/mL murine recombinant, affinity-purified MCSF.
Determination of cell growth
KB and VFB cells were seeded (10 000 cells/well in 96-well plates) in DMEM supplemented with 10% FBS, in the absence or the presence of 30-μmol/L PD98059 (Calbiochem-Novabiochem, La Jolla, CA) dissolved in dimethyl sulfoxide (DMSO), and incubated for 3 days. Medium was removed, and 10% ice-cold trichloroacetic acid was added and removed after 1 hour at 4°C; cell monolayers were then washed 5 to 6 times with water and allowed to dry. Sulforodamine-B (Sigma-Aldrich, Milan, Italy) solution (0.4% in 1% acetic acid; 75 μL/well) was then added. After 30 minutes at room temperature, cell monolayers were washed 5 to 6 times with 1% acetic acid and allowed to dry. The cell-bound sulforodamine-B was solubilized in 150-μL/well 10-mmol/L Tris and the absorbance of solution spectrophotometrically measured (595 nm). Cell numbers corresponding to absorbance values were calculated according to a titration curve. Cell numbers determined after a 3-hour incubation (time necessary to allow cell adhesion after plating) were considered as time-zero values.
Antibodies
Antibodies included mouse monoclonal α-pERK (Biotechnology, Santa Cruz, CA), reacting with phospho-Tyr(Y)204 and phospho-Y185 of ERK1 and ERK2, respectively; rabbit polyclonal α-pERK1/pERK211 12 (New England Biolabs, Hitchin, UK), reacting with phospho-Thr202 and phospho-Y204 of ERK1 and with phospho-Thr183 and phospho-Y185 of ERK2; rabbit polyclonal α-ERK1 (Santa Cruz), reacting with ERK1 and, to a lower extent, ERK2; rabbit polyclonal α-pMEK1/2 (New England Biolabs), reacting with MEK1/2 only when activated by phosphorylation at Ser217/221; rabbit polyclonal α-MEK1/2 (New England Biolabs); α-mouse immunoglobulin (Ig)G and α-rabbit IgG secondary antibodies, horseradish peroxidase (HRP)-conjugated, for immunoblotting (Sigma); and Cy3-conjugated donkey α-rabbit IgG secondary antibody for immunostaining (Chemicon International, Temecula, CA).
Determination of ERK1/2 and MEK1/2 expression and phosphorylation
Cells were incubated for 16 to 18 hours in DMEM supplemented with 10% FBS. Culture plates were then placed on ice, cell monolayers rapidly washed 3 times with ice-cold phosphate-buffered saline (PBS) containing 100-μmol/L orthovanadate, cells removed by scraping in 1 mL of ice-cold PBS containing 100-μmol/L orthovanadate, and collected by centrifugation (300g, 10 minutes, 4°C). Cell pellet was solubilized in ice-cold lysis buffer (5-mmol/L ethylenediaminetetraacetic acid, 50-mmol/L NaCl, 10 mM of Tris, 1% Triton X-100, 50-mmol/L NaF, 30-mmol/L Na4P2O7, 1-mmol/L Na3VO4, 1-mmol/L phenylmethylsulfonylfluoride, 0.1-U/mL aprotinin, 4-μg/mL pepstatin A, and 10-μg/mL N-tosyl phenylalanine chloromethyl-ketone) and Triton-insoluble material removed by centrifugation (20 000g, 30 minutes, 4°C). Protein concentration in supernatants was determined, and 30 μg aliquots of each sample were diluted with 4×Laemmli buffer and boiled for 10 minutes in the presence of 100-mmol/L 2-mercaptoethanol.
Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 12.5% or 15% minigels and then transferred onto nitrocellulose membranes (Hybond-ECL, Amersham Life Science, Little Chalfont, UK) by electroblotting. To determine ERK1/2 tyrosine phosphorylation, membranes were blocked in PBS containing 0.1% Tween 20 (TPBS) and 1% bovine serum albumin (BSA) (3 to 5 hours at room temperature), incubated in a 1:1000 dilution of mouse or rabbit α-pERK in TPBS/1%BSA (16 to18 hours, 4°C), and then incubated in a 1:5000 dilution of HRP-conjugated antimouse IgG in TPBS/5%BSA or in a 1:10 000 dilution of HRP-conjugated antirabbit IgG in TPBS/2%BSA (1 hour, 4°C). Antibody-coated protein bands were visualized by ECL detection. To determine ERK1/2 expression, membranes were stripped, blocked, and reprobed by incubating in a 1:1000 dilution of rabbit α-ERK in TPBS/5%BSA (1 hour at room temperature) and then in a 1:10 000 dilution of HRP-conjugated α-rabbit IgG in TPBS/2%BSA (1 hour at room temperature). To determine MEK1/2 phosphorylation, membranes were blocked in TPBS/1%BSA (3 to 5 hours at room temperature), incubated in a 1:1000 dilution of rabbit α-phospho-MEK1/2 in TPBS/1%BSA (16 to 18 hours at room temperature), and then incubated in a 1:10 000 dilution of HRP-conjugated antirabbit IgG in TPBS/2%BSA (1 hour at room temperature). To determine MEK1/2 expression, membranes were stripped, blocked, and reprobed by incubating in a 1:1000 dilution of rabbit α-MEK1/2 in TPBS/5%BSA (1 hour at room temperature) and then in a 1:10 000 dilution of HRP-conjugated α-rabbit IgG in TPBS/2%BSA (1 hour at room temperature).
Immunocytochemistry
Cells were seeded in 6-well plates, cultured for 24 hours in DMEM supplemented with 10% FBS, and incubated or not with 30-μmol/L PD98059 for 90 minutes. Immunocytochemistry was performed as suggested by New England Biolabs. Briefly, after a wash with PBS, cell monolayers were fixed by an incubation for 20 minutes at 4°C in PBS containing 3% paraformaldehyde. After 3 washes of 5 minutes each with PBS containing 1% Triton X-100 (PBST), cells were incubated for 45 to 60 minutes in PBST containing 5.5% horse serum (Celbio). Cells were then incubated in a 1:250 dilution of rabbit α-pERK or in a 1:500 dilution of rabbit α-ERK in PBS/3%BSA (70 μL per sample; overnight, 4°C). Cells were then washed (2×15 minutes) with PBST and incubated in a 1:200 dilution of Cy3-conjugated α-rabbit IgG in PBST/3%BSA (1 hour at room temperature). Cells were finally washed (3×5 minutes) with PBST and examined by epifluorescence with excitation-emission filters for rhodamine. Specificity was checked (not shown) by (1) fixing cells with 4% paraformaldehyde (20 minutes at room temperature) or with methanol/acetone (3:7 vol/vol, 10 minutes, −20°C), which produced the same results and (2) incubating with secondary antibody alone, which did not produce any significant fluorescence.
Results
Increased constitutive activation of ERK1 and ERK2 in macrophages overexpressing v-fes
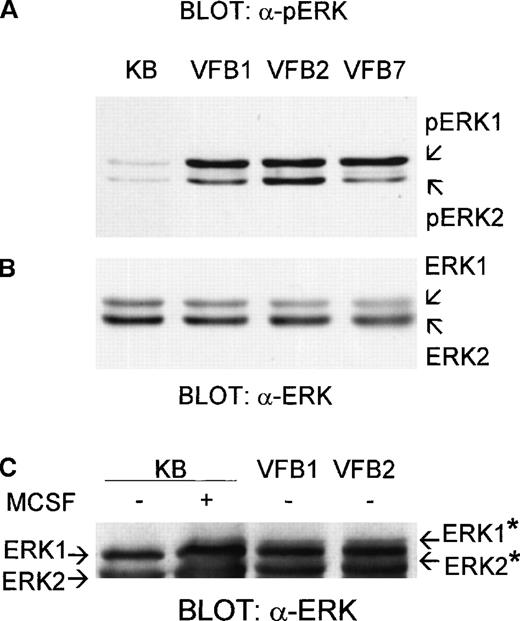
To investigate the involvement of the MAPK pathway in the v-fes–induced macrophage growth, the level of ERK phosphorylation in v-fes–overexpressing clones of BAC-1.2F5 cells was determined using an antibody raised against both phosphorylation sites required for activation of ERK1 and ERK2 (Figure1A). In control, mock-infected KB cells, incubated for 16 to 18 hours in the absence of MCSF to suppress mitogenic stimuli, ERK1 and ERK2 were found weakly phosphorylated, like in BAC-1.2F5 parental cells.13 14 All v-fes–overexpressing clones exhibited a hyperphosphorylation of ERK1 and ERK2. Densitometry of bands obtained in 4 independent experiments revealed that the average phosphorylation of ERK1 was about 5-fold, and of ERK2 3-fold, higher in v-fes–overexpressing clones than in KB cells. Identical results were obtained using an antibody reacting with the phospho-Y204 and phospho-Y185 of ERK1 and ERK2, respectively (not shown). The amount of ERK proteins was comparable in all clones and KB cells (Figure 1B). The detection of ERK hyperphosphorylation in all v-fes–overexpressing clones excluded that it was a random effect of the infection procedure. Figure1C shows that the electrophoretic mobility of ERK1 and ERK2 was constitutively reduced in v-fes–overexpressing clones to the same extent as in MCSF-stimulated KB cells.
Constitutive phosphorylation of ERK1 and ERK2 in v-fes–overexpressing macrophages.
VFB or KB cells were incubated for 16 to 18 hours in DMEM supplemented with 10% FBS and lysed. Equal amounts of proteins were separated by SDS-PAGE in a 12.5% gel and immunoblotted with polyclonal α-pERK antibody (A) or, after stripping, α-ERK antibody (B) or in a 15% gel and immunoblotted with α-ERK antibody (C). Arrows indicate the positions of ERK, pERK, or ERK* (migration-retarded ERK) bands.
Constitutive phosphorylation of ERK1 and ERK2 in v-fes–overexpressing macrophages.
VFB or KB cells were incubated for 16 to 18 hours in DMEM supplemented with 10% FBS and lysed. Equal amounts of proteins were separated by SDS-PAGE in a 12.5% gel and immunoblotted with polyclonal α-pERK antibody (A) or, after stripping, α-ERK antibody (B) or in a 15% gel and immunoblotted with α-ERK antibody (C). Arrows indicate the positions of ERK, pERK, or ERK* (migration-retarded ERK) bands.
Increased constitutive activation of MEK in macrophages overexpressing v-fes
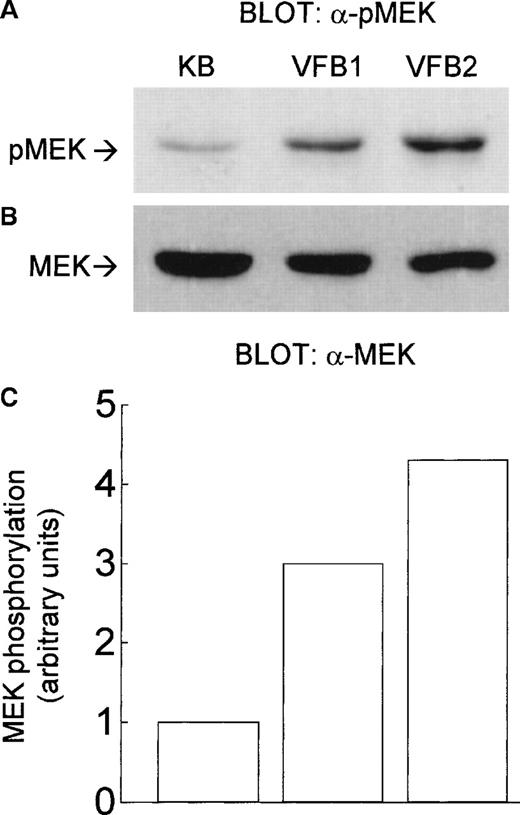
Because ERK1 and ERK2 are activated by MEK, we tested whether MEK is also activated in v-fes–overexpressing clones. Using an antibody reacting with phosphorylated/activated MEK, MEK phosphorylation was found constitutively increased in these clones with respect to KB cells (Figure 2A). The amount of MEK protein was comparable in the clones and KB cells (Figure 2B). MEK phosphorylation was estimated, by densitometry of bands, to be 3-fold higher in the VFB1 clone and 4.2-fold higher in the VFB2 clone than in KB cells (Figure 2C).
Constitutive phosphorylation of MEK in v-fes–overexpressing macrophages.
VFB or KB cells were incubated for 16 to 18 hours in DMEM supplemented with 10% FBS and lysed. Equal amounts of proteins were separated by SDS-PAGE in a 12.5% gel and immunoblotted with α-pMEK1/2 antibody (A) or, after stripping, with α-MEK antibody (B). The graph in C represents the -fold increase of MEK phosphorylation/activity in VFB with respect to KB cells, as determined by band densitometry (arbitrary units, MEK phosphorylation of KB cells being assumed = 1); values referring to MEK phosphorylation (A) were normalized on the basis of those of MEK expression (B).
Constitutive phosphorylation of MEK in v-fes–overexpressing macrophages.
VFB or KB cells were incubated for 16 to 18 hours in DMEM supplemented with 10% FBS and lysed. Equal amounts of proteins were separated by SDS-PAGE in a 12.5% gel and immunoblotted with α-pMEK1/2 antibody (A) or, after stripping, with α-MEK antibody (B). The graph in C represents the -fold increase of MEK phosphorylation/activity in VFB with respect to KB cells, as determined by band densitometry (arbitrary units, MEK phosphorylation of KB cells being assumed = 1); values referring to MEK phosphorylation (A) were normalized on the basis of those of MEK expression (B).
Nuclear localization of ERK in macrophages overexpressing v-fes
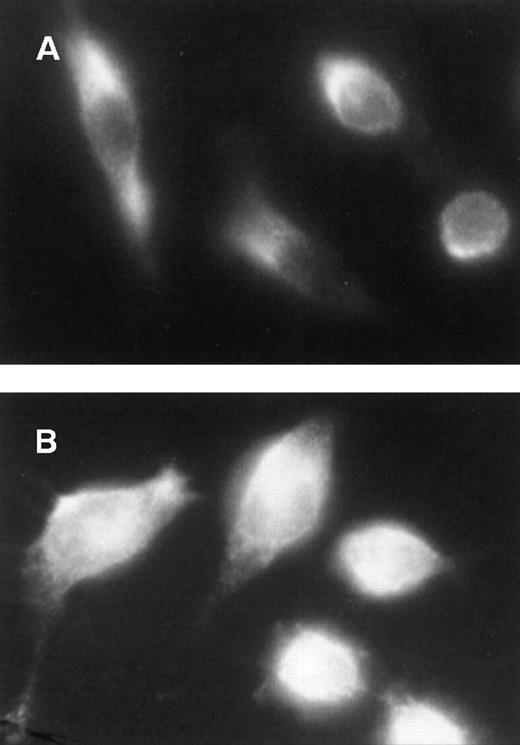
In resting cells, ERK is mostly cytoplasmic,6 and mitogenic stimuli cause the translocation of activated ERK into the nucleus.5 6 We therefore expected ERK to be constitutively present in the nucleus of v-fes–overexpressing cells. Immunocytochemistry with anti-ERK antibodies showed that ERK was mainly located in the cytoplasm of unstimulated KB cells (Figure3A), while it was detectable in both the nucleus and the cytoplasm of v-fes–overexpressing cells (Figure 3B).
Constitutive nuclear localization of ERK in v-fes–overexpressing macrophages.
KB (A) or VFB (B) cells were incubated for 16 to 18 hours in DMEM supplemented with 10% FBS and immunostained with α-ERK rabbit antibody, followed by Cy3-conjugated α-rabbit antibody.
Constitutive nuclear localization of ERK in v-fes–overexpressing macrophages.
KB (A) or VFB (B) cells were incubated for 16 to 18 hours in DMEM supplemented with 10% FBS and immunostained with α-ERK rabbit antibody, followed by Cy3-conjugated α-rabbit antibody.
Inhibition of v-fes–dependent mitogenesis and ERK hyperphosphorylation by PD98059
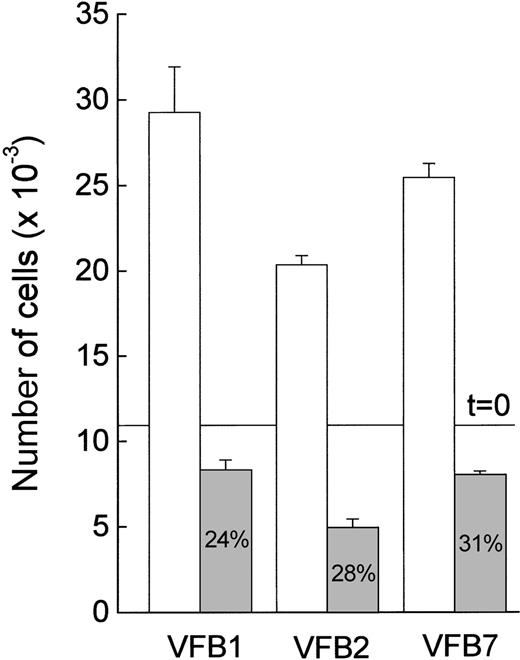
The relationship between v-fes overexpression and the involvement of the MAPK pathway in mitogenesis was further investigated by measuring cell growth in the presence of PD98059. This is a specific MEK inhibitor that acts by binding inactive MEK, thereby preventing its activation by upstream kinases.15 After a 3-day incubation with PD98059, the growth of v-fes–overexpressing clones was strongly inhibited (Figure 4). DMSO alone did not significantly interfere with cell growth (not shown).
Effect of PD98059 on the growth of v-fes–overexpressing macrophages.
VFB cells were plated in 96-well culture plates at 10 000 cells/well, and the accuracy of plating was verified 3 hours later (t = 0 line). Cells were incubated in DMEM supplemented with 10% FBS in the absence (white columns) or the presence (gray columns) of 30-μmol/L PD98059 and counted 3 days later. Data are means ± SEM of triplicate samples of a typical experiment.
Effect of PD98059 on the growth of v-fes–overexpressing macrophages.
VFB cells were plated in 96-well culture plates at 10 000 cells/well, and the accuracy of plating was verified 3 hours later (t = 0 line). Cells were incubated in DMEM supplemented with 10% FBS in the absence (white columns) or the presence (gray columns) of 30-μmol/L PD98059 and counted 3 days later. Data are means ± SEM of triplicate samples of a typical experiment.
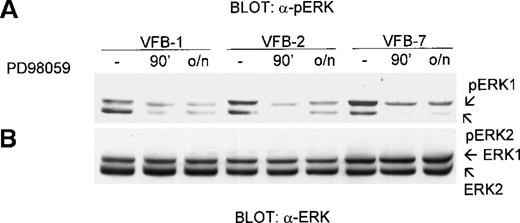
The effect of PD98059 on ERK phosphorylation in v-fes–overexpressing clones is shown in Figure5A. The 90 minutes or overnight treatment of cells with PD98059 markedly inhibited ERK phosphorylation under conditions in which ERK expression was not altered (Figure 5B). DMSO alone had no effect on ERK phosphorylation (not shown). ERK phosphorylation, as determined by densitometry of bands obtained in 3 independent experiments, was reduced by PD98059, on the average, by 70%. Higher doses of PD98059 (60 μM) produced the same results (not shown). The low constitutive level of ERK phosphorylation in KB cells was reduced further by PD98059 treatment for either 90 minutes or overnight (not shown).
Effect of PD98059 on ERK phosphorylation in v-fes–overexpressing macrophages.
VFB cells were incubated for 16 to 18 hours in DMEM supplemented with 10% FBS and treated or not for 30 minutes or overnight (o/n) with 30-μmol/L PD98059. Equal amounts of cell lysates were separated by SDS-PAGE in a 12.5% gel and immunoblotted with polyclonal α-pERK antibody (A) or, after stripping, α-ERK antibody (B).
Effect of PD98059 on ERK phosphorylation in v-fes–overexpressing macrophages.
VFB cells were incubated for 16 to 18 hours in DMEM supplemented with 10% FBS and treated or not for 30 minutes or overnight (o/n) with 30-μmol/L PD98059. Equal amounts of cell lysates were separated by SDS-PAGE in a 12.5% gel and immunoblotted with polyclonal α-pERK antibody (A) or, after stripping, α-ERK antibody (B).
Effects of PD98059 on nuclear localization of ERK and pERK
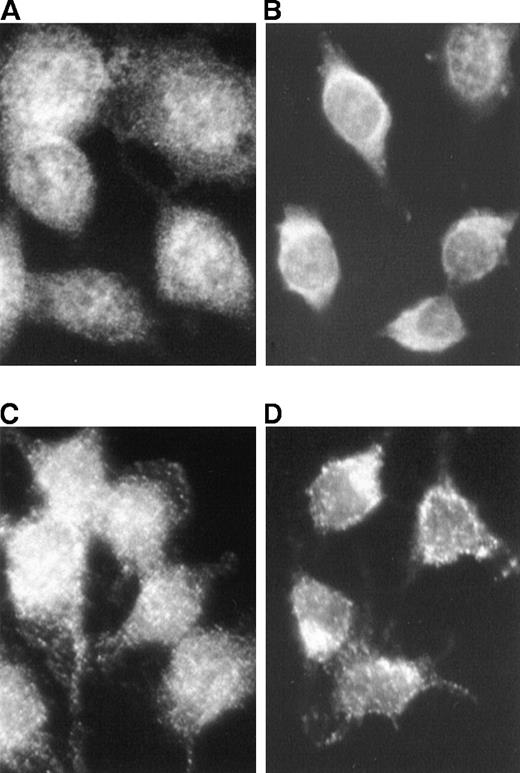
The effect of PD98059 on the constitutive presence of ERK in the nucleus of v-fes–overexpressing cells was determined. Immunocytochemistry with both anti-ERK and anti-pERK antibodies showed a marked reduction of ERK (Figure 6B) and pERK (Figure 6D) in the nuclei of cells treated with PD98059 for 90 minutes in comparison with untreated cells (Figures 6A and 6C, respectively).
Effect of PD98059 on ERK and pERK localization in v-fes–overexpressing macrophages.
VFB cells were incubated for 16 to 18 hours in DMEM supplemented with 10% FBS and treated (B, D) or not (A, C) for 90 minutes with 30-μmol/L PD98059. Immunostaining was performed with α-ERK (A, B) or polyclonal α-pERK rabbit antibody (C, D), followed by Cy3-conjugated α-rabbit antibody.
Effect of PD98059 on ERK and pERK localization in v-fes–overexpressing macrophages.
VFB cells were incubated for 16 to 18 hours in DMEM supplemented with 10% FBS and treated (B, D) or not (A, C) for 90 minutes with 30-μmol/L PD98059. Immunostaining was performed with α-ERK (A, B) or polyclonal α-pERK rabbit antibody (C, D), followed by Cy3-conjugated α-rabbit antibody.
Discussion
In macrophages, the substrates of Fes so far identified are involved in cell adhesion and cell-cell signaling but not mitogenesis.2 3 We report here the first biochemical characterization of the linkage between Fes and mitogenesis in macrophages, showing that the MEK/ERK pathway mediates the growth factor–independent proliferation of macrophages overexpressing v-fes. Both ERK1 and ERK2 were constitutively phosphorylated in all v-fes– (Figure 1) as well as c-fes– (E. Rovida, unpublished results) overexpressing macrophages, indicating that this is an effect of the Fes kinase itself and not only of its oncogenically activated counterpart.
The possibility of a direct effect of Fes on ERK, explaining its constitutive phosphorylation, was evaluated. The finding that MEK was also constitutively phosphorylated in Fes-overexpressing cells suggests that ERK phosphorylation depends on the activation of the MEK/ERK cascade. This was confirmed by the reduction to 30% (a value in keeping with those obtained by others) of ERK phosphorylation following treatment with the MEK inhibitor PD98059. The effect of PD98059 also led us to exclude that ERK activation depends on the inhibition by overexpressed Fes of phosphatase(s) normally controlling ERK phosphorylation. The fact that ERK phosphorylation is still phosphatase-regulated was also indicated by the observation (data not shown) that in the presence of tyrosine-phosphatase inhibitors the level of ERK phosphorylation in v-fes–overexpressing clones was much higher than that shown in Figure 1A and identical in clones and KB cells.
The findings reported in this paper are also relevant to the understanding of the role of MEK/ERK pathway in macrophages, where a link between ERK activation and proliferation has not been conclusively established.14,16,17 ERK activation follows MCSF treatment in BAC-1.2F5 cells,14 and ERK activity was found 2-fold higher in cycling than in growth-arrested bone marrow macrophages, while the MEK inhibitor PD98059 reduced MCSF-stimulated DNA synthesis.18 On the other hand, we previously found that, in v-raf–overexpressing MCSF-independent clones of BAC.12F5 cells, the level of ERK activation is not constitutively higher than in parental cells, while ERK activation induced by MCSF in parental cells is suppressed.16 Furthermore, ERK activity appears to control cyclin D1 messenger RNA and c-myc RNA expression in MCSF-treated NIH-3T3 cells expressing ectopic MCSF receptor but not in MCSF-stimulated bone marrow macrophages.18 19
The prolonged presence of activated ERK in the nucleus correlates with cell cycle progression and mitogenesis. In our study, ERK was constitutively present in the nucleus of v-fes–overexpressing macrophages, and PD98059 reduced the nuclear amount of both pERK and ERK. These findings are in keeping with the involvement of MEK/ERK cascade in the mitogenic effect of v-fes overexpression. Furthermore, nuclear retention of ERK requires not only ERK activation but also neosynthesis of ERK-anchoring proteins.20 The fact that ERK was able to exit the nucleus following PD98059 treatment indicates that the mechanisms controlling its transport back to the cytoplasm are not impaired. Thus, the constitutive presence of ERK in the nucleus of v-fes–overexpressing macrophages is a regulated event that depends on a sustained MEK activation.
The link between ERK activation and proliferation of v-fes–overexpressing macrophages is further supported by the PD98059-induced reduction of cell growth to 30% of control, a value quantitatively similar to that of the long-sustained effect of PD98059 on ERK phosphorylation. The fact that in v-fes–overexpressing macrophages the level of ERK activation was moderate is very well in keeping with previous results indicating that a moderate activation of the MAPK cascade promotes proliferation, whereas its strong activation causes cell cycle arrest. This was shown in murine fibroblasts,21-23 small-cell lung carcinoma lines,24 and human prostate carcinoma cells.25
Acknowledgment
The authors thank Professor Massimo Olivotto, Department of Experimental Pathology and Oncology, University of Florence, for moral and material support during all phases of this work.
Supported by grants from the Italian Ministero della Università e della Ricerca Scientifica e Tecnologica (funds 60% and 40%), Associazione Italiana per la Ricerca sul Cancro, and Regione Toscana (Progetto Qualità).
Reprints:Persio Dello Sbarba, Dipartimento di Patologia e Oncologia Sperimentali, Università di Firenze, viale G.B. Morgagni 50, 50134 Firenze, Italy; e-mail: persio@unifi.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal