Abstract
In normal T-cell development, IL-7 plays a nonredundant role as an antiapoptic factor by regulating Bcl-2 expression in pro-T cells. In the current study, we addressed the roles of IL-7 and related cytokines as apoptosis-modulating factors in precursor T-cell acute lymphoblastic leukemia (T-ALL). To this end, leukemic blasts from pediatric patients with T-ALL were prospectively investigated as to their responsiveness to IL-7, IL-4, and IL-2 (in terms of modulation of spontaneous apoptosis, assessed by flow cytometry), cytokine receptor expression profiles, and expression levels of Bcl-2 and Bax proteins. IL-7, in contrast to IL-4 and IL-2, was highly efficient in apoptosis inhibition , and this effect correlated with the expression levels of IL-7R chain and with the up-regulation of Bcl-2 protein expression (P< .0001). Subclassification of T-ALL samples (n = 130) according to their in vitro IL-7 responses revealed that IL-7 refractory samples were more frequently positive for CD34 (P< .0001) and the myeloid-associated antigen CD33 (P= .01), whereas IL-7 responsiveness was associated with an expression of more mature differentiation-associated T-cell antigens (CD1a, surface CD3, CD4/8; P < .05). Furthermore, the extent of apoptosis inhibition by IL-7 in vitro quantitatively correlated with early cytoreduction as determined by the prednisone peripheral blood response on day 8 and cytoreduction in the marrow on day 15 (n = 87;P < .05). Multivariate analysis of the apoptosis-related parameters investigated, including spontaneous apoptosis, its inhibition by IL-7, and expression levels of Bcl-2 and Bax, showed that only IL-7 responsiveness has an independent impact on early cytoreduction (P < .05), thus indicating a potential prognostic relevance of IL-7 sensitivity in T-ALL.
In normal hematopoiesis, responsiveness of progenitor cells to cytokine signaling for proliferation, death, and survival strongly depends on their lineage commitment and differentiation state.1-3 Normal T-lineage development has recently been shown to depend crucially on the so-called common γ chain of the IL-2 receptor (IL-2Rγ) shared by the receptors for cytokines IL-2, IL-4, IL-7, IL-9, and IL-15.4 Early T-cell development was found to be severely impaired in IL-2Rγ chain knock-out mice,4and the absence or markedly reduced number of T cells in patients with X-linked severe combined immunodeficiency (X-SCID) has been associated with γ-chain mutations.5 However, most of these cytokines seem to be functionally redundant because mice deficient in single cytokines revealed unimpaired lymphopoiesis.6,7 The only exception is IL-7, which, when deficient in mice, has been shown to result in a drastically reduced number of thymocytes, though with a normal subset distribution.8,9 The critical role of IL-7 for early T-cell development in humans has also been demonstrated in chimeric human–mouse thymus organ culture models in which antibodies blocking IL-7 and IL-7R have been used.3 10
The analysis of thymocytes from IL-7–deficient mice disclosed that their developmental arrest occurs at the triple-negative (CD3−, CD4−, CD8−) stages.9 These cells revealed increased rates of spontaneous apoptosis associated with decreased levels of the anti-apoptotic protein Bcl-2 and increased levels of the proapoptotic Bcl-2 homolog Bax.11,12 Treatment of triple-negative thymocytes with IL-7 resulted in the up-regulation of Bcl-2 expression and the inhibition of apoptosis.12Moreover, the overexpression of Bcl-2 in IL-7–deficient mice largely restored normal T-cell development, suggesting that IL-7 may exert its survival function by the up-regulation of Bcl-2.11
Acute leukemia results from a clonal expansion of hematopoietic progenitor cells that have undergone malignant transformation at distinct stages of differentiation.13 Because leukemia cells may retain certain features of their normal counterparts, their characterization with respect to cytokine responsiveness can provide valuable information about the differentiation state of malignant cells and their dependence on the microenvironment. Previous studies14-16 investigated the effects of IL-7, IL-4, and IL-2 regarding the induction of proliferation in childhood T-lineage acute lymphoblastic leukemia (T-ALL), and their potential as growth factors has been pointed out. However, the roles of these cytokines as apoptosis-modulating factors in T-ALL have not yet been studied in detail.
Therefore, in the current study we investigated cytokine responsiveness, receptor expression profiles, and the expression of apoptosis-regulating Bcl-2 and Bax proteins in leukemic blasts from pediatric patients with T-ALL. IL-7, in contrast to IL-4 and IL-2, was highly efficient in apoptosis inhibition, and this effect correlated with the expression levels of the IL-7Rα chain and the up-regulation of Bcl-2 protein expression. Given these findings, a large series of T-ALL samples was classified according to in vitro IL-7 responses and examined for immunophenotypic features (n = 130) and early therapy response in vivo (n = 87). Our data demonstrating significant correlations of IL-7 responsiveness in vitro with maturational stage and early response to initial therapy in vivo point to a potential prognostic relevance for the determination of IL-7 sensitivity in T-ALL.
Patients, materials, and methods
Patient population
Leukemic samples from 130 children with T-lineage ALL who were enrolled in the ALL–Berlin-Frankfurt-Münster (BFM) 95 trial were prospectively investigated for the current study. Diagnosis of ALL was based on morphology and on cytochemical and immunophenotypic features.17 18
All leukemic samples were characterized by immunophenotype. Immunophenotyping was carried out on leukemic blasts isolated by standard Ficoll–Hypaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation, and cell-surface as well as intracytoplasmic antigens were detected by a panel of monoclonal antibodies either by direct or indirect immunofluorescence techniques as previously described.19,20 The criteria for marker positivity and for the subclassification of T-lineage ALL (pro-, pre-, cortical, and mature T-ALL) were adopted from the guidelines proposed by the European Group for the Immunological Characterization of Leukemias.18 In the current study, 28 samples were subclassified as pro-T–/pre-T–ALL, 79 samples as cortical T-ALL, and 23 samples as mature T-ALL.
Data on early responses to initial therapy, as measured by the in vivo corticosteroid response in peripheral blood on day 8 and the response in the bone marrow on day 15, were available for 87 and 62 patients, respectively. Therapy in the first 14 days consisted of the administration of intrathecal methotrexate (on day 1), prednisone (daily, in rapidly increasing dosages from day 1), vincristine and daunorubicin (on day 8), and asparaginase (on day 12).21 If a patient had more than 1000 blood blasts/μL on day 8, it was defined as a prednisone-poor response.
Cell culture
Freshly obtained leukemic blasts were purified either from bone marrow (n = 113) or peripheral blood (n = 17) patient samples by density gradient centrifugation using Ficoll–Hypaque separation (Pharmacia). Cell viability was always more than 90%, as determined by trypan blue or propidium iodide (PI) exclusion (Sigma, Deisenhofen, Germany). All samples contained more than 90% leukemic cells based on morphologic and immunophenotypic criteria.
Leukemic cells were maintained in RPMI 1640 (Biochrom, Berlin, Germany) standard medium containing 2 mmol/LL-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin and supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco BRL, Paisley, UK). Functional assays (spontaneous apoptosis and its modulation by cytokines) performed with samples from several patients in the presence and absence of FCS showed similar results.
To assess spontaneous apoptosis, 0.5 × 106 leukemic cells/well were cultured in 96-well microtiter plates (Nunc, Roskilde, Denmark) in standard medium at 37°C in a humidified atmosphere of 5% CO2 in air. To investigate cytokine-mediated effects, leukemic cells were cultured in the presence of recombinant human cytokines IL-7 (25 ng/mL), IL-4 (50 ng/mL), or IL-2 (25 ng/mL) (Pharma Biotechnologie Hannover, Hannover, Germany) at concentrations found to be optimal in different T-cell systems22 23 and in our own titration experiments (not shown). In several experiments, blocking anti–IL-7 rabbit polyclonal antibodies (10 μg/mL; Pharma Biotechnologie Hannover) were used.
Assessment of apoptosis and cell cycle distribution
To determine the extent of apoptosis, cells were stained with fluorescein isothiocyanate (FITC)-conjugated annexin V and PI using the annexin V kit (Immunotech, Marseille, France) as recommended by the manufacturer. Thereafter, samples were analyzed by flow cytometry (FACScan; Becton Dickinson, San Jose, CA) for the presence of viable (annexin V- and PI-negative), early apoptotic (annexin V-positive, PI-negative), and late apoptotic (annexin V- and PI-positive) cells. The extent of apoptosis (N%) was quantified as the percentage of annexin V-positive cells. The extent of spontaneous apoptosis was calculated as the difference of N% values in leukemic samples before and after culture in medium, ΔNspont. Leukemic cell responsiveness to cytokines was evaluated in terms of cytokine-specific apoptosis modulation, which was calculated as the difference between the extent of apoptosis after culturing in the absence and presence of the respective cytokine (ΔNIL). In a number of experiments (n = 38), apoptotic cells were identified, in addition to the annexin/PI method, by their subdiploid DNA content.24
To study cell cycle distributions, cells were fixed and stained as described elsewhere.24 Briefly, a pellet of 5 × 105 cells was fixed by adding 2 mL ice-cold 70% ethanol for at least 1 hour at 4°C. After washing, the cells were resuspended in 0.5 mL phosphate-buffered saline containing 50 μg/mL PI, pH 7.5. After treatment with 10 μL of 10 mg/mL RNase (type I-A; Boehringer Mannheim, Mannheim, Germany) for 30 minutes at room temperature in the dark, the cells were stored at 4°C until flow cytometric analysis. Cell cycle analysis was carried out using either CellQuest (Becton Dickinson) or ModFit LT (Verity, Topsham, ME) software.
Using flow cytometry, 10 000 and 20 000 cells were characterized for apoptosis and cell cycle distribution analysis, respectively. All tests were performed in duplicate. In addition to flow cytometric analysis, the total number of viable cells in a series of samples was estimated by trypan blue exclusion.
Assessment of surface expression of interleukin receptors
IL-2Rα (CD25), IL-7Rα (CDw127), and IL-2Rγ (CD132) were detected by direct immunofluorescence using mouse monoclonal antibodies conjugated either with FITC (CD25, clone 3G-10; Medac, Hamburg, Germany) or phycoerythrin (CDw127, clone R34.34; Immunotech; CD132, clone AG184; Pharmingen, Hamburg, Germany). IL-4Rα was detected by indirect immunofluorescence using a specific monoclonal antibody (CDw124, clone S4-56C9; Immunotech) and FITC-conjugated goat antimouse polyclonal antibodies (Medac). Isotype-matched irrelevant mouse antibodies (Immunotech) were used as negative controls to determine background fluorescence. Antigen expression was quantified by flow cytometry in units of molecules of equivalent soluble fluorochrome (MESF) using calibration beads as fluorescence standards (DAKO FluoroSpheres; DAKO, Glostrup, Denmark).
Assessment of Bcl-2 and Bax expression
To evaluate the expression of the intracellular proteins Bcl-2 and Bax, leukemic cells were fixed and permeabilized using the Fix & Perm kit (An-der Grub, Kaumberg, Austria) according to the manufacturer's instructions. Bcl-2 antigen was detected by the FITC-conjugated anti–Bcl-2 antibody clone 124 (DAKO). FITC-conjugated irrelevant mouse antibodies of the appropriate immunoglobulin isotype (Immunotech) were used as negative controls.
To detect Bax, Bax-specific rabbit polyclonal antibodies raised against synthetic peptide sequences (I-19; Santa Cruz Biotechnology, Santa Cruz, CA) and FITC-conjugated goat antirabbit serum (Medac) as a secondary staining reagent were used. For negative controls, cells were stained with Bax antibody in the presence of the blocking synthetic peptide (provided by the manufacturer, Santa Cruz Biotechnology) at saturation conditions (10 μg peptide vs 1 μg antibody), as determined by peptide titration experiments (data not shown).
Immunofluorescence analysis was performed on a FACScan (Becton Dickinson). In cell samples with ongoing apoptosis, only antigen expression in viable and early apoptotic cells has been considered; late apoptotic cells might have had reduced protein content because of cell disintegration. Viable and early apoptotic cells were differentiated from late apoptotic cells by the higher forward-scatter and lower side-scatter intensities.25 26
Antigen expression was quantified by flow cytometry in MESF units using calibration beads as fluorescence standards (DAKO). Relative change of expression (RCE) of Bcl-2 and Bax resulting from culturing in standard medium was quantified as the ratio RCEspont = MESFmedium/MESF0h. IL-7–specific RCE of Bcl-2 and Bax was calculated from the ratio of antigen expression levels in IL-7–treated and untreated cells, so that RCEIL-7 = MESFIL-7/MESFmedium.
Statistical analysis
Mean values are given as mean ± SEM. Differences (Pvalues) were evaluated using the 2-tailed, nonparametric Mann–WhitneyU test for continuous variables and the Fisher exact test for categorical variables. Associations (correlation coefficient [r] and probability [P]) of the extent of spontaneous or IL-7–modulated apoptosis with protein expression levels in leukemic cells and blast counts in T-ALL patients were evaluated using Spearman correlation statistics. Trend lines were calculated using linear regression statistics. Differences were considered significant for P < .05. Statistical analysis was performed using SPSS software.
Results
IL-7, but not IL-4 or IL-2, is a highly potent inhibitor of spontaneous apoptosis in T-ALL
Leukemic samples were investigated for their responsiveness to cytokines according to the inhibition or enhancement of spontaneous apoptosis (Figure 1). To determine the optimal incubation time periods, leukemic cell samples (n = 16) were initially examined by flow cytometry after incubation periods of 24 and 72 hours. In all patients, cytokine responses in terms of apoptosis modulation and cell cycle distribution changes (see below) measured after 24 and 72 hours were similar. However, the extent of spontaneous cell death, measured after 24 hours in individual samples, was highly variable (range, 6%-75%; mean, 33% ± 3%). Therefore, samples with high rates of spontaneous apoptosis could not be evaluated after 72 hours (data not shown). In addition, the total number of viable cells was estimated after 3 and 7 days of culture. At day 7, leukemic samples contained 50% to 100% of dead cells, and the total number of cells did not exceed 20% of the initial number of 5 × 105 cells/well. Consequently, 24-hour incubation periods were found to be optimal for standardizing cytokine response studies in a larger series of T-ALL samples.
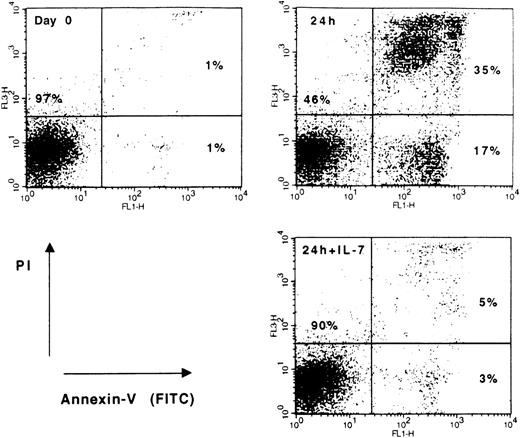
Flow cytometric analysis of spontaneous apoptosis and cytokine effects in leukemic cells from a patient with cortical T-ALL.
Blast cells were either cultured in medium alone or treated with 25 ng/mL rIL-7 for 24 hours. Freshly isolated (day 0) and cultured (24 hours; 24 hours +IL-7) cells were stained with annexin V-FITC and PI to differentiate between viable (lower-left quadrant) and apoptotic (lower- and upper-right quadrants) cells. Data shown are representative of T-ALL responsive to IL-7.
Flow cytometric analysis of spontaneous apoptosis and cytokine effects in leukemic cells from a patient with cortical T-ALL.
Blast cells were either cultured in medium alone or treated with 25 ng/mL rIL-7 for 24 hours. Freshly isolated (day 0) and cultured (24 hours; 24 hours +IL-7) cells were stained with annexin V-FITC and PI to differentiate between viable (lower-left quadrant) and apoptotic (lower- and upper-right quadrants) cells. Data shown are representative of T-ALL responsive to IL-7.
Apoptosis-modulating effects of IL-7, IL-4, and IL-2 were investigated in leukemic samples from 38 patients. As Table1 indicates, IL-2 was unable to inhibit apoptosis in leukemic blasts, and IL-4 prevented spontaneous apoptosis in only a few (7 of 37) patients. By contrast, IL-7 inhibited apoptosis (ΔNIL-7, >5%) in most T-ALL (24 of 38 patients; 63%) patients. Apoptosis induction by IL-2 and IL-4 was observed in 1 and 2 patients, respectively (Table 1).
Differential effects of cytokines on spontaneous apoptosis in T-ALL
| Apoptosis-modulating effects* . | IL-2 (%) . | IL-4 (%) . | IL-7 (%) . |
|---|---|---|---|
| Inhibition | 0/37 (0)† | 7/37 (19) | 24/38 (63) |
| No effect | 36/37 (97) | 28/37 (76) | 14/38 (37) |
| Induction | 1/37 (3) | 2/37 (5) | 0/38 (0) |
| Apoptosis-modulating effects* . | IL-2 (%) . | IL-4 (%) . | IL-7 (%) . |
|---|---|---|---|
| Inhibition | 0/37 (0)† | 7/37 (19) | 24/38 (63) |
| No effect | 36/37 (97) | 28/37 (76) | 14/38 (37) |
| Induction | 1/37 (3) | 2/37 (5) | 0/38 (0) |
Cells were incubated in the presence and absence of cytokines for 24 hours at 37°C and were assessed for apoptosis by flow cytometry, and the cytokine-specific apoptosis modulation (ΔN) was calculated as the difference between apoptosis extents in the presence and absence of the corresponding cytokine. Experiments were performed at least twice for each leukemic cell sample, with a mean value of standard deviation (SD) of 2%. The 2.5-fold SD-value (ie, ΔNIL-7 = 5%) was used as a threshold value for the cytokine-induced modulation of apoptosis.
Number of patients/total number of patients (percentage of patients).
In individual samples, the inhibiting effect of IL-4 coincided with that of IL-7 in almost all (6 of 7) patients. In 1 patient (patient 25), IL-4 and IL-7 demonstrated opposite (pro-apoptotic and anti-apoptotic, respectively) effects on spontaneous apoptosis. Most samples (17 of 24) were only responsive to IL-7.
To demonstrate the specificity of IL-7 effects, we used cytokine-specific blocking antibodies against IL-7 during cell culturing in the presence or absence of IL-7. These antibodies successfully prevented the inhibition of spontaneous apoptosis by IL-7 but did not affect spontaneous apoptosis, indicating that IL-7 was not produced by the blast cells themselves (data not shown).
IL-7, IL-4, and IL-2 do not affect cell cycle distribution in T-ALL samples
In addition to their apoptosis-modulating capacity, cytokines were tested for their ability to influence proliferation kinetics. Evaluation of the cell cycle distribution disclosed a low initial rate of proliferation that did not significantly change after 24-hour incubation in the culture medium (Table 2). No spontaneous proliferation after in vitro was observed except in patient 38, in whom the percentage of cells in S and G2/M phases increased from 12% to 22%.
Proliferative activity of T-ALL cells
| . | 0 h . | Medium . | +IL-2 . | +IL-4 . | +IL-7 . |
|---|---|---|---|---|---|
| % Cells in S, G2/M phases* | 10.3 ± 1.2 | 9.3 ± 1.2 | 8.7 ± 1.2 | 10.0 ± 1.2 | 9.9 ± 1.4 |
| Range (%) | 2-26 | 0-29 | 2-27 | 1-32 | 1-38 |
| No. patients | 36 | 38 | 34 | 38 | 38 |
| . | 0 h . | Medium . | +IL-2 . | +IL-4 . | +IL-7 . |
|---|---|---|---|---|---|
| % Cells in S, G2/M phases* | 10.3 ± 1.2 | 9.3 ± 1.2 | 8.7 ± 1.2 | 10.0 ± 1.2 | 9.9 ± 1.4 |
| Range (%) | 2-26 | 0-29 | 2-27 | 1-32 | 1-38 |
| No. patients | 36 | 38 | 34 | 38 | 38 |
Leukemic cells were analyzed for DNA content before and after incubation in the standard medium or with cytokines for 24 hours at 37°C. Cell cycle analysis was performed by flow cytometry on ethanol-fixed, RNase-treated, and PI-stained cells. Proliferative activity was quantified as percentage of cells in the S and G2/M phases.
Average values (mean ± SEM) are presented. Experiments were performed at least twice for each leukemic cell sample.
The presence of cytokines in the culture medium did not affect cell cycle distribution in most leukemic cell samples (Table 2). Marked increases (>5%) of cells in the S and G2/M phases after 24-hour incubation with cytokines were observed in a few patients (3 of 38 with IL-7 and 2 of 37 with IL-4). The increased proliferation in these patients, however, did not prevent a decrease in the overall number of viable cells from spontaneous apoptosis, as assessed after 3 and 7 days of incubation (data not shown).
Cell surface expression of IL-7R, IL-4R, IL-2R, and IL-2Rγ
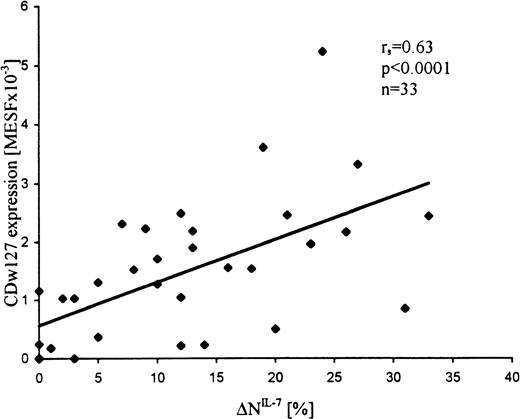
Expression levels of IL-7Rα, IL-4Rα, IL-2Rα, and IL-2Rγ were examined, in addition to the functional studies, in a subset of samples from T-ALL patients (n = 33, n = 21, n = 21, and n = 12, respectively). Expression values of IL-7Rα were highly variable and correlated with the extent of apoptosis inhibition by IL-7 (r = 0.63; P < .0001; Figure2). Most (15 of 21) T-ALL samples were negative for the expression of IL-4Rα, but IL-4 responding samples revealed significantly higher expression levels than nonresponding samples (mean MESF × 10−3, 0.69 ± 0.25 vs 0.07 ± 0.07, respectively; P = .02). In contrast to IL-7R and IL-4R, almost all (15 of 19) leukemic samples expressed, in spite of their unresponsiveness to IL-2, low but distinct levels of IL-2Rα (mean MESF × 10−3, 0.37 ± 0.1; range, 0-1.26). In addition, all samples studied were positive for IL-2Rγ (n = 12; mean MESF × 10−3, 1.8 ± 0.2). Interestingly, in most samples, IL-2Rγ expression levels were relatively similar (MESF × 10−3; range, 1.5-3.0) and did not correlate with the responsiveness of leukemic blasts to IL-7. In a T-ALL sample with relatively low IL-2Rγ expression (0.3 MESF × 10−3), neither IL-7 response nor IL-7Rα expression was observed.
Expression levels of IL-7R (CDw127) in T-ALL cells correlate with the apoptosis-inhibiting effects of IL-7 (▵NIL-7).
Leukemic cells were incubated in the presence and absence of IL-7 for 24 hours at 37°C and assessed for apoptosis by flow cytometry, and the cytokine-specific apoptosis modulation (ΔN) was calculated as the difference between the extent of apoptosis in the presence and absence of the cytokine. CDw127 expression was quantified in MESF units using calibration beads.
Expression levels of IL-7R (CDw127) in T-ALL cells correlate with the apoptosis-inhibiting effects of IL-7 (▵NIL-7).
Leukemic cells were incubated in the presence and absence of IL-7 for 24 hours at 37°C and assessed for apoptosis by flow cytometry, and the cytokine-specific apoptosis modulation (ΔN) was calculated as the difference between the extent of apoptosis in the presence and absence of the cytokine. CDw127 expression was quantified in MESF units using calibration beads.
Bcl-2 and Bax involvement in the IL-7 inhibition of spontaneous apoptosis
Constitutive expression levels of Bcl-2 and Bax were determined by flow cytometry as shown in Figure 3. All samples (n = 45) expressed Bcl-2 and Bax with mean values of (4.9 ± 0.8) × 103 MESF and (186 ± 18) × 103 MESF, respectively. Expression levels in individual samples were highly variable (0.5-25 × 103 MESF for Bcl-2 and 40-553 × 103 MESF for Bax), which, however, failed to correlate with the extent of spontaneous apoptosis or apoptosis inhibition by IL-7 (data not shown).
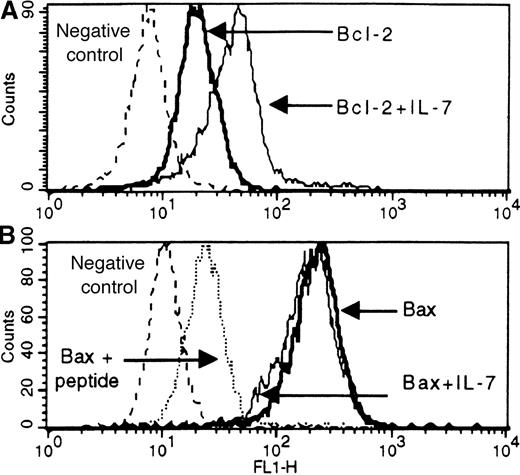
Flow-cytometric detection of Bcl-2 (A) and Bax (B) antigens in untreated and IL-7-treated leukemic cells from a patient with cortical T-ALL.
Blast cells were fixed, permeabilized, and stained by either direct or indirect immunofluorescence for Bcl-2 (mouse monoclonal antibody) or Bax (rabbit polyclonal antibodies against Bax peptide sequences), respectively. Solid lines correspond to antigen staining in untreated and IL-7-treated cells (bold and thin solid lines, respectively). Dotted lines correspond to background fluorescence of cells stained either with irrelevant antibodies (A and B) or with Bax antibody blocked with an excess of the corresponding specific peptide (B). Expression changes representative of T-ALL responsive to IL-7 are shown.
Flow-cytometric detection of Bcl-2 (A) and Bax (B) antigens in untreated and IL-7-treated leukemic cells from a patient with cortical T-ALL.
Blast cells were fixed, permeabilized, and stained by either direct or indirect immunofluorescence for Bcl-2 (mouse monoclonal antibody) or Bax (rabbit polyclonal antibodies against Bax peptide sequences), respectively. Solid lines correspond to antigen staining in untreated and IL-7-treated cells (bold and thin solid lines, respectively). Dotted lines correspond to background fluorescence of cells stained either with irrelevant antibodies (A and B) or with Bax antibody blocked with an excess of the corresponding specific peptide (B). Expression changes representative of T-ALL responsive to IL-7 are shown.
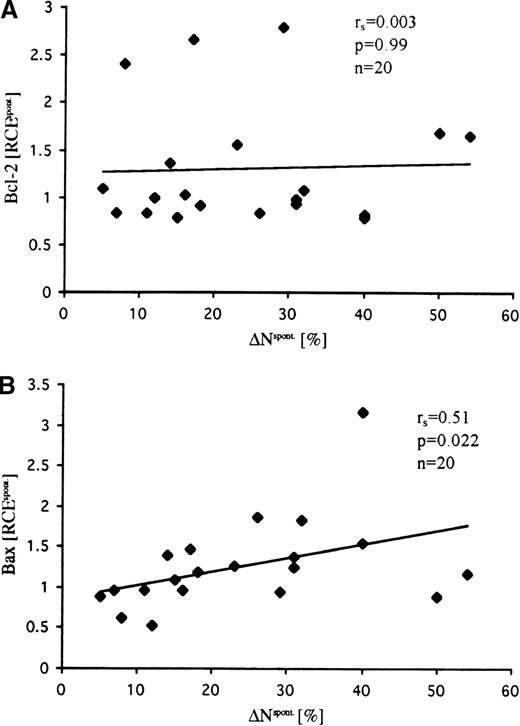
In contrast to the constitutive expression levels, changes of the Bax expression after in vitro culturing of T-ALL cells were associated with the extent of spontaneous apoptosis (r = 0.51; P= .02) (Figure 4). However, changes in neither Bcl-2 levels (Figure 4) nor Bcl-2/Bax ratios (not shown) correlated with spontaneous apoptosis.
Relative changes of expression (RCEspont) of Bcl-2 (A) and Bax (B) in T-ALL cells after culturing in standard medium for 24 hours.
RCEspont values for Bax, but not Bcl-2, positively correlated with the extent of spontaneous apoptosis (ΔNspont). RCE values were calculated as the ratio RCEspont = MESFmedium/MESF0h. Bcl-2 and Bax expression were assessed by flow cytometry as described in Figure 3 and were quantified in MESF units using calibration beads as fluorescence standards.
Relative changes of expression (RCEspont) of Bcl-2 (A) and Bax (B) in T-ALL cells after culturing in standard medium for 24 hours.
RCEspont values for Bax, but not Bcl-2, positively correlated with the extent of spontaneous apoptosis (ΔNspont). RCE values were calculated as the ratio RCEspont = MESFmedium/MESF0h. Bcl-2 and Bax expression were assessed by flow cytometry as described in Figure 3 and were quantified in MESF units using calibration beads as fluorescence standards.
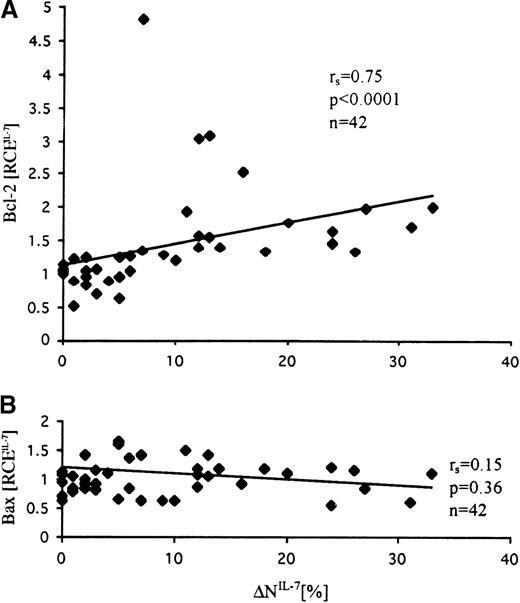
To investigate the involvement of Bcl-2 and Bax in the IL-7–induced inhibition of spontaneous apoptosis, we examined their expression levels in IL-7–treated cells in relation to those in untreated cells (Figure 5). Bcl-2 expression after IL-7 treatment increased significantly when in comparison with untreated samples, and these changes highly correlated with the extent of apoptosis reduction by IL-7 (r = 0.75; P < .0001) (Figure 5A). In contrast to Bcl-2, IL-7–induced changes of Bax were relatively low and did not correlate with the effect of IL-7, though a tendency to a negative correlation was observed (Figure 5B). Moreover, examination of the Bcl-2/Bax ratios did not reveal a better correlation with IL-7 effect than the analysis of Bcl-2 levels alone (r = 0.55; P = .0002).
Correlation between IL-7–induced changes of Bcl-2 (A) and Bax (B) expression levels and extent of IL-7–specific apoptosis inhibition (▵NIL-7).
Changes of Bcl-2 but not Bax positively correlated with the anti-apoptotic effect of IL-7. IL-7–specific relative changes of expression of Bcl-2 and Bax were calculated from the ratio of antigen expression levels in IL-7–treated and untreated cells (RCEIL-7 = MESFIL-7/MESFmedium). Bcl-2 and Bax expression were assessed by flow cytometry as described in Figure 3 and were quantified in MESF units using calibration beads as fluorescence standards.
Correlation between IL-7–induced changes of Bcl-2 (A) and Bax (B) expression levels and extent of IL-7–specific apoptosis inhibition (▵NIL-7).
Changes of Bcl-2 but not Bax positively correlated with the anti-apoptotic effect of IL-7. IL-7–specific relative changes of expression of Bcl-2 and Bax were calculated from the ratio of antigen expression levels in IL-7–treated and untreated cells (RCEIL-7 = MESFIL-7/MESFmedium). Bcl-2 and Bax expression were assessed by flow cytometry as described in Figure 3 and were quantified in MESF units using calibration beads as fluorescence standards.
Unresponsiveness to IL-7 is associated with an immature or aberrant immunophenotype and with lower spontaneous apoptosis
To investigate the diagnostic and clinical relevance of IL-7 responsiveness in vitro, we examined prospectively a series of 130 patients with immunophenotypically characterized T-ALL. Only few (10 of 130) patients could not be evaluated because of the absence of spontaneous apoptosis (ΔNspont, <5%). Of the 120 samples that revealed different extents of spontaneous apoptosis (range, 7%-75%), 64 (53%) were sensitive to IL-7 (ΔNIL-7, >5%), whereas 56 were IL-7 refractive (ΔNIL-7, ≤5%). Interestingly, IL-7–sensitive T-ALL samples revealed a significantly higher extent of spontaneous apoptosis when compared with IL-7–refractive samples (39% vs 28%; P = .002).
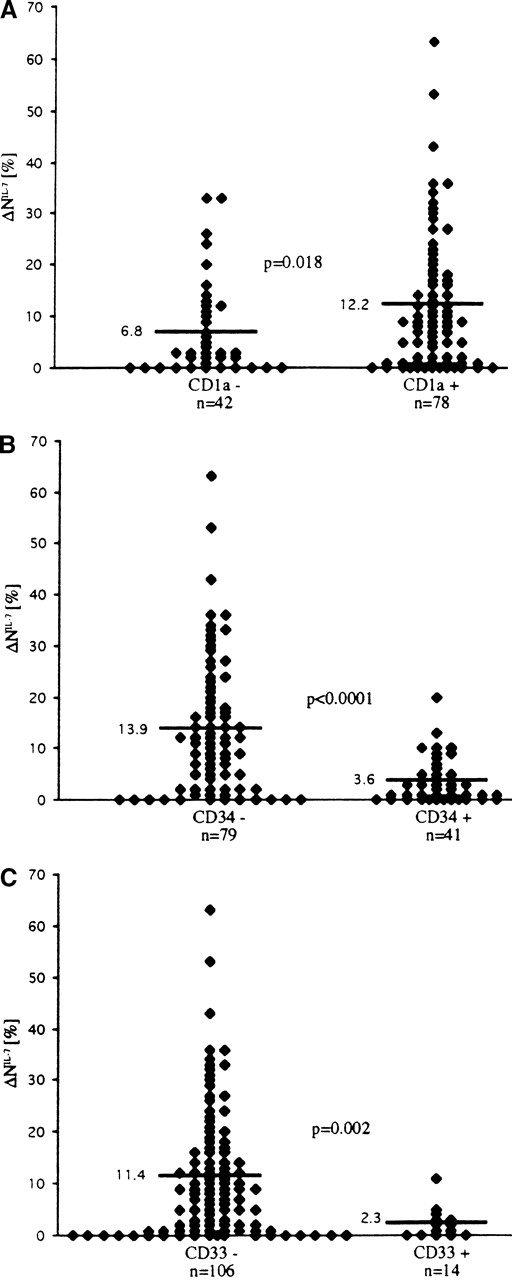
There was no correlation between responsiveness to IL-7 and initial laboratory and clinical parameters of the 130 patients with T-ALL, including white blood cell count, hemoglobin level, platelet count, age, organomegaly, and mediastinal mass. Furthermore, we studied whether the sensitivity of T-ALL blasts to IL-7 in terms of spontaneous apoptosis inhibition might be associated with a particular immunophenotype pattern. A significant positive correlation with the response to IL-7 was found for CD1a (P < .05; Figure6), surface (s)CD3 (P < .05), CD8 (P < .01), and CD4/CD8 coexpression (P < .05). An even stronger, but negative, correlation was observed between the expression of CD34 and IL-7 responsiveness (P < .0001; Figure6). Blast cells exhibiting aberrant expression of the myeloid antigen CD33 were mainly refractory to IL-7 (P < .01; Figure 6).
Correlation of sensitivity to IL-7 in terms of inhibition of spontaneous apoptosis (▵NIL-7) with expression of surface markers in T-ALL cells.
Antigen positivity was determined by a 20% cutoff criterion—that is, a sample was considered positive for the surface antigen if more than 20% of the leukemic cells expressed fluorescence intensity greater than 98% of the negative control cells.
Correlation of sensitivity to IL-7 in terms of inhibition of spontaneous apoptosis (▵NIL-7) with expression of surface markers in T-ALL cells.
Antigen positivity was determined by a 20% cutoff criterion—that is, a sample was considered positive for the surface antigen if more than 20% of the leukemic cells expressed fluorescence intensity greater than 98% of the negative control cells.
In addition, we investigated the correlation between IL-7Rα expression levels and immunophenotypic features associated with more mature differentiation stages (n = 33). We observed higher IL-7Rα expression levels in the T-ALL samples positive for CD1a, CD4, CD8, and CD3 and negative for CD34 and CD33, with cortical T-ALL revealing the highest and pro-T-ALL and pre-T-ALL revealing the lowest expression values (mean MESF × 10−3, 1.1 ± 0.3 vs 1.7 ± 0.2 vs 1.4 ± 0.6 for 8, 17, and 8 patients with pro-/pre-, cortical, and mature T-ALL, respectively). However, the observed differences were not statistically significant (P > .05). Leukemic cell samples with extremely low levels of spontaneous apoptosis (ΔNspont, <5%) did not reveal any particular phenotype or subtype (4, 3, and 2 samples of pro-/pre-, cortical, and mature T-ALL, respectively).
Response to IL-7 correlates with a good early response to initial therapy
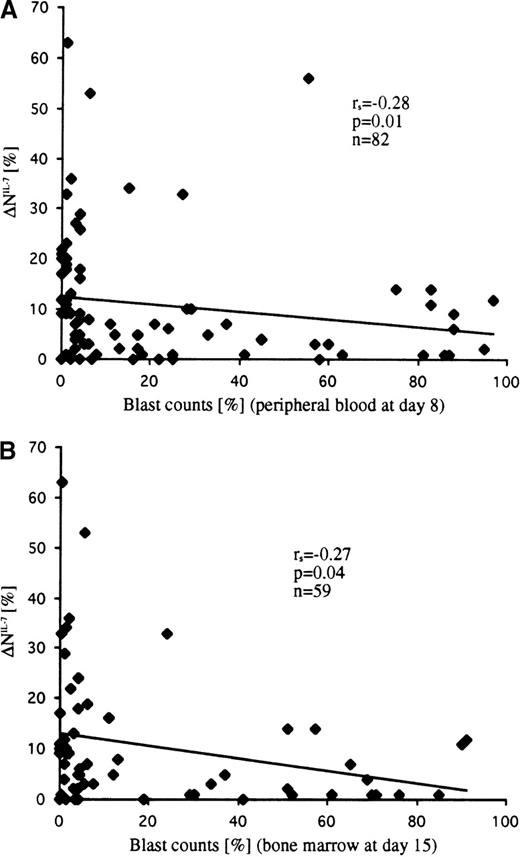
Within the subgroup of patients with T-ALL showing spontaneous apoptosis in vitro (n = 120), analysis of the available data on the rate of early cytoreduction revealed significant differences between IL-7–responding and IL-7–nonresponding samples. Both parameters—blast counts in peripheral blood at day 8 (r = −0.28; P = .01; n = 82) and in bone marrow at day 15 (r = −0.27; P = .04; n = 59)—were negatively associated with the extent of apoptosis inhibition by IL-7 (ie, leukemic cells with higher IL-7 sensitivity were more sensitive to the initial therapy) (Figure 7). This correlation was observed in T-ALL patients irrespective of their immunophenotypic features. The only exception was the TcRγ/δ+ T-ALL subgroup, who had highly variable but IL-7–independent responses to initial therapy (r = 0.03; P= .9; n = 13). Interestingly, the exclusion of TcRγ/δ+ T-ALL from the analysis considerably improved the correlation parameters (r = −0.38 and P = .001; r = −0.37 and P = .009; day 8 and day 15, respectively). Similarly, a statistically significant difference was observed between T-ALL patients classified as good and poor responders to prednisone (cut-off criterion, >1000 leukemic blasts/μL or ≤1000 leukemic blasts/μL in peripheral blood at day 8) when patients with TcRγ/δ+ T-ALL were not considered (Table3).
Correlation of IL-7–specific apoptosis inhibition (▵NIL-7) with the percentage of leukemic blasts at day 8 in peripheral blood (A) and at day 15 in bone marrow (B).
Blast cell counts inversely correlated with the extent of apoptosis inhibition.
Correlation of IL-7–specific apoptosis inhibition (▵NIL-7) with the percentage of leukemic blasts at day 8 in peripheral blood (A) and at day 15 in bone marrow (B).
Blast cell counts inversely correlated with the extent of apoptosis inhibition.
IL-7-specific inhibition of spontaneous apoptosis (▵N) in leukemic cells from T-ALL patients
| . | Prednisone response . | P . | |
|---|---|---|---|
| Good . | Poor . | ||
| Total | |||
| ΔN (%) n (pts) | 12.5 ± 1.9 53 | 7.8 ± 2.1 29 | 0.10 |
| TcRγ/δ+ | |||
| ΔN (%) n (pts) | 13.7 ± 3.4 6 | 20.0 ± 6.9 7 | 0.45 |
| TcRγ/δ− | |||
| ΔN (%) n (pts) | 12.4 ± 2.1 47 | 3.9 ± 0.8 22 | 0.02 |
| . | Prednisone response . | P . | |
|---|---|---|---|
| Good . | Poor . | ||
| Total | |||
| ΔN (%) n (pts) | 12.5 ± 1.9 53 | 7.8 ± 2.1 29 | 0.10 |
| TcRγ/δ+ | |||
| ΔN (%) n (pts) | 13.7 ± 3.4 6 | 20.0 ± 6.9 7 | 0.45 |
| TcRγ/δ− | |||
| ΔN (%) n (pts) | 12.4 ± 2.1 47 | 3.9 ± 0.8 22 | 0.02 |
T-ALL patients were subgrouped according to expression of TcRγ/δ and response to initial therapy (prednisone response). The in vivo response was evaluated by blast counting in peripheral blood after the 7-day prednisone prephase and 1 dose of intrathecal methotrexate on day 1. Good and poor response was defined by 1000 blasts/μL peripheral blood cut-off criterion.
Notably, the small group of patients with T-ALL who did not show spontaneous apoptosis in vitro revealed a considerably higher, though not a statistically significant, mean number of blast counts (%) on day 8 (results available in 5 of 10 patients) than patients with T-ALL who exhibited spontaneous apoptosis (33 ± 15 vs 22 ± 3, respectively). Within the latter group of patients with T-ALL, however, no correlation was found between the extent of spontaneous apoptosis and blast counts on day 8. With respect to blast counts on day 15, a tendency to a negative correlation with the extent of spontaneous apoptosis was observed (r = −0.24; P = .076; n = 59). Analysis of constitutive expression levels of Bcl-2 and Bax in T-ALL patients tested (n = 45) also did not reveal a significant correlation with early cytoreduction parameters, though Bcl-2 levels showed a tendency to a positive correlation with blast counts on days 8 and 15 (mean values, r = 0.20 and P = .17). Interestingly, in the multivariate analysis of apoptosis-related parameters of T-ALL patients tested in this study (ie, extent of spontaneous apoptosis, the extent of apoptosis inhibition by IL-7, and the expression levels of Bcl-2 and Bax), only IL-7 responsiveness revealed an independent impact on early cytoreduction and prednisone response parameters (P < .05).
Discussion
In the current study, T-ALL cells from pediatric patients were characterized according to their cytokine responsiveness in terms of apoptosis modulation in vitro. IL-7, but not IL-2 or IL-4, was found to be a highly potent inhibitor of apoptosis in T-ALL blasts. The extent of apoptosis inhibition by IL-7 correlated with the expression levels of IL-7Rα and was strongly associated with an up-regulation of Bcl-2. Most interesting, the classification of a large series of T-ALL cells according to their in vitro IL-7 responsiveness revealed significant differences between IL-7–sensitive and IL-7–refractive leukemic samples with respect to the expression of maturation-associated and myeloid markers and early responses to therapy.
In normal T-cell development, thymocyte responsiveness to cytokines has been found to depend strongly on thymocyte maturational stage (reviewed in Zlotnik and Moore3). Although thymocytes at the most immature pro-T-cell stage of intrathymic development selectively respond to IL-7 but not to IL-2 or IL-4,27 they lose the ability to respond to cytokines after entering a cortical, double-positive (CD4+CD8+) differentiation stage.28 After positive and negative selection, mature single-positive (CD4+CD8− or CD4− CD8+) thymocytes not only regain their IL-7-responsiveness but acquire the ability to respond to IL-2 and IL-4.3 Therefore, our findings demonstrating selective responsiveness to IL-7 in approximately two thirds of patients suggest that the cytokine responsiveness profile in T-ALL largely corresponds to that of pro-T thymocytes.
Cytokines have been shown able not only to inhibit apoptosis but also to induce it.1 IL-2 and IL-4, but not IL-7, were reported to induce apoptosis in thymocytes29,30 and activated T cells.31 Highly cytotoxic effects caused by the induction of apoptosis by IL-4 were found in B-lineage ALL.32 33 In our study, we observed, albeit rarely, an induction of apoptosis in T-ALL blasts by IL-2 and IL-4, but not by IL-7 (2 cases for IL-4 and 1 case for IL-2).
Although pathways of cytokine-mediated inhibition of apoptosis are poorly understood, there is abundant evidence that members of the Bcl-2 family are involved in the regulation of these processes. Overexpression of Bcl-2 delays cytokine deprivation-triggered apoptosis in growth factor-dependent cell lines, whereas overexpression of Bax induces or enhances spontaneous cell death without additional factors.34-36 Studies on the constitutive expression of Bcl-2 and Bax in fresh leukemic cells reveal correlations between the extent of spontaneous apoptosis and the expression levels of these proteins in acute myeloid leukemia (AML), B-lineage ALL, and chronic lymphocytic leukemia.25,32,37-39 However, we did not observe clear-cut correlations between Bcl-2 expression levels and the extent of spontaneous apoptosis in a relatively large series of ALL (n = 95)24 and AML (n = 100)40 patients. In the current study, the constitutive expression of Bcl-2 and Bax did not correlate with the extent of either spontaneous or IL-7 modulated apoptosis in T-ALL. Recently, changes of expression levels after cell treatment rather than constitutive expression levels of untreated cells have been suggested to be associated with the apoptosis-regulative function of Bcl-2 family members.41 A correlation between increased levels of Bcl-2 and cytokine-induced inhibition of apoptosis was observed in IL-7–treated mouse normal pro-T cells, malignant T-lymphoma cells, and human activated T lymphocytes.11,12,42-44 Apoptosis-associated up-regulation of Bax and its prevention by IL-7 was reported for pro-T cells.12 However, Bax expression levels did not change in human activated T cells42 and in mouse malignant T-lymphoma cells.44 In the current study, we found that expression changes of Bax, but not of Bcl-2, were positively correlated with the extent of spontaneous apoptosis in T-ALL. By contrast, IL-7–induced effects were strongly correlated with changes of Bcl-2 (P < .0001) but not Bax expression levels. Interestingly, consideration of the Bcl-2/Bax ratios, which have been shown to better predict apoptosis-related events in several but not all cell systems,45-47 showed a weak influence on the correlation parameters found for Bcl-2 alone. Therefore, our data suggest differential involvement of Bcl-2 and Bax in the induction of spontaneous apoptosis in vitro and its modulation by IL-7 in T-ALL.
The specific Bcl-2–dependent role of IL-7 as an anti-apoptotic factor in T-ALL in vitro resembles its crucial role in the normal early T-cell development. Mice lacking IL-7 or IL-7Rα genes, in contrast to IL-2– and IL-4–deficient mice, have been found to be severely deficient in thymocyte development.8,9 Analysis of thymocytes from IL-7–deficient mice disclosed their developmental arrest at the triple-negative (CD3−, CD4−, and CD8−) stages.9 Freshly isolated triple-negative cells from IL-7–deficient mice showed spontaneous apoptosis in vitro, which could be inhibited by the addition of IL-7.48 Because Bcl-2–deficient mice also revealed impaired development of lymphoid lineages49 and Bcl-2 overexpression in IL-7–deficient mice largely restored the maturation of thymocytes,11 it has been suggested that IL-7 exerts its anti-apoptotic function by up-regulating or maintaining Bcl-2 levels in pro-T cells.48 Interestingly, the IL-7–specific inhibition of spontaneous apoptosis in vitro has not been accompanied by changes of cell cycle distribution in the subpopulation of isolated pro-T cells, as demonstrated by flow cytometry.12 Based on these data, Kim et al12 suggested IL-7 to be a trophic rather than a growth factor in early T-cell development. Similarly, our flow cytometric analysis, which showed that IL-7 was not able to affect cell cycle distribution in T-ALL samples, points to the role of IL-7 as survival or trophic, but not as proliferative, at least for the bulk of T-ALL cells.
Comparison of the IL-7 response in vitro with the clinical response in vivo revealed a better prednisone response and early cytoreduction of IL-7 responding cells. Mechanisms underlying this correlation are unclear. The extent of spontaneous apoptosis, though significantly increased in IL-7–sensitive cells, did not reveal a statistically significant impact on early cytoreduction in univariate analysis. The latter observation is in line with studies in pediatric patients with ALL that did not find a correlation between the extent of spontaneous apoptosis and response to initial therapy.50 In contrast to ALL, several studies in AML demonstrated the predictive value of the extent of spontaneous apoptosis.40,51 We also did not observe a predictive significance of Bcl-2 expression levels, though the observed tendency to a positive correlation with blast counts is in line with previous reports on Bcl-2 in T-ALL.52 Moreover, a multivariate analysis of the investigated apoptosis-related parameters, including spontaneous apoptosis, its inhibition by IL-7, and expression levels of Bcl-2 and Bax, showed that only IL-7 responsiveness has an independent impact on early cytoreduction (P < .05), indicating a potential prognostic relevance of IL-7 sensitivity in T-ALL.
Spontaneous death of leukemic cells observed in vitro might be in part caused by a deprivation of humoral and cellular factors capable of protecting these cells from apoptosis in vivo. Because glucocorticoids are known to exert powerful anti-inflammatory effects from the suppression of cytokine gene expression,53 prednisone treatment in vivo might result in the overall down-regulation of cytokine production, including leukemia survival factors. In survival factor-dependent leukemic cells, this effect would result in the induction of apoptosis by cytokine deprivation and might account for the observed correlation between IL-7 effects in vitro and early cytoreduction in vivo. This effect of apoptosis induction by the down-regulation of survival factors would represent an antileukemic mechanism that is in addition to the well-known effect of apoptosis induction by glucocorticoids and other therapeutic drugs directly in target cells.54 55
The nature of survival factors in T-ALL remains largely unknown, though our data point to the putative role of IL-7 as a T-ALL survival factor. Further studies, including the investigation of patients' IL-7 serum levels and the application of cytokine blocking antibodies during in vitro cultivation of T-ALL blasts with autologous serum or stroma cells, are necessary to clarify the role of IL-7.
Our analysis of IL-7 response of T-ALL cells in the context of their phenotypic features revealed an association between IL-7 unresponsiveness and an immature phenotype characterized by the expression of CD34 and CD33. Conversely, IL-7–sensitive cells were found to express preferentially cortical and mature T-cell antigens. These correlations point to IL-7 responsiveness as a factor that could be involved in differentiation and maturation processes of leukemic T cells. Because leukemic cells are thought to originate from either transformed multipotential stem cells or from lineage-restricted progenitor cells,13,56 57 IL-7–refractive T-ALL cells could arise from relatively immature pluripotent progenitor cells, whereas IL-7–sensitive blasts might originate from more differentiated hematopoietic progenitors already committed to the lymphoid lineage.
IL-7Rα expression revealed only a tendency to correlate with immunophenotype and, therefore, could not account for the observed correlation between IL-7 responsiveness and immunophenotype. Moreover, considerable variability in IL-7 responsiveness, observed in T-ALL samples with similar levels of receptor expression (Figure 2), indicates a complex regulation of signal transduction pathways in leukemic blasts. Although molecular pathways of cytokine signaling and their influence on spontaneous apoptosis are poorly understood, the early signaling events triggered by engagement of the IL-7 receptor have been shown to be intimately linked to a functional tyrosine-specific protein kinase pathway and to involve stimulation of the inositol phospholipid turnover in human fetal thymocytes as well as in T-lineage ALL blasts.14,58 Numerous transcription factors are subsequently activated, including Stat5 and c-myc.59 60 It would be of interest to analyze whether potential defects in these signal transduction pathways are involved in the differential responsiveness of T-ALL cells to IL-7.
Normal TcRγ/δ+ cells represent a distinct T-cell population with respect to requirement for IL-7.61IL-7R–mediated signaling has been shown to be crucial for normal TcRγ/δ+ cell development and to be responsible for the rearrangement of TcRγ genes.62 However, because Bcl-2 overexpression has not been associated with the rescue of TcRγ/δ+ T cells in IL-7R–deficient mice, it has been suggested that IL-7 is involved in the generation rather than the survival of TcRγ/δ+ cells.11 Interestingly, our examination of IL-7 response in TcRγ/δ+ T-ALL, a rare subgroup accounting for approximately 15% of T-ALL,63did not reveal any correlation between IL-7 response and therapy response, indicating that IL-7 may be irrelevant in this subgroup as a survival factor in vivo.
Maturational stage of leukemic T lymphoblasts has been suggested as a means for subgrouping patients with T-lineage ALL (reviewed in Uckun et al64). In the ALL–BFM 86 trial, children with CD1a-positive cortical immunophenotypes disclosed significantly better in vivo responses to prednisone and longer durations of event-free survival than those with immature (pro-/pre-T) or mature sCD3+ CD1a− phenotypes.65 A detailed update of 316 children enrolled in the ALL–BFM 86 and the ALL–BFM 90 trials66 and recent data from the German COALL multicenter studies67 confirmed the better in vivo responses to prednisone and the more favorable outcomes of patients with cortical T-ALL compared with other immunophenotypic subsets. This indicates pharmacologic differences in responsiveness to cytotoxic treatment among T-ALL maturational subgroups. In view of these findings, our data on the correlation between IL-7 sensitivity and immunophenotype suggest that the susceptibility of leukemic blasts to apoptosis modulation by IL-7 may represent a valuable in vitro parameter reflecting distinct developmental stages of T-ALL, with differing accessibility to apoptotic programs. Furthermore, early cytoreduction in vivo, which has been shown to be an independent prognostic factor in pediatric patients with ALL,66,68 69revealed a quantitative correlation with the extent of IL-7–mediated apoptosis inhibition in vitro, thus suggesting that the classification of T-ALL according to IL-7 sensitivity may have prognostic significance. However, studies with larger numbers of patients and longer follow-up times are obviously needed to elucidate the predictive value of this parameter and its impact on the event-free and overall survival in childhood T-ALL.
In conclusion, our results show that IL-7, but not IL-2 or IL-4, is a potent inhibitor of spontaneous apoptosis in T-ALL. Similar to the recently described effects of IL-7 in pro-T cells during normal T-cell development,11 48 the IL-7–specific anti-apoptotic signaling in T-ALL blasts was associated with an up-regulation of Bcl-2 expression. From a clinical point of view, our findings demonstrating a correlation between IL-7–induced apoptosis inhibition in vitro and immunophenotype, and the early response to therapy in vivo, suggest that IL-7 responsiveness may be a useful surrogate marker reflecting differential survival factor dependence, apoptosis regulation, and treatment response in T-ALL.
Acknowledgments
We thank Karin Ganzel, Mathilde Martin, Karin Liebezeit, and Grit Czerwony for their valuable technical assistance, and we thank all the clinicians who provided samples for our investigations.
Supported in part by the Deutsche Leukämie–Forschungshilfe (grant no. DLFH-98.04) and by the Deutsche Jose Carreras Leukämie–Stiftung (grant no. DJCLS-98/NAT-3).
L.K. and V.R. contributed equally to this work.
Reprints:Leonid Karawajew, Robert–Rössle Clinic, Humboldt University Berlin, Lindenberger Weg 80, D-13125 Berlin, Germany; e-mail: karawajew@rrk-berlin.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal