Abstract
Although several CXC chemokines have been shown to induce angiogenesis and play roles in tumor growth, to date, no member of the CC chemokine family has been reported to play a direct role in angiogenesis. Here we report that the CC chemokine, monocyte chemotactic protein 1 (MCP-1), induced chemotaxis of human endothelial cells at nanomolar concentrations. This chemotactic response was inhibited by a monoclonal antibody to MCP-1. MCP-1 also induced the formation of blood vessels in vivo as assessed by the chick chorioallantoic membrane and the matrigel plug assays. As expected, the angiogenic response induced by MCP-1 was accompanied by an inflammatory response. With the use of a rat aortic sprouting assay in the absence of leukocytic infiltrates, we ruled out the possibility that the angiogenic effect of MCP-1 depended on leukocyte products. Moreover, the direct effect of MCP-1 on angiogenesis was consistent with the expression of CCR2, the receptor for MCP-1, on endothelial cells. Assessment of supernatant from a human breast carcinoma cell line demonstrated the production of MCP-1. Treatment of immunodeficient mice bearing human breast carcinoma cells with a neutralizing antibody to MCP-1 resulted in significant increases in survival and inhibition of the growth of lung micrometastases. Taken together, our data indicate that MCP-1 can act as a direct mediator of angiogenesis. As a chemokine that is abundantly produced by some tumors, it can also directly contribute to tumor progression. Therefore, therapy employing antagonists of MCP-1 in combination with other inhibitors of angiogenesis may achieve more comprehensive inhibition of tumor growth.
Several members of the CXC chemokine subfamily that contain an ELR motif, including interleukin-8 (IL-8) NAP-2, ENA-78, and GROα, mediate angiogenic responses, whereas the ELR−CXC chemokines IP-10 and MIG are reported to be angiostatic, demonstrating the diverse roles chemokines can play in this process.1-5 The sole reported exception of the ELR− chemokines is SDF-1α, which, despite lacking an ELR motif, is angiogenic.6,7 Although, to date, no members of the CC chemokine family have been proven to play a direct role in angiogenesis, several lines of evidence have suggested that the CC chemokine, monocyte chemotactic protein 1 (MCP-1), can contribute to angiogenesis, attributable mostly to its chemotactic effect on monocytes. For example, MCP-1 together with IL-8 is co-expressed during the initial stages of wound healing.8 Additionally, infusion of MCP-1 into rabbits after femoral artery occlusion enhances lateral and peripheral conductance because of enhanced vessel growth either by augmentation of monocyte accumulation or by an unknown effect of MCP-1 on endothelial and smooth muscle cells.9 We, therefore, investigated if these effects of MCP-1 were mediated by eliciting direct responses of endothelial cells or, as it has been proposed, by an indirect effect because of its chemotactic activity on monocytes.
MCP-1 is encoded by a single gene, which is well conserved in several species, including human, mouse, and rat.10,11 Human MCP-1 is active in all these species; therefore, human MCP-1 can be tested in cross-species bioassays. Although MCP-1 exerts chemotactic activity for several cell types, including monocytes, T lymphocytes, basophils, and NK cells,12-17 it is not known if it is chemotactic for endothelial cells. Moreover, the expression of CCR2 on endothelial cells, the only seven-transmembrane G protein-coupled receptor for MCP-1, has not yet been reported.
MCP-1 is abundantly produced in a variety of inflammatory diseases, such as atherosclerosis and rheumatoid arthritis.18,19 The MCP-1 gene is also expressed during early stages of melanoma, and it is also produced in metastatic lesions.20,21 The expression of the MCP-1 gene in tumor parenchyma has been correlated with the degree of invasiveness of human breast carcinomas.21 Moreover, the concentration of MCP-1 in the urine was shown to be correlated with the degree of tumor malignancy.22 Because MCP-1 is abundantly produced by tumors, we tested whether MCP-1 contributes directly toward tumor angiogenesis by a mechanism independent of monocyte recruitment. In contrast to a recent report23 implicating that the angiogenic effects of MCP-1 were due to monocytic infiltrates, we have found that MCP-1 is a direct mediator of angiogenesis, as measured by its ability to induce in vitro endothelial cell migration, endothelial cell sprouting from aortal rings in the absence of an inflammatory response, and in vivo angiogenesis in the matrigel plug assay. Furthermore, blocking of MCP-1 activity in immunodeficient mice bearing human breast carcinomas resulted in significant prolongation of the survival of tumor-bearing mice. These results demonstrate that MCP-1 can exert direct effects promoting angiogenesis.
Materials and methods
Chemokines and antibodies
Recombinant human MCP-1, vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF) were purchased from Pepro Tech, Inc (Rocky Hill, NJ). Endothelial cell growth supplement (ECGS) was purchased from Sigma (St Louis, MO). Monoclonal anti-human CCR2 was kindly provided by Dr C. Martinez (Centro National de Biotecnologia, Madrid, Spain). Polyclonal antibody to human MCP-1, named Ab 279, was purchased from R&D. Rabbit immunoglobulin G (IgG; Calbiochem, San Diego, CA) and mouse IgG (Coulter, Miami, FL) were used as the negative controls.
Cell culture
Human umbilical cord vein endothelial cells (HUVECs) were isolated from neonatal umbilical cords. Human dermal microvascular endothelial cells (HMECs) were either obtained from Clonetics or isolated from preputial skin.24 Endothelial cell preparations were tested for their expression of CD31 and von Willebrand factor by flow cytometry, and preparations containing less than 2% contaminating cell types were selected for further studies. All endothelial cell types were cultured on collagen type I coated plastic wells (Biocoat, Becton Dickinson, Bedford, MA), in EGM medium (Clonetics, Walkersville, MD) containing 5% fetal calf serum (FCS), VEGF (10 ng/mL), bFGF (10 ng/mL), glutamine (2 mmol/L), and gentamicin (100 U/mL). All experiments were performed using subcultures between the second and seventh passage. The MDA-231 human breast carcinoma cell line was obtained from ATCC and grown in RPMI 1640 medium containing 5% FCS, glutamine (2 mmol/L), and penicillin-streptomycin (100 U/mL).
Flow cytometric analysis
Indirect immunofluorescence was performed on HMECs and HUVECs by exposing cells to saturating amounts of mouse antibodies to human CCR2. Fluorescein-conjugated F(ab)2 fragments of goat anti-mouse (Sigma) diluted 1:50 was used as the secondary antibody. After staining, cells were analyzed using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA).
Endothelial cell migration assay
HMEC and HUVEC chemotaxis was performed using micro-Boyden chambers as described.6 Briefly, polycarbonate filters of 5 μm pore size (Nucleopore, NeuroProbe, Cabin John, MD) were coated with fibronectin (10 μg/mL; Sigma) overnight at 4°C. Binding buffer containing 1.0% bovine serum albumin in RPMI 1640 with or without various amounts of MCP-1 was placed in the lower compartment of the chamber, and 0.5 × 106 cells/mL resuspended in binding medium were then added to the upper compartment. The chambers were incubated for 4 hours at 37°C. After the filters were removed, the upper surface was scraped, fixed with methanol, and stained with Leukostat (Fisher Scientific, Pittsburgh, PA). Membranes were analyzed using the BIOQUANT program (R & M Biometrics, Inc, Nashville, TN), and the results were expressed as the mean number of migrated cells/10 fields at 10× magnification. For inhibitory assays, MCP-1 antibody was added together with MCP-1 in the lower compartment of the chamber. Each sample was tested in triplicate. Chemotaxis and inhibition of chemotaxis experiments were performed 5 times.
Rat aortic ring assay
Rat aortic rings were prepared as previously described.6The thoracic and abdominal aorta was obtained from 100- to 150-g male Sprague-Dawley rats (Taconic, Germantown, NY). Excess perivascular tissue was removed, transverse sections (1 to 2 mm) were made, and the resulting aortic rings were then washed in medium 199 (Gibco BRL, Life Technology, Grand Island, NY). The rings were then embedded in matrigel (Beckton Dickinson, Bedford, MA) in 8-well chamber slides (Nalge Nunc International, Milwaukee, WI) so that the lumen was parallel to the base of the slide. After the matrigel gelled, serum-free medium (endothelial basal medium supplemented with antibiotics) with or without different concentrations of MCP-1 (1-100 ng/mL) was added to each well, and the slides were incubated at 37°C, with 5% CO2, for 3 days (n = 6 per dose). ECGS was used as the positive control at concentrations of 200 μg/mL. After the incubation period, the rings were fixed, stained, and photographed. The ring assay was repeated 2 times.
Chick chorioallantoic membrane (CAM) assay
Ovalbumin (4 mL) was removed from 3-day-old embryonated eggs (Truslow Farms, Charlestown, MD). Thereafter, windows were opened for each egg and coated with tape, and eggs were incubated at 37°C. On day 10, five μL of distilled water containing different amounts of MCP-1 were applied in the center of quartered 13-mm diameter plastic coverslips (Thermanox, Nalge NUNC International) and let dry for 10 minutes. Each coverslip was placed on the chorioallantoic membrane of the chick, and the eggs were incubated at 37°C for 3 days. The assay was scored and photographed on the 13th embryonic day. EGF and water were used as positive and negative controls, respectively. Twenty eggs were used in total for each data point. A positive score for angiogenesis was made when vessels appeared to radiate from the spot in the coverslip to which the stimulant was applied. The scores are reported as a percentage of positive CAMs at each dilution.
In vivo matrigel plug angiogenesis assay
Matrigel (9 mg/mL; 0.3 mL/mouse) alone or mixed with different concentrations of MCP-1 was injected subcutaneously into the flank of C57BL/6 mice. For angiogenesis inhibition, the mice were injected intraperitoneally with antibody to MCP-1 or control rabbit IgG (35 μg/mouse) on days 1, 3, and 6. On day 7, mice were sacrified, and plugs were removed and fixed in 3.7% formaldehyde/phosphate-buffered saline (PBS), paraffin embedded, and Giemsa-stained slides were photographed. The experiment was repeated 2 times with 8 mice per group in each experiment.
In vivo tumor studies
CB-17 severed combine immune deficient (SCID) mice were used at 6 to 8 weeks of age and purchased from the animal production area (NCI-FCRDC, Frederick, MD). Animal housing and management were in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, Institute of Laboratory Animal Resources, National Research Council, 1996), and the protocol used was approved by the NCI-FCRDC Animal Care and Use Committee. For survival experiments, SCID mice were injected intravenously with 20 μL of anti-ASGM1 (Wako Chemicals, Richmond, VA) on day 0, and 3 × 105 MDA231 human breast carcinoma cells were injected intravenously on day 1. Antibody to MCP-1 (Ab 279) (25 μg/mouse, 1 mg/kg) and control rabbit IgG were given intraperitoneally to the mice on days 4, 8, 12, 16, 20, 24, and 28. Survival was monitored daily, and moribund mice were euthanized. For experimental metastasis experiments, mice from both groups were sacrificed on the 35th day after intravenous injection of the tumor cells. Lungs were extracted and fixed in formalin. At this point few, if any, macrometastases were detected, and micrometastases were quantitated. Histological sections were stained with hematoxylin and eosin, and tumor micrometastasis was quantitated using the Bioquant Program, counting the total tissue area per field 40× field (D1). The micrometastasis present within the same field were gated, and the area within the gates was measured (D2). The metastatic index was calculated by the ratio D2/D1. A minimum of 20 fields was analyzed per slide, and 8 mice were used per group in each experiment. The experiment was repeated 2 times. The survival experiment was repeated 3 times with 10 mice per group in each experiment.
Cell proliferation assay
MDA-31 was resuspended at 1 × 106 cells/mL of proliferation medium (RPMI, 1% FCS, 2 mmol/L glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin). Cell suspensions (100 μL/well) were placed in 96-well plates and stimulated with different concentrations of MCP-1 in the presence or absence of antibody to MCP-1 (10 μg/mL). Plates were incubated at 37°C in 5% CO2 for 24, 48, or 72 hours. To determine cell proliferation, cells were loaded with 3H-thymidine (0.5-1 μCi/well) 10 hours before uptake determination. After the incubation plates were kept at −70°C overnight, the plates were thawed at room temperature and harvested, and 3H-thymidine incorporation was counted with the use of a beta counter.
Results
MCP-1 is chemotactic for human microvascular and umbilical vein endothelial cells
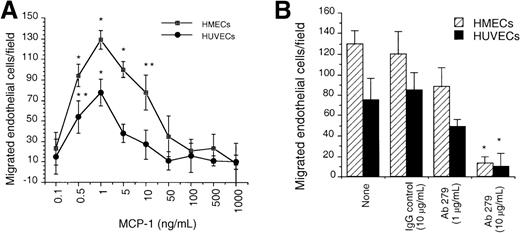
Given earlier observations regarding the ability of ELR+CXC chemokines and SDF-1α to induce neovessel formation, which was consistent with their ability to induce migration of endothelial cells, we first evaluated the capacity of human endothelial cells from either HUVECs or HMECs to respond to MCP-1 by in vitro chemotaxis assay. We observed a dose-dependent chemotactic response for both cell types toward MCP-1. The maximal chemotactic response for each cell type was observed at 1 ng/mL of MCP-1 (Figure 1A). To assess the specificity of the chemotactic response of endothelial cells toward MCP-1, we used a blocking polyclonal antibody to human MCP-1. This antibody specifically inhibited the chemotactic response of HUVECs and HMECs to MCP-1 when used at 10 μg/mL (Figure 1B). These data demonstrate that endothelial cells migrate to very low doses of MCP-1.
In vitro chemotaxis of HMECs and HUVECs toward MCP-1 is inhibited by anti-MCP-1.
(A) The migration of HMECs and HUVECs toward different concentrations of MCP-1 was quantitated as the number of cells per 10× field as described in the “Materials and methods” section. (B) Inhibition of the chemotactic responses of HMECs and HUVECs toward MCP-1 by the mAb 279. MCP-1 was used at the dose that induced maximal chemotactic responses (1 ng/mL). The migration toward medium alone (basal migration) was subtracted. *P < .001; **P < .025. One representative experiment is shown. The assay was repeated 5 times.
In vitro chemotaxis of HMECs and HUVECs toward MCP-1 is inhibited by anti-MCP-1.
(A) The migration of HMECs and HUVECs toward different concentrations of MCP-1 was quantitated as the number of cells per 10× field as described in the “Materials and methods” section. (B) Inhibition of the chemotactic responses of HMECs and HUVECs toward MCP-1 by the mAb 279. MCP-1 was used at the dose that induced maximal chemotactic responses (1 ng/mL). The migration toward medium alone (basal migration) was subtracted. *P < .001; **P < .025. One representative experiment is shown. The assay was repeated 5 times.
HMECs and HUVECs express CCR2
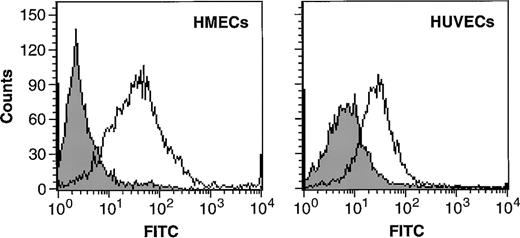
The chemotactic response of HUVECs and HMECs to MCP-1 prompted us to investigate the expression of CCR2, the receptor for MCP-1, on endothelial cells. By using immunofluorescence, we found CCR2 on the cell surface of both HMECs and HUVECs (Figure2). The mean fluorescence intensity was 62 (±14) for HMECs versus 37 (±9) for HUVECs, indicating that CCR2 is more abundantly expressed on the cell surface of HMECs than on HUVECs. The expression of CCR2 on HMECs was threefold lower than levels found on human monocytes (data not shown).
HMECs and HUVECs express CCR2.
Flow cytometric analysis of CCR2 expression on the cell surface of HMECs and HUVECs. Cells were incubated with either IgG control (filled histograms) or with CCR2 Ab (empty histograms). The figure shows binding of the antibody to CCR2 compared with that of the negative control.
HMECs and HUVECs express CCR2.
Flow cytometric analysis of CCR2 expression on the cell surface of HMECs and HUVECs. Cells were incubated with either IgG control (filled histograms) or with CCR2 Ab (empty histograms). The figure shows binding of the antibody to CCR2 compared with that of the negative control.
MCP-1 induces angiogenesis in vivo
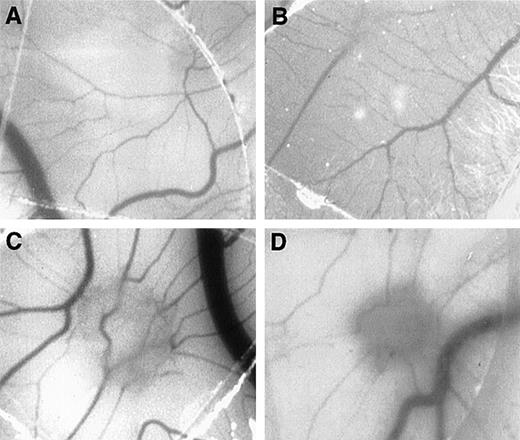
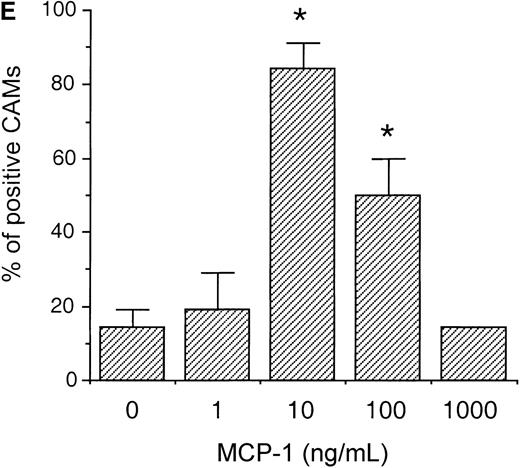
To evaluate whether MCP-1 could exhibit angiogenic activities in vivo, we tested different concentrations of MCP-1, ranging from 1 to 1000 ng/mL, using the CAM assay. As shown in Figure3, MCP-1 at concentrations of 10 ng/mL (Figure 3C) and 100 ng/mL (Figure 3D) induced the typical radial formation of vessels characteristic of other well-known angiogenic factors, such as epidermal growth factor (EGF). The scores of the angiogenic response of MCP-1 were 80% and 50% positive CAMs when used at 10 ng/mL and 100 ng/mL, respectively. The negative control showed less than 15% positivity. No significant angiogenic responses were observed above the negative control level when MCP-1 was used at 1 ng/mL or at 1000 ng/mL (Figure 3E). An inflammatory response, as indicated by an area with increased opacity on the coverslip, however, was also observed in association with the angiogenesis induced by MCP-1. These data demonstrate that MCP-1 has angiogenic effects in vivo.
MCP-1 promotes angiogenesis in the chick CAM assay.
Plastic coverslips containing 1 (B), 10 (C), or 100 (D) ng of lyophylized MCP-1 were placed on the chorioallantoic membrane of 10-day-old embryos. Distilled water, which was used as a solvent, served as the negative control (A). The percentage of positive CAMs for each MCP-1 concentration was scored (E). Twenty embryonated eggs were used for each data point. *P < .005, as assessed by Studentt test.
MCP-1 promotes angiogenesis in the chick CAM assay.
Plastic coverslips containing 1 (B), 10 (C), or 100 (D) ng of lyophylized MCP-1 were placed on the chorioallantoic membrane of 10-day-old embryos. Distilled water, which was used as a solvent, served as the negative control (A). The percentage of positive CAMs for each MCP-1 concentration was scored (E). Twenty embryonated eggs were used for each data point. *P < .005, as assessed by Studentt test.
Antibody to MCP-1 inhibits the angiogenic effect of MCP-1 in the in vivo matrigel plug assay
We also evaluated the effect of MCP-1 using the in vivo matrigel plug assay. Mice were injected with matrigel alone or with MCP-1 containing matrigel subcutaneously in the flank. Histologic sections of the matrigel plugs indicated a significant angiogenic effect induced by MCP-1 when used at concentrations of 10 or 100 ng/mL in contrast to matrigel alone (Figure 4, and data not shown). We next studied if the angiogenic effect of MCP-1 could be inhibited specifically by an antibody to MCP-1. As shown in Figure 4C, anti-MCP-1 significantly inhibited the angiogenesis induced by 100 ng/mL of MCP-1 to a level similar to that observed in the control matrigel plugs lacking MCP-1. Moreover, injections of the control antibody did not inhibit this angiogenic effect (Figure 4D). However, as observed using the CAM assay, an inflammatory reaction was also observed in the matrigel plugs that contained MCP-1, which consisted predominantly of monocytes with few neutrophils. This inflammatory response was also inhibited by the MCP-1 antibody but not by rabbit IgG control antibody. These data demonstrate that MCP-1 is angiogenic, but it is not clear if this effect is direct or via the inflammatory cells.
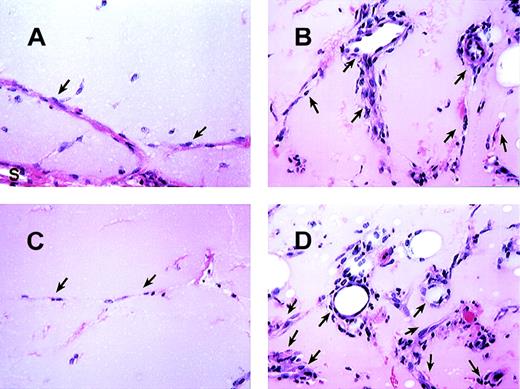
MCP-1 induced angiogenesis in matrigel plugs.
The histologic appearance of matrigel plug sections after 7 days with or without MCP-1. (A) Matrigel plug section without MCP-1. (B) Matrigel plug containing 100 ng/mL of MCP-1. (C and D) The effects of Ab to MCP-1 (C) and control rabbit Ab (D) on matrigel plugs containing 100 ng/mL of MCP-1. As indicated, formation of vessels is basely observed in matrigel alone. “S” indicates stroma surrounding the plugs. Arrows point to endothelial cells forming vessels. All figures are at 340× magnification. One representative field from 2 experiments is shown.
MCP-1 induced angiogenesis in matrigel plugs.
The histologic appearance of matrigel plug sections after 7 days with or without MCP-1. (A) Matrigel plug section without MCP-1. (B) Matrigel plug containing 100 ng/mL of MCP-1. (C and D) The effects of Ab to MCP-1 (C) and control rabbit Ab (D) on matrigel plugs containing 100 ng/mL of MCP-1. As indicated, formation of vessels is basely observed in matrigel alone. “S” indicates stroma surrounding the plugs. Arrows point to endothelial cells forming vessels. All figures are at 340× magnification. One representative field from 2 experiments is shown.
MCP-1–induced rat aortic endothelial cell sprouting
Because in our in vivo angiogenesis assays, as well as in the CAM and the matigel plug assays MCP-1 angiogenic effects were accompanied by monocytic infiltration, we sought to investigate the possibility that the observed angiogenesis was leukocyte dependent. We, therefore, tested the effect of MCP-1 using the ex vivo rat aortic ring sprouting assay, which allows the detection of angiogenesis in the absence of an inflammatory response. Transverse sections of rat aorta tissue embedded in collagen were cultured with MCP-1 as described in the “Materials and methods” section, and thereafter examined for the degree of sprouting vessels. Cell culture medium and ECGS medium were used as negative and positive controls, respectively. As shown in Figure 5 and Table1, MCP-1 stimulated numerous capillary sprouts at concentrations between 5 ng/mL (nmol/L) and 50 ng/mL. Thus, MCP-1 can induce endothelial cell sprouting at nanomolar concentrations from rat aortic rings in the absence of inflammatory infiltrates, indicating a direct effect in promoting angiogenesis.
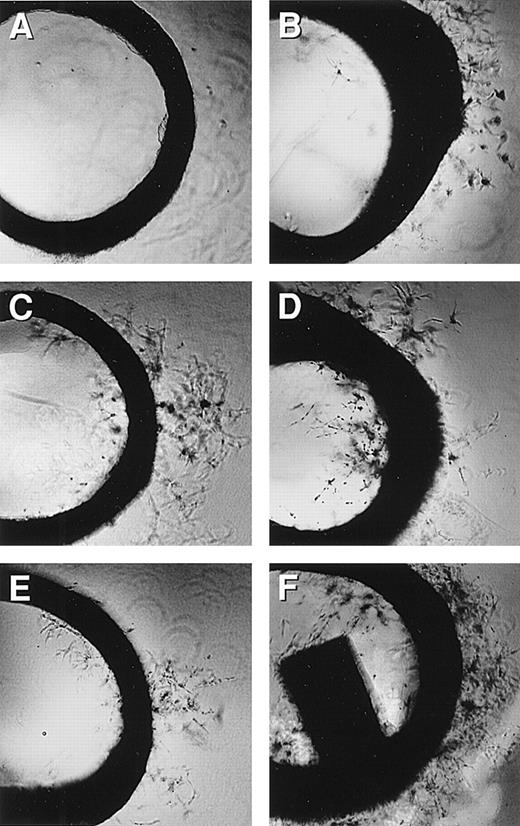
Rat aortic ring assay.
Rat aortic ring capillary sprouting in response to MCP-1 (1 ng/mL). Capillary sprouting occurred from the edge of the ring. (A) Negative control, (B) MCP-1 at 0.5 ng/mL, (C) MCP-1 at 5 ng/mL, (D) MCP-1 at 50 ng/mL, (E) MCP-1 at 500 ng/mL, and (F) EGF at 100 ng/mL was used as the positive control; 4× magnification. Note that MCP-1-induced endothelial cell sprout in a dose-responsive manner. One representative of 2 experiments is shown.
Rat aortic ring assay.
Rat aortic ring capillary sprouting in response to MCP-1 (1 ng/mL). Capillary sprouting occurred from the edge of the ring. (A) Negative control, (B) MCP-1 at 0.5 ng/mL, (C) MCP-1 at 5 ng/mL, (D) MCP-1 at 50 ng/mL, (E) MCP-1 at 500 ng/mL, and (F) EGF at 100 ng/mL was used as the positive control; 4× magnification. Note that MCP-1-induced endothelial cell sprout in a dose-responsive manner. One representative of 2 experiments is shown.
MCP-1 induces rat endothelial cell sprouting in a dose-dependent manner
| Experiment number . | Aortic rings . | MCP-1 concentration (ng/mL) . | ECGS (100 ng/mL) . | ||||
|---|---|---|---|---|---|---|---|
| 0 . | 0.5 . | 5 . | 50 . | 500 . | |||
| 1 | 1 | — | — | + | ++ | + | +++ |
| 2 | — | ++ | ++ | +++ | + | + | |
| 3 | — | + | — | ++ | + | ++ | |
| 4 | — | + | — | + | — | + | |
| 5 | — | + | ++ | + | — | + | |
| 6 | — | — | + | — | — | — | |
| 2 | 1 | + | ++ | + | ++ | ++ | ++++ |
| 2 | — | + | ++ | +++ | + | +++ | |
| 3 | + | — | — | +++ | — | ++++ | |
| 4 | — | — | + | ++ | — | ++ | |
| 5 | — | + | ++ | + | — | ++++ | |
| 6 | — | — | — | — | — | ++ | |
| Experiment number . | Aortic rings . | MCP-1 concentration (ng/mL) . | ECGS (100 ng/mL) . | ||||
|---|---|---|---|---|---|---|---|
| 0 . | 0.5 . | 5 . | 50 . | 500 . | |||
| 1 | 1 | — | — | + | ++ | + | +++ |
| 2 | — | ++ | ++ | +++ | + | + | |
| 3 | — | + | — | ++ | + | ++ | |
| 4 | — | + | — | + | — | + | |
| 5 | — | + | ++ | + | — | + | |
| 6 | — | — | + | — | — | — | |
| 2 | 1 | + | ++ | + | ++ | ++ | ++++ |
| 2 | — | + | ++ | +++ | + | +++ | |
| 3 | + | — | — | +++ | — | ++++ | |
| 4 | — | — | + | ++ | — | ++ | |
| 5 | — | + | ++ | + | — | ++++ | |
| 6 | — | — | — | — | — | ++ | |
Induction of endothelial cell sprouting by different concentrations of MCP-1 was compared with that observed in the presence of medium alone (negative control) or ECGS (positive control). Each concentration was tested by sextuples, and the aortic rings were scored as follows: —, no sprouting; +, low sprouting levels; ++, 25%-50% sprouting; +++, 50%-75% sprouting; ++++, more than 75% sprouting. The scores of 2 separate experiments are shown. ECGS, endothelial cell growth supplement.
Blocking MCP-1 enhances the survival of SCID mice bearing MDA-231 human breast carcinoma cells
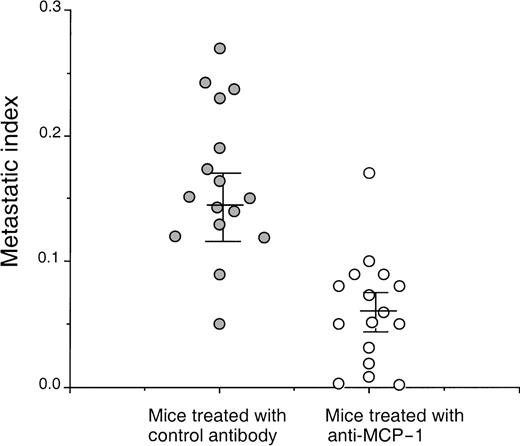
On the basis of the fact that MCP-1 is abundantly produced by tumor cells, we wanted to evaluate the contribution of MCP-1 toward tumor growth. We, therefore, selected a human breast carcinoma cell line MDA-231 to study the effect of a MCP-1 antibody on tumor growth. The MDA-231 cell line produced approximately 6500 pg of MCP-1/mL, when cells were grown at a concentration of 0.5 × 106 cells/mL of RPMI for 24 hours as determined by enzyme-linked immunosorbent assay (data not shown). MDA-231 cells were then injected intravenously into SCID mice, as described in the “Materials and methods” section. As shown in Figure 6, administration of MCP-1 antibody significantly increased the survival of SCID mice bearing MDA-231 carcinoma tumors in contrast to mice treated with control antibody (P < .024). Neither administration of exogenous MCP-1 nor antibodies to MCP-1 had an effect on the growth of MDA-231 cells in vitro (Table 2). Analysis of the metastatic lesions in the lungs revealed that the experimental micrometastases therein were significantly smaller and lower in number when treated with the MCP-1 antibody than in the control antibody group (Figure 7). As shown in Figure 8, grading analysis of the lung metastasis by calculating the total area invaded by the tumor in each mouse indicated that the group of mice treated with control antibody exhibited about 2.5 times more metastases than the group of mice treated with anti-MCP-1. The metastatic index of control-treated mice was 0.146 (SEM ± 0.027), whereas the metastatic index of anti-MCP-1–treated mice was 0.057 (SEM ± 0.011; P < .005). These data demonstrate that the size and number of metastatic lesions formed in the presence of antibody to MCP-1 are reduced, and increases in survival were observed, indicating that MCP-1 has a role in tumor progression.
Blocking MCP-1 increases the survival of SCID mice bearing MDA-231 human breast carcinoma cells.
MDA231 human breast cancer cells (3 × 105) were injected intravenously on day 1. Antibodies, including rabbit IgG and anti-MCP-1 (25 μg/mouse, 1 mg/kg), were given intraperitoneally to the mice on days 4, 8, 12, 16, 20, 24, and 28. Ten mice were included in each group. Their survival was monitored daily. The differences in the median values among the 2 groups are greater than would be expected by chance. There is a statistically significant difference (P < .024) as assessed by the log-rank test. One representative experiment of 3 experiments is shown.
Blocking MCP-1 increases the survival of SCID mice bearing MDA-231 human breast carcinoma cells.
MDA231 human breast cancer cells (3 × 105) were injected intravenously on day 1. Antibodies, including rabbit IgG and anti-MCP-1 (25 μg/mouse, 1 mg/kg), were given intraperitoneally to the mice on days 4, 8, 12, 16, 20, 24, and 28. Ten mice were included in each group. Their survival was monitored daily. The differences in the median values among the 2 groups are greater than would be expected by chance. There is a statistically significant difference (P < .024) as assessed by the log-rank test. One representative experiment of 3 experiments is shown.
MCP-1 has no effect on the in vitro growth of MDA-231 cells
| MCP-1 (ng/mL) . | 3H-thymidine incorporation (CPM) . | |
|---|---|---|
| No antibody . | Anti-MCP-1 (10 μg/mL) . | |
| 0 | 45 260 (±4011) | 48 269 (±2300) |
| 1 | 41 670 (±3999) | 44 500 (±1890) |
| 10 | 44 380 (±3457) | 43 012 (±3889) |
| 100 | 48 000 (±7001) | 43 991 (±5012) |
| MCP-1 (ng/mL) . | 3H-thymidine incorporation (CPM) . | |
|---|---|---|
| No antibody . | Anti-MCP-1 (10 μg/mL) . | |
| 0 | 45 260 (±4011) | 48 269 (±2300) |
| 1 | 41 670 (±3999) | 44 500 (±1890) |
| 10 | 44 380 (±3457) | 43 012 (±3889) |
| 100 | 48 000 (±7001) | 43 991 (±5012) |
The proliferation of MDA-231 cells was tested in the presence of different concentrations of exogenous MCP-1 and compared with that observed in the presence of medium alone. The effect of an MCP-1 antibody on MDA-231 cellular proliferation was also tested, as measured by 3H-thymidine incorporation after 48 hours of MCP-1 stimulation. Each MCP-1 concentration was tested in triplicate. The average results of 3 experiments are shown ± SEM. Statistical analysis was performed, using analysis of variance relative to the medium alone.
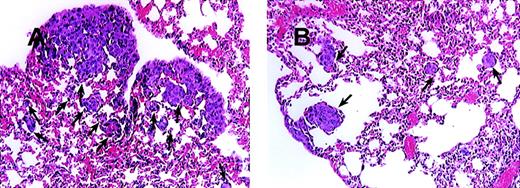
Blocking of MCP-1 inhibited lung tumor metastases of SCID mice bearing MDA-231 human tumor breast cancer cells.
SCID mice were injected intravenously with 20 μL of antiserum to ASGM1 on day 0, and 3 × 105 MDA231 human breast carcinoma cells were injected intravenously on day 1. Antibodies, including rabbit IgG (panel A) and 279 Ab (Panel B) at 25 μg/mouse (1 mg/kg), were given intraperitoneally to the mice on days 4, 8, 12, 16, 20, 24, and 28. For experimental metastasis experiments, mice from both groups were sacrificed on the 35th day after intravenous injection of the tumor cells. Lungs were extracted and fixed in formalin. Histological sections were stained with hematoxylin and eosin to evaluate tumor metastases. Photographs were taken at 150× magnification. The arrows indicate metastatic lesions. One representative field from 2 experiments is shown.
Blocking of MCP-1 inhibited lung tumor metastases of SCID mice bearing MDA-231 human tumor breast cancer cells.
SCID mice were injected intravenously with 20 μL of antiserum to ASGM1 on day 0, and 3 × 105 MDA231 human breast carcinoma cells were injected intravenously on day 1. Antibodies, including rabbit IgG (panel A) and 279 Ab (Panel B) at 25 μg/mouse (1 mg/kg), were given intraperitoneally to the mice on days 4, 8, 12, 16, 20, 24, and 28. For experimental metastasis experiments, mice from both groups were sacrificed on the 35th day after intravenous injection of the tumor cells. Lungs were extracted and fixed in formalin. Histological sections were stained with hematoxylin and eosin to evaluate tumor metastases. Photographs were taken at 150× magnification. The arrows indicate metastatic lesions. One representative field from 2 experiments is shown.
Anti-MCP-1 inhibited lung tumor metastasis in mice bearing MDA-231 tumors.
SCID mice were injected intravenously with 20 μL of antiserum to ASGM1, on day 0, and 3 × 105 MDA231 human breast carcinoma cells were injected intravenously on day 1. Antibodies, including rabbit IgG and anti-MCP-1 at 25 μg/mouse (1 mg/kg), were given intraperitoneally to the mice on days 4, 8, 12, 16, 20, 24, and 28. For experimental metastasis experiments, mice from both groups were sacrificed on the 35th day after intravenous injection of the tumor cells. Lungs were extracted and fixed in formalin. Histological sections were stained with hematoxylin and eosin. By using the Bioquant Program, the area of the total tissue per field 40× field (D1) was determined. The micrometastases present within the same field were gated, and the area within the gates was counted (D2). The metastatic index was calculated by the ratio D2/D1. A minimum of 20 fields was analyzed per slide in a blinded manner, and 8 mice were used per group in each experiment. The experiment was repeated 2 times. The figure shows the data obtained from the 2 separate experiments. The mean and SEM for each group are indicated. Statistical analysis between the 2 groups was performed using Student t test (P < .005).
Anti-MCP-1 inhibited lung tumor metastasis in mice bearing MDA-231 tumors.
SCID mice were injected intravenously with 20 μL of antiserum to ASGM1, on day 0, and 3 × 105 MDA231 human breast carcinoma cells were injected intravenously on day 1. Antibodies, including rabbit IgG and anti-MCP-1 at 25 μg/mouse (1 mg/kg), were given intraperitoneally to the mice on days 4, 8, 12, 16, 20, 24, and 28. For experimental metastasis experiments, mice from both groups were sacrificed on the 35th day after intravenous injection of the tumor cells. Lungs were extracted and fixed in formalin. Histological sections were stained with hematoxylin and eosin. By using the Bioquant Program, the area of the total tissue per field 40× field (D1) was determined. The micrometastases present within the same field were gated, and the area within the gates was counted (D2). The metastatic index was calculated by the ratio D2/D1. A minimum of 20 fields was analyzed per slide in a blinded manner, and 8 mice were used per group in each experiment. The experiment was repeated 2 times. The figure shows the data obtained from the 2 separate experiments. The mean and SEM for each group are indicated. Statistical analysis between the 2 groups was performed using Student t test (P < .005).
Discussion
Here we report that MCP-1, a CC chemokine, can directly mediate angiogenesis. By using endothelial cells of different origins, including HUVECs and HMECs, we demonstrated that MCP-1 induced endothelial cell migration in a dose-responsive manner. This chemotactic response was inhibited by a neutralizing antibody to MCP-1. The ability of HUVECs and HMECs to respond to MCP-1 was further supported by the detection of CCR2 on the endothelial cell surface. The expression of CCR2 by endothelial cells and their responsiveness toward MCP-1 prompted us to analyze the effect of MCP-1 toward angiogenesis. The angiogenic effect of MCP-1 was clearly evident in both the in vivo matrigel plug assay and CAM assays and was appropriately inhibited by a neutralizing antibody to MCP-1. The associated inflammatory responses and previously established observations that mononuclear cell products can also act as angiogenic mediators25-27 led to the use of the rat aortic ring assay, which allowed us to evaluate angiogenic effects in the absence of an inflammatory response. MCP-1 induced rat aortic endothelial cell sprouting in a dose-responsive manner. Thus, MCP-1 can act as a direct mediator of angiogenesis.
Our data regarding the angiogenic effects of MCP-1 are in agreement with an early report by Ito et al.9 in which MCP-1 administration was found to increase lateral and peripheral conductance in rabbits with femoral occlusion. This phenomenon involved the enhancement of capillary sprouting induced by either monocyte accumulation or as an unknown direct effect of MCP-1 on endothelial cells. Furthermore, MCP-1 was found to be abundantly expressed by perivascular smooth muscle cells during the proliferative phase of hemangiomas, in contrast to involuting hemangiomas that did not express MCP-1.28 Additionally, dexamethasone and interferon (IFN)α, two agents commonly used to treat the proliferative phase of hemangioma growth, also down-regulated MCP-1 messenger RNA (mRNA) expression by smooth muscle cells in vitro.28 Because MCP-1 is abundantly found during the initial stage of wound healing, it follows that MCP-1 contributes not only indirectly but also directly toward wound healing. However, the observation that knockout mice deficient for either MCP-1 or CCR2 did not reveal detectable impairments regarding vascularization29-31 might be due to the redundancy of angiogenic pathways that support this process.
Given our data reported above and previous reports32,33 on the production of MCP-1 by several tumor cell types, questions were raised regarding the possibilities that MCP-1 production actually can contribute to tumor growth and that this chemokine might be a contributor to tumor angiogenesis. Chemokines have previously been demonstrated to promote the progression of other human tumors.1-3 Treatment of SCID mice bearing human breast carcinoma cells with antibody to MCP-1 significantly enhanced their survival and inhibited the growth of tumor metastases in the lungs in about 2.5-fold when compared with control antibody-treated mice. These results are in line with a previous report by Nakashima et al.20 in which transfection of MCP-1 into a murine colon adenocarcinoma cell line increased lung metastases by augmentation of neovascularization. Several studies have been conducted to evaluate MCP-1 gene therapy in murine tumor models with controversial results.34-36 Although the underlying basis of this divergence is unclear, one can speculate that different responses might be obtained with different tumor types, which might exhibit heterogeneity in their cytokine repertoire. In addition, it is important to recall that chemokine responses are dose dependent; in our case, MCP-1 exerted major in vivo angiogenic effects at doses between 10 and 100 ng/mL but not at higher doses. This principle can also be exemplified by other chemokines, such as GRO-β, a well-known angiogenic factor, that was shown to inhibit blood vessel formation in the CAM assay and cornea vascularization induced by bFGF when used at high concentrations.37
In addition to dose-dependent effects, another aspect to consider, regarding MCP-1, is that it has multifunctional activities that might conflict with one another. On one hand, the capacity of MCP-1 to induce angiogenesis directly and, on the other, its capacity to induce macrophage infiltration into tumor tissue could have disparate effects in tumor immunity.38 Although the reported roles of MCP-1 as a factor conferring immunity against the tumors are controversial,39 40 in our studies, we cannot address this issue, because we used SCID mice that limit us regarding the study of antitumor immunity. And despite our observation that antibody therapy directed against MCP-1 seemed to have a beneficial effect on the growth of experimental tumor metastases, we were unable to cure the mice bearing MDA 231 tumors. This situation might be due to the broad repertoire of other angiogenic and growth factors produced by this tumor, including VEGF, IL-8, GROα, etc. We are currently continuing to use MCP-1 therapy in combination with antibodies to other angiogenic factors produced by these tumors in an attempt to more efficiently block tumor growth. Anti-MCP-1 therapy will also be tested in several human carcinomas, including breast carcinoma cell lines, and it follows that carcinomas responsive to anti-MCP-1 treatment will be selected for therapy in combination of anti-MCP-1 with other antibodies to different angiogenic factors produced by these tumors.
Acknowledgments
The authors thank Dr Carlos Martinez (Centro National de Biotecnologia, Madrid, Spain) for kindly providing the antibody to CCR2. We also thank Dr Robert Wiltrout for critically reviewing the manuscript and for helpful suggestions and Dr Lloyd Kincer of Bioquant Inc for assistance regarding the method used for the measurement of micrometastases.
Supported by the National Cancer Institute; R.S. was supported by Abgenix, Inc. By acceptance of this article, the publisher or recipient acknowledges the right of the U.S. Government to retain a non-exclusive, royalty-free license in and to any copyright covering the article.
Reprints:William J. Murphy, LLB, DBS, NCI-Frederick Cancer Research and Development Center, Bldg 567, Rm 209, Frederick, MD 21702-1201; e-mail: murphyw@ncifcrf.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal