Abstract
The authors investigated the roles of PI3-kinase and PLC-γ in stimulation by Steel Factor (SLF) through c-Kit. c-Kit mutants YF719, YF728, and a YF719/YF728 double mutant were expressed in 32D myelomonocytic cells. KitYF719 fails to recruit PI3-kinase after stimulation with SLF, whereas KitYF728 fails to stimulate PLC-γ phosphorylation or mobilize Ca++. Both single mutants responded mitogenically to soluble SLF (sSLF) in a manner indistinguishable from wild type (WT), although sSLF failed to stimulate or promote the survival of cells expressing the double mutant. In contrast, although cells expressing WT or YF719 were mitogenically stimulated by membrane-bound SLF (mSLF), stimulation of cells expressing KitYF728 was impaired. Similarly, cells expressing WT or YF719 receptors were stimulated by plate-bound anti-Kit antibodies, whereas cells expressing the YF728 receptor were not stimulated. Neomycin sulfate, a PLC antagonist, inhibited cells expressing YF719 receptors stimulated by sSLF. Neomycin also inhibited cells expressing the WT receptor that were stimulated by mSLF or immobilized anti-Kit antibodies but did not inhibit stimulation of cells expressing WT or YF719 receptors by sSLF. 32D cells expressing KitWT, KitYF719, or KitYF728 were injected into mice and the presence of cells was evaluated by colony assays 6 to 7 weeks later. Although both KitWT and KitYF719 expressing cells could be recovered from the spleen and bone marrow, recovery of KitYF728 cells from these organs was severely reduced. These results indicate that Kit tyrosine 728 is of particular importance for mitogenic stimulation by mSLF or immobilized ligand and is required for full maintenance of cells in vivo, likely through activation of PLC-γ.
Introduction
c-Kit, the gene product of the W locus, is a receptor tyrosine kinase that regulates the survival, growth, and differentiation of hemopoietic stem cells, mast cells, germ cells, and melanocytes.1-4 Steel Factor (SLF), the gene product of the Sl locus, is the ligand for c-Kit. Mutations in either gene exhibit similar defects in their target tissues, highlighting the complimentary nature of the ligand-receptor interaction.5-8
SLF is expressed on stromal cells or fibroblasts as a membrane-bound molecule. Soluble SLF (sSLF) is generated by chymase cleavage of the extracellular portion of the growth factor.9-12 Increasing evidence indicates that the 2 forms of the ligand stimulate qualitatively different responses.13 For example, in vitro, combinations of other factors with membrane-bound SLF (mSLF) but not sSLF support the survival of long-term hemopoietic progenitors and erythroblastic leukemia cells14-17 and stimulate erythropoietic development.18 Stromal cells and fibroblasts from Steel-Dickie mice (Sld) secrete sSLF but do not express mSLF. Mice with this mutation exhibit hemopoietic, coat color, and germ cell defects, suggesting that mSLF plays a unique biologic role in these tissues.19 In vivo, the concentration of sSLF in serum is such (3 ng/mL) that it is likely in a monomeric form, and thus limited in its mitogenic potential.20 Therefore, mSLF is presumably the relevant physiologic form of the ligand in vivo.
Although the biochemical basis for the differences between sSLF and mSLF are not completely understood, some signaling differences between the 2 ligand isoforms have been observed. After ligand binding, signal transduction cascades are initiated by the stimulation of receptor autophosphorylation on key tyrosine residues.21 In the case of sSLF, tyrosine phosphorylation of c-Kit is rapid (within minutes), followed by a decline in phosphorylation. This decline in phosphorylation coincides with receptor internalization and endocytosis,22 leading ultimately to receptor degradation. In contrast, phosphorylation of c-Kit by mSLF persists over much longer periods.23 This persistence in tyrosine phosphorylation was attributed to the enhanced stability of the c-Kit receptor on the cell surface after mSLF stimulation, likely because of the prevention of receptor internalization by this form of ligand.
Autophosphorylation of receptor tyrosine kinases generates binding sites that recruit SH2-containing proteins to the receptor. For c-Kit, stimulation with sSLF results in recruitment of PI3-kinase, PLC-γ, and other signal transducing molecules.24 Activated PI3-kinase phosphorylates phosphatidylinositol lipids on the D3 position of the inositol ring.25 This enzyme and its phosphorylated lipid products have been implicated in a number of cellular responses, including membrane ruffling,26,27chemotaxis,28-31 adhesion to fibronectin,32receptor internalization22,33,34 and maintenance of cell survival, and mitogenesis.35-37 Activation of PLC-γ stimulates the hydrolysis of PIP2 to inositol tris-phosphate (IP3) and diacylglycerol (DAG). IP3 promotes the mobilization of Ca++ from internal stores, followed by Ca++ influx from the extracellular milieu, whereas DAG activates protein kinase C (PKC).38 A functional role for PLC-γ in a number of different systems has been observed (reviewed by Kamat and Carpenter39). PLC-γ activation was shown to be required for platelet-derived growth factor (PDGF)-stimulated mitogenesis and monocytic differentiation of myeloid progenitor FDC-P2 cells.40 In addition, a truncated form of c-Kit has been implicated in oocyte activation after fertilization, and this activity was inhibited by PLC-γ inhibitors or Ca++chelators.41 Furthermore, we have previously demonstrated that the blockade of PLC-γ-stimulated Ca++ influx can reverse SLF-mediated survival signals in bone marrow-derived mast cells.42 Therefore, both PI3-kinase, PLC-γ, and their second messengers link receptor activation with a variety of distal responses.
Valius and Kazlauskas43 tested the functional relevance of PI3-kinase and PLC-γ activation using PDGF receptors (PDGFRs) in which all the intrinsic tyrosine phosphorylation sites were mutated and then added back individually. They demonstrated that adding back either the PLC-γ binding site or the PI3-kinase binding site in the PDGFR is sufficient to mediate PDGF-stimulated mitogenesis.43 In addition, other studies have shown that cellular responses such as chemotaxis and adhesion require either PI3-kinase or PLC-γ.44-46 Therefore, despite their different biochemical activities, PLC-γ and PI3-kinase appear to stimulate overlapping or “redundant” cellular responses.
Given the apparent redundancy between PI3-kinase and PLC-γ, our objective was to evaluate the requirement for activation of either of these enzymes after stimulation by soluble or membrane-bound SLF in vitro and support of leukemogenesis in vivo. Cells expressing receptors with either the PI3-kinase SH2-binding site or the PLC-γ SH2-binding site mutated to prevent recruitment of these signaling proteins (KitYF719 and KitYF728 receptors, respectively) were tested for their ability to respond to sSLF, mSLF, or plate-bound anti–c-Kit antibodies. We found that, although both mutants responded equally to sSLF, KitYF728-expressing cells demonstrated impaired responses to either mSLF or plate-bound anti-Kit antibodies. Neomycin sulfate, a PLC antagonist, inhibited cellular stimulation by mSLF or immobilized anti–c-Kit antibodies, but not sSLF. Furthermore, the KitYF728 receptor is impaired in its ability to support leukemogenesis of 32D cells in vivo. These results therefore demonstrate that, although not required for stimulation by sSLF, Kit tyrosine 728 is essential for mitogenic stimulation by mSLF, by an immobilized ligand such as plate-bound anti–c-Kit antibodies, and for leukemic growth in vivo, likely through its role in activating PLC-γ.
Materials and methods
Cell culture and transfection
The gp + amphotrophic NIH 3T3 packaging cells (gift from Dr R. Rottapel, Toronto, Ontario) were grown in Dulbecco's modified Eagle's medium (Life Technologies Inc, Burlington, Ontario) supplemented with 10% fetal bovine serum (FBS) and antibiotics. The 32D cells (gift from Dr Joel Greenberger, Pittsburgh, PA) are an interleukin-3(IL-3)–dependent, c-Kit negative myelomonocytic cell line.47 They were routinely grown in RPMI (Life Technologies, Inc) supplemented with 10% FBS and 2% supernatant from WEHI-3 cells as a source of IL-3. All cell cultures contained 55 μmol/L β-mercaptoethanol and antibiotics (both Sigma, Oakville, Ontario).
The complementary DNAs (cDNAs) (obtained from Dr R. Rottapel) for wild-type (wt) murine c-kit or mutants in which either tyrosine 719 was replaced with phenylalanine (YF719) or tyrosine 728 was replaced with phenylalanine (YF728) were cloned into LXSN retroviral expression vectors. This retroviral vector also contains the neo gene that confers resistance to the antibiotic G418. The LXSN YF728 construct was used to generate a YF719/YF728 double mutant by using a directed mutagenesis by the polymerase chain reaction (PCR) method. Briefly, synthetic primers containing both the YF719 mutation and an EcoRI site were combined with flanking primers and amplified to produce 2 fragments, overlapping in the region of the mutating primer. The 2 fragments were isolated and reamplified with the flanking primers. The resulting 1 kilobase (kb) fragment was then TA cloned and verified by restriction digest, followed by digestion with ApaI andSalI to produce a 630-base pair (bp) fragment for subsequent ligation and subcloning into the LXSN Kit YF728 vector. Both junctions and mutations were verified by sequencing.
These vectors were transfected into gp + a NIH 3T3 packaging cells by lipofectamine treatment (Gibco, Grand Island, NY) and selected in 1 mg/mL G418 (Gibco). The 32Ds were cocultured with a confluent layer of pooled, irradiated (20 Gy) gp + a transfectants for 24 hours. The nonadherent 32Ds were removed and cultured for an additional 2 to 5 days in IL-3, followed by selection for c-Kit positive cells in 1 mg/mL G418 and IL-3. The c-Kit–positive 32D cells were further enriched by cell sorting (FacStar Plus, Becton Dickinson, Franklin Lakes, NJ). Surface expression of c-Kit was detected using biotinylated-SLF and streptavidin-conjugated phycoerythrin (Jackson, Westgrove, PA) as described previously.22 Stable populations of c-Kit–expressing 32Ds were then cloned by limiting dilution. The c-Kit–negative 32D-neo cells that express only the neo gene were generated with a similar process that used the empty LXSN vector.
Reagents
Recombinant murine sSLF was produced as described 22and assessed for purity by silver staining and Western blot or was purchased from Biosource (Camarillo, CA). Bioactivity was always assayed on c-Kit–positive cells by 3H-thymidine incorporation. Neomycin sulfate was purchased from Calbiochem (La Jolla, CA).
Western blotting
For the analysis of receptor autophosphorylation and recruitment of p85, 5 × 106 32D infectants were starved overnight in RPMI + 0.5% FBS. Cells were then washed in phosphate-buffered saline containing FBS (PBS-FBS) 3 times, followed by stimulation with 500 ng/mL SLF for 2.5 minutes at 37°C. Cells were immediately washed in ice-cold PBS-FBS, then lysed in lysis buffer containing 50 mmol/L Tris (pH 7.0), 1% NP-40, 20 mmol/L EDTA with the following inhibitors: 200 μmol/L sodium orthovanadate, 20 mmol/L NaF, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin, and 10 μg/mL leupeptin (all Sigma). Lysates were then spun at 10 000 rpm for 20 minutes, and supernatants were incubated with 50 μL of a 20% Protein A slurry (Pharmacia Biotech Inc, Baie D'Urfe, Quebec) and 5 μL of Rb125, a polyclonal rabbit antibody raised against the intracellular portion of the c-Kit protein (gift from Dr Herman Ziltener, Vancouver, British Columbia). Lysates were incubated for 2 hours at 4°C, and the beads were washed 3 times in lysis buffer with inhibitors. Beads were resuspended in loading buffer with β2-mercaptoethanol and boiled for 5 minutes. Released proteins were resolved on a 7.5% acrylamide gel, transferred to nitrocellulose, and blocked in TBS plus 3% gelatin and 0.5% Tween 20. Blots were incubated with 4G10 antiphosphotyrosine antibody (UBI, Lake Placid, NY) in tris-buffered saline (TBS) plus 1% gelatin and 0.5% Tween 20 at a dilution of 1:1000 for 1 hour, followed by goat antimouse-conjugated horseradish peroxidase secondary antibodies27 at a dilution of 1:5000 and visualized with chemiluminescence (NEN Life Science Products). Blots were stripped by acid treatment and reprobed with rabbit polyclonal anti–c-Kit antisera at a dilution of 1:500 or with rabbit polyclonal anti-p85 antisera (Santa Cruz Biotechnology, Santa Cruz, CA), followed by incubation with protein-A–horseradish peroxidase (Amersham Corp, Mississauga, Ontario) at a dilution of 1:30 000. Visualization was again by chemiluminescence. For the analysis of PLC-γ phosphorylation, cells were starved in RPMI + ITS Liquid Media Supplement (Sigma) + 0.05% BSA for 6 hours. Cells were spun down and resuspended at a concentration of 2 × 107/mL. A 2x solution of RPMI + ITS ± SLF (2 μg/mL) preheated to 37°C was added to an equal volume of cells and incubated for 5 minutes in a 37°C waterbath. After the incubation, samples were removed and placed on ice. Cells were washed in ice-cold PBS twice and lysed by freezing and thawing in a buffer containing 50 mmol/L Tris (pH 7.0), 1% NP40, 50 mmol/L EDTA, protease inhibitor cocktail (Roche, Laval, Quebec), phosphatase inhibitor cocktail (Sigma), 200 μmol/L sodium orthovanadate, 20 mmol/L NaF, and 1 mmol/L PMSF. Cells were frozen in a dry ice ethanol bath, thawed on ice, and pelleted in a microfuge at 10 000 rpm for 20 minutes at 4°C. The supernatant was recovered and 50 μL of a 50% protein A slurry, precoated with anti-PLCγ1 antisera (Pharmingen, Mississauga, Ontario) was added. The samples were incubated 2 hours at 4°C with rotation and the beads were washed 5 times in lysis buffer. After the final wash, the beads were resuspended in gel-loading buffer that contained β2-mercaptoethanol and boiled, and the samples were resolved on a 6% polyacrylamide gel. The proteins were transferred to nitrocellulose that was blocked in TBST containing 1% gelatin (Bio-Rad, Mississauga, Ontario), incubated with antiphosphotyrosine antibody (4G10; Upstate Biotechnology) at 0.5 μg/mL in a 1% gelatin solution overnight at room temperature. Secondary antibody, development of the blot, stripping, and reprobing with anti-PLCγ1 antibody was performed as described previously.
Calcium mobilization measurements
The 32D infectants were starved in RPMI with no FBS for 2 hours at 37°C, washed 3 times in Ca++ mobilization buffer, (140 mmol/L NaCl, 4 mmol/L KCl, 1.8 mmol/L CaCl2 · 2H2O, 0.8 mmol/L Mg2SO4 · 7H2O, 1 mmol/L KH2PO4, and 10 mmol/L glucose, pH 8) and then incubated with 10 μmol/L Indo-1 am ester dye (Molecular Probes, Eugene, OR) plus 0.03% pluronic acid (Molecular Probes) for 1 hour at 37°C. Cells were then washed in the same buffer at pH 7.4 and resuspended at a volume of 1 × 107 cells/mL. Cells were kept at room temperature and, for each Ca++ measurement, warmed to 37°C and kept at this temperature for the duration of the 400-second run. SLF was added at a concentration of 200 ng/mL after 30 seconds, and Ca++ mobilization was monitored using flow cytometry by following the ratio of bound-to-unbound fluorophore over time.
Stimulation assays
For bioassays with soluble SLF, 32D infectants were washed 3 times in RPMI + 0.5% FBS and then plated in the same medium with sSLF at a density of 2.5 × 104 cells per well in 96-well flat-bottom plates. The X9/D3 cells are Sl/Sl4 cells that have been transfected with an expression vector that produces only the membrane-bound form of SLF. For Sl/Sl4 or X9/D318 coculture assays, the adherent cells were treated with mitomycin C (1.8 μg/mL) for 2 hours at 37°C, washed 3 times with PBS, trypsinized, counted, and then plated in 96-well plates that had been precoated with 0.1% gelatin (Sigma) at a concentration of 1 × 104 cells per well. The cells were allowed to adhere for 4 to 6 hours. The 32D cells were washed 3 times in RPMI + 0.5% FBS and starved of growth factor for 5 hours. The 32D cells were then added to the wells at a density of 2 × 104 cells per well in medium containing 2% FBS final concentration. For the immobilized anti–c-Kit antibody assays, 96-well plates (Nunc) were coated with 10 μg/mL of a mouse antirat monoclonal antibody (Jackson) overnight at 4°C. Excess antibody was then washed from the plate, the plate was blocked with PBS plus 1% FBS, followed by the addition of varying concentrations of ACK-2 (gift from Dr S. Nishikawa) in PBS-FBS.48 Plates were incubated for 2 hours at 4°C and washed several times, then 2 × 104 32D infectants were added to each well. Neomycin sulfate, when used, was preincubated with the 32D cells for 20 minutes. In all cases, after 18 hours of stimulation, 0.037 MBq (1 μCi) of 3H-thymidine was added to each well for 6 hours. Cells were then harvested and incorporated radioactivity was determined by scintillation counting. The degree of stimulation was determined by calculating the ratio of radioactivity incorporated by the infectants in the presence of the SLF-expressing stromal cells to radioactivity incorporated by the infectants in the presence of stromal cells not expressing SLF after subtracting off counts obtained with the stromal cells alone, according to the formula:
This formula takes into account variations in background from cell to cell and also any nonspecific support of the 32D cells by stromal cells not related to SLF. Plate-coated ACK-2 results were plotted as a measure of incorporation with both secondary and ACK-2 over incorporation with secondary alone.
Leukemic potential
The 5 × 106 32D infectants in PBS were injected intravenously into 5- to 7-week-old female C3H/He mice. Six to 7 weeks later, the mice were killed, and spleen cells and bone marrow cells from both femurs were removed. Single cell suspensions were prepared and counted with a hemocytometer. To determine the 32D-Kit cell content, 1 × 105 cells were plated in RPMI containing 0.3% agar plus 10% FBS, 4% IL-3, and 0.5 mg/mL G418. The plates were incubated for 10 days, after which the number of colonies was determined. Control experiments have determined that the plating efficiency of 32D cells and the various infectants was approximately 30%.
Results
Expression of c-Kit on 32D cells
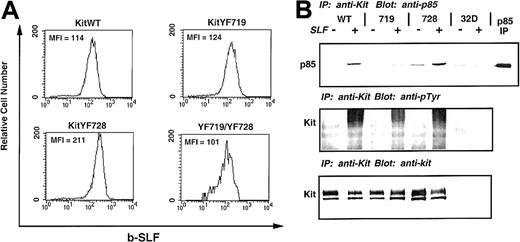
To evaluate the role of PI3-kinase and PLC-γ activation by c-Kit after stimulation by sSLF or mSLF, we transferred WT and c-Kit receptors mutated at recruitment sites for these enzymes into 32D cells. The 32D cells are a c-Kit negative, IL-3–dependent myelomonocytic cell line that have been shown previously to express c-Kit on infection with retroviral vectors containing the c-kit gene.47 The 32D cells were infected with KitWT, KitYF719, and KitYF728 retroviral constructs, selected, and sorted for c-Kit–positive cells by FACS. Single clones were generated and tested for c-Kit expression by flow cytometry. As shown in Figure1A, 32D cells infected with either KitWT, KitYF719, KitYF728, or a double mutant, KitYF719/YF728, express detectable levels of c-Kit on their surface. The mean fluorescence intensities are approximately the same, indicating equivalent levels of receptor on the cell surface of all infectants.
Expression and tyrosine phosphorylation of 32D infectants.
The 32D cells were infected with the indicated c-kitconstructs, selected, sorted, and cloned. These clones were then measured for receptor levels by flow cytometry using biotinylated-SLF, followed by streptavidin-PE. Levels of c-Kit receptor on KitWT, KitYF719, KitYF728, and KitYF719/YF728 cells were approximately equivalent as measured by the mean fluorescence intensity (MFI). Uninfected 32D cells are represented by the shaded histogram. (B): c-Kit receptors from KitWT, KitYF719, KitYF728, and 32D cells were either not stimulated (−) or stimulated (+) with SLF for 2.5 minutes at 37°C. Receptors were then precipitated and resolved by 7.5% SDS-PAGE, followed by transfer to nitrocellulose and blotting with antiphosphotyrosine antibodies (middle panel). Blots were then stripped and reprobed with anti-p85 antibodies (upper panel). Equal levels of Kit were precipitated in each sample as evidenced by stripping and reprobing with anti–c-Kit antibodies (bottom panel).
Expression and tyrosine phosphorylation of 32D infectants.
The 32D cells were infected with the indicated c-kitconstructs, selected, sorted, and cloned. These clones were then measured for receptor levels by flow cytometry using biotinylated-SLF, followed by streptavidin-PE. Levels of c-Kit receptor on KitWT, KitYF719, KitYF728, and KitYF719/YF728 cells were approximately equivalent as measured by the mean fluorescence intensity (MFI). Uninfected 32D cells are represented by the shaded histogram. (B): c-Kit receptors from KitWT, KitYF719, KitYF728, and 32D cells were either not stimulated (−) or stimulated (+) with SLF for 2.5 minutes at 37°C. Receptors were then precipitated and resolved by 7.5% SDS-PAGE, followed by transfer to nitrocellulose and blotting with antiphosphotyrosine antibodies (middle panel). Blots were then stripped and reprobed with anti-p85 antibodies (upper panel). Equal levels of Kit were precipitated in each sample as evidenced by stripping and reprobing with anti–c-Kit antibodies (bottom panel).
Tyrosine phosphorylation of 32D c-Kit and recruitment of p85
We evaluated the ability of the WT and mutant c-Kit receptors to undergo autophosphorylation on ligand binding by exposing the infectants to sSLF, followed by immunoprecipitation with anti-Kit antisera and immunoblotting with antiphosphotyrosine antibodies. As shown in Figure 1B, KitWT, KitYF719, and KitYF728 were tyrosine phosphorylated on stimulation with sSLF (middle panel). When this blot was stripped and reprobed with antibodies to the p85 regulatory domain of PI3-kinase, it was found that p85 coimmunoprecipitated with KitWT and KitYF728 receptors but not KitYF719 receptors. This is in accordance with previous results which demonstrated that Y719 is part of the consensus binding site for PI3-kinase.49 50 The amount of precipitated c-Kit (bottom panel) was found to be similar for all 3 infectants as determined by stripping and reprobing the blot with anti–c-Kit antibodies (Rb 125). We failed to observe immunoprecipitated c-Kit protein or tyrosine phosphorylation in uninfected 32D cells. Therefore, all 3 of these infectants demonstrate kinase activity as exhibited by tyrosine phosphorylation on stimulation with sSLF, and only KitWT and KitYF728 receptors coimmunoprecipitate p85.
KitYF728 receptors do not induce Steel Factor-stimulated PLC-γ recruitment or Ca++ mobilization
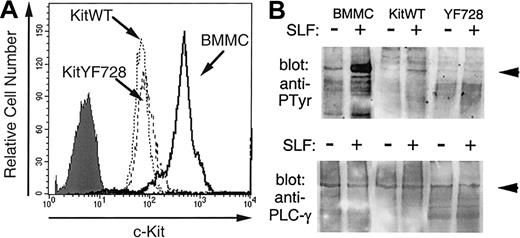
Residue Y728 in murine c-Kit is part of a short sequence of amino acids that closely matches the consensus sequence identified for the PLC-γ SH2 binding domain.51,52 It has been previously demonstrated that PLC-γ is recruited to the c-Kit receptor and becomes tyrosine phosphorylated after stimulation by sSLF.53 54 To confirm the involvement of Y728 in activating PLC-γ, we investigated the tyrosine phosphorylation of PLC-γ1 in response to SLF stimulation. Preliminary experiments confirmed that PLC-γ1 was expressed in both 32D cells and bone marrow-derived mast cells (BMMCs). We also observed that in contrast to BMMCs, 32D cells expressed very low levels of PLC-γ2. Therefore, phosphorylation of this isoenzyme was not analyzed. The 32D cells expressing KitWT or KitYF728 were stimulated with sSLF for 5 minutes, PLC-γ1 was immunoprecipitated and then analyzed by Western blotting with antiphosphotyrosine antibodies. As an additional control, PLC-γ1 from sSLF-stimulated BMMCs was analyzed in a similar fashion. As shown in Figure 2B PLC-γ1 tyrosine phosphorylation was increased in response to sSLF in both BMMCs and 32DkitWT cells. The degree of phosphorylation was considerably greater in BMMCs than in 32D-kitWT cells likely because of the higher c-Kit receptor levels on BMMCs (Figure 2A). In contrast, no additional tyrosine phosphorylation of PLC-γ1 was observed in lysates from 32D-KitYF728 cells in response to sSLF.
SLF-stimulated tyrosine phosphorylation of PLC-γ.
(A) Expression of c-Kit on 32D-kitWT and KitYF728 cells were compared with BMMCs. Cells were incubated with anti-cKit antibody 2B8 and analyzed by flow cytometry. The shaded histogram represents BMMCs with secondary antibody alone. Both 32D infectants exhibited a similar histogram when stained with secondary alone. (B) PLC-γ1 from BMMCs, 32D-KitWT, or 32D-KitYF728 cells stimulated for 5 minutes with sSLF was immunoprecipitated, resolved by SDS-PAGE, transferred to nitrocellulose, and blotted with antiphosphotyrosine antibodies (upper panel). The blot was then stripped and reprobed with anti-PLCγ1 antisera. This experiment was repeated 3 times with similar results.
SLF-stimulated tyrosine phosphorylation of PLC-γ.
(A) Expression of c-Kit on 32D-kitWT and KitYF728 cells were compared with BMMCs. Cells were incubated with anti-cKit antibody 2B8 and analyzed by flow cytometry. The shaded histogram represents BMMCs with secondary antibody alone. Both 32D infectants exhibited a similar histogram when stained with secondary alone. (B) PLC-γ1 from BMMCs, 32D-KitWT, or 32D-KitYF728 cells stimulated for 5 minutes with sSLF was immunoprecipitated, resolved by SDS-PAGE, transferred to nitrocellulose, and blotted with antiphosphotyrosine antibodies (upper panel). The blot was then stripped and reprobed with anti-PLCγ1 antisera. This experiment was repeated 3 times with similar results.
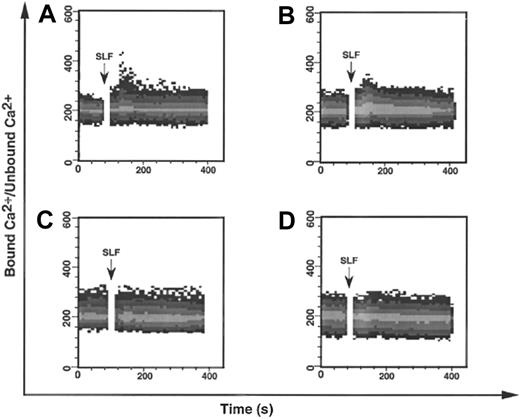
To further investigate the effect of the YF728 mutation, we followed Ca++ mobilization, a downstream consequence of PLC-γ activation.38 KitWT, KitYF719, KitYF728, and uninfected 32D cells were loaded with INDO-1, a fluorescent Ca++chelating agent at 37°C. Cells were then stimulated with sSLF and analyzed for Ca++ mobilization by flow cytometry. In the case of either KitWT or KitYF719 (Figure3A,B), a modest Ca++mobilization is observed after stimulation with sSLF. This increase is comparable to that observed by other groups.55 We observed that neither uninfected 32D cells nor KitYF728 32D cells responded to sSLF by mobilizing Ca++. These results are consistent with an inability of the YF728 mutant to activate PLC-γ.
SLF-stimulated mobilization of Ca++ in 32D infectants.
The 32D cells infected with KitWT (A), KitYF719 (B), or KitYF728 (C), or uninfected (D) were loaded with 10 μmol/L INDO-1 for 1 hour at 37°C, followed by analysis by FACStar plus for Ca++mobilization. 200 ng/mL SLF was added after 30 seconds to INDO-1 loaded cells. Ca++ mobilization was followed over time as a ratio of bound Ca++ signal/unbound Ca++signal.
SLF-stimulated mobilization of Ca++ in 32D infectants.
The 32D cells infected with KitWT (A), KitYF719 (B), or KitYF728 (C), or uninfected (D) were loaded with 10 μmol/L INDO-1 for 1 hour at 37°C, followed by analysis by FACStar plus for Ca++mobilization. 200 ng/mL SLF was added after 30 seconds to INDO-1 loaded cells. Ca++ mobilization was followed over time as a ratio of bound Ca++ signal/unbound Ca++signal.
KitWT, KitYF719, and KitYF728 but not KitYF719/YF728 32D cells respond to soluble Steel Factor
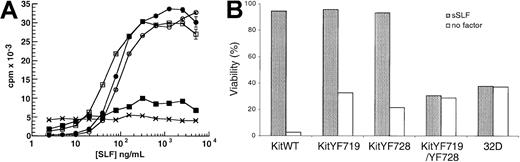
Both PI3-kinase and PLC-γ have been implicated in growth factor receptor-mediated mitogenesis,35-37,39,40,56 and we have previously demonstrated that PLC-γ–stimulated Ca++influx is critical for SLF-dependent cell survival.42 We determined the effect of the KitYF719 and KitYF728 mutations on SLF-stimulated mitogenesis by incubating KitWT-, KitYF719-, and KitYF728-expressing 32D cells with varying concentrations of sSLF and measuring thymidine incorporation. In addition to these 3 cell types, 32D cells expressing the KitYF719/YF728 double mutant were also tested. As shown in Figure 4A, the 3 cell lines 32D-KitWT, KitYF719, and KitYF728 responded equally well to sSLF (empty circles, filled circles, and empty squares, respectively). In contrast, the KitYF719/YF728 double-mutant 32D cells (filled squares) failed to respond to sSLF, exhibiting counts that were not significantly different than those obtained with the uninfected 32D c-Kit–negative cells (crosses). A failure to incorporate thymidine may represent either loss of cell viability or simple growth arrest. To distinguish between these 2 possibilities, we determined the proportion of viable cells in the cultures after incubation in the presence or absence of sSLF. As shown in Figure 4B, SLF maintains the viability of 32D cells expressing the WT receptor or receptors with either the YF719 or YF728 mutations. In contrast, cells expressing the double mutant do not remain viable in sSLF. These data are therefore consistent with the requirement for either PI3-kinase or PLC-γ activation for survival and mitogenic signals. These results are also in agreement with those of Valius and Kazlauskas43 who demonstrated that either the PI3-kinase or the PLC-γ binding sites were sufficient to restore PDGF-mediated mitogenesis, but that receptors bearing mutations at both of these sites were mitogenically inert.
Stimulation of 32D infectants with sSLF.
(A) 32D cells infected with KitWT (○), KitYF719 (●), KitYF728 (■), KitYF719/YF728 (▪), or uninfected (×) were incubated with various concentrations of sSLF for 18 hours at 37°C, followed by a 6 hours 3H-thymidine pulse. Cells were then harvested and incorporated counts were determined by scintillation counting. Error bars represent the standard error determined from triplicate measurements. (B) 32D infectants were incubated with sSLF overnight. Percentage (%) viability was determined by scoring ability to exclude trypan blue. Viability of all cells in the presence of WEHI cm was 93% to 97%.
Stimulation of 32D infectants with sSLF.
(A) 32D cells infected with KitWT (○), KitYF719 (●), KitYF728 (■), KitYF719/YF728 (▪), or uninfected (×) were incubated with various concentrations of sSLF for 18 hours at 37°C, followed by a 6 hours 3H-thymidine pulse. Cells were then harvested and incorporated counts were determined by scintillation counting. Error bars represent the standard error determined from triplicate measurements. (B) 32D infectants were incubated with sSLF overnight. Percentage (%) viability was determined by scoring ability to exclude trypan blue. Viability of all cells in the presence of WEHI cm was 93% to 97%.
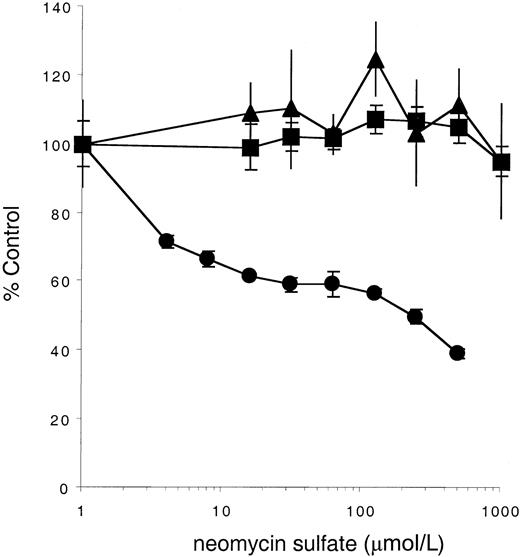
Neomycin sulfate inhibits stimulation of KitYF719- but not KitWT- or KitYF728-expressing cells by soluble Steel Factor
Our observation that the YF719/YF728 double c-Kit mutant does not mediate a survival or mitogenic signal in response to sSLF suggests that in the absence of PI3-kinase recruitment, PLC-γ activation is required for cell support. To further confirm this requirement, cells expressing either the WT or mutant receptors were stimulated in the presence or absence of neomycin sulfate, an antagonist of PLC activity.57 As shown in Figure5, neomycin concentrations as high as 500 μmol/L have little effect on stimulation by sSLF of 32D cells expressing the KitWT or KitYF728 receptor. In contrast, 32D cells expressing KitYF719 are inhibited by neomycin sulfate. The IC50 is approximately 100 μmol/L, a concentration previously reported to be inhibitory for PLC-dependent processes.57-61 These data therefore support the conclusion that, in the absence of PI3-kinase recruitment, PLC activation is required for a full mitogenic signal by sSLF.
Neomycin sulfate inhibits stimulation of KitYF719- but not KitWT- or KitYF728-expressing cells.
32D cells infected with KitWT (▪), KitYF719 (●), or KitYF728 (▴) were incubated with 150 ng/mL sSLF and varying concentrations of neomycin sulfate for 18 hours at 37°C, followed by a 6 hours3H-thymidine pulse. Cells were then harvested and incorporated counts were determined by scintillation counting. Percentage control refers to incorporated counts observed in the absence of neomycin sulfate. Error bars represent the standard error determined from triplicate measurements.
Neomycin sulfate inhibits stimulation of KitYF719- but not KitWT- or KitYF728-expressing cells.
32D cells infected with KitWT (▪), KitYF719 (●), or KitYF728 (▴) were incubated with 150 ng/mL sSLF and varying concentrations of neomycin sulfate for 18 hours at 37°C, followed by a 6 hours3H-thymidine pulse. Cells were then harvested and incorporated counts were determined by scintillation counting. Percentage control refers to incorporated counts observed in the absence of neomycin sulfate. Error bars represent the standard error determined from triplicate measurements.
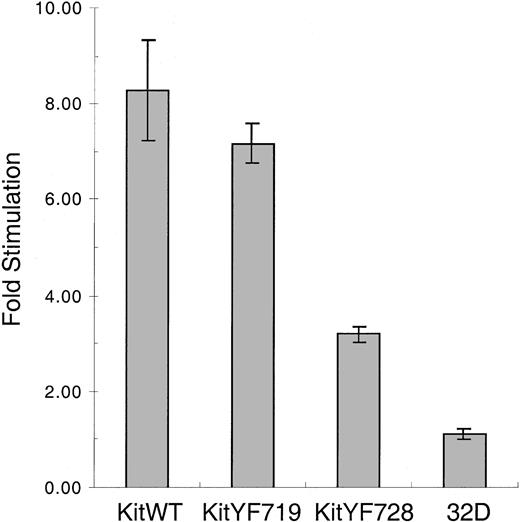
Response of KitYF728 receptors to membrane-bound Steel Factor is impaired
We next determined the ability of c-KitWT and mutant receptors to be stimulated by mSLF. We used X9/D3 stromal cells as a source of mSLF.18 These cells were generated by transfecting SLF-negative Sl/Sl4 cells with an expression vector encoding a form of murine SLF that produces only the membrane bound and not the soluble form of the ligand. X9/D3 cells were treated with mitomycin C, plated, and then cocultured with KitWT, KitYF719, KitYF728, or uninfected 32D cells. As shown in Figure6, both KitWT and KitYF719 32D infectants are stimulated 7- to 8-fold by coculture with X9/D3 cells above stimulation obtained with SLF-negative Sl/Sl4 cell cocultures. This pattern of stimulation has been observed in a number of experiments that used other mSLF-expressing cell lines as well (not shown). In contrast, the KitYF728 32D infectants were stimulated at most 3-fold by X9/D3 cells above that seen using SLF-negative Sl/Sl4 cells as the stimulus. This experiment revealed that, although 32D-KitYF728 cells are fully stimulated by sSLF, these cells are poorly stimulated by mSLF, suggesting that PLC-γ activation may be critical for responding to the membrane-bound isoform of SLF.
Stimulation of 32D infectants with mSLF on X9/D3 stromal cells.
32D infectants were cocultured with mSLF-expressing X9/D3 stromal cells. KitWT, KitYF719, KitYF728, and 32D uninfected cells were incubated with X9/D3 cells for 18 hours, followed by a 6 hours3H-thymidine pulse. Cells were then harvested and incorporated counts were determined by scintillation counting. Fold stimulation represents stimulation of cells on X9/D3 cells compared with stimulation observed on SLF-negative parental Sl/Sl4cells according to the formula in “Materials and methods.” Incorporation levels for fibroblasts alone were typically 9000 to 10 000 cpm, whereas cocultures resulted in 12 000 to 19 000 cpm depending on the cell type. Error bars represent the standard error determined from triplicate measurements.
Stimulation of 32D infectants with mSLF on X9/D3 stromal cells.
32D infectants were cocultured with mSLF-expressing X9/D3 stromal cells. KitWT, KitYF719, KitYF728, and 32D uninfected cells were incubated with X9/D3 cells for 18 hours, followed by a 6 hours3H-thymidine pulse. Cells were then harvested and incorporated counts were determined by scintillation counting. Fold stimulation represents stimulation of cells on X9/D3 cells compared with stimulation observed on SLF-negative parental Sl/Sl4cells according to the formula in “Materials and methods.” Incorporation levels for fibroblasts alone were typically 9000 to 10 000 cpm, whereas cocultures resulted in 12 000 to 19 000 cpm depending on the cell type. Error bars represent the standard error determined from triplicate measurements.
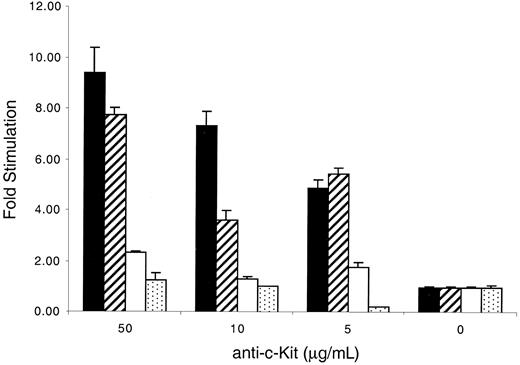
KitWT and KitYF719 receptors but not KitYF728 receptors respond to plate-bound anti–c-Kit antibodies
An alternate method that mimics stimulation by mSLF is the use of plate-bound c-Kit–specific antibodies.62 We therefore used this form of stimulation, which is not complicated by the presence of other cellular factors, to investigate the response of cells bearing WT and mutant receptors. We found that c-Kit–positive 32D cells failed to be stimulated by rat antimouse c-Kit–specific monoclonal antibody ACK-2 alone or mouse-antirat IgG alone (data not shown). However, when both plate-bound antirat antibodies, followed by ACK-2, were used together, both 32D-KitWT and KitYF719 cells responded to the plate-bound antibodies in a concentration-dependent fashion with maximal stimulation of 8- to 9-fold above background (Figure7). In contrast, KitYF728 cells exhibited stimulation no more than 2- to 3-fold above background. Therefore, although 32D-KitYF728 cells respond to sSLF, they fail to fully respond to plate-bound anti-Kit antibodies. Given the previous observation that plate-bound anti–c-Kit antibodies have been shown to mimic mSLF,62 this experiment is consistent with a requirement for PLC-γ activation after stimulation with mSLF or immobilized ligand.
Stimulation of 32D infectants with plate-bound anti–c-Kit antibodies.
The 96-well plates were coated with 10 μg/mL mouse-antirat antibodies for 2 hours at 4°C, followed by coating with various concentrations of anti–c-Kit antibodies (ACK-2). KitWT (filled), KitYF719 (hatched), KitYF728 (empty), and 32D uninfected cells (stippled) were then added to coated plates for 18 hours, followed by a 6 hours3H-thymidine pulse. Cells were then harvested and incorporated counts were determined by scintillation counting. Stimulation of 32D cells with both ACK-2 and secondary antibodies was measured as fold stimulation over counts obtained from stimulation with secondary antibodies alone. Incorporation stimulated by ACK-2 varied from 2000 to 7000 cpm, depending on the cell type. Incorporation by the infectants in the absence of ACK-2 was always less than 1000 cpm. Similar results were obtained if fold stimulation was measured over counts obtained from ACK-2 alone. Error bars represent the standard error determined from triplicate measurements.
Stimulation of 32D infectants with plate-bound anti–c-Kit antibodies.
The 96-well plates were coated with 10 μg/mL mouse-antirat antibodies for 2 hours at 4°C, followed by coating with various concentrations of anti–c-Kit antibodies (ACK-2). KitWT (filled), KitYF719 (hatched), KitYF728 (empty), and 32D uninfected cells (stippled) were then added to coated plates for 18 hours, followed by a 6 hours3H-thymidine pulse. Cells were then harvested and incorporated counts were determined by scintillation counting. Stimulation of 32D cells with both ACK-2 and secondary antibodies was measured as fold stimulation over counts obtained from stimulation with secondary antibodies alone. Incorporation stimulated by ACK-2 varied from 2000 to 7000 cpm, depending on the cell type. Incorporation by the infectants in the absence of ACK-2 was always less than 1000 cpm. Similar results were obtained if fold stimulation was measured over counts obtained from ACK-2 alone. Error bars represent the standard error determined from triplicate measurements.
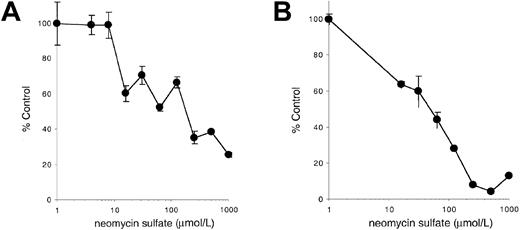
Neomycin sulfate inhibits stimulation by membrane-bound Steel Factor or immobilized anti–c-Kit antibodies
Our results indicate that PLC-γ activation may be critical for responding to the membrane-bound isoform of SLF or to immobilized agonists such as anti–c-Kit antibodies. To further investigate this possibility, we determined the effect of the PLC antagonist neomycin sulfate on stimulation of c-Kit–positive cells by mSLF, sSLF, or immobilized anti–c-Kit antibodies. As shown previously in Figure 5, addition of neomycin sulfate has only a minimal effect on the ability of sSLF to stimulate 32D-KitWT cells. In contrast as shown in Figure8, neomycin sulfate specifically inhibits the ability of fibroblasts or immobilized anti–c-Kit antibodies to support these cells. Furthermore, the IC50 in both cases is approximately 100 μmol/L, a concentration consistent with previously reported concentrations required to inhibit PLC activation58 59 and the concentration required to inhibit stimulation of YF719 cells by sSLF (Figure 5). These results therefore provide further evidence that PLC activation is important for cells stimulated by mSLF but not sSLF.
Effect of neomycin sulfate on stimulation by mSLF, or immobilized anti–c-Kit antibodies.
(A) 32D cells expressing KitWT were incubated on X9/D3 cells or (B) on immobilized ACK-2 anti-Kit antibodies as described in “Materials and methods” in the presence of varying concentrations of neomycin sulfate for 18 hours, followed by a 6 hours pulse with3H-thymidine. Cells were then harvested and incorporated counts were determined by scintillation counting. Percentage control refers to incorporated counts observed in the absence of neomycin sulfate. Error bars represent the standard error determined from triplicate measurements.
Effect of neomycin sulfate on stimulation by mSLF, or immobilized anti–c-Kit antibodies.
(A) 32D cells expressing KitWT were incubated on X9/D3 cells or (B) on immobilized ACK-2 anti-Kit antibodies as described in “Materials and methods” in the presence of varying concentrations of neomycin sulfate for 18 hours, followed by a 6 hours pulse with3H-thymidine. Cells were then harvested and incorporated counts were determined by scintillation counting. Percentage control refers to incorporated counts observed in the absence of neomycin sulfate. Error bars represent the standard error determined from triplicate measurements.
32D-KitYF728 cells have impaired leukemic potential
Our results indicate that PLC-γ activation is particularly important for supporting Kit-positive cells by mSLF. Because mSLF is likely the more important form of the growth factor in vivo, our results predict that cells dependent on c-Kit–SLF interactions for in vivo growth may also be dependent on PLC-γ activation in vivo. The 32D cells are normally nonleukemogenic. However, transfer and expression of c-kit in these cells renders them responsive to SLF and leukemogenic.47 We therefore tested the leukemic potential of the 32D cells expressing Kit-WT, YF719, and YF728 receptors. The 5 × 106 cells were injected intravenously into unirradiated syngeneic C3H/He mice. Six to 7 weeks later, the presence of 32D cells in the spleen and bone marrow was quantitated using colony formation in agar as an assay. The presence of cells in these organs has been found to be a reliable indicator for the eventual development of lethal leukemia47 (Sittaro and Berger; unpublished data, June 2000). As summarized in Tables1 and2, 32D-KitWT and KitYF719 cells were readily detected in the spleen and bone marrow of inoculated mice (P values of .001 for WT vs control, andP = .034 and .002 for KitYF719 vs control). Tumor load for YF719-expressing cells appeared to be reduced compared with WT-expressing cells; however, this difference was not statistically significant. Furthermore, mice inoculated with these cells will develop massive splenomegaly, extensive infiltration of liver and lymph nodes, and eventual death (Sittaro and Berger; unpublished data, June 2000). In contrast, recovery of 32D-KitYF728 cells from the spleen and bone marrow was severely diminished (P values of .03, .004, and .001 vs KitWT in spleen; P values of .012 and .004 vs WT in bone marrow). Diminished recovery of cells was observed for 2 independent 32D-KitYF728 clones as well as an uncloned, heterogenous population of 32D-KitYF728 cells derived by sorting for Kit-positive cells after large scale infection with the KitYF728 virus. It is therefore unlikely that the diminished recovery of KitYF728 cells in vivo is due to the variation in leukemic potential among different clones. Furthermore, we have extended these observations for as long as 12 weeks after inoculation and have observed that even at this late time point, recovery of YF728 cells is severely diminished (Berger and Sittaro; unpublished data, June 2000). Taken together, these results therefore suggest that tyrosine 728 is of particular importance in supporting 32D cells in vivo, likely because of the contribution of this residue in activating PLC-γ.
Recovery of 32D cells from spleen of mice
| Experimental group . | n . | Cellularity × 10−7 . | G418R cfu/5 × 105cells . | G418R cfu/spleen . | P value vs ctl . | P value vs KitWT . |
|---|---|---|---|---|---|---|
| PBS | 10 | 6.7 | 0.0034 | 0.063 | n/a | .001 |
| 32D neo | 6 | 4.5 | 0.017 | 1.4 | n/a | .001 |
| 32D-KitWT | 16 | 11 | 200 | 41 299 | .001 | n/a |
| 32D-KitYF719 | 6 | 9.5 | 7.3 | 1 534 | .034 | .16 |
| 32D-KitYF728(cl1) | 5 | 6.8 | 4.2 | 81.7 | .262 | .03 |
| 32D-KitYF728(cl2) | 11 | 8.8 | 0.026 | 1.1 | .962 | .004 |
| 32D-KitYF728(sorted) | 5 | 9.5 | 0.1 | 18.2 | .551 | .001 |
| Experimental group . | n . | Cellularity × 10−7 . | G418R cfu/5 × 105cells . | G418R cfu/spleen . | P value vs ctl . | P value vs KitWT . |
|---|---|---|---|---|---|---|
| PBS | 10 | 6.7 | 0.0034 | 0.063 | n/a | .001 |
| 32D neo | 6 | 4.5 | 0.017 | 1.4 | n/a | .001 |
| 32D-KitWT | 16 | 11 | 200 | 41 299 | .001 | n/a |
| 32D-KitYF719 | 6 | 9.5 | 7.3 | 1 534 | .034 | .16 |
| 32D-KitYF728(cl1) | 5 | 6.8 | 4.2 | 81.7 | .262 | .03 |
| 32D-KitYF728(cl2) | 11 | 8.8 | 0.026 | 1.1 | .962 | .004 |
| 32D-KitYF728(sorted) | 5 | 9.5 | 0.1 | 18.2 | .551 | .001 |
Single cell suspensions from spleen of inoculated mice were assayed for 32D cell content 5 to 7 weeks after inoculation. The PBS control refers to mice injected with phosphate-buffered saline only. Cellularity refers to the arithmetic average of cell number per spleen samples. G418R colony-forming units (cfu) were enumerated 10 days after plating in agar. The reported numbers are the geometric means of each group. P values were determined using the unpaired, 2-tailed Student t test.
Recovery of 32D cells from bone marrow of mice
| Experimental group . | n . | Cellularity × 10−6 . | G418R cfu/5 × 105cells . | G418R cfu/femur . | P value vs ctl . | P value vs KitWT . |
|---|---|---|---|---|---|---|
| PBS | 10 | 4.1 | 0.00093 | 0.0031 | n/a | .001 |
| 32D neo | 6 | 1.7 | 0.00081 | 0.0024 | n/a | .001 |
| 32D-KitWT | 16 | 2.9 | 180 | 877 | .001 | n/a |
| 32D-KitYF719 | 6 | 4.1 | 58.2 | 373 | .002 | .347 |
| 32D-KitYF728(cl1) | 5 | 3.4 | 0.00083 | 0.0036 | .92 | .012 |
| 32D-KitYF728(cl2) | 11 | 2.8 | 0.049 | 0.2 | .329 | .004 |
| 32D-KitYF728(sorted) | 5 | 3.3 | 0.011 | 0.2 | .25 | .004 |
| Experimental group . | n . | Cellularity × 10−6 . | G418R cfu/5 × 105cells . | G418R cfu/femur . | P value vs ctl . | P value vs KitWT . |
|---|---|---|---|---|---|---|
| PBS | 10 | 4.1 | 0.00093 | 0.0031 | n/a | .001 |
| 32D neo | 6 | 1.7 | 0.00081 | 0.0024 | n/a | .001 |
| 32D-KitWT | 16 | 2.9 | 180 | 877 | .001 | n/a |
| 32D-KitYF719 | 6 | 4.1 | 58.2 | 373 | .002 | .347 |
| 32D-KitYF728(cl1) | 5 | 3.4 | 0.00083 | 0.0036 | .92 | .012 |
| 32D-KitYF728(cl2) | 11 | 2.8 | 0.049 | 0.2 | .329 | .004 |
| 32D-KitYF728(sorted) | 5 | 3.3 | 0.011 | 0.2 | .25 | .004 |
Single cell suspensions from bone marrow of inoculated mice were assayed for 32D cell content 5 to 7 weeks after inoculation. The PBS control refers to mice injected with phosphate-buffered saline only. Cellularity refers to the arithmetic average of cell number per femur in the bone marrow samples. G418R colony-forming units (cfu) were enumerated 10 days after planting in agar. The reported numbers are the geometric means of each group. P values were determined using the unpaired, 2-tailed Student ttest.
Discussion
In this study, we have investigated the role of 2 c-Kit–associated signaling molecules, PI3-kinase and PLC-γ, in mitogenic stimulation by sSLF and mSLF. We found that KitWT and the mutants KitYF719 and KitYF728 transmitted equivalent mitogenic signals in response to sSLF. In contrast, KitYF719/YF728, a receptor with both PI3-kinase and PLC-γ binding sites mutated, failed to respond to sSLF. As well, we showed that neomycin sulfate, a PLC antagonist, inhibits mitogenic stimulation by sSLF in cells expressing the KitYF719 mutant, but not the KitWT or KitYF728 receptors. These observations indicate that either PI3-kinase or PLC-γ activation (but not both) is required for a mitogenic response to sSLF. Because Valius and Kazlauskas43 observed a similar dependence for stimulation through the PDGF receptor, a requirement for either PI3-kinase or PLC-γ activation may be a common characteristic of mitogenic stimulation by other growth factor receptors as well. More recently, Timokhina et al63 observed a similar form of redundancy between PI3-kinase and c-src activation after stimulation of c-Kit with sSLF. Taken together, these results all suggest that a variety of pathways may be found to be “redundant” when stimulated by soluble forms of ligand.
We extended these observations by investigating the response of these c-Kit mutants to mSLF. We observed that, although both KitWT and KitYF719 transmitted mitogenic signals in response to mSLF, KitYF728, the mutant that does not activate PLC-γ, was impaired in its ability to transmit a mitogenic signal. A similar response by these receptors was also observed when immobilized anti–c-Kit antibodies were used as the stimulus. In further agreement with these results, we observed that the PLC antagonist neomycin sulfate differentially inhibited the ability of mSLF or immobilized anti–c-Kit antibodies to stimulate c-Kit–positive cells. These data therefore indicate that, although PLC-γ activation is not absolutely required in response to sSLF, it is essential when the stimulus is mSLF or an immobilized ligand. Because mSLF is likely the relevant physiologic form of the c-Kit ligand,18,19,64 these results indicate that PLC-γ activation may play a unique and indispensable role in the support of c-Kit–positive cells by SLF in vivo. In agreement with this hypothesis, we found that 32D cells expressing the c-KitYF728 mutant were significantly impaired in their ability to survive in vivo, whereas cells expressing the KitWT or KitYF719 receptors were able to survive and expand. In light of these results, it is of interest that the Wv allele produces a c-Kit receptor that is unable to activate PLC-γ but retains the ability to activate PI3-kinase.65 Thus, some of the phenotypic defects observed in the Wv mouse may reflect the importance of PLC-γ activation in vivo.
A number of studies have demonstrated cross-regulation of PLC-γ activity by products of PI3-kinase. For instance, PI3-kinase inhibitors such as wortmannin or LY294002 can suppress PLC-γ–stimulated calcium signals in diverse cellular systems.26,54,66-68 As well, Falasca et al69 demonstrated that PI3,4,5P3 could bind to the PH domain of PLC-γ1 promoting its targeting to the plasma membrane. Rameh et al68 demonstrated that PI3,4,5P3could also bind to the C-terminal SH2 domain of PLC-γ1, thus stimulating its dissociation from the PDGF receptor and presumably enhancing its ability to access substrates. These researchers further observed that coactivation of PLC-γ and PI3-kinase by the WT PDGF receptor resulted in a 40% greater peak Ca++ response compared with a PDGF receptor that could only activate PLC-γ. In the 32D-Kit infectants used in this study, the low receptor levels preclude an extensive biochemical analysis of the effects of PI3-kinase activation on PLC-γ activity. Nevertheless, it is interesting to note that cells expressing the YF719 mutant do exhibit slightly decreased stimulation by immobilized ligand and decreased tumor load in vivo compared with WT. Therefore, it is possible that the cross-regulation observed in a variety of systems in vitro may also be important in vivo.
Our results highlight the importance of PLC-γ in stimulation by mSLF or immobilized ligand, but they do not address why these forms of stimulation fail to support Kit-positive cells in the absence of PLC activation. Given that, in the absence of PLC activation, PI3-kinase is able to support cells stimulated by sSLF, it is possible that mSLF or immobilized ligand fail to fully activate elements of the PI3-kinase–dependent pathway. This possibility is currently under investigation.
Although this study has focused on the roles of PI3-kinase and PLC-γ in mitogenic stimulation by SLF, the c-Kit receptor is known to activate a number of other signaling molecules, including theras70 and c-srcpathways.71 Our observation of a clear correlation between stimulation by mSLF, immobilized antibody, and recovery of 32D cells in vivo suggests that these forms of in vitro stimulation are appropriate models for investigating Kit signaling as it occurs in vivo.
Acknowledgments
We wish to thank Drs R. Rottapel, J. Greenberger, and H. Ziltener for cell lines and reagents. We also wish to acknowledge the excellent assistance of R. Kapur, D. Bouchard with the Ca++ measurements, and R. Chow with the vector production and sequencing.
Supported by grants from the National Cancer Institute of Canada and the Leukemia Research Fund of Canada to S.A.B. and from the NIH (2RO1 DK 48605) to D.A.W.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stuart A. Berger, Arthritis and Immune Disorder Research Centre, 620 University, Suite 700, Toronto, Ontario, Canada, M5G 2M9; e-mail: berger@oci.utoronto.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal