Abstract
Somatic mutations of the receptor tyrosine kinase Flt3 consisting of internal tandem duplications (ITD) occur in 20% of patients with acute myeloid leukemia. They are associated with a poor prognosis of the disease. In this study, we characterized the oncogenic potential and signaling properties of Flt3 mutations. We constructed chimeric molecules that consisted of the murine Flt3 backbone and a 510-base pair human Flt3 fragment, which contained either 4 different ITD mutants or the wild-type coding sequence. Flt3 isoforms containing ITD mutations (Flt3-ITD) induced factor-independent growth and resistance to radiation-induced apoptosis in 32D cells. Cells containing Flt3-ITD, but not those containing wild-type Flt3 (Flt3-WT), formed colonies in methylcellulose. Injection of 32D/Flt3-ITD induced rapid development of a leukemia-type disease in syngeneic mice. Flt3-ITD mutations exhibited constitutive autophosphorylation of the immature form of the Flt3 receptor. Analysis of the involved signal transduction pathways revealed that Flt3-ITD only slightly activated the MAP kinases Erk1 and 2 and the protein kinase B (Akt) in the absence of ligand and retained ligand-induced activation of these enzymes. However, Flt3-ITD led to strong factor-independent activation of STAT5. The relative importance of the STAT5 and Ras pathways for ITD-induced colony formation was assessed by transfection of dominant negative (dn) forms of these proteins: transfection of dnSTAT5 inhibited colony formation by 50%. Despite its weak constitutive activation by Flt3-ITD, dnRas also strongly inhibited Flt3-ITD–mediated colony formation. Taken together, Flt3-ITD mutations induce factor-independent growth and leukemogenesis of 32D cells that are mediated by the Ras and STAT5 pathways.
Introduction
The receptor tyrosine kinase Flt31-3and its ligand FL4-6 play an important role in survival and self-renewal of early hematopoietic progenitors and monocytic precursors and in early lymphoid development.7-9 The expression of Flt3 on leukemic cell lines and samples from patients with acute leukemia has been extensively studied. Flt3 is expressed on most acute leukemias of myeloid and B-lymphoid origin.10,11 Incubation of leukemic blasts with FL results in enhanced DNA synthesis in some, but not all cases of acute myeloid leukemia (AML)10-15 and in a reduced rate of spontaneous apoptosis of AML blasts.16 Chronic overexpression of Flt3 ligand in hematopoietic progenitors has been shown to induce hematologic malignancies after a long latency period.17Somatic mutations involving a set of in-frame internal tandem duplications (ITDs) occur at the end of exon 11 of the Flt3 gene. These result in the duplication of a stretch of several amino acids in the juxtamembrane region of the receptor.18 ITD mutations are detectable in about 20% of AML samples18 but not in normal hematopoiesis,19 and they are associated with high leukemic cell numbers in patients with acute promyelocytic leukemia.20 The presence of ITD mutations of Flt3 on AML blasts indicates a very poor prognosis.21-23 So far, little is known about the functional consequences of ITD mutations. Expression of ITD-containing Flt3 in COS-7 cells resulted in its constitutive autophosphorylation.20 Our own results indicated that constitutive autophosphorylation of Flt3 is detectable in about 10% of AML cases.24 However, autophosphorylation did not correlate with ITD mutations. The growth of AML blasts with ITD mutations in stroma cell cultures was inhibited compared with blasts not containing ITD mutations.25
In this study, we show that ITD mutations of Flt3 (Flt3-ITD) have oncogenic potential. ITD mutations confer factor-independent growth and radiation resistance onto 32D cells. Also, they induce colony growth in semisolid media, which is not supported by Flt3 ligand in 32D cells transfected with wild-type Flt3 (Flt3-WT). When injected into syngeneic hosts, Flt3-ITD, but not Flt3-WT, leads to the rapid development of a disease resembling leukemia. Analysis of the signaling properties of Flt3-ITD reveals weak constitutive activation of Ras and Akt with ligand-dependent augmentation and strong constitutive activation of STAT5, a novel feature of Flt3 signaling. STAT5 is not activated by a ligand-bound wild-type receptor. Analyses by dominant negative (dn) constructs show an important role of Ras and STAT5 for ITD-induced colony formation. Taken together, ITD mutations of Flt3 have powerful transforming potential in myeloid cells mediated by Ras and aberrant STAT5 activation.
Materials and methods
Reagents
Recombinant human FL and recombinant murine interleukin 3 (IL-3) were purchased from Pepro Tech (Rocky Hill, NJ). Polyclonal rabbit antimouse Flt3 antibodies and the antiphosphoSTAT5 antibody were purchased from Upstate Biotechnology (Lake Placid, NY) and from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal rat antimouse Flt3 antibody was obtained from Pharmingen (San Diego, CA). Monoclonal mouse antiphosphotyrosine antibody (PY20) was obtained from Transduction Laboratories (Lexington, KY). AntiphosphoErk2, anti-Erk2, and total anti-STAT5a/b antibodies were obtained from Santa Cruz Biotechnology. AntiphosphoAkt and anti-Akt were purchased from New England Biolabs (Beverly, MA). Anti-ras antibody was obtained from Upstate Biotechnology. The Annexin-V FITC kit was obtained from Immunotech (Marseille, France).
Cell lines
The IL-3–dependent murine myeloid cell line 32Dcl3 (kindly provided by Dr Felicitas Rosenthal, Freiburg, Germany) was cultured in RPMI 1640, supplemented with 10% WEHI-conditioned medium as a source of IL-3, 10% fetal calf serum (FCS), and antibiotics at 37°C with 5% CO2.
Construction of expression plasmids and transfection into 32Dcl3 cells
ITD mutations were detected in patients with AML as described previously.24 The membrane proximal region of 4 mutated Flt3 receptor sequences, as well as wild-type human Flt3 derived from the Oci-AML5 cell line, were amplified by reverse transcriptase–polymerase chain reaction (RT-PCR) using PfuDNA polymerase. The sense primer was 5′-GCA CAT CTT GTG AGA CGA TCC-3′ and the antisense primer was 5′-CAC CAT AGC AAC AAT ATT CAA AAA TC-3′, yielding a product corresponding to nucleotides 1562 to 2092 of the published sequence of human Flt3.3 One of 2SspI sites of the PCR products, which is unique to the human Flt3 sequence, was removed by site-directed mutagenesis without alteration of the resulting amino acid code. ABsmBI/SspI fragment of these PCR products spanning nt1567 to 2077 was substitutionally introduced into the sequence of murine Flt3 (provided by Dr I. Lemischka, Princeton, NJ). The sequences of all mutants were confirmed by DNA sequencing. These constructs were cloned into an expression vector (pAL) under the control of the 5′LTR of the Moloney murine sarcoma virus (MoMSV), derived from pLXSN (Clontech, Palo Alto, CA). Ten micrograms of plasmid DNA of either plasmid and 1 μg pMAM/BSD (Kaken Pharmaceutical, Japan) were cotransfected into 32Dcl3 cells by electroporation. Cells were selected with 15 μg/mL blasticidin (Invitrogen, Groningen, The Netherlands) in IL-3 supplemented culture. Polyclonal cell lines were used for further experiments. The ovine STAT5/Y694F mutant (dnSTAT5)26 was subcloned into an expression vector under the control of the 5′LTR of the MoMSV (pOPALI), derived from pOPI3CAT (Stratagene, La Jolla, CA), which contained a neomycin resistance marker.
Lac inducible system
The 32Dcl3 were first transfected with pLAM-LacR, which was derived from pMAM and expresses the Lac repressor. The 32D/pLAM-LacR were further transfected with pOPRSVI (Stratagene) containing RasS17N (dnRas), the expression of which is suppressed by the Lac repressor in the uninduced state, and induced by the addition of isopropyl thiogalactose (IPTG). Highly inducible dnRas-expressing clones were selected by limiting dilution. One of the clones was cotransfected with the pAL/Flt3 constructs and pPur (Clontech) as a selection marker, and puromycin-selected polyclonal lines were used for further experiments. The expression of dnRas was induced by incubating the cell lines with 0.5 mmol/L IPTG for 24 hours, and the expression was verified by Western blotting with anti-ras Ab (Upstate Biotechnology).
Flow cytometry
Cells were preincubated for 15 minutes at 4°C with mouse IgG and subsequently stained for 15 minutes with the indicated antibody. The cells were washed twice in phosphate-buffered saline (PBS)/0.1% bovine serum albumin (BSA) and analyzed with a FACSCalibur (Becton Dickinson, Heidelberg, Germany).
Immunoprecipitation and Western blot analysis
The 32Dcl3 cells transfected with Flt3 constructs were starved from IL-3 and serum for 12 hours in 0.5% FCS. Subsequently, cells were resuspended in 1 mL medium for 10 minutes at 37°C with or without 100 ng/mL FL, washed once with ice-cold PBS, and lysed with buffer containing 50 mmol/L HEPES (pH 7.4), 10% glycerol, 150 mmol/L NaCl, 1% Triton X-100, 1 mmol/L EDTA, 1 mmol/L EGTA, 50 μmol/L ZnCl, 25 mmol/L NaF, proteinase inhibitors (Complete; Boehringer, Mannheim, Germany), 1 μmol/L pepstatin, and 1 mmol/L sodium orthovanadate. Cell lysates were clarified at 20 000g for 20 minutes. For immunoprecipitation, cell lysates were incubated with rabbit polyclonal antibody to murine Flt3 (Upstate Biotechnology) and with Protein A/G-Plus-Sepharose (Santa Cruz Biotechnology). The immunoprecipitates were washed 3 times with lysis buffer. Immunoprecipitates as well as total lysates were resuspended in SDS sample buffer, heated, and separated by SDS PAGE. Gels were blotted on Immobilon P membrane (Millipore, Bedford, MA) and stained with the indicated antibody. Antibody binding was detected by incubation with an horseradish peroxidase (HRP)-labeled secondary antibody, followed by chemiluminescence detection (ECL-Plus, Amersham Pharmacia Biotech, Uppsala, Sweden).
3H thymidine incorporation
A total of 1 × 104 32D cells was IL-3 and serum starved (0.5% FCS) for 12 hours. Subsequently, cells were placed in 200 μL medium supplemented with the indicated concentrations of FL. After a 24-hour incubation at 37°C, 0.037 MBq (1 μCi)3H thymidine was added to each well, and cells were incubated for an additional 8 hours. Cells were harvested on glass fiber filters, and β-emission of the bound DNA was analyzed with a scintillation counter. Experiments were repeated at least 3 times. Each data point represents the mean ± standard deviation of 4 wells.
Radiation-induced apoptosis
A total of 2.5 × 105 cells was starved from IL-3 and serum for 3 hours, placed in 24-well plates, and exposed to 5 Gy γ-irradiation. Immediately after irradiation, cells were supplemented with FL (100 ng/mL), IL-3 (1 ng/mL), or no factor. Cell viability was analyzed using the Annexin-V assay. Cells staining negative for both Annexin-V and propidium iodide were counted as viable cells.
Electrophoretic mobility shift assay
The 32D cells exposed to the indicated cytokines were washed with ice-cold PBS containing 0.5 mmol/L PMSF and 0.2 mmol/L NaVO3 and lysed in 500 μL cold buffer I (20 mmol/L HEPES, pH 7.9, 0.2% [vol/vol] Nonidet P-40, 10% [vol/vol] glycerol, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L DTT, 0.2 mmol/L NaVO3, 0.5 mmol/L PMSF, 10 μg/mL leupeptin, and 10 μg/mL aprotinin). After incubation on ice for 10 minutes, cell lysates were centrifuged at 14 000 rpm for 10 minutes at 2°C. The pellets were resuspended in 100 μL buffer II, containing 20 mmol/L HEPES (pH 7.9), 20% (vol/vol) glycerol, 420 mmol/L NaCl, 2 mmol/L EDTA, 1.5 mmol/L MgCl2, 0.5 mmol/L DTT, 2 mmol/L NaVO3, 0.5 mmol/L PMSF, 10 μg/mL leupeptin, and 10 μg/mL aprotinin, and incubated for 30 minutes at 4°C. After centrifugation at 14 000 rpm for 20 minutes at 2°C, the supernatant was dialyzed against buffer III (20 mmol/L HEPES [pH 7.9], 20% [vol/vol] glycerol, 100 mmol/L NaCl, 2 mmol/L EDTA, 0.5 mmol/L DTT, 2 mmol/L NaVO3, 0.5 mmol/L PMSF) for 4 hours at 4°C. The DNA mobility shift assay was performed by incubating 10 μg nuclear extract with 1 μg poly(dI-dC) and 20 000 cpm of the end-labeled, double-stranded probe in binding buffer (10 mmol/L Tris [pH 7.5], 20% [vol/vol] glycerol, 50 mmol/L NaCl, 1 mmol/L DTT, and 1 mmol/L EDTA) in a final volume of 20 μL. The binding reaction was incubated for 20 minutes at 25°C. The double-stranded oligonucleotide was 5′-AGA TTT CTA GGA ATT CAA TCC-3′, harboring the consensus binding site for STAT5.27 DNA-protein complexes were run on a 4% nondenaturing polyacrylamide gel carried out in 0.5x Tris-Borate-EDTA buffer. The gel was dried and exposed to film. DNA supershift assays were performed by incubation of the nuclear proteins with a specific antibody directed against STAT5a/b (Santa Cruz Biotechnology, 2 μg each per assay) for 15 minutes at 25°C before the addition of the probe.
Clonal growth in methylcellulose
To analyze clonal growth, 1 mL of a culture mixture containing Iscove modified Dulbecco medium (IMDM; Life Technologies, Grand Island, NY), 1% methylcellulose, and 10% FCS was plated on a 35-mm culture dish in the presence of FL (20 to 200 ng/mL), IL-3 (1 ng/mL), or no cytokine. Stably transfected 32Dcl3 cells were seeded at a concentration of 3 × 102 or 3 × 103 cells per dish. The assays were plated as quadruplicates, and colonies were counted on day 6. The numbers given show representative results of one of at least 3 independent experiments per construct. To analyze the effect of the expression of dnSTAT5 on the colony growth, 32Dcl3/Flt3-ITD cells were electroporated with either 20 μg pOPALI/STAT5Y694F or the same amount of pOPALI/CAT. One day after the electroporation, cells were seeded at a concentration of 1 × 105 cells per dish in 1 mL of a culture mix containing IMDM, 1% methylcellulose, 20% FCS, and 0.6 mg/dL G418. The colonies were counted on day 8.
In vivo tumorigenesis assay
Eight- to 10-week-old female C3H/HeJ mice, which were syngeneic with 32Dcl3 cells, were used for the in vivo tumorigenesis experiments. The 1 × 106 cells were inoculated by intravenous injection. Moribund animals were killed. Bone marrow cells were extracted from the femur and stained with Pappenheim stain. The experimental protocols had been reviewed and approved by the local Animal Experimentation Committee.
Results
Construction and expression of the chimeric mutant Flt3 receptors
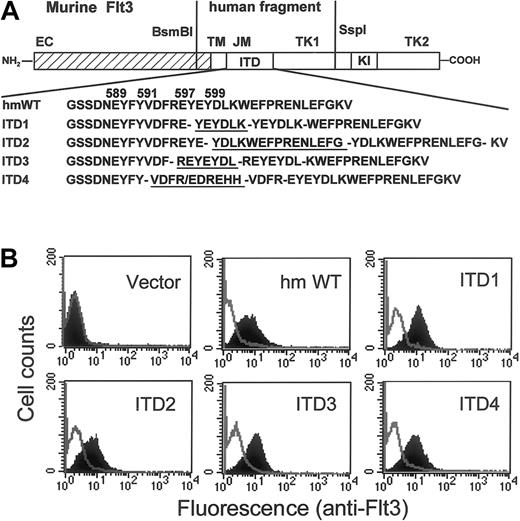
We have previously identified Flt3-ITD mutations from patients with AML.24 To examine the role of ITD mutations in leukemogenesis, we constructed mouse Flt3 complementary DNA (cDNA) mutants containing the mutated human Flt3 sequences (Figure1A). Four different ITD mutations were cloned into the mouse backbone. These mutations were chosen because of their variable involvement of tyrosine residues and differences in the lengths of the duplication. The wild-type human Flt3 cDNA of this region was amplified from OCI-AML5. All constructs were introduced into the IL-3–dependent myeloid progenitor cell line 32D under the control of the MoMSV 5′LTR. After selection of the bulk culture for 14 days in the presence of IL-3, stable expression of murine Flt3 was established as shown in Figure 1B. All Flt3-ITD mutant receptors were expressed at comparable levels with the wild-type human/murine chimera Flt3 (Flt3-WT) or with wild-type murine Flt3 (data not shown). The parental or mock transfected 32D lines did not express Flt3 (Figure1B).
Construction of Flt3 internal tandem duplications and their expression in 32D cells.
(A) A human Flt3 fragment spanning nt1624 to 2134, containing either wild-type or 4 different ITD mutations, was substituted for the corresponding murine Flt3 sequences. The predicted amino acid sequence from either the wild-type or the 4 different ITDs is shown. Duplicated sequences are underlined. EC indicates extracellular domain; TM, transmembrane domain; JM, juxtamembrane domain; TK, tyrosine kinase domain; KI, kinase insert; hmWT, human/mouse wild-type; ITD, internal tandem duplication. (B) Expression of Flt3 receptors on the surface of 32D cells were analyzed by staining with PE-conjugated monoclonal rat antimouse Flt3 antibody. The shaded areas show staining with anti-Flt3; the blank areas show the isotype control.
Construction of Flt3 internal tandem duplications and their expression in 32D cells.
(A) A human Flt3 fragment spanning nt1624 to 2134, containing either wild-type or 4 different ITD mutations, was substituted for the corresponding murine Flt3 sequences. The predicted amino acid sequence from either the wild-type or the 4 different ITDs is shown. Duplicated sequences are underlined. EC indicates extracellular domain; TM, transmembrane domain; JM, juxtamembrane domain; TK, tyrosine kinase domain; KI, kinase insert; hmWT, human/mouse wild-type; ITD, internal tandem duplication. (B) Expression of Flt3 receptors on the surface of 32D cells were analyzed by staining with PE-conjugated monoclonal rat antimouse Flt3 antibody. The shaded areas show staining with anti-Flt3; the blank areas show the isotype control.
Factor-independent DNA synthesis of 32D/Flt3-ITD
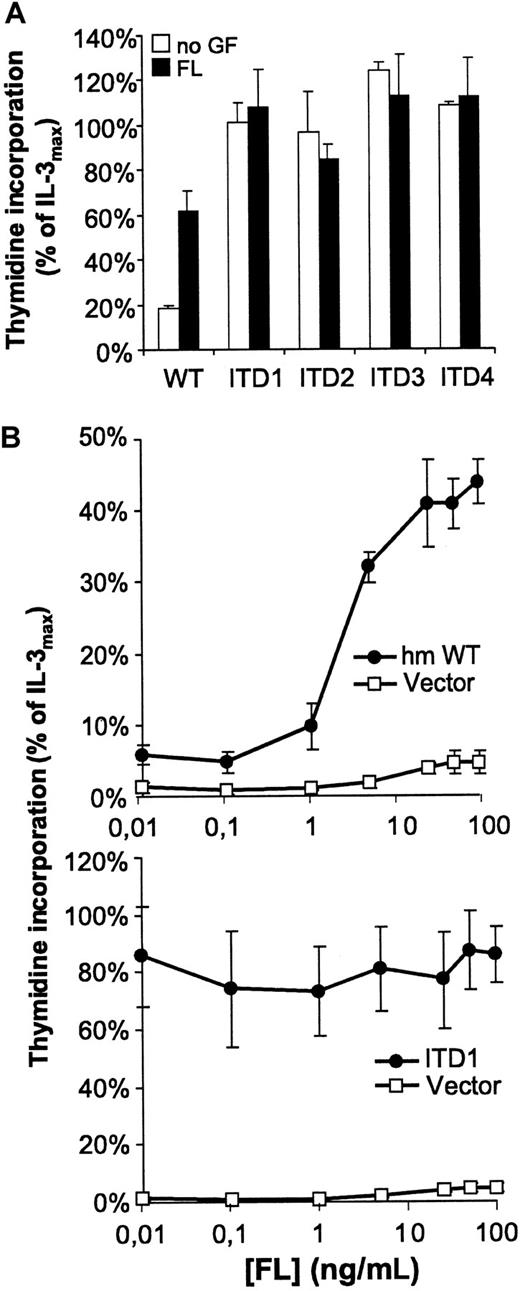
We analyzed the DNA-synthesis of the different mutants by3H thymidine incorporation. The parental 32Dcl3 line did not respond to FL, whereas the Flt3-WT transfectant (32D/Flt3-WT) grew dependent on either IL-3 or FL. However, all cell lines containing Flt3-ITD (32D/Flt3-ITD) proliferated independently of exogenously added IL-3 or FL (Figure 2). In addition, FL did not further increase the rate of DNA synthesis in 32D/Flt3-ITD. Thymidine incorporation of 32D/Flt3-ITD was much higher compared with Flt3-WT cells stimulated with maximal levels of FL. As shown in Figure2A, DNA synthesis of ITD-containing 32D cells without exogenously added growth factors approximately equaled their synthesis rate under the influence of maximal IL-3 stimulation (10 ng/mL).
Ligand-independent 3H thymidine incorporation by Flt3-ITD mutants.
Cells were starved for 12 hours from IL-3 and serum and stimulated with FL (100 ng/mL or indicated concentrations), IL-3 (10 ng/mL), or no cytokine. Data are shown as percentage of thymidine incorporation compared with thymidine incorporation of the respective cell line under IL-3 stimulation. Note the different scale of the y-axis in the 2 diagrams of B.
Ligand-independent 3H thymidine incorporation by Flt3-ITD mutants.
Cells were starved for 12 hours from IL-3 and serum and stimulated with FL (100 ng/mL or indicated concentrations), IL-3 (10 ng/mL), or no cytokine. Data are shown as percentage of thymidine incorporation compared with thymidine incorporation of the respective cell line under IL-3 stimulation. Note the different scale of the y-axis in the 2 diagrams of B.
Resistance to radiation-induced apoptosis of 32D/Flt3-ITD
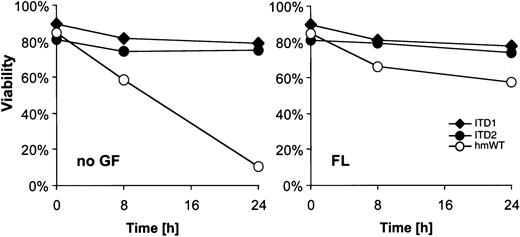
It had been reported that FL mediates survival of early hematopoietic precursors as well as AML cells.16 28Therefore, we examined the role of ITD mutants in DNA damage-induced apoptosis. As shown in Figure 3, 32D/Flt3-WT were highly sensitive to γ-irradiation, with less than 10% viable cells 24 hours after irradiation. On FL exposure, up to 60% of 32D/Flt3-WT were rescued from apoptosis. Interestingly, cells transfected with Flt3-ITD were entirely resistant to irradiation-induced apoptosis. The resistance was independent of FL presence or absence.
Ligand-independent resistance against radiation-induced apoptosis.
Cells were starved for 3 hours and exposed to 5 Gy γ-irradiation. Cells that were Annexin-V and propidium iodide-negative were counted as viable cells. Viability was calculated as a percentage of these cells over the total cell population. FL concentration was 100 ng/mL.
Ligand-independent resistance against radiation-induced apoptosis.
Cells were starved for 3 hours and exposed to 5 Gy γ-irradiation. Cells that were Annexin-V and propidium iodide-negative were counted as viable cells. Viability was calculated as a percentage of these cells over the total cell population. FL concentration was 100 ng/mL.
Clonal growth of 32D/Flt3-ITD in methylcellulose
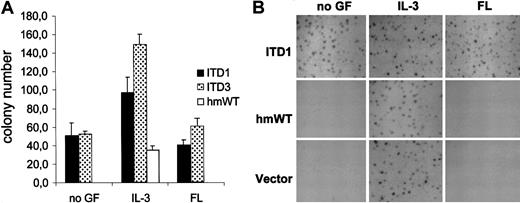
To further analyze the effect of Flt3-ITD mutants on proliferation and survival, we examined the ability of 32D/Flt3-ITD cells to form colonies. The 32D cells transfected with the empty vector produced colonies in the presence of IL-3, but not in the presence of FL (Figure4). Surprisingly, Flt3-WT could not support clonal growth of the 32D cells even in the presence of up to 200 ng/mL FL (Figure 4A). To confirm these findings, we repeated the experiment with 10 times the number of cells per dish. In addition, appropriate expression of Flt3 on the surface of the cells was checked immediately before plating. We still did not detect any colonies, evidencing that Flt3-WT did not allow clonal growth of 32D cells in methylcellulose (Figure 4B). Results obtained with wild-type mouse Flt3 were identical (data not shown). In stark contrast, Flt3-ITD led to colony formation of 32D cells, even in the absence of IL-3 and FL (Figure 4). Taken together, these results indicate that signaling of Flt3-ITD may elicit a signal quality different from the wild-type isoforms of Flt3.
Clonal growth of 32D cells in methylcellulose.
(A) Transfected 32Dcl3 cells were plated at a concentration of 300 cells per dish. Colonies were counted on day 6. The assays were plated as quadruplicates. The numbers given show the results of one of at least 3 independent experiments per construct, which all gave similar results. IL-3; 1 ng/mL, FL; 20 ng/mL. (B) Transfected cells were plated at 3000 cells per dish under the indicated growth conditions. Photographs of the dishes were taken on day 6. IL-3, 1 ng/mL; FL, 200 ng/mL.
Clonal growth of 32D cells in methylcellulose.
(A) Transfected 32Dcl3 cells were plated at a concentration of 300 cells per dish. Colonies were counted on day 6. The assays were plated as quadruplicates. The numbers given show the results of one of at least 3 independent experiments per construct, which all gave similar results. IL-3; 1 ng/mL, FL; 20 ng/mL. (B) Transfected cells were plated at 3000 cells per dish under the indicated growth conditions. Photographs of the dishes were taken on day 6. IL-3, 1 ng/mL; FL, 200 ng/mL.
Flt3-ITD induce leukemogenesis of 32D cells in vivo
Previously, we have shown that a mutation in the catalytic domain of Flt3 leads to factor-independent receptor autophosphorylation, proliferation, and survival of 32D cells.24 However, when we injected cells carrying these mutations into syngeneic mice, they developed obvious disease very late, with a latency period of several months (M.M., R.S., H.S., unpublished data). Therefore, we investigated whether Flt3-ITD mutants enhance leukemogenesis of 32D cells in vivo. The 32D/Flt3-ITD1, 32D/Flt3-ITD2, 32D/Flt3-WT, and 32D/mock cells were intravenously injected into syngeneic mice. In all but one of the mice injected with 32D/Flt3-ITD1 or 32D/Flt3-ITD2, a disease resembling leukemia developed, with massive enlargement of spleen and liver and infiltration of the bone marrow by blastlike cells (Figure5). Most animals injected with these cells died within 5 weeks after injection (Figure6). Mice injected with 32D/mock or 32D/Flt3-WT did not develop obvious disease up to 3 months after injection. After this time, 2 of 10 animals injected with 32D/Flt3-WT developed a similar disease, as did the animals injected with the ITD-containing cells, and died.
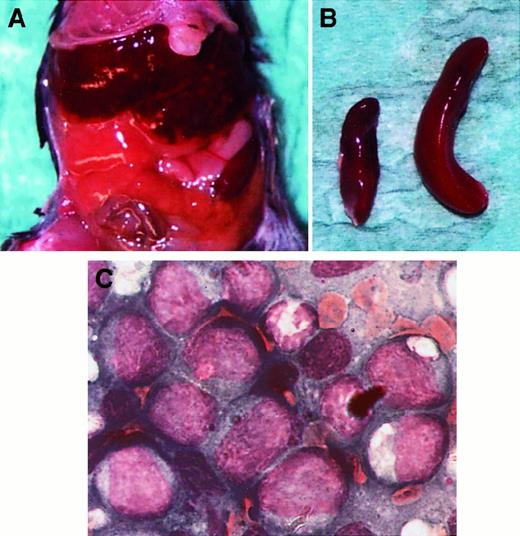
Development of leukemia-like disease in 32D/Flt3-ITD–injected mice.
Female C3H/HeJ mice, aged 8 to 10 weeks, were used for the in vivo tumorigenesis experiments. The 1 × 106 cells were inoculated intravenously. The figure shows an animal at autopsy 28 days after injection of 32D cells containing ITD2. (A) The abdomen of the mouse showing a grossly enlarged liver. (B) The explanted spleen (right) is shown; for size comparison, the spleen of an uninjected animal (left) was photographed simultaneously. (C) Bone marrow smear, Pappenheim stain.
Development of leukemia-like disease in 32D/Flt3-ITD–injected mice.
Female C3H/HeJ mice, aged 8 to 10 weeks, were used for the in vivo tumorigenesis experiments. The 1 × 106 cells were inoculated intravenously. The figure shows an animal at autopsy 28 days after injection of 32D cells containing ITD2. (A) The abdomen of the mouse showing a grossly enlarged liver. (B) The explanted spleen (right) is shown; for size comparison, the spleen of an uninjected animal (left) was photographed simultaneously. (C) Bone marrow smear, Pappenheim stain.
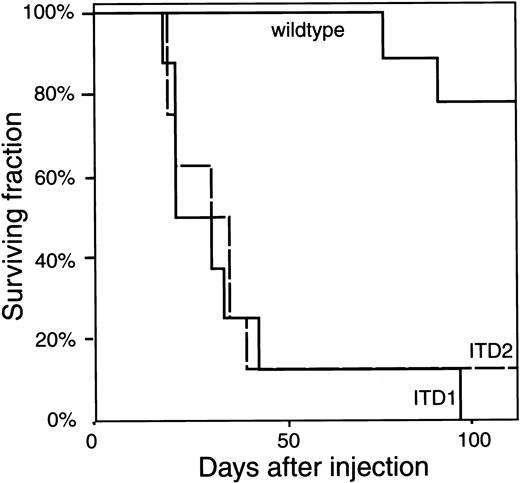
Kaplan-Meier plot of the mice injected with 32D/Flt3.
The fraction of surviving mice is plotted over the time after injection. All but one mouse injected with ITD1 or ITD2 developed the disease (described in the text and in Figure 5) within 4 to 5 weeks after injection and died. Mice injected with wild-type Flt3 survived without obvious disease until day 77 and day 92, when 2 of 10 mice developed the identical disease and also died.
Kaplan-Meier plot of the mice injected with 32D/Flt3.
The fraction of surviving mice is plotted over the time after injection. All but one mouse injected with ITD1 or ITD2 developed the disease (described in the text and in Figure 5) within 4 to 5 weeks after injection and died. Mice injected with wild-type Flt3 survived without obvious disease until day 77 and day 92, when 2 of 10 mice developed the identical disease and also died.
Constitutive activation of the Flt3 receptor and ligand-dependent activation of MAPK and Akt in 32D/Flt3-ITD
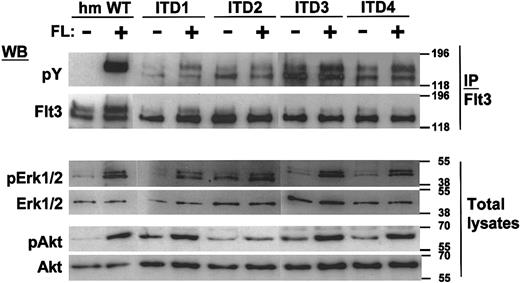
To analyze the signaling properties of Flt3-ITD in comparison to Flt3-WT, we first studied the autophosphorylation status of the Flt3 mutants in transfected 32D cells. The cells were IL-3 and serum starved for 12 hours and FL stimulated in the absence of serum for 10 minutes. From the cell lysates, Flt3 was immunoprecipitated and immunoblotted with an antiphosphotyrosine moAb or an anti-Flt3 polyclonal antibody. Interestingly, we detected aberrant and constitutive autophosphorylation of the lower band of the mutant Flt3 isoforms, which corresponds to its immature, possibly intracellular form (Figure7).29 In contrast, the mature form seems to retain its ligand-specific autophosphorylation pattern, at least in some cases. Also, the balance between the immature and the mature forms of the receptor was disturbed, because only weak expression of the mature form of the receptor was seen on Flt3 immunoblots.
Constitutive tyrosine phosphorylation of Flt3 and ligand-dependent activation of MAP kinase and Akt.
After a 12-hour starvation period, 1 × 107 cells were stimulated for 10 minutes in 1 mL medium with or without 100 ng/mL FL for 10 minutes as indicated. Immunoprecipitates with anti-Flt3 were performed where indicated, and the immunoprecipitates or total cell lysates were separated by SDS-PAGE. After blotting, the blots were stained with the indicated activation-specific antibodies. pErk recognizes Erk1 (p44), and Erk2 (p42), phosphorylated on tyrosine 204; pAkt is specific for Akt phosphorylated on serine 473. Subsequently, the blots were stripped and stained with the indicated protein-specific antibodies to demonstrate equal loading: tErk is specific for total Erk2 (p42) and does not recognize Erk1.
Constitutive tyrosine phosphorylation of Flt3 and ligand-dependent activation of MAP kinase and Akt.
After a 12-hour starvation period, 1 × 107 cells were stimulated for 10 minutes in 1 mL medium with or without 100 ng/mL FL for 10 minutes as indicated. Immunoprecipitates with anti-Flt3 were performed where indicated, and the immunoprecipitates or total cell lysates were separated by SDS-PAGE. After blotting, the blots were stained with the indicated activation-specific antibodies. pErk recognizes Erk1 (p44), and Erk2 (p42), phosphorylated on tyrosine 204; pAkt is specific for Akt phosphorylated on serine 473. Subsequently, the blots were stripped and stained with the indicated protein-specific antibodies to demonstrate equal loading: tErk is specific for total Erk2 (p42) and does not recognize Erk1.
To examine downstream signals, total cell lysates of all 4 ITD mutant cell lines were subjected to immunoblotting with antibodies specific to the activation status of signal transduction molecules. The loading conditions of the gels were verified by antibodies detecting the molecules independent of their activation state. To detect MAPK activation, we used an anti-pErk antibody, which is specific for Erk1/2 phosphorylated on tyrosine 204. To detect the activation of PI3K-dependent pathways, we analyzed the activation of Akt, a serine/threonine protein kinase downstream of PI3K, which has been implicated in cell survival. The anti-pAkt–specific antibody detects Akt, which is phosphorylated at serine 473. As shown in Figure 7, Erk1/2 and Akt were activated ligand dependently in 32D/Flt3-ITD cells, with slightly increased basal levels of phosphorylation. Only one ITD construct, ITD2, led to significant constitutive activation of the Erk proteins. Taken together, the ITD mutations induced factor-independent autophosphorylation of the immature form of Flt3. In the absence of ligand, they did not induce strong activation of Erk proteins or of Akt.
Constitutive activation of STAT5 in 32D/Flt3-ITD
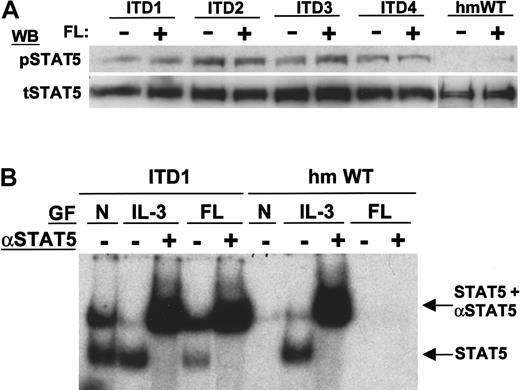
To analyze STAT5 activation, we first used an antibody specific for STAT5a/b phosphorylated on tyrosine 694. Flt3-WT induced very weak phosphorylation of STAT5 after FL stimulation (Figure8A) However, the ITD mutants induced strong constitutive phosphorylation of STAT5. To confirm the activation of STAT5 in a different assay system, we performed electrophoretic mobility shift assays using a STAT5-specific probe (Figure 8B). On stimulation with IL-3, both WT and ITD mutants showed similar DNA binding activity of STAT5. Only 32D/Flt3-ITD cells showed constitutive DNA binding activity, and even FL stimulation could not induce STAT5 DNA binding in 32D/Flt3-WT. The DNA binding activity of 32D/Flt3-ITD was confirmed to be STAT5 by supershift analyses with an anti-STAT5 antibody.
Constitutive activation of STAT5 by Flt3-ITD.
(A) Detection of STAT5 activation by immunoblot. Total cell lysates were separated by SDS-PAGE and immunoblotted with anti-pSTAT5, which reacts with STAT5 phosphorylated on tyrosine 694, or antitotal STAT5 as indicated. (B) Electrophoretic mobility shift assay of STAT5. Nuclear extracts were incubated with a double-stranded 32P-labeled oligonucleotide containing the consensus STAT5 binding sequence. Results from 2 constructs are shown: ITD1 (lanes 1-5) and Flt3-WT (lanes 6-10). The stimulation conditions of the cells before lysis are indicated: no growth factors added (N, lanes 1 and 6), 10 ng/mL IL-3 for 10 minutes (IL-3, lanes 2, 3 and 7, 8), and 100 ng/mL FL (FL, lanes 4, 5 and 9, 10). To verify the presence of STAT5 in the shifted oligonucleotide/protein complex, a supershift assay was performed with an anti-STAT5a/b antibody as indicated (lanes 3, 5 and 8, 10). Note that no shift or supershift is visible in unstimulated and FL-stimulated wild-type Flt3 cells, whereas shifted complexes containing STAT5 can be seen in ITD1 cells.
Constitutive activation of STAT5 by Flt3-ITD.
(A) Detection of STAT5 activation by immunoblot. Total cell lysates were separated by SDS-PAGE and immunoblotted with anti-pSTAT5, which reacts with STAT5 phosphorylated on tyrosine 694, or antitotal STAT5 as indicated. (B) Electrophoretic mobility shift assay of STAT5. Nuclear extracts were incubated with a double-stranded 32P-labeled oligonucleotide containing the consensus STAT5 binding sequence. Results from 2 constructs are shown: ITD1 (lanes 1-5) and Flt3-WT (lanes 6-10). The stimulation conditions of the cells before lysis are indicated: no growth factors added (N, lanes 1 and 6), 10 ng/mL IL-3 for 10 minutes (IL-3, lanes 2, 3 and 7, 8), and 100 ng/mL FL (FL, lanes 4, 5 and 9, 10). To verify the presence of STAT5 in the shifted oligonucleotide/protein complex, a supershift assay was performed with an anti-STAT5a/b antibody as indicated (lanes 3, 5 and 8, 10). Note that no shift or supershift is visible in unstimulated and FL-stimulated wild-type Flt3 cells, whereas shifted complexes containing STAT5 can be seen in ITD1 cells.
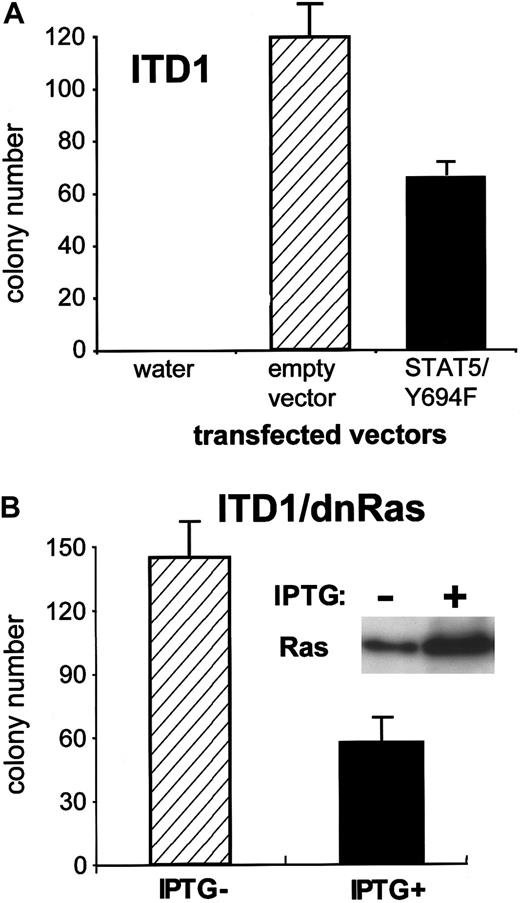
Effects of dominant negative Ras and STAT5 on ITD-induced colony formation
To examine the consequences of STAT5 activation in 32D/Flt3-ITD, we analyzed the effects of dnSTAT5 on the colony formation of 32D/Flt3-ITD. After transfection of STAT5Y694F (dnSTAT5), the colony formation of 32D/Flt3-ITD1 was reduced by approximately 50%, compared with the transfection of a control vector (Figure9A).
Effects of dominant negative constructs on colony formation induced by Flt3-ITD.
(A) Equal amounts of an empty control vector and a dominant negative STAT5 (Y694F) were electroporated into 32D cells containing ITD1. One day after electroporation, cells were plated at a concentration of 1 × 105 cells per dish in the presence of 0.6 mg/mL G418 and no cytokine. Numbers of colonies were counted at day 8. Data shown are representative of 3 independent experiments. (B) Inducible expression of dn-Ras. ITD1/dnRas were polyclonal cell lines, which were made by transfection of Flt3-ITD1 into the 32D/dnRas clone. Cells were treated with 0.5 mmol/L IPTG for 24 hours, and total cell lysates were subjected to Western blot with anti-Ras Ab. These cells were subjected to a colony assay as described in Figure 4, under the presence or absence of IPTG.
Effects of dominant negative constructs on colony formation induced by Flt3-ITD.
(A) Equal amounts of an empty control vector and a dominant negative STAT5 (Y694F) were electroporated into 32D cells containing ITD1. One day after electroporation, cells were plated at a concentration of 1 × 105 cells per dish in the presence of 0.6 mg/mL G418 and no cytokine. Numbers of colonies were counted at day 8. Data shown are representative of 3 independent experiments. (B) Inducible expression of dn-Ras. ITD1/dnRas were polyclonal cell lines, which were made by transfection of Flt3-ITD1 into the 32D/dnRas clone. Cells were treated with 0.5 mmol/L IPTG for 24 hours, and total cell lysates were subjected to Western blot with anti-Ras Ab. These cells were subjected to a colony assay as described in Figure 4, under the presence or absence of IPTG.
As shown in Figure 7, we observed weak MAPK activation by some of the ITD mutations in the absence of exogenously added Flt3 ligand. Also, inactivation of STAT5 did not abrogate the Flt3-ITD–induced colony growth completely. Therefore, we were interested in examining the relative role of the Ras/MAPK pathway for the function of the ITD mutations. We used the Lac-inducible system, in which the expression of dnRas (Ras S17N) was repressed by the Lac repressor. Repression was relieved by IPTG, leading to highly inducible expression of the dominant negative construct, as shown in Figure 9B. The colony formation induced by the ITD forms of Flt3 was strongly inhibited by dnRas induction, as shown in Figure 9B for ITD1. Similar results were obtained with ITD2 (data not shown), which had caused considerable constitutive activation of MAPK in the pErk blot (Figure 7). IPTG alone did not show any effect on cell lines transfected with the empty control vector.
Discussion
In this report, we demonstrate that internal tandem repeat mutations of Flt3, which are readily detectable in about 20% of cases with AML18,30 and which indicate a poor prognosis of the disease,21-23 elicit transforming ability in the factor-dependent murine myeloid cell line 32D. Our analyses of the autophosphorylation pattern of the Flt3 mutations indicate that ITD mutations cause constitutive autophosphorylation of Flt3. We also demonstrate that the observed activation of Flt3 is sufficient to mediate proliferation and survival in the absence of ligand, even if the mature form of the receptor is only marginally autophosphorylated. Furthermore, the mutant receptors alter cellular behavior in a way not seen with ligand-activated wild-type Flt3.
The mechanism of Flt3 activation by mutation in the juxtamembrane domain remains elusive. Constitutive activation of the close relative Kit by point mutations and small deletions in the juxtamembrane region has been studied extensively,31,32 showing that ligand-independent dimerization occurs in the cellular membrane. Mutations in the kinase domain of Kit cause intracellular dimerization and activation of the receptor.33 Recently, it has been reported that in the Kit juxtamembrane domain, methionine 552 to isoleucine 563 form a putative alpha-helical conformation, and that substitution of any of 4 different amino acids sitting along the hydrophobic side of the alpha-helix leads to receptor activation.34 We also were interested in juxtamembrane deletion mutants of Flt3 and constructed a deletion mutant, in which all 4 potential tyrosine autophosphorylation sites were eliminated. This mutant also showed ligand-independent receptor autophosphorylation, proliferation, and apoptosis resistance, suggesting that the conformational change of the juxtamembrane domain, due to either addition or deletion, leads to receptor activation and similar biologic effects (M.M., R.F., H.S., manuscript in preparation). Taken together, ITD mutations in the Flt3 juxtamembrane region seem to disturb the conformation of a tertiary structure, which normally exerts inhibitory control over unoccupied receptor tyrosine kinases. The ITD mutations significantly shorten the latency period, which is required for Flt3 to transform 32D cells into a fully oncogenic cell line in vivo. The low, but detectable transforming ability of induced wild-type Flt3 expression is in accordance with the ability of c-kitto induce leukemogenesis in 32D cells, with a long latency period.35 The additional effect of the ITD mutations most likely depends on the ligand-independent activation of Flt3.
We found that all types of Flt3-ITD do not only confer constitutive activation of the major biologic functions of Flt3, but also induce additional functions in 32D cells. Of particular interest is the ability of ITD mutations to support clonal growth. The surprising difference between activated wild-type Flt3 and the ITD mutations could be attributable to a complementary signal necessary for the Flt3 response in semisolid media, but not in suspension culture. The ITD mutations obviously bypass the necessity for this. They seem to provide an intracellular signal different from activated wild-type receptors.
We analyzed intermediates of several signaling pathways and could indeed find qualitative differences in the signals provided by wild-type and ITD receptors.
Our results show that Flt3 activates Akt, which has been shown to be an enzyme regulated by phosphoinositide products of PI3 kinase. The importance of PI3 kinase signaling for Flt3-mediated proliferation and survival is controversial, because the binding of the enzyme to Flt3 has not been demonstrated with certainty. On the other hand, it has been recently shown that Flt3 activates PI3 kinase by alternative mechanisms.36 37 Thus, our results showing the activation of Akt by wild-type Flt3 are in line with the latter experiments.
We and others have shown that the Ras/Raf/MAP kinase pathways are involved in proliferation and survival induced by ligand-activated wild-type Flt3.38-40 Although the ITD mutants induced autonomous proliferation and survival of 32D cells, constitutive activation of MAP kinase and Akt was only weak in most of the ITD-containing cell lines.
In contrast, STAT5 is strongly and constitutively activated by all ITD mutations tested, whereas STAT5 phosphorylation by wild-type Flt3 is very weak and does not translate into its enhanced DNA binding after Flt3 stimulation. After the initial submission of this paper, the constitutive activation of STAT5 by ITD mutations and the lack of STAT5 activation by wild-type Flt3 has been independently described by others,41 confirming our results of differential signaling between wild-type and ITD-mutated Flt3.
Our results provide the first evidence for the relative importance of the different signaling pathways for Flt3/ITD function: both Ras and STAT5 are important for Flt3/ITD-induced colony formation of 32D cells. Transfection of dominant negative STAT5 does not inhibit colony growth completely. One explanation could be that other signaling pathways, possibly other STAT proteins, can substitute for STAT5. The relative importance of Ras-dependent signaling pathways is unexpected, given the low constitutive activation of MAPK by the Flt3 mutations. On the other hand, wild-type Flt3 strongly activates the Ras/MAPK pathway, but does not induce colony formation (Figures 4 and 7). Obviously, basic activity of the Ras/MAPK pathway is a necessary, but not sufficient, condition for 32D cells to grow in colonies. In recent reports, it was shown that STAT proteins are coactivated by Ras-dependent signals: expression of dominant negative Ras in a T-cell line inhibited IL-2–induced STAT5 activity on a β-casein promotor.42Also, full STAT3 activation has been shown to be dependent on Rac, which is downstream of Ras.43 Thus, by inhibiting Ras function, we inhibit the activation of STAT5 and other STAT proteins of potential importance for Flt3/ITD-induced colony formation, a possible explanation for its relative importance for transformation of 32D cells. Taken together, our results show that the ITD mutations activate signaling pathways differently from wild-type Flt3, which results in a different phenotype of 32D cells in vitro and in vivo.
In conclusion, we provide evidence that frequently occurring Flt3 mutations from patients with AML are leukemogenic in 32D cells. The leukemogenic effect is based on several intracellular signals of these receptor mutants, which involve the basal Ras activation and the constitutive activation of STAT5. Future work will have to elucidate these signaling pathways in still more detail. This will hopefully result in a more sophisticated picture of how proliferation and survival of leukemic blasts are regulated and how they may be subjected to specific therapeutic interventions.
Acknowledgments
We thank Dr I. Lemischka for providing the murine Flt3 cDNA, and Dr F. Rosenthal for providing the 32D cells. We thank Astrid Kolkmeyer, Corinna Dördelmann, and Steffi Wetzlich for their excellent technical assistance.
Supported by grant Se 600/2-2 from the Deutsche Forschungsgemeinschaft and Se 119804 and KR 229838 from the IMF Münster.
This paper represents partial fulfillment of the requirements for the PhD program of R.F. and for the MD program of R.S. and C.S. at the University of Münster.
M.M. and R.F. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hubert Serve or Wolfgang E. Berdel, Department of Medicine/ Hematology and Oncology, Medizinische Universitätsklinik Münster, Albert-Schweitzer-Strasse 33, 48129 Münster, Germany; e-mail: serve@uni-muenster.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal