Abstract
We analyzed engraftment of unrelated-donor (URD) bone marrow in 5246 patients who received transplants facilitated by the National Marrow Donor Program between August 1991 and June 1999. Among patients surviving at least 28 days, 4% had primary graft failure (failure to achieve an absolute neutrophil count > 5 × 108/L before death or second stem-cell infusion). Multivariate logistic regression analysis showed that engraftment was associated with marrow matched at HLA-A, HLA-B, and DRB1; higher cell dose; younger recipient; male recipient; and recipient from a non–African American ethnic group. More rapid myeloid engraftment was associated with marrow serologically matched at HLA-A and HLA-B, DRB1 match, higher cell dose (in non-T-cell–depleted cases), younger recipient, recipient seronegativity for cytomegalovirus (CMV), male donor, no methotrexate for graft-versus-host disease prophylaxis, and transplantation done in more recent years. A platelet count higher than 50 × 109/L was achieved by 47% of patients by day 100. Conditional on survival to day 100, survival at 3 years was 61% in those with platelet engraftment at day 30, 58% in those with engraftment between day 30 and day 100, and 33% in those without engraftment at day 100 (P < .0001). Factors favoring platelet engraftment were higher cell dose, DRB1 allele match, recipient seronegativity for CMV, HLA-A and HLA-B serologically matched donor, and male donor. Secondary graft failure occurred in 10% of patients achieving initial engraftment, and 18% of those patients are alive. These data demonstrate that quality of engraftment is an important predictor of survival after URD bone marrow transplantation.
Introduction
The establishment of an international network of bone marrow donor registries has allowed increasing clinical use of unrelated-donor (URD) bone marrow for treatment of a range of malignant and nonmalignant disorders.1 Stable engraftment of donor hematopoiesis is essential for a successful outcome of bone marrow transplantation (BMT). Studies in recipients of marrow from non–HLA-identical family members showed that graft failure increases with increasing HLA disparity.2-4 Because unrelated donors are phenotypically, not genotypically, matched at the major HLA loci, they are likely to have additional disparities at major and minor histocompatibility loci that will increase the difficulties in engraftment. In an initial study by the National Marrow Donor Program (NMDP), graft failure was evaluated by calculating the probability of both initial myeloid engraftment and secondary marrow failure. In this early investigation, the probability of engraftment within 100 days after BMT was 94% ± 3%.1 An accelerated rate of engraftment was associated with use of HLA-matched marrow and with T-cell depletion of the graft. In this study, we describe in detail the frequencies and kinetics of myeloid and platelet engraftment in a large, well-characterized cohort of patients receiving URD BMT facilitated by the NMDP. The data show that quality of engraftment is an important predictor of survival after URD BMT.
Patients and methods
The NMDP
The NMDP was established in 1986 through a contract from the United States Navy to the American Red Cross. Responsibility for the contract was transferred to the National Heart, Lung, and Blood Institute in February 1989 and to the Health Resources and Services Administration in November 1994. The policies and procedures of the NMDP were described previously.5 6
Patients and transplant procedure
The study population consisted of 5246 patients who underwent URD BMT facilitated by the NMDP between August 1991 and June 1999. Recipients who had previously undergone transplantation were excluded from the study, as were 71 patients for whom data submitted by the transplant center were incomplete. All patients included in the study received bone marrow stem cells; no primary peripheral blood stem-cell transplantations were performed during the study period. Patient characteristics are summarized in Table 1 and Table 2. The most frequent diagnosis was chronic myelogenous leukemia. Median patient age was 29 years (range, 0-66 years), and median donor age was 36 years (range, 18-60 years).
Characteristics of recipients of unrelated-donor transplants
| Disease . | No. of patients . | Median (range) age, y . | No. (%) female . |
|---|---|---|---|
| CMLCP | 1223 | 37 (2-59) | 527 (43) |
| CML2CP | 147 | 34 (4-55) | 59 (40) |
| CMLAP | 295 | 38 (4-62) | 113 (38) |
| CMLBP | 78 | 37 (5-57) | 23 (29) |
| ALL1 | 228 | 16 (0-53) | 100 (44) |
| ALL2 | 385 | 13 (0-54) | 171 (44) |
| ALL3+ | 139 | 12 (1-40) | 51 (37) |
| ALL/RLPS | 262 | 23 (0-54) | 89 (34) |
| AML1 | 231 | 27 (0-65) | 102 (44) |
| AML2 | 280 | 26 (1-58) | 126 (45) |
| AML3+/RLPS | 599 | 34 (0-62) | 262 (44) |
| MDS | 476 | 37 (0-63) | 211 (44) |
| Other leukemia | 132 | 26 (0-66) | 53 (40) |
| Other malignant disease | 237 | 37 (2-58) | 81 (34) |
| SAA | 198 | 16 (0-52) | 77 (39) |
| Other nonmalignant disease | 336 | 3 (0-48) | 126 (38) |
| Total | 5246 | 29 (0-66) | 2171 (41) |
| Disease . | No. of patients . | Median (range) age, y . | No. (%) female . |
|---|---|---|---|
| CMLCP | 1223 | 37 (2-59) | 527 (43) |
| CML2CP | 147 | 34 (4-55) | 59 (40) |
| CMLAP | 295 | 38 (4-62) | 113 (38) |
| CMLBP | 78 | 37 (5-57) | 23 (29) |
| ALL1 | 228 | 16 (0-53) | 100 (44) |
| ALL2 | 385 | 13 (0-54) | 171 (44) |
| ALL3+ | 139 | 12 (1-40) | 51 (37) |
| ALL/RLPS | 262 | 23 (0-54) | 89 (34) |
| AML1 | 231 | 27 (0-65) | 102 (44) |
| AML2 | 280 | 26 (1-58) | 126 (45) |
| AML3+/RLPS | 599 | 34 (0-62) | 262 (44) |
| MDS | 476 | 37 (0-63) | 211 (44) |
| Other leukemia | 132 | 26 (0-66) | 53 (40) |
| Other malignant disease | 237 | 37 (2-58) | 81 (34) |
| SAA | 198 | 16 (0-52) | 77 (39) |
| Other nonmalignant disease | 336 | 3 (0-48) | 126 (38) |
| Total | 5246 | 29 (0-66) | 2171 (41) |
CMLCP indicates chronic myelogenous leukemia in first chronic phase; CML2CP, CML in 2nd chronic phase; CMLAP, CML in accelerated phase; CMLBP, CML in blast phase; ALL1, acute lymphoblastic leukemia in first complete remission (CR); ALL2, ALL in 2nd CR; ALL3+, ALL in 3rd or higher CR; RLPS, relapse; AML1, acute myelogenous leukemia in first CR; AML2, AML in 2nd CR; AML3+, AML in 3rd or higher CR; MDS, myelodysplastic syndrome; and SAA, severe aplastic anemia.
Characteristics of donor-recipient pairs
| Characteristic . | No. (%) of pairs . |
|---|---|
| Race/ethnic group of recipient | |
| Caucasian | 4439 (85) |
| Hispanic | 343 (7) |
| African American | 265 (5) |
| Asian/Pacific Islander | 159 (3) |
| Native American | 10 (0) |
| Other/Unknown | 30 (1) |
| Sex (recipient/donor) | |
| M/M | 1967 (37) |
| F/F | 1058 (20) |
| M/F | 1108 (21) |
| F/M | 1113 (21) |
| CMV status (recipient/donor) | |
| +/+ | 1009 (19) |
| +/− | 1477 (28) |
| −/+ | 880 (17) |
| −/− | 1772 (34) |
| Unknown | 108 (2) |
| HLA matching | |
| A, B match/DRB1 match | 3652 (70) |
| A, B match/DRB1 potential match | 276 (5) |
| A, B match/DRB1 allele mismatch | 409 (8) |
| A, B match/DR major Mismatch | 60 (1) |
| A or B mismatch/DRB1 known match | 742 (14) |
| A or B mismatch/DRB1 potential match | 56 (1) |
| A or B mismatch/DRB1 allele mismatch | 49 (1) |
| A or B mismatch/DR major mismatch | 2 (0) |
| Characteristic . | No. (%) of pairs . |
|---|---|
| Race/ethnic group of recipient | |
| Caucasian | 4439 (85) |
| Hispanic | 343 (7) |
| African American | 265 (5) |
| Asian/Pacific Islander | 159 (3) |
| Native American | 10 (0) |
| Other/Unknown | 30 (1) |
| Sex (recipient/donor) | |
| M/M | 1967 (37) |
| F/F | 1058 (20) |
| M/F | 1108 (21) |
| F/M | 1113 (21) |
| CMV status (recipient/donor) | |
| +/+ | 1009 (19) |
| +/− | 1477 (28) |
| −/+ | 880 (17) |
| −/− | 1772 (34) |
| Unknown | 108 (2) |
| HLA matching | |
| A, B match/DRB1 match | 3652 (70) |
| A, B match/DRB1 potential match | 276 (5) |
| A, B match/DRB1 allele mismatch | 409 (8) |
| A, B match/DR major Mismatch | 60 (1) |
| A or B mismatch/DRB1 known match | 742 (14) |
| A or B mismatch/DRB1 potential match | 56 (1) |
| A or B mismatch/DRB1 allele mismatch | 49 (1) |
| A or B mismatch/DR major mismatch | 2 (0) |
Donor-recipient pairs that appeared matched at DRB1 by serologic determination or intermediate-resolution DRB1 typing but in which either the donor or the recipient did not have high-resolution DRB1 typing were considered a potential match.
CMV indicates cytomegalovirus.
Patients received a variety of preparative regimens determined by the individual transplant centers and based on requirements for the treatment of the specific underlying disease (Table3). Most recipients (79%) received total-body irradiation (TBI); 20% were given chemotherapy alone. TBI was fractionated in all but 199 cases; the median total fractionated dose was 1320 cGy (range, 200-1500 cGy). Chemotherapy drugs administered as part of the preparative regimen included cyclophosphamide, busulfan, cytosine arabinoside, etoposide, and thiotepa. Methods for providing prophylaxis against graft-versus-host disease (GVHD) varied according to the transplant center (Table 3). The graft was depleted of T-cells in 25% of cases.
Characteristics of bone marrow transplantations
| Characteristic . | Value . |
|---|---|
| Median cell dose per kg | 2.43 × 108 |
| Median (range) donor age, y | 36 (18-60) |
| Median (range) recipient age, y | 29 (0-66) |
| Radiation therapy, no. (%) | |
| None | 1072 (20) |
| Total body | 4129 (79) |
| Thoracoabdominal | 4 (0) |
| Total lymphoid/nodal | 26 (0) |
| Other | 15 (0) |
| Graft-versus-host disease prophylaxis, no. (%) | |
| T-cell depletion | 1293 (25) |
| Methotrexate | 3779 (72) |
| Cyclosporine A | 4298 (82) |
| Growth-factor administration, no. (%) | 3487 (66) |
| Time marrow collected before BMT, no. (%)3-150 | |
| < 12 hours | 2054 (41) |
| 12-24 hours | 2064 (41) |
| > 24 hours | 860 (17) |
| Characteristic . | Value . |
|---|---|
| Median cell dose per kg | 2.43 × 108 |
| Median (range) donor age, y | 36 (18-60) |
| Median (range) recipient age, y | 29 (0-66) |
| Radiation therapy, no. (%) | |
| None | 1072 (20) |
| Total body | 4129 (79) |
| Thoracoabdominal | 4 (0) |
| Total lymphoid/nodal | 26 (0) |
| Other | 15 (0) |
| Graft-versus-host disease prophylaxis, no. (%) | |
| T-cell depletion | 1293 (25) |
| Methotrexate | 3779 (72) |
| Cyclosporine A | 4298 (82) |
| Growth-factor administration, no. (%) | 3487 (66) |
| Time marrow collected before BMT, no. (%)3-150 | |
| < 12 hours | 2054 (41) |
| 12-24 hours | 2064 (41) |
| > 24 hours | 860 (17) |
BMT indicates bone marrow transplantation.
Collection time was unknown for 268 patients.
Donor selection and processing of bone marrow
The characteristics of the donors are shown in Table2. The matching of donors and recipients was based on serologic typing for HLA-A and HLA-B, performed according to standard techniques. Typing at DRB1 was done with molecular typing in most (94%) of cases. Disparity between DR serotypes was considered a DR major mismatch. Donor-recipient pairs that appeared to be matched serologically or by intermediate-resolution DRB1 typing but in which either the recipient or the donor did not have high-resolution DRB1 typing were considered a potential match. In analyses, those matched at DRB1 by high-resolution molecular typing and those with a potential match were considered to be matched. A total of 3652 donor-recipient pairs (70%) were matched at HLA-A, HLA-B, and DRB1. HLA-C typing was not available for most donor-recipient pairs and was not included in the analysis.
Marrow was infused without manipulation in 1785 patients (34%). In 3461 patients (66%), it was manipulated for the following reasons: volume reduction (281 patients [5%]), red-cell depletion for ABO blood group incompatibility (1488 patients [28%]), T-cell depletion to prevent GVHD (1293 patients [25%]), plasma depletion (531 patients [10%]), and no reason given (62 patients [1%]). Values do not add up to 100% because more than one reason for manipulation was recorded in some cases.
In recording the time from marrow collection to infusion, all reported times were adjusted to Greenwich Mean Time. Complete and consistent data were available for 4978 (95%) of patients. Marrow was infused less than 12 hours after collection in 2054 (41%) of patients, 12 to 24 hours after collection in 2064 (41%), and more than 24 hours after collection in 860 (17%).
Data collection
The transplantations were performed at 132 transplant centers. Median length of follow-up was 712 days (range, 16-2192 days). Data were collected prospectively from transplant centers at defined time points. The day of myeloid engraftment was defined as the first of 3 consecutive days on which the absolute neutrophil count (ANC) exceeded 5 × 108/L at any time after transplantation. Patients with initial engraftment in whom severely hypocellular marrow or an ANC below 5 × 108/L recurred in the absence of relapse were considered to have secondary graft failure. Platelet recovery was considered to have occurred when the platelet count was 50 × 109/L, without transfusion, for 7 days. This time point was selected because this platelet value is a clinically protective platelet level. Additionally, lower levels (eg, 20 × 109/L) are influenced by the need to administer platelets for minor procedures or by individual physician practice and may less accurately reflect achievement of durable engraftment.
Acute GVHD of the skin, gut, and liver was reported by individual transplant centers, and an overall GVHD grade was calculated at the NMDP coordinating center.7 Chronic GVHD was assessed as limited (mild skin involvement only) or extensive.
Data were validated for accuracy and consistency at the NMDP by using cross-validation of fields and validation of laboratory values against range values. Reporting centers were queried about inconsistencies and corrections made in consultation with those centers.
Statistical analysis
Probabilities of engraftment and secondary failure were calculated by using the cumulative incidence method.8Second transplantations were treated as a competing risk. Relapse was also treated as a competing risk for secondary graft failure. Analysis of graft failure included only patients for whom initial engraftment and measured time from initial engraftment—not from infusion—were reported. Median times for engraftment and graft failure were defined as the earliest time that the conditional probability of the event, given that it occurred within 2 years (the ratio of the cumulative incidences), reached 50%. P values for univariate comparisons of the cumulative incidences were calculated for particular time points by using the maximum likelihood estimate for the variance of the cumulative incidence.
Survival rates were calculated with the Kaplan-Meier9method, with censoring at the time of last follow-up assessment. Kaplan-Meier survival curves were compared by using the log-rank test.10 Causes of death in various subpopulations were compared by using the likelihood-ratio χ2 statistic. Primary causes of death were compared by using the likelihood-ratio χ2 square statistic, with P values adjusted for multiple comparisons by using the method of Bonferroni.
Multivariate analyses were performed with the Cox proportional hazards regression model.11 Indicators for disease stage (defined in Table 1) and transplant center were included in every model, regardless of whether they were statistically significant. Other risk factors were determined by a stepwise selection procedure, with inclusion if the χ2 statistic achieved a significance level of 0.05. Factors considered were donor and recipient age, sex, cytomegalovirus (CMV) status, and race; HLA-A and HLA-B serologic matching; DRB1 allele matching (or potential match based on low-resolution HLA typing); cell dose; T-cell depletion; conditioning regimen; year of infusion; interval from diagnosis to BMT; and time from marrow collection to infusion. Because cell dose for T-cell–depleted and T-cell–replete grafts varies, the models also checked for a potential interaction between these factors. When the interaction term was significant, cell dose was tested separately for T-cell–depleted and T-cell–replete cases. For secondary graft failure, the time from infusion to initial engraftment was also considered for the model.
For myeloid engraftment, the effect of T-cell depletion was deemed to be inconsistent with the proportional hazards assumption. The baseline hazard functions were therefore stratified according to this variable. P values obtained from multivariate analyses were based on the Wald χ2 statistic. Additional regression models were fit to assess the effect of one outcome on another (eg, platelet recovery to predict survival). This was done by treating the former variable as a time-dependent indicator variable.
Logistic regression was also used to repeat the analysis of myeloid engraftment, ignoring the time to event. Results were compared with those from the proportional hazards model and differences were noted. All analyses were done with SAS statistical software, version 6.12 (SAS Institute Inc, Cary, NC).
Results
Myeloid engraftment
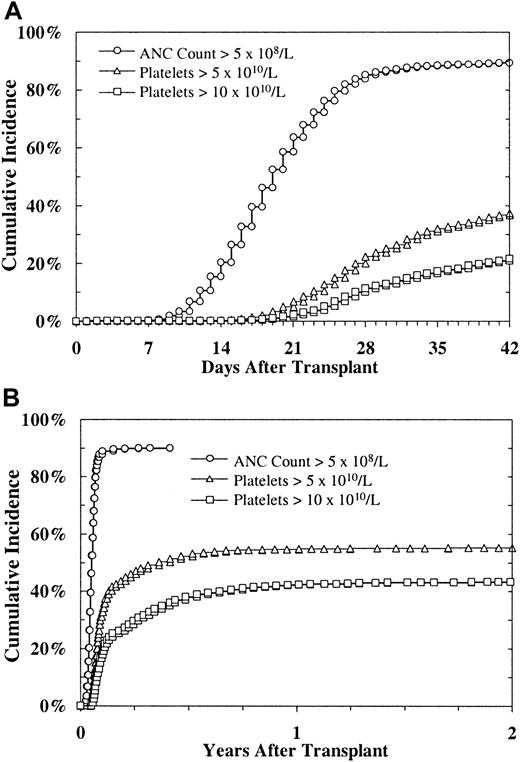
Myeloid engraftment (ANC > 5 × 108/L) occurred a median of 18 days after marrow infusion (Figure1). Overall, 84% of patients achieved an ANC above 5 × 108/L by day 28. Four percent (192/4770) of all patients had primary graft failure (defined as failure to achieve an ANC > 5 × 108/L before death or second stem-cell infusion in patients who survived at least 28 days). Four percent (191/5246) of all patients received a subsequent infusion of stem cells for delayed engraftment; 27% (51/191) of those patients are still alive.
Platelet engraftment (platelet count > 50 × 109/L) and neutrophil engraftment (ANC > 5 × 108/L) after URD BMT. (A) 0 to 42 days. (B) 0 to 2 years.
Platelet engraftment (platelet count > 50 × 109/L) and neutrophil engraftment (ANC > 5 × 108/L) after URD BMT. (A) 0 to 42 days. (B) 0 to 2 years.
Univariate analysis showed that myeloid engraftment occurred more slowly in recipients of marrow with a serologic mismatch at HLA-A or HLA-B than in recipients of marrow matched at HLA-A and HLA-B (median, 19 days versus 18 days after BMT). Similarly, 86% of recipients given HLA-A and HLA-B serologically matched marrow achieved an ANC above 5 × 108/L by day 28, whereas this ANC level was achieved in only 82% of recipients of marrow with an HLA-A or HLA-B serologic mismatch (P = .0006). Myeloid engraftment was also slower in recipients of marrow with a DRB1 mismatch.
Analysis of myeloid engraftment in recipients of non-T-cell–depleted and T-cell–depleted marrow grafts suggested that T-depleted marrow engrafts more rapidly (median day of ANC > 5 × 108/L, 15 days versus 19 days after BMT). However, the number of patients who eventually achieved engraftment was comparable (89% versus 89% with engraftment at day 42; Pnot significant). Despite this, a second stem-cell infusion given because of graft failure was more frequent in those who received T-cell–depleted grafts (7% versus 2%; P < .0001 by χ2 testing). Myeloid engraftment occurred more slowly in patients receiving methotrexate for GVHD prophylaxis, although the rates of engraftment by day 42 were similar in those who received methotrexate and those who did not (90% versus 89%; P not significant). It should be noted, however, that the use of methotrexate and the use of T-cell–replete marrow were highly correlated: 90% of those given T-cell–replete marrow also received methotrexate, whereas only 16% of those given T-cell–depleted marrow also received methotrexate.
Multivariate Cox proportional hazards regression analysis of myeloid engraftment showed that factors favoring rapid engraftment were HLA-A and HLA-B serologically matched marrow, DRB1 matched marrow, higher cell dose (in recipients of T-cell–replete marrow only), younger age, recipient seronegativity for CMV, male donor, no methotrexate for GVHD prophylaxis, and transplantation in more recent years (Table4). Because the Cox model incorporated both speed and incidence of engraftment, a logistic regression analysis was performed to identify risk factors for achieving or not achieving engraftment, irrespective of time to engraftment. In this analysis, HLA-A and HLA-B serologic match, DRB1 match, and higher cell dose were favorable factors for engraftment, indicating that engraftment is achieved more frequently and more rapidly in recipients of a closely matched marrow given a higher cell dose. In addition, engraftment was more likely to occur in younger recipients, male recipients, and non–African American recipients.
Results of multivariate Cox proportional hazards regression analysis of risk factors in relation to myeloid engraftment (absolute neutrophil count > 5 × 108/L)
| Factor . | RR . | 95% CI . | P value . | Favorable characteristic . |
|---|---|---|---|---|
| Recipient age (per decade) | 1.04 | 1.01-1.06 | .008 | Younger |
| HLA-A and HLA-B matching | 1.20 | 1.10-1.30 | .0001 | Matched |
| DRB1 match4-150 | 1.16 | 1.04-1.28 | .006 | Matched |
| Recipient CMV serologic status | 0.92 | 0.86-0.98 | .006 | Negative |
| Donor sex | 0.91 | 0.86-0.97 | .004 | Male |
| Cell dose (non–T-cell depleted per 108/kg) | 1.10 | 1.08-1.13 | .0001 | Higher dose |
| Methotrexate prophylaxis for GVHD | 0.64 | 0.58-0.71 | .0001 | No methotrexate |
| Year of marrow infusion | ||||
| 1991-1995 | 1.00 | NA | NA | |
| 1996-1997 | 1.24 | 1.15-1.34 | .0001 | Recent infusion |
| 1998-1999 | 1.13 | 1.05-1.22 | .001 | Recent infusion |
| Factor . | RR . | 95% CI . | P value . | Favorable characteristic . |
|---|---|---|---|---|
| Recipient age (per decade) | 1.04 | 1.01-1.06 | .008 | Younger |
| HLA-A and HLA-B matching | 1.20 | 1.10-1.30 | .0001 | Matched |
| DRB1 match4-150 | 1.16 | 1.04-1.28 | .006 | Matched |
| Recipient CMV serologic status | 0.92 | 0.86-0.98 | .006 | Negative |
| Donor sex | 0.91 | 0.86-0.97 | .004 | Male |
| Cell dose (non–T-cell depleted per 108/kg) | 1.10 | 1.08-1.13 | .0001 | Higher dose |
| Methotrexate prophylaxis for GVHD | 0.64 | 0.58-0.71 | .0001 | No methotrexate |
| Year of marrow infusion | ||||
| 1991-1995 | 1.00 | NA | NA | |
| 1996-1997 | 1.24 | 1.15-1.34 | .0001 | Recent infusion |
| 1998-1999 | 1.13 | 1.05-1.22 | .001 | Recent infusion |
RR indicates relative risk; CI, confidence interval; CMV, cytomegalovirus; GVHD, graft-versus-host disease; and NA, not available.
Includes potential DRB1 match.
Platelet recovery
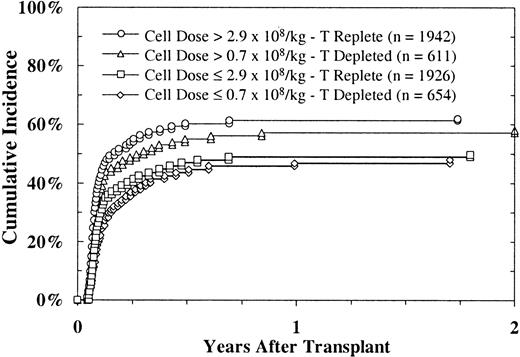
Platelet recovery (platelet count > 50 × 109/L, without transfusion) occurred a median of 32 days after marrow infusion in those who achieved recovery (Figure 1). A platelet count higher than 50 × 109/L was achieved at day 100 by 47% of all patients transplanted and by 70% of those surviving at day 100; 55% of all patients transplanted and 95% of those surviving at 2 years achieved platelet counts of 50 000 or higher. In univariate analysis, favorable factors influencing platelet recovery were a higher cell dose (Figure2), DRB1 matched marrow, HLA-A and HLA-B serologically matched marrow, recipient seronegativity for CMV, female recipient, male donor, younger recipient, and recipient of T-cell–replete marrow. Multivariate regression analysis of platelet recovery showed that factors favoring engraftment were higher cell dose, DRB1 matching, recipient seronegativity for CMV, HLA-A and HLA-B serologically matched marrow, and male donor (Table5).
Platelet recovery (platelet count > 50 × 109/L) after URD BMT, according to cell dose, in patients given T-cell–replete and T-cell– depleted marrow.
Platelet recovery (platelet count > 50 × 109/L) after URD BMT, according to cell dose, in patients given T-cell–replete and T-cell– depleted marrow.
Results of multivariate Cox proportional hazards regression analysis of risk factors in relation to platelet engraftment (platelet count > 50 × 109/L)
| Factor . | RR . | 95% CI . | P value . | Favorable characteristic . |
|---|---|---|---|---|
| HLA-A and HLA-B match | 1.31 | 1.17-1.47 | .0001 | Match |
| DRBI match5-150 | 1.55 | 1.34-1.78 | .0001 | Match |
| Recipient CMV serologic status | 0.88 | 0.81-0.95 | .0009 | Negative |
| Donor sex | 0.92 | 0.85-0.99 | .03 | Male |
| Cell dose (per 108/kg) | 1.12 | 1.10-1.15 | .0001 | Higher dose |
| Factor . | RR . | 95% CI . | P value . | Favorable characteristic . |
|---|---|---|---|---|
| HLA-A and HLA-B match | 1.31 | 1.17-1.47 | .0001 | Match |
| DRBI match5-150 | 1.55 | 1.34-1.78 | .0001 | Match |
| Recipient CMV serologic status | 0.88 | 0.81-0.95 | .0009 | Negative |
| Donor sex | 0.92 | 0.85-0.99 | .03 | Male |
| Cell dose (per 108/kg) | 1.12 | 1.10-1.15 | .0001 | Higher dose |
RR indicates relative risk; CI, confidence interval; CMV, cytomegalovirus.
Includes potential DRB1 match.
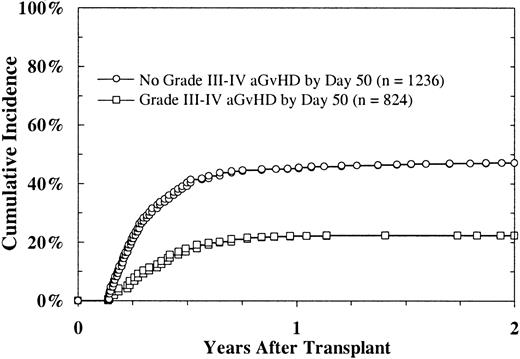
To investigate the effect of GVHD on platelet recovery, we examined platelet recovery in patients who had not achieved a platelet count higher than 50 × 109/L, without transfusion, by day 50. The data showed a reduced likelihood of platelet recovery in patients with severe acute GVHD (grades II-IV) compared with those without this disorder (22% versus 47% at 2 years; P < .0001; Figure3). In a multivariate regression analysis in which severe acute and chronic GVHD were considered time-dependent indicators, acute GVHD was strongly predictive of slower platelet recovery (P < .0001). Chronic GVHD showed a borderline association with platelet recovery (P = .04).
Platelet recovery (platelet count > 5 × 109/L) after URD BMT, according to onset of acute GVHD, in patients without platelet recovery before day 50.
Platelet recovery (platelet count > 5 × 109/L) after URD BMT, according to onset of acute GVHD, in patients without platelet recovery before day 50.
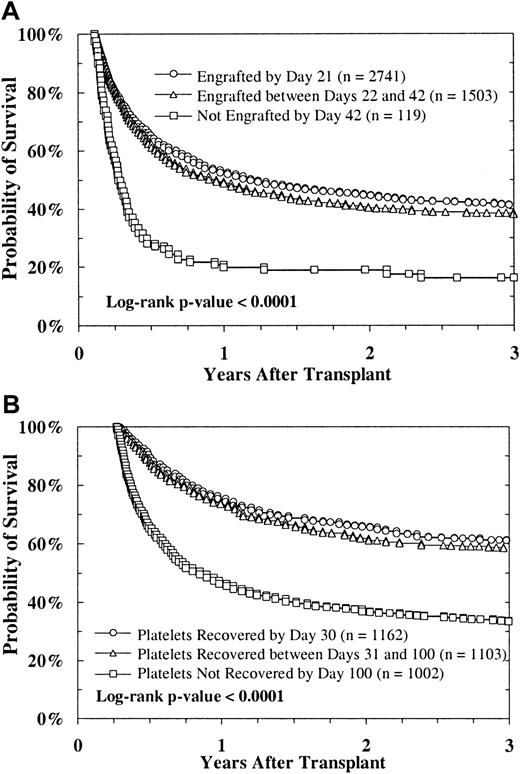
Myeloid engraftment, platelet recovery, and survival
The survival rate was similar in patients achieving myeloid engraftment before day 28 after BMT and in those achieving engraftment between day 28 and day 42, but it was reduced in patients who survived but did not have engraftment by day 42 (n = 119; Figure4A). Platelet recovery also predicted survival; the association was significant (Figure 4B). Conditional on survival to day 100, the survival rate at 3 years was 61% in patients with platelet recovery at day 30, 58% in those who had recovery between day 31 and day 100, and 33% in those who did not have recovery at day 100 (P < .0001). Multivariate regression analyses that controlled for risk factors, including disease stage and transplant center, and considered platelet-recovery status and acute and chronic GVHD as time-dependent indicators confirmed these results. A regression model for survival that included myeloid and platelet recovery as time-dependent indicators showed a greater reduction in relative risk (RR) with platelet recovery (RR, 0.32; 95% confidence interval [CI], 0.29-0.35) than with myeloid engraftment (RR, 0.43; 95% CI, 0.37-0.49).
Survival after URD BMT.
Survival shown according to time of myeloid engraftment (ANC > 5 × 108/L) (A) and according to platelet recovery (platelet count > 50 × 109/L), conditional on survival to day 100 (B).
Survival after URD BMT.
Survival shown according to time of myeloid engraftment (ANC > 5 × 108/L) (A) and according to platelet recovery (platelet count > 50 × 109/L), conditional on survival to day 100 (B).
To gain further understanding of the relation between platelet recovery and survival, we determined the primary cause of death in patients who have died, according to time of platelet recovery (Table6). The frequency of nonrelapse mortality was 61% in patients who had recovery of platelets by day 30 after BMT, 61% in those who had recovery between day 31 and day 100, and 79% in those who did not have recovery by day 100 (P < .0001 by χ2 testing). Results were similar when the analysis was restricted to deaths occurring one year after BMT. Hemorrhage was an infrequent cause of death in all categories, indicating that the relation between platelet recovery and survival is indirect.
Causes of death after day 100 after bone marrow transplantation, according to time to platelet recovery
| Cause of death . | Platelet level recovered by day 30 . | Platelet level recovered by day 31-100 . | Platelet level not recovered by day 100 . | P value . |
|---|---|---|---|---|
| Recurrent or residual leukemia | 142 (39) | 156 (39) | 124 (21) | <.0001 |
| Acute or chronic GVHD | 39 (11) | 45 (11) | 88 (15) | NS |
| Bacterial infection | 22 (6) | 22 (6) | 36 (6) | NS |
| ARDS | 24 (7) | 18 (5) | 38 (7) | NS |
| Fungal infection | 23 (6) | 33 (8) | 71 (12) | NS |
| Other pneumonia | 21 (6) | 24 (6) | 49 (8) | NS |
| Secondary malignancy | 7 (2) | 5 (1) | 4 (1) | NS |
| CMV pneumonia | 9 (3) | 4 (1) | 19 (3) | NS |
| Hemorrhage | 10 (3) | 10 (3) | 25 (4) | NS |
| Graft failure | 2 (1) | 3 (1) | 6 (1) | NS |
| Other | 62 (17) | 77 (19) | 128 (22) | NS |
| Cause of death . | Platelet level recovered by day 30 . | Platelet level recovered by day 31-100 . | Platelet level not recovered by day 100 . | P value . |
|---|---|---|---|---|
| Recurrent or residual leukemia | 142 (39) | 156 (39) | 124 (21) | <.0001 |
| Acute or chronic GVHD | 39 (11) | 45 (11) | 88 (15) | NS |
| Bacterial infection | 22 (6) | 22 (6) | 36 (6) | NS |
| ARDS | 24 (7) | 18 (5) | 38 (7) | NS |
| Fungal infection | 23 (6) | 33 (8) | 71 (12) | NS |
| Other pneumonia | 21 (6) | 24 (6) | 49 (8) | NS |
| Secondary malignancy | 7 (2) | 5 (1) | 4 (1) | NS |
| CMV pneumonia | 9 (3) | 4 (1) | 19 (3) | NS |
| Hemorrhage | 10 (3) | 10 (3) | 25 (4) | NS |
| Graft failure | 2 (1) | 3 (1) | 6 (1) | NS |
| Other | 62 (17) | 77 (19) | 128 (22) | NS |
Unless otherwise indicated, values are number of patients and, in parentheses, frequency expressed as a percentage of those in that category who have died. The total numbers of patients in each category who have died are 361 in the group with recovery of platelet levels at day 30, 397 in the group with recovery between day 31 and day 100, and 588 in the group without recovery at day 100.
NS indicates not significant; GVHD, graft-versus-host disease; ARDS, adult respiratory distress syndrome; CMV, cytomegalovirus.
Factors influencing secondary graft failure
Of the 4716 patients in whom initial engraftment (ANC > 5 × 108/L) occurred, 456 had a subsequent decline in ANC to below 5 × 108/L, at a median of 53 days (range, 3-813 days) after initial engraftment. The cumulative incidence of secondary graft failure at 2 years after initial engraftment was 10%. Of note, the decline in ANC was transient in 245 patients, in whom recovery of ANC values indicating engraftment occurred a median of 7 days (range, 1-170 days) after the decline. In 16 patients, engraftment followed by a subsequent decline in ANC below 5 × 108/L occurred more than a year after BMT (range, 377-833 days), not in association with relapse. Statistical analyses of secondary graft failure were censored at 2 years because only one event occurred beyond 2 years and it was considered to be an outlier. In univariate analysis, lower cell dose, DRB1 mismatch, recipient seropositivity for CMV, African American ethnic group, T-cell depletion, and speed of initial engraftment (before or after day 28) were associated with secondary graft failure. In multivariate analysis, lower cell dose, older donor, DRB1 mismatch, recipient seropositivity for CMV, African American ethnic group, Hispanic ethnic group, and initial engraftment after day 28 were associated with secondary graft failure (Table7). In this analysis, T-cell depletion was not a risk factor for secondary graft failure after adjustment for cell dose, conditional on initial engraftment.
Results of multivariate Cox proportional hazards regression analysis of risk factors in relation to secondary graft failure
| Factor . | RR . | 95% CI . | Pvalue . | Favorable characteristic . |
|---|---|---|---|---|
| Donor age (per decade) | 1.20 | 1.08-1.34 | .0008 | Younger |
| Recipient CMV serologic status | 1.32 | 1.08-1.61 | .007 | Negative |
| Caucasian race | 1.00 | NA | NA | Reference group |
| African American ethnic group | 1.79 | 1.23-2.61 | .002 | Non–African American |
| Hispanic ethnic group | 1.89 | 1.30-2.75 | .0008 | Non-Hispanic |
| Cell dose (per 108/kg) | 0.86 | 0.80-0.92 | .0001 | Higher dose |
| Initial engraftment after day 28 | 1.82 | 1.32-2.52 | .0003 | Earlier engraftment |
| Factor . | RR . | 95% CI . | Pvalue . | Favorable characteristic . |
|---|---|---|---|---|
| Donor age (per decade) | 1.20 | 1.08-1.34 | .0008 | Younger |
| Recipient CMV serologic status | 1.32 | 1.08-1.61 | .007 | Negative |
| Caucasian race | 1.00 | NA | NA | Reference group |
| African American ethnic group | 1.79 | 1.23-2.61 | .002 | Non–African American |
| Hispanic ethnic group | 1.89 | 1.30-2.75 | .0008 | Non-Hispanic |
| Cell dose (per 108/kg) | 0.86 | 0.80-0.92 | .0001 | Higher dose |
| Initial engraftment after day 28 | 1.82 | 1.32-2.52 | .0003 | Earlier engraftment |
RR indicates relative risk; CI, confidence interval; CMV, cytomegalovirus.
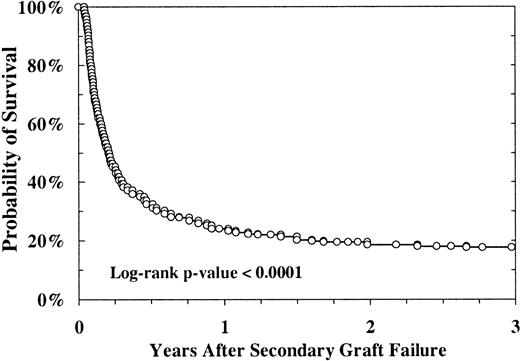
Secondary graft failure was associated with poor survival; only 18% of patients were still alive 3 years after this event (Figure5). The causes of death were infection (34%), acute or chronic GVHD (24%), toxicity (19%), relapse (12%), graft failure (6%), and other (5%). Seventy of the 456 patients with secondary graft failure received a second stem-cell infusion and 21 of them (30%) are still alive.
Time from marrow collection to infusion
To determine whether prolonged marrow-transport times have an adverse effect on clinical endpoints, we analyzed survival, myeloid engraftment, and platelet recovery, according to time from marrow collection to infusion (0-12 hours, n = 2083; 12-24 hours, n = 2035; 24-36 hours, n = 727; and > 36 hours, n = 133). In multivariate analysis, survival was marginally reduced in those who received marrow 12 to 24 hours (RR, 1.11; 95% CI, 1.02-1.21;P = .02) and 24 to 36 hours (RR, 1.18; 95% CI, 1.03-1.35;P = .02) after collection compared with those who received marrow less than 12 hours after collection, but it was not different in those who received marrow transported for more than 36 hours (RR, 1.04; 95% CI, 0.81-1.34; P = .76). Myeloid engraftment was similar among patients who received marrow up to 36 hours after collection. Those given marrow more than 36 hours after it was collected actually had more rapid engraftment than who received marrow sooner (RR, 1.27; 95% CI, 1.04-1.55; P = .02). Platelet recovery was similar among patients who received marrow 12 hours, 12 to 24 hours, and more than 36 hours after collection, but it was reduced in those who received marrow 24 to 36 hours after collection (RR, 0.85; 95% CI, 0.74-0.99; P = .03). Taken together, these data suggest that a delay in infusion of marrow beyond 36 hours after it is collected is not associated with adverse clinical consequences. However, because only a small number of patients in this study received marrow more than 36 hours after collection, these results should be viewed with caution.
Discussion
Durable engraftment of donor cells is an essential step in a successful stem-cell transplantation. Previous studies showed that in recipients of marrow from non-HLA–identical family members, graft failure increases with increasing HLA disparity.2-4 In an analysis of 1199 patients who received transplants from a family member, Anasetti et al2 found that the frequency of graft failure (defined as failure to achieve an ANC of 108/L by day 14 or a subsequent decline to < 108/L) was 2% in recipients of genotypically identical grafts and 12.3% in recipients of grafts from non-HLA–identical family members (0-3 antigens mismatched). Similarly, Szydlo et al,4 in reporting on 2055 transplants from genotypically matched donors, mismatched family-member donors, and URDs for the International Bone Marrow Transplant Registry, noted a frequency of graft failure (defined as absence of evidence of hematologic recovery on day 21 or recurrent pancytopenia) of 1% after transplantation from a genotypically matched donor and 9% after transplantation from an URD. In this study, we analyzed myeloid and platelet engraftment of URD marrow in a large series of patients who underwent transplantation facilitated by the NMDP.
Engraftment after BMT needs to be prompt to minimize the duration of neutropenia and to be durable. In this study, the median day of myeloid engraftment was 18 days after BMT, which is not different from that reported after sibling-donor transplantation.12-14However, a significant percentage of patients had not achieved myeloid engraftment by day 28, and 4% of all patients had primary graft failure. Delay of myeloid engraftment beyond 42 days was associated with reduced survival. Analysis of factors that might be adjusted to improve myeloid engraftment showed that engraftment was impaired in recipients of an HLA-A or HLA-B mismatched graft. These findings are in agreement with data from studies of transplants from nongenotypically identical family-member donors in which increasing HLA disparity was associated with increased primary and late engraftment failure.2-4 An increase in the size of the NMDP donor registry, together with improvements in techniques for HLA typing that would better identify mismatched donor-recipient pairs, might reduce the frequency of delayed engraftment in future transplantations. The typing in this study was serologic typing for class I loci and molecular typing for class II loci. As molecular typing methods are increasingly applied to class I loci and typing is expanded to include additional loci (eg, HLA-C), additional information on the importance of HLA matching may become available.
In this study, T-cell depletion of the marrow graft was associated with more rapid myeloid engraftment, perhaps because fewer patients who received T-cell–depleted grafts were also given methotrexate prophylaxis, which was associated with slower engraftment. However, the frequency of failure of primary engraftment was not increased in recipients of T-cell–depleted grafts. These data contrast with those in a previous study in recipients of T-cell–depleted sibling-donor grafts, in whom engraftment was also prompt but frequencies of failed engraftment were increased in the absence of prophylaxis for rejection.14 Four percent of patients in the current study received a second stem-cell infusion because of poor primary engraftment and less than one third of those patients are still alive—findings that emphasize the importance of achieving prompt primary engraftment. Univariate analysis of risk factors for secondary graft failure initially showed T-cell depletion to be an adverse risk factor. However, after adjustment for cell dose, T-cell depletion was no longer an adverse risk factor. It is possible that cell loss during processing may have an adverse effect on the durability of engraftment.
Analysis of platelet recovery showed that the median day of engraftment was 32 days after BMT. However, cumulative incidence curves for platelet recovery showed a striking plateau, with similar frequencies of recovery at 1 and 2 years after transplantation. One possible reason for this delay in platelet recovery is GVHD, and univariate and multivariate models confirmed that severe acute GVHD was strongly predictive of slower platelet recovery. In multivariate analysis, higher cell dose was associated with improved platelet recovery, as was reported in a previous study.15 16 Also, matching at HLA-A, HLA-B, and DRB1 was associated with improved platelet recovery. Improved HLA matching, together with use of higher cell doses, are 2 possible strategies for improving platelet recovery.
In contrast to our findings regarding speed of myeloid engraftment, use of methotrexate did not influence platelet recovery. This result may indicate that platelet recovery reflects graft function as well as the primary achievement of engraftment and is influenced by later factors, such as GVHD and infection, in addition to earlier factors, such as prophylaxis for GVHD. Our analysis suggests some improvement in time to myeloid engraftment in younger patients and in patients given transplants in more recent years but no improvement in the likelihood of engraftment (on logistic regression analysis) or platelet recovery. For clinical outcomes, the likelihood of engraftment is a more important endpoint than is a difference of a few days in time to engraftment.
In this study, we showed for the first time that platelet recovery is a strong predictor of survival after URD BMT and is a stronger predictor of outcome than is myeloid engraftment. In our patients, platelet recovery likely served as a composite surrogate marker for several other factors that influence survival (eg, acute and chronic GVHD, immune reconstitution, and viral infections), since our analysis of causes of death showed that hemorrhage was an infrequent cause of treatment failure, even in those with delayed platelet recovery. Data from previous studies of recipients of grafts from sibling donors indicate that in patients with chronic GVHD, thrombocytopenia is associated with a poor outcome.17 However, in our study, platelet recovery remained a strong predictor of survival even when statistical adjustments were made for the occurrence of GVHD. These results indicate that although GVHD does influence platelet recovery, the prognostic importance of platelet recovery is not just a reflection of GVHD activity. Whatever the reason for these findings, time to platelet recovery provides a valuable clinical indicator of likely outcome.
Secondary graft failure (as judged by hematologic criteria) occurred in 10% of patients in this study, and approximately half of them had a subsequent recovery. Survival was poor among these patients, and new strategies are needed to reduce and treat secondary graft failure.
Male sex of the donor was a favorable risk factor for myeloid and platelet engraftment, with no statistical interaction between the sex of the donor and that of the recipient. Previous studies of recipients of sibling-donor transplants showed reduced engraftment with male donors, and this effect was observed in both male and female recipients.13,14 Previous analysis of the first 462 recipients of URD transplants facilitated by the NMDP did not show that sex influenced engraftment.1 Similarly, analysis of engraftment in recipients of nongenotypically matched transplants did not show an important effect of donor or recipient sex.2-4Because of these differing data, further follow-up and examination of additional data sets are needed to determine whether there is a biologically important difference in engraftment of transplants from female compared with male donors.
In summary, this study demonstrated the important influence of HLA matching on primary engraftment after URD BMT. We showed that platelet recovery is an important predictor of survival, with excellent outcomes occurring in patients with early recovery but significantly reduced survival rates in those with a poor or delayed recovery. Platelet recovery has strong prognostic significance and may be a composite indicator of immunologic events such as GVHD, immune reconstitution, and active infection.
Supported in part by an educational grant from Baxter Corporation, the Children's Cancer Research Fund (S.M.D.), and the Jock Adams Fund (J.H.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
S. M. Davies, MMC 422, 420 Delaware Street SE, Minneapolis, MN 55455; e-mail: davie008@tc.umn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal