Abstract
An understanding of mechanisms regulating hematopoietic stem cell engraftment is of pivotal importance to the clinical use of cultured and genetically modified transplants. Human cord blood (CB) cells with lymphomyeloid repopulating activity in NOD/SCID mice were recently shown to undergo multiple self-renewal divisions within 6 days in serum-free cultures containing Flt3-ligand, Steel factor, interleukin 3 (IL-3), IL-6, and granulocyte colony-stimulating factor. The present study shows that, on the fifth day, the transplantable stem cell activity is restricted to the G1fraction, even though both colony-forming cells (CFCs) and long-term culture-initiating cells (LTC-ICs) in the same cultures are approximately equally distributed between G0/G1and S/G2/M. Interestingly, the G0 cells defined by their low levels of Hoechst 33342 and Pyronin Y staining, and reduced Ki67 and cyclin D expression (representing 21% of the cultured CB population) include some mature erythroid CFCs but very few primitive CFCs, LTC-ICs, or repopulating cells. Although these findings suggest a cell cycle–associated change in in vivo stem cell homing, the cultured G0/G1 and S/G2/M CD34+ CB cells exhibited no differences in levels of expression of VLA-4, VLA-5, or CXCR-4. Moreover, further incubation of these cells for 1 day in the presence of a concentration of transforming growth factor β1 that increased the G0/G1 fraction did not enhance detection of repopulating cells. The demonstration of a cell cycle–associated mechanism that selectively silences the transplantability of proliferating human hematopoietic stem cells poses both challenges and opportunities for the future improvement of ex vivo–manipulated grafts.
Introduction
Transplants of hematopoietic cells have assumed an important role in the treatment of many malignancies. They also hold much promise for future clinical applications involving hematopoietic stem cell–based gene therapy, tolerance induction to facilitate allogeneic or xenogeneic organ transplants, and other situations in which hematopoietic stem cell purification and/or expansion before transplantation would be desirable. Recently, considerable progress has been made in the characterization of human hematopoietic cells with long-term multilineage engraftment activity in various xenogeneic models using either irradiated immunodeficient mice1-4 or fetal sheep5 as hosts. In addition, a number of specific molecules, including VLA-4,6-9 VLA-5,10 and CXCR4,11 have been implicated in the mechanism by which hematopoietic stem cells exit from the blood into the extravascular compartment of the marrow, both in syngeneic and xenogeneic hosts.
Conditions that support the proliferation of primitive human hematopoietic cells in vitro readily support very large (more than 100-fold) amplifications of many progenitor cell types.12These may be accompanied by self-renewal divisions of the transplantable hematopoietic stem cells initially present in such cultures, as shown by their ability to be retrovirally transduced13-16 (which requires passage of cells through mitosis17,18), and the results of high-resolution dye tracking analyses.19,20 However, to date, only modest net expansions of transplantable stem cells in vitro have been demonstrable.21-23 In cultures of both murine24-26 and human stem cells,27 an initial dramatic loss of repopulating activity has been found to precede execution of a first division. At later times in cultures of murine cells, sequential oscillations of repopulating activity have been observed that correlated in time with the synchronous cell cycle passage of an initially highly purified stem cell population.28 In addition, similarly enriched populations of murine fetal liver stem cells have shown a disproportionate association of activity in the G0/G1subset.29 These findings have suggested that proliferating hematopoietic stem cells in the S/G2/M phases of the cell cycle may have a reduced ability to engraft. The present studies were designed to directly examine the validity of this hypothesis for transplantable human stem cells stimulated to proliferate in vitro. For this, we used 5-day expansion cultures of CD34+ human cord blood (CB) cells maintained under conditions that do not stimulate a first division of CD34+CD38− CB cells until day 330 and yet result in more than 60% of the transplantable stem cells detectable on day 6 having completed at least 3 divisions.19 In addition, we asked whether a proportion of the CB stem cells that had divided might spontaneously re-enter G0, since previous studies had suggested asymmetry in the cell cycle duration of mitogenically activated CD34+CD38− human hematopoietic cells.31 32
Materials and methods
Cells
CB from healthy, full-term infants delivered by cesarean section was collected in heparinized 50-mL tubes. Low-density (less than 1.077 g/mL) cells were isolated by Ficoll-Hypaque gradient centrifugation (Pharmacia, Uppsala, Sweden) and cryopreserved in a solution of 90% fetal calf serum (FCS, StemCell Technologies, Vancouver, BC, Canada) and 10% DMSO (Sigma Chemicals, St Louis, MO). As required, cells from multiple CB were thawed, cells expressing surface antigens characteristic of various mature hematopoietic lineages (lin+ cells) removed immunomagnetically, and the lin− CB cells obtained stained with anti-CD34(8G12)-FITC antibody (from Dr P. Lansdorp, Terry Fox Laboratory) and propidium iodide (PI, Sigma) before isolation of PI−CD34+ cells (more than 99% purity) using a FACStar+ cell sorter (Becton Dickinson, San Jose, CA) as described.19
Healthy bone marrow cells, used for preparing adherent stromal cell layers,33 were obtained as leftover material from allogeneic marrow harvests at our center, or were from healthy cadaveric donors (Northwest Tissue Center, Seattle, WA). All human material was acquired with informed consent and used according to approved institutional protocols.
Short-term suspension cultures
CD34+ CB cells were first cultured overnight in serum-free medium (BIT 9500, StemCell), supplemented with 10−4 mol/L 2-mercaptoethanol (2-ME, Sigma), 40 μg/mL low-density lipoproteins (LDL, Sigma), and 50 ng/mL human thrombopoietin (TPO, Genentech, Palo Alto, CA) at 105cells/mL. The cells were then washed twice in serum-free medium and cultured for another 4 days in fresh serum-free medium, this time supplemented with 2-ME, LDL, 100 ng/mL Flt3-ligand (FL, Immunex Corp, Seattle, WA), 100 ng/mL Steel factor (SF, purified from media conditioned by COS cells transiently transfected in the Terry Fox Laboratory with human SF complementary DNA [cDNA]), and 20 ng/mL IL-3 (Novartis, Basel, Switzerland), 20 ng/mL interleukin 6 (IL-6) (Cangene, Mississauga, ON, Canada), and 20 ng/mL granulocyte colony-stimulating factor (G-CSF) (StemCell). After 48 hours, the cultures were diluted 2-fold by addition of an equal volume of fresh medium with the same growth factors. In 3 experiments, the influence of transforming growth factor beta (TGF-β1) on the cell cycle and engraftment potential of cultured CB cells was analyzed. In these experiments, one fifth of the cells from each culture were transferred into secondary cultures for an additional 24 hours under the following conditions: in medium with FL + SF + IL-3 + IL-6 + G-CSF (as previously described) either with or without 5 ng/mL TGF-β1 (R&D Systems Ltd, Minneapolis, MN), or in LTC medium (Myelocult, StemCell) supplemented with 10−6 mol/L hydrocortisone (Sigma) on preestablished, first passage human marrow feeder layers prepared as described previously33 either with or without the 5 growth factors and/or 5 ng/mL TGF-β1.
Isolation and analysis of cultured hematopoietic cells in different cell cycle phases
Staining of cells with Hoechst 33342 (Hst, Molecular Probes, Eugene, OR) with or without Pyronin Y (Py, Sigma) was performed as previously described.34 Briefly, cultured CB cells were washed once in Hanks solution plus 2% FCS (HF), incubated in HF containing 10 μmol/L Hst at 37°C for 45 minutes, and Py was then added to give a final concentration of 2.5 μg/mL, followed by an additional 45 minutes incubation at 37°C with FITC-conjugated anti-CD34 antibody added for the last 20 minutes. The cells were then washed twice with HF containing 1 μg/mL PI, 10 μmol/L Hst, and 2.5 μg/mL Py, resuspended in HF with 10 μmol/L Hst and 2.5 μg/mL Py, and all cells (both CD34+ and CD34−) sorted using gates previously established to distinguish adult marrow G0, G1, and S/G2/M cells on the basis of their Ki-67 and cyclin D expression, and DNA content assessed by 7AAD staining.34 In the present experiments, distinction of G0/G1 and S/G2/M cells isolated using these same gates was confirmed by PI staining.35 The purity of the separately isolated G0 and G1 populations was determined by reanalysis of the initially sorted populations in 5 experiments. Because of the considerable loss of Hst and Py staining intensity that occurred during the first sort, the gates for this reanalysis had to be adjusted accordingly. This was performed using an aliquot of the same original cells that had been stained and passed through the FACS to collect PI− cells without further subdivision. The gates established with these cells to give an equivalent proportion of G0 and G1 cells as had been obtained originally were then used to determine the purity of the sorted G0 and G1 cells.
For the studies of adhesion molecule expression, cultured cells were stained with Hst only and either anti–CD34-FITC and anti–CXCR4-PE (Pharmingen, Mississauga, ON, Canada), or anti–CD34-PE (Becton Dickinson) and anti–VLA-4-FITC (Immunotech, Marseille, France) or anti–VLA-5-FITC (Immunotech), or appropriate isotype control antibodies.
For analysis of intracellular expression of the D cyclins, cultured cells were first sorted into G0 and G1populations as described above, and these cells were then fixed and stained with FITC-conjugated antibodies against cyclins D1, D2, and D3 (Pharmingen) according to the manufacturer's directions.
Reverse transcriptase–polymerase chain reaction analysis of cyclin D3 and Ki67 transcripts
Transcript levels in G0 and G1populations were compared using a semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) procedure described in detail previously.36 37 Briefly, 104 cells from each population were lysed in 50 μL of GIT buffer (5 mol/L guanidine isothiocyanate, 20 mmol/L 1,4-dithiothreitol [DTT], 25 mmol/L sodium citrate [pH = 7.0], 0.5% sarcosyl) and nucleic acids precipitated using ethanol. The RNA was then redissolved in 5.8 μL of RNase-free water plus 0.2 μL of oligo (dT) primer (1 μg/mL) (60 mer: 5′CATGTCGTCCAGGCCGCTCTGGACAAAATATGAATTCT24), heated to 70°C for 10 minutes, quenched on ice, and mixed with 2 μL of 5X RT buffer (GIBCO/BRL, Grand Island, NY), 1 μL of 0.1 mol/L DTT, 0.2 μL of 25 mmol/L (deoxynucleotide triphosphate) dNTPs (GIBCO/BRL), 0.5 μL of placental RNase inhibitor (10 U/μL), 0.5 μg nuclease-free bovine serum albumin (BSA) (Boehringer Mannheim, Laval, QC, Canada), and 0.5 μL of Superscript II (GIBCO/BRL). The mixture was incubated at 42°C for 1 hour and heat-inactivated at 70°C for 10 minutes. After ethanol precipitation, the pellet was resuspended in 5.5 μL of tailing solution (1 μL of 5X tailing buffer [GIBCO/BRL], 0.5 μL of 100 mmol/L dATP, 3.5 μL of water, and 0.5 μL of 15 U/mL terminal deoxynucleotidyl transferase [GIBCO/BRL] for 15 minutes at 37°C, and then heated to 70°C for 10 minutes. Aliquots of this solution were subjected to a PCR in 50 mmol/L KCl; 5 mmol/L MgCl2, 0.5 μg/mL BSA, 1 mmol/L dNTPs, 1 μL of gene 32 (Pharmacia), and 5 units of Taq polymerase (GIBCO/BRL). The cDNA was then amplified for the first 25 cycles (1 minute at 94°C, 2 minutes at 55°C, and 10 minutes at 72°C, except for the first cycle, in which the annealing temperature was 37°C instead of 55°C). To maintain the linearity of the PCR, one tenth of the volume of the reaction mixtures from the initial PCRs was then mixed with an additional 5 units of Taq polymerase before starting the second 25 cycles. After electrophoresis in 1% agarose gel, the cDNAs were transferred to nylon membranes (Hybond-N, Amersham, Buckinghamshire, United Kingdom) and hybridized with specific cDNA probes as follows. The cDNA for human Ki-67 (Kon-21 as) was obtained from Dr T. Scholzen (Borstel Research Institute, Borstel, Germany) and digested withBamH1/Not1. The cDNA for human cyclin D3 was obtained from Dr A. Arnold (University of Connecticut, Farmington, CT) and was digested with EcoR1. For Southern analyses, each filter was hybridized with probes labeled with 32P using a random primer kit (GIBCO/BRL) and hybridized for 12 to16 hours at 45°C in a solution of 40% formamide, 50 mmol/L NaPO4, 0.5% SDS, 5X SSPE, 5X Denhardt's solution, 0.25 mg/mL denatured salmon sperm DNA, and 100 mg/mL sodium dextran. The hybridized filters were then washed at room temperature in 2X SSC plus 0.1% SDS, followed by consecutive washes in 0.2X SSC plus 0.1% SDS, first at 50°C and then at 55°C. Specific signals were quantitated using a phosphoimager (Storm 860, Molecular Dynamics, Sunnyvale, CA) with MD APPS software. Each measurement was corrected for the signal level obtained in the RT− control that was hybridized to the same filter under the same conditions.
In vitro progenitor assays
Colony-forming cell (CFC) and 6-week long-term culture-initiating cell (LTC-IC) frequencies were determined on cells harvested from the various types of cultures or in the various fractions of cells isolated by FACS using standard procedures, as described previously.38
In vivo repopulation studies
NOD/LtSz-scid/scid (NOD/SCID) mice, originally obtained from Dr L. Schultz (The Jackson Laboratory, Bar Harbor, ME), were bred and maintained in the animal facility of the British Columbia Cancer Research Center (Vancouver, BC, Canada) under sterile conditions in microisolator cages and were provided exclusively with autoclaved food and water. Mice were irradiated with 350 cGy of total body137Cs γ-rays at 6 to12 weeks of age and then started on acidified drinking water supplemented with 100 mg/L ciprofloxacine (Bayer AG, Leverkusen, Germany) for the duration of the experiments. Test cells were injected intravenously into the irradiated mice, together with 106 irradiated (1500 cGy) human bone marrow cells as carrier cells. Mice were killed 6 to 8 weeks after transplant, and the cells from both tibiae and femurs of each mouse collected in HF. The cells were then incubated with 5% human serum and 2.4G2 (an antimouse Fc receptor antibody39) to decrease nonspecific antibody binding. Separate aliquots of cells were then stained for 30 minutes at 4°C, either with antihuman CD34 (8G12)–FITC plus antihuman CD19-PE and antihuman CD20-PE antibodies (Becton Dickinson) to detect human (CD34−CD19/20+) pre-B cells or with antihuman CD15-FITC (Becton Dickinson) and antihuman CD66b-FITC (Pharmacia Biotech, Baie d-Urfe, PQ) plus antihuman CD45-PE (Becton Dickinson) and antihuman CD71-PE antibodies (OKT9 from Dr P. Lansdorp) for the detection of mature human (CD45/71+CD15/66b+) myeloid cells. A detection limit of more than or equal to 5 human lymphoid cells (CD34−CD19/20+) and/or more than or equal to 5 human myeloid cells (CD45/71+ CD15/66b+) per 20 000 PI− cells analyzed was used to identify positively engrafted mice using gates set to exclude more than 99.99% of nonspecifically stained PI− cells incubated with irrelevant isotype-matched control antibodies labeled with the corresponding fluorochromes, as previously described.40Mice were then categorized as those containing either no human cells or human lymphoid cells only, those containing both human lymphoid and myeloid cells (L + M), and those containing any human cells, ie, mice containing only human lymphoid cells with or without human myeloid cells (L ± M) as no mice were found to contain only human myeloid cells. Frequencies of injected lymphomyeloid stem cells (referred to as competitive repopulating units or CRUs21 40) were calculated from the proportions of mice in a given experiment, or set of identical experiments, that did not contain both human lymphoid and human myeloid cells (ie, the first category) using Poisson statistics and the method of maximum likelihood with the assistance of the L-calc software (StemCell).
Statistical analyses
The results are shown as mean values ± SEM from independent experiments. Differences between groups were assessed using the Student t test.
Results
Cell cycle distribution of CD34+ cells and progenitors in 5-day cord blood expansion cultures
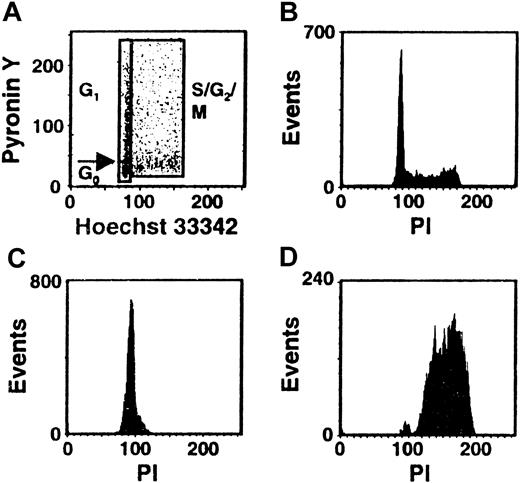
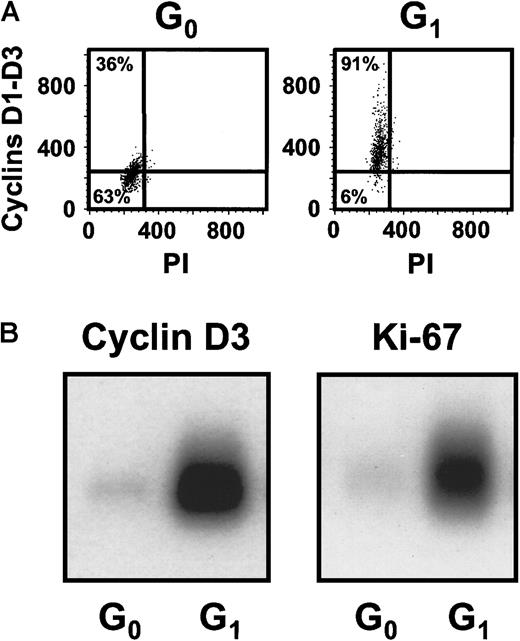
Cultures of CD34+ CB cells were incubated in serum-free medium with TPO only for the first day and then with FL + SF + IL-3 + IL-6 + G-CSF for the next 4 days to mimic the protocol used previously to demonstrate the execution of at least 3 self-renewal divisions of the input CRUs after a total of 6 days of culture under these conditions.19 Comparison of the numbers of CFCs and LTC-ICs at the end of the 5 days of culture in the present experiments with corresponding input values showed that these progenitor populations had increased 40 ± 6-fold and 9 ± 6-fold, respectively (n = 12). Using the gates illustrated in Figure 1, 21% ± 3% of the 5-day cultured CB cells were classified as in G0(HstloPylo), 36% ± 4% as in G1(HstloPyhi) and 43% ± 3% as in S/G2/M (Hsthi) (n = 6, Figure2). The purities of the combined G0/G1 and the remaining S/G2/M populations, as determined by PI staining, were 94% ± 1% for the G0/G1 cells and 95% ± 1% for the S/G2/M cells (n = 9, Figure 1C,D). The purities of the G0 versus G1 populations, as assessed by resorting, were 96% ± 2% and 90% ± 3%, respectively (n = 3). The validity of the gates used to distinguish G0and G1 cells was confirmed both by FACS analysis of their differential intracellular expression of D cyclins (49% ± 7% of the G0 cells did not contain detectable levels of any of the D cyclins, whereas only 8% ± 1% of the G1 cells were D cyclin negative, n = 5, Figure3A) and by semiquantitative RT-PCR detection of reduced Ki-67 and cyclin D3 transcript levels in the G0 population (n = 3, Figure 3B).
Distribution of the total population of cultured CB cells in different phases of the cell cycle.
CD34+ CB cells were cultured for 5 days in serum-free medium containing TPO (1day) and FL + SF + IL-3 + IL-6 + G-CSF (4 days) and then stained with Hst and Py and analyzed by FACS using the gates shown. A representative profile is shown in panel A. Corresponding FACS profiles are also shown for the total cultured CB cells (B) and the isolated G0/G1 (C) and S/G2/M (D) fractions after restaining of the fixed cells with PI.
Distribution of the total population of cultured CB cells in different phases of the cell cycle.
CD34+ CB cells were cultured for 5 days in serum-free medium containing TPO (1day) and FL + SF + IL-3 + IL-6 + G-CSF (4 days) and then stained with Hst and Py and analyzed by FACS using the gates shown. A representative profile is shown in panel A. Corresponding FACS profiles are also shown for the total cultured CB cells (B) and the isolated G0/G1 (C) and S/G2/M (D) fractions after restaining of the fixed cells with PI.
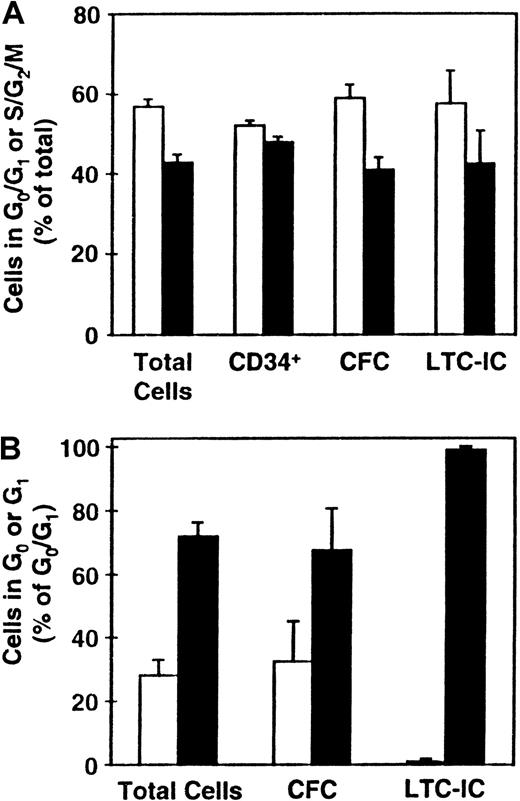
Distribution of various progenitor cell types between the different phases of the cell cycle.
CD34+ CB cells were cultured (as described in the legend to Figure 1) and the distributions of total cells and LTC-ICs (n = 9), CFCs (n = 8), and CD34+ cells (n = 5) between G0/G1 (■) and S/G2/M (▪) determined (A). (B) Corresponding distributions of these cells (except for those expressing CD34) between G0 (■) and G1 (▪) (n = 3).
Distribution of various progenitor cell types between the different phases of the cell cycle.
CD34+ CB cells were cultured (as described in the legend to Figure 1) and the distributions of total cells and LTC-ICs (n = 9), CFCs (n = 8), and CD34+ cells (n = 5) between G0/G1 (■) and S/G2/M (▪) determined (A). (B) Corresponding distributions of these cells (except for those expressing CD34) between G0 (■) and G1 (▪) (n = 3).
Differential expression of the D cyclins and Ki-67 between the sorted G0 and G1 cells.
(A) Representative FACS profiles for G0 and G1cells isolated from the same type of 5-day cultures described in Figure1 after staining for intracellular cyclins D1, D2, and D3. (B) A representative Southern blot analysis of cDNAs for cyclin D3 and Ki-67 generated from sorted G0 and G1 cells from the cultures described above.
Differential expression of the D cyclins and Ki-67 between the sorted G0 and G1 cells.
(A) Representative FACS profiles for G0 and G1cells isolated from the same type of 5-day cultures described in Figure1 after staining for intracellular cyclins D1, D2, and D3. (B) A representative Southern blot analysis of cDNAs for cyclin D3 and Ki-67 generated from sorted G0 and G1 cells from the cultures described above.
Cultured G0/G1 and S/G2/M cells were additionally stained for CD34 expression and aliquots also assayed for CFCs and LTC-ICs. As shown in Figure 2A, the proportions of CD34+ cells in these 2 fractions were approximately equal (which was significantly different from the distribution of the total cells, P < .03). In contrast, the distribution of CFCs and LTC-ICs between the G0/G1 and S/G2/M populations more closely mirrored the total cell distribution (P > .1). Separate comparison of the proportions of CFCs in G0 versus G1 showed these also to mirror the total cell distribution (P = .8). However, the types of CFCs found in the G0 and G1 fractions were different with primitive CFCs being found almost exclusively in the G1 fraction. Most (more than 95%) of the LTC-ICs were also found to be present in the G1 fraction, which was significantly different from the total cell distribution between the G0 and G1fractions (P < .03). Taken together, these findings suggest a preferential ability of more mature cell types to enter G0 under the conditions prevalent in these cultures after 5 days of incubation.
Competitive repopulating units in 5-day cord blood expansion cultures are detected exclusively in the G1population
In 3 of the experiments described in Figure 2A, the remaining cells in the separately isolated G0/G1 and S/G2/M fractions of the day 5 cultured CB cells (more than 90% of each) were injected into irradiated NOD/SCID mice to examine their content of human cells with in vivo lymphoid and/or myeloid repopulating activity. As shown in Table1, engraftment after 6 to 8 weeks with both myeloid and lymphoid human cells was seen only in mice transplanted with G0/G1 cells in all 3 experiments, and in only 4 of 17 mice was any engraftment (lymphoid only) by the cultured human S/G2/M cells obtained. Because not all mice injected with G0/G1 cells were found to contain human myeloid and lymphoid cells, the data could be used to estimate the frequency of CRUs in the G0/G1 fraction of the cultured CB cells. The value obtained was 1 CRU/106 G0/G1cells (with a range defined by ± SEM of 1 per 8 × 105 to 1.6 × 106 cells). A similar calculation for the S/G2/M population (assuming less than one mouse was positive) gave a value of less than 1 CRU/107S/G2/M cells. This suggests a difference of at least 10-fold in the number of CRU detectable in the 2 fractions.
Differential repopulating activity of G0/G1 and S/G2/M cells present in 5-day cultures of CD34+ cord blood cells
| Experiment no. . | G0/G1 . | S/G2/M . | ||||
|---|---|---|---|---|---|---|
| Cells per mouse* (×106) . | Fraction of positive mice . | Cells per mouse*(×106) . | Fraction of positive mice . | |||
| L+ + M+ (% human cells)† . | L+ ± M+ . | L+ + M+ . | L+ ± M+ . | |||
| 1 | 1.5 | 2/6 (0.6, 0.3) | 6/6 | 0.73 | 0/6 | 4/6 |
| 2 | 1.0 | 6/6 (1.3, 10.9, 0.7, 1.5, 0.6, 1.5) | 6/6 | 0.65 | 0/6 | 0/6 |
| 3 | 0.78 | 3/5 (1.1, 0.2, 0.6) | 5/5 | 0.46 | 0/5 | 0/5 |
| Experiment no. . | G0/G1 . | S/G2/M . | ||||
|---|---|---|---|---|---|---|
| Cells per mouse* (×106) . | Fraction of positive mice . | Cells per mouse*(×106) . | Fraction of positive mice . | |||
| L+ + M+ (% human cells)† . | L+ ± M+ . | L+ + M+ . | L+ ± M+ . | |||
| 1 | 1.5 | 2/6 (0.6, 0.3) | 6/6 | 0.73 | 0/6 | 4/6 |
| 2 | 1.0 | 6/6 (1.3, 10.9, 0.7, 1.5, 0.6, 1.5) | 6/6 | 0.65 | 0/6 | 0/6 |
| 3 | 0.78 | 3/5 (1.1, 0.2, 0.6) | 5/5 | 0.46 | 0/5 | 0/5 |
More than 90% of all cells in each fraction from the entire culture were injected into 6 irradiated NOD/SCID mice in each experiment.
% of human CD45/CD71+ cells in the bone marrow of those mice found to be positive 6 weeks after transplant (L+= at least 5 of 20 000 cells were human CD34−CD19/20+, M+ = at least 5 of 20 000 cells were human CD45/71+CD15/66b+cells).
In the 3 experiments in which G0 and G1 cells were isolated as separate populations from 5-day cultures (Figure 2B), the majority of the cells were again injected into NOD/SCID mice to assess their repopulating activity. As shown in Table2, only mice injected with G1cells showed any engraftment with human cells. Calculation of the CRU frequency in the G1 fraction gave a value of 1 per 7 × 105 G1 cells (with a range defined by ± SEM of 1 per 105 to 9 × 105cells). Based on the detection limit of CRUs in the G0fraction (where none were seen), and the different sizes of the 2 fractions in terms of total cells, the G1 fraction was estimated to have contained at least 85% of the detectable CRUs present in the cultures.
Lack of repopulating activity in the G0 cells present in 5-day cultures of CD34+ cord blood cells
| Experiment no. . | G1 . | G0 . | ||||
|---|---|---|---|---|---|---|
| Cells per mouse* (×106) . | Fraction of positive mice . | Cells per mouse*(×106) . | Fraction of positive mice . | |||
| L+ + M+ (% human cells)† . | L+ ± M+ . | L+ + M+ . | L+ ± M+ . | |||
| 1 | 1.0 | 5/6 (2.1, 1.9, 3.4, 0.5, 4.4) | 6/6 | 0.2 | 0/6 | 0/6 |
| 2 | 0.8 | 5/6 (11, 3, 12, 20, 9) | 6/6 | 0.3 | 0/6 | 0/6 |
| 3 | 0.9 | 3/6 (0.6, 0.5, 1.5) | 6/6 | 0.6 | 0/6 | 0/6 |
| Experiment no. . | G1 . | G0 . | ||||
|---|---|---|---|---|---|---|
| Cells per mouse* (×106) . | Fraction of positive mice . | Cells per mouse*(×106) . | Fraction of positive mice . | |||
| L+ + M+ (% human cells)† . | L+ ± M+ . | L+ + M+ . | L+ ± M+ . | |||
| 1 | 1.0 | 5/6 (2.1, 1.9, 3.4, 0.5, 4.4) | 6/6 | 0.2 | 0/6 | 0/6 |
| 2 | 0.8 | 5/6 (11, 3, 12, 20, 9) | 6/6 | 0.3 | 0/6 | 0/6 |
| 3 | 0.9 | 3/6 (0.6, 0.5, 1.5) | 6/6 | 0.6 | 0/6 | 0/6 |
More than 90% of all cells in each fraction from the entire culture were injected into 6 irradiated NOD/SCID mice in each experiment.
% of human CD45/CD71+ cells in the bone marrow of those mice found to be positive 6 weeks after transplant (L+= at least 5 of 20 000 cells were human CD34−CD19/20+, M+ = at least 5 of 20 000 cells were human CD45/71+CD15/66b+cells).
Expression of VLA-4, VLA-5, CD44, and CXCR-4 does not differ between cultured cord blood cells in G0/G1 and S/G2/M
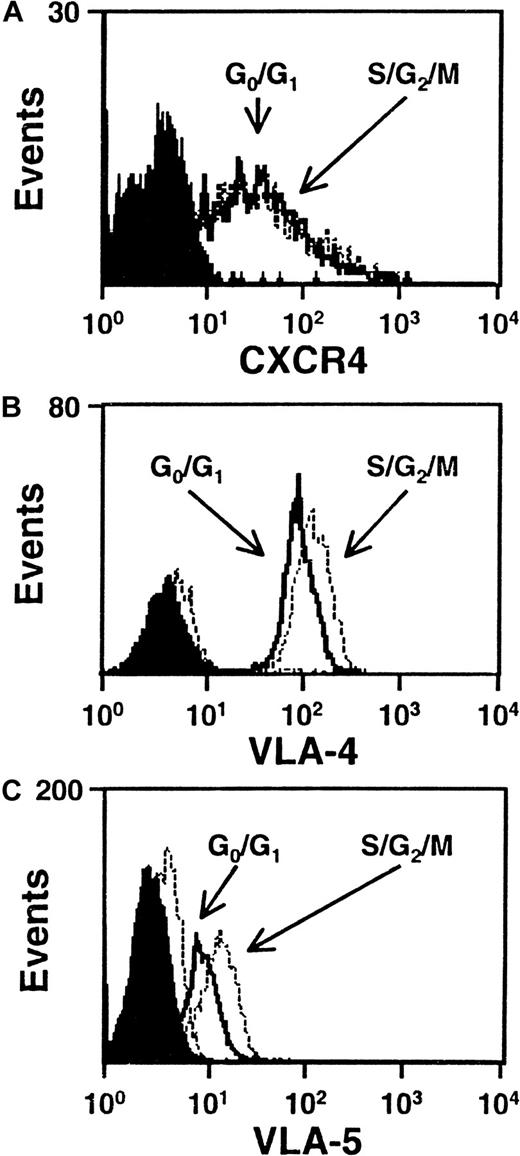
We next asked whether any differences in the level of expression of VLA-4, VLA-5, CXCR-4, or CD44 could be seen on G0/G1 and S/G2/M cells isolated from the same type of 5-day expansion cultures of CD34+ CB cells. Figure 4 shows FACS profiles for the first 3 of these adhesion molecules on the CD34+ subset of cells from both populations in representative experiments. All 3 adhesion molecules were consistently found to be highly expressed on the CD34+ cells examined with no differences seen between the G0/G1 and S/G2/M populations (VLA-4, n = 5; VLA-5, n = 3; CXCR4, n = 6). Similar results were obtained when the total cells were analyzed for CD44 expression (n = 3, data not shown). Because of the intense staining of CD44, it was not possible to perform this analysis on an additionally stained CD34+ subset.
CXCR4, VLA-4 and VLA-5 are expressed at similar levels by cells in G0/G1 and S/G2/M.
Representative FACS histograms of intracellular CXCR4 (A), VLA-4 (B), and VLA-5 (C) staining in cultured CD34+G0/G1 (solid lines) and S/G2/M (dotted lines) CB cells. G0/G1 and S/G2/M cells were purified by Hst sorting after 5 days of culture (see legend to Figure 1) and were then stained with anti–CD34-FITC and anti–CXCR4-PE, or anti–CD34-PE and anti–VLA-4-FITC or anti–VLA-5-FITC. Solid and dotted histograms show the control data for the G0/G1 and S/G2/M cells, respectively, stained with irrelevant isotype-matched control antibodies. The S/G2/M cells showed a higher green autofluorescence than the G0/G1cells.
CXCR4, VLA-4 and VLA-5 are expressed at similar levels by cells in G0/G1 and S/G2/M.
Representative FACS histograms of intracellular CXCR4 (A), VLA-4 (B), and VLA-5 (C) staining in cultured CD34+G0/G1 (solid lines) and S/G2/M (dotted lines) CB cells. G0/G1 and S/G2/M cells were purified by Hst sorting after 5 days of culture (see legend to Figure 1) and were then stained with anti–CD34-FITC and anti–CXCR4-PE, or anti–CD34-PE and anti–VLA-4-FITC or anti–VLA-5-FITC. Solid and dotted histograms show the control data for the G0/G1 and S/G2/M cells, respectively, stained with irrelevant isotype-matched control antibodies. The S/G2/M cells showed a higher green autofluorescence than the G0/G1cells.
Exposure of previously cultured cord blood cells to TGF-β1 for one day has no effect on their repopulating activity
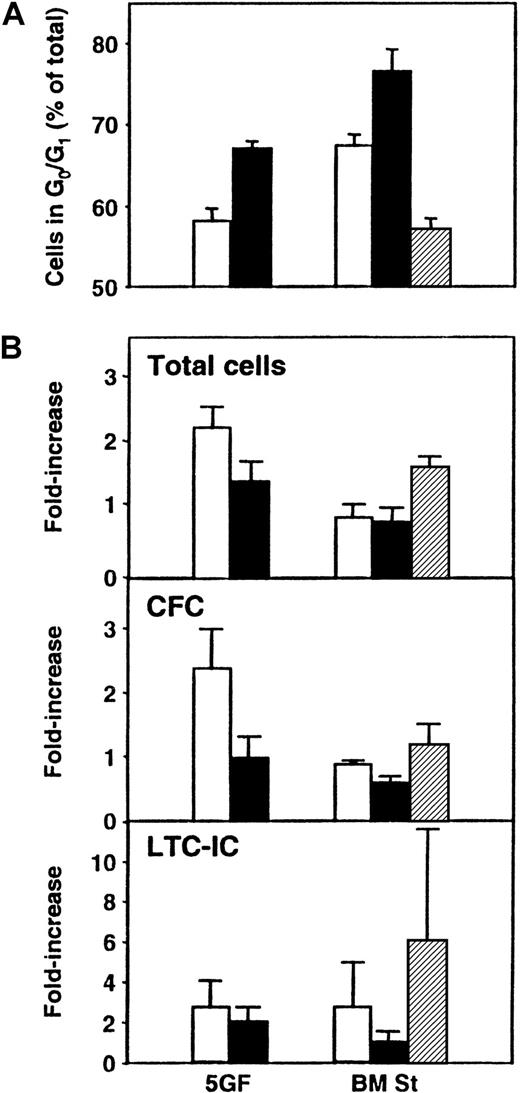
In a final series of experiments, we asked whether exposure of cultured CB cells to TGF-β1 could be used to enhance the detection of transplantable stem cells by forcing their arrest and hence their accumulation in G0/G1. Accordingly, CD34+ CB cells were first cultured for 5 days under the same conditions as previously stated, and then all their progeny were transferred without further separation to different types of secondary cultures for an additional 24 hours (± TGF-β1 in the presence or absence of stroma). The cells were then harvested, and cell cycle analyses using PI staining were performed. In addition, CFCs, LTC-ICs, and repopulating activity in NOD/SCID mice were measured. As shown in Figure 5, exposure of the cells to this concentration of TGF-β1 increased the size of the G0/G1 population to the greatest extent in the presence of bone marrow stroma (76% ± 3% G0/G1 cells with TGF-β1 vs 67% ± 1% without, P < .03), although an increase was also seen when the cells were incubated with FL + SF + IL-3 + IL-6 + G-CSF (5GF) plus TGF-β1 in the absence of stroma (67% ± 1% with TGF-β1 vs 58% ± 3% without, P < .01, n = 3). However, when both stroma and the 5GF were present, there was no increase in G0/G1 cells above the levels seen with 5GF alone (P > .05). In another 3 experiments, the effect of TGF-β1 exposure on the proportion of cells entering G0 was analyzed. Exposure to TGF-β1 in the presence of stroma increased the ratio of G1 to G0 cells from 1.1 ± 0.1 to 3.8 ± 0.7. In the absence of stroma but in the presence of the 5GF, the increase in this ratio was more modest (from 1.7 ± 0.3 to 4.2 ± 0.7,P < .03). In the suspension cultures, the proportion of total cells defined as apoptotic (because they contained less than 2n DNA content) was also increased after exposure to TGF-β1(21% ± 6% with TGF-β1 vs 8% ± 4% without,P = .07). However, in the presence of stroma, the proportion of apoptotic cells was the same, whether or not TGF-β1 was added (13% ± 5% with TGF-β1vs 12% ± 7% without). The total cell number and the number of CFCs were slightly, but not significantly (P > .05), decreased when TGF-β1 was present. LTC-IC values were more variable but, on average, showed no differences between the different groups (P > .05). Similar results were obtained when cells from these cultures were assayed in NOD/SCID mice. As shown in Table3, there was no difference (P > .05) in the percentage of mice repopulated with lymphoid and myeloid cells (or the average level of engraftment, data not shown) after transplantation of cells cultured in the presence or absence of TGF-β1.
Effect of TGF-β1 exposure on cultured CB cells.
Panel A shows the proportion of total cells in G0/G1 after 5 days of culture of CD34+ CB cells in the presence of FL, SF, IL-3, IL-6, and G-CSF (5GF) and then for an additional 24 hours in 5 new culture conditions: in medium plus 5 GF (as previously) either without (■) or with TGF-β1 (▪), or in LTC medium on preestablished bone marrow stroma layers (BM St) either without (■) or with TGF-β1 (▪), or with 5GF (▨). Cell cycle analyses were performed by FACS after staining of fixed cells with PI. Panel B compares the number of total cells, CFCs, and LTC-ICs in the same 3 experiments.
Effect of TGF-β1 exposure on cultured CB cells.
Panel A shows the proportion of total cells in G0/G1 after 5 days of culture of CD34+ CB cells in the presence of FL, SF, IL-3, IL-6, and G-CSF (5GF) and then for an additional 24 hours in 5 new culture conditions: in medium plus 5 GF (as previously) either without (■) or with TGF-β1 (▪), or in LTC medium on preestablished bone marrow stroma layers (BM St) either without (■) or with TGF-β1 (▪), or with 5GF (▨). Cell cycle analyses were performed by FACS after staining of fixed cells with PI. Panel B compares the number of total cells, CFCs, and LTC-ICs in the same 3 experiments.
Lack of effect of short-term exposure to TGF-β1 on the repopulating activity of cultured CD34+ cord blood cells
| Experiment no. . | Suspension culture + 5 GF . | Suspension culture + 5 GF + TGF-β1 . | BM stroma . | BM stroma + 5 GF . | BM stroma + TGF-β1 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cells per mouse3-150 (×106) . | L+ + M+ . | Cells per mouse3-150 (×106) . | L+ + M+ . | Cells per mouse3-150 (×106) . | L+ + M+ . | Cells per mouse3-150 (×106) . | L+ + M+ . | Cells per mouse3-150 (×106) . | L+ + M+ . | |
| 1 | 3.0 | 7/8 | 2.1 | 7/8 | 1.5 | 8/8 | 2.1 | 7/8 | 0.9 | 6/6 |
| 2 | 3.3 | 5/10 | 1.6 | 7/10 | 0.8 | 3/9 | 1.8 | 3/10 | 0.9 | 4/7 |
| 3 | 1.5 | 8/8 | 1.2 | 5/8 | 0.8 | 5/7 | 1.2 | 6/6 | 0.8 | 3/5 |
| Total | 20/26 (77%) | 19/26 (73%) | 16/24 (67%) | 16/26 (62%) | 13/18 (72%) | |||||
| Experiment no. . | Suspension culture + 5 GF . | Suspension culture + 5 GF + TGF-β1 . | BM stroma . | BM stroma + 5 GF . | BM stroma + TGF-β1 . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cells per mouse3-150 (×106) . | L+ + M+ . | Cells per mouse3-150 (×106) . | L+ + M+ . | Cells per mouse3-150 (×106) . | L+ + M+ . | Cells per mouse3-150 (×106) . | L+ + M+ . | Cells per mouse3-150 (×106) . | L+ + M+ . | |
| 1 | 3.0 | 7/8 | 2.1 | 7/8 | 1.5 | 8/8 | 2.1 | 7/8 | 0.9 | 6/6 |
| 2 | 3.3 | 5/10 | 1.6 | 7/10 | 0.8 | 3/9 | 1.8 | 3/10 | 0.9 | 4/7 |
| 3 | 1.5 | 8/8 | 1.2 | 5/8 | 0.8 | 5/7 | 1.2 | 6/6 | 0.8 | 3/5 |
| Total | 20/26 (77%) | 19/26 (73%) | 16/24 (67%) | 16/26 (62%) | 13/18 (72%) | |||||
TGF-β1 indicates transforming growth factor β1; BM, bone marrow.
More than 90% of all the cells from each culture were injected into a minimum of 8 irradiated NOD/SCID mice in each experiment. 5GF = FL + SF + IL-3 + IL-6 + G-CSF, as described in “Materials and methods.”
Discussion
In this study, we have shown that human CB cells transiting the S/G2/M phases of the cell cycle after 5 days of growth factor stimulation, first by TPO (1 day) and then by IL-3, IL-6, G-CSF, SF, and FL (4 days), do not repopulate the bone marrow of irradiated NOD/SCID mice. Several lines of evidence indicate that this is due to a reversible silencing of the engraftment potential of stem cells present in these populations. First, the frequencies of CD34+cells, CFCs, and LTC-ICs were approximately equally distributed between the S/G2/M and G0/G1 fractions, indicating that all these cell types were dividing asynchronously. The proportionate representation in both fractions of LTC-ICs, a cell type considered to be closely related to CRUs,13 further suggests that cells with the developmental potential of CRUs would be similarly distributed between Go/G1 and S/G2/M. Second, we have previously shown that after one more day under the same culture conditions as used in the present studies, the majority of all the cells, including the lymphomyeloid repopulating cells, have already completed at least 3 divisions,19 even though very few are likely to have completed a first division before the third day,30suggesting an average cell cycle time of less than 24 hours. Moreover, separate assessment of the functional activities of the G0and G1 cells isolated from the same 5-day cultures showed no evidence of any repopulating cell (or LTC-IC) re-entry into G0 once their proliferation had been initiated. This contrasts with the situation found for the mature erythroid CFCs being produced, many of which could be found in the G0 fraction. The LTC-IC results also appear to differ from those reported by Gothot et al.41 However, their methods differed from those used here in 2 important ways. First, they did not include FL in the cytokine cocktail in which the cells were cultured, which we42,43 and others44 have found to be critical for optimal recruitment into division of the most primitive human cells. Second, they measured CFC progenitors in a 2-week stroma-free culture, whereas we measured CFC progenitors using a stroma-containing culture system and a 6-week read-out. Although these 2 assays may measure similar frequencies of progenitors in some cell suspensions,45 we believe they do not detect the same progenitor because significant differences in progenitor frequency can be revealed under a number of circumstances (eg, in mobilized blood populations, even when the duration of the stroma-containing LTC-IC assay cultures is prolonged beyond 5 weeks).46,47 Thus, it seems likely that the majority of the proliferating CB cells detectable after 6 days in our cultures as transplantable stem cells19would have been transiting S/G2/M the previous day, ie, when the present analyses were performed.
These findings prompted us to look for cell cycle–associated changes in levels of expression of several molecules that have been implicated in homing mechanisms used by primitive hematopoietic cells. Although these are still not well defined, a variety of in vivo studies have suggested a role of β1-integrins, CXCR4, and CD44 in hematopoietic stem cell homing.6,7,10,11,48 Nevertheless, in the present study, we did not obtain any evidence of a quantitative change in expression of VLA-4, VLA-5, CXCR4, or the glycoprotein CD44, when G1/G0 and S/G2/M cells were compared, even when the CD34+ subset was analyzed separately. At first glance, these findings seem to contradict those of Yamaguchi et al49 who reported a lower expression of VLA-4 on unstimulated CD34+ adult bone marrow cells in G0/G1. This possible discrepancy may, however, be due to the different ontogenic origins of the cells analyzed in the 2 studies. Our failure to discern any difference in the level of expression of adhesion molecules does not, of course, rule out other potential cell cycle–associated changes in their activity (eg, altered ligand affinities). For example, a modulation of the affinity status of VLA-4 by inside-out signaling has been documented to occur when CFC progenitors are incubated with granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-3, and SF.50-52 In addition, it should be noted that any changes unique to cells with stem cell activity would not have been resolved by our studies because of the low frequency of stem cells even within the CD34+subset.21 22
As a first attempt to explore the possibility of improving engraftment by a TGF-β1–mediated arrest of proliferating repopulating cells, we investigated the effect of a 24-hour exposure of the cultured cells to this cytokine. This included incubating the cells under conditions that we have reproducibly shown can block the S-phase entry of high proliferative potential CFCs53,54 and was based also on other reports indicating an ability of TGF-β1 to inhibit the proliferation of primitive hematopoietic cells, including CB cells, in a variety of settings.53-58 Although the conditions used here were effective in increasing the proportion of total cells in G0/G1, TGF-β1–treated cells did not show any increase in repopulating activity. These results are, in fact, similar to those reported by Garbe et al59 who failed to demonstrate an effect of TGF-β1 on the yield of LTC-ICs (in contrast to reducing CFC numbers) in short-term suspension cultures of CD34+ cells isolated from human mobilized peripheral blood. They are also similar to those reported in a recent study of growth factor–stimulated stem cells isolated from the adult mouse bone marrow, where an additional 1 day of culture in the presence of TGF-β also did not improve their transplantability.25Whether these findings indicate a unique insensitivity of proliferating CRUs to TGF-β1, or the need for different exposure conditions will require additional experiments to determine. In this regard, it is interesting to note that we have recently found that in vivo administration of TGF-β can enhance the detection of human fetal liver-derived CRUs regenerating in NOD/SCID mice.60
The observed engraftment defect of proliferating human hematopoietic stem cells during their passage through the S/G2/M phases of the cell cycle and the failure of these cells to enter G0, at least before the end of 5 days of culture in the presence of FL, SF, IL-3, IL-6, and G-CSF, may have a considerable negative impact on the clinical utility of current ex vivo expansion or retroviral marking protocols that depend on maximal induction of stem cell cycling. Indeed, continuous improvement in the proportion of transduced stem cells obtained is well known to be associated with significant losses in stem cell yields relative to the number initially present.30 The average duration of the cell cycle of Rhodaminelo/Hstlo mouse bone marrow cells, which are highly enriched in stem cells, has been shown to decrease from 36 to 12 hours when these cells are stimulated to proliferate in suspension culture.61 If a relatively fixed duration of S phase of approximately 7 to 8 hours, a G2 phase of 2 hours, and 1 hour for M is assumed for mammalian cells in general, this would imply a very short (1 to 2 hours) G1 phase for proliferating murine hematopoietic stem cells. Similar data are not available for human stem cells, although it is unlikely that the duration of the cell cycle is longer than 18 hours, based on studies of growth factor–stimulated LTC-IC turnover rates in vitro.35 It is therefore reasonable to anticipate that strategies able to reversibly arrest human stem cells might significantly enhance the activity of proliferating stem cells, even though our preliminary efforts using short-term exposure to TGF-β1 did not prove successful. We also do not yet know whether mechanisms that regulate homing may remain suboptimal when rapidly cycling stem cells transit G1, as suggested by the reduction in repopulating activity exhibited by G0 stem cells first entering G1.27 Further studies are thus needed to improve our understanding of the cell cycle–associated modulation of hematopoietic stem cell engraftment described here to overcome this potentially important clinical drawback to the use of proliferating populations for transplantation applications.
Finally, the present findings may also have important relevance to leukemic stem cell populations where engraftment may be limited to, or preferentially associated with, a quiescent compartment.34,62,63 This raises the possibility that proliferating leukemic stem cells may share the same engraftment defect as their healthy counterparts. If supported, this would imply that the removal of leukemic cells from autologous transplants might be less essential than currently believed, because the leukemic stem cells appear to comprise very small subpopulations within the leukemic clone,64-66 and the majority of these may be proliferating.34,67 68 On the other hand, transplantability may also impose a functional requirement on the detection of malignant stem cells that underestimates the number of such cells present that may have been able to sustain or even regenerate disease in situ.
Acknowledgments
We thank Maya Sinclaire and Ken Lee for assistance with the animal work; Gayle Thornbury, Giovanna Cameron, and Rick Zapf for assistance in cell sorting; the staff of the Stem Cell Assay Service for initial hematopoietic cell processing; and Yvonne Yang for manuscript preparation.
Supported by grants from the National Cancer Institute of Canada (NCIC) with funds from the Terry Fox Run and from the NIH (P01 HL 55435). H.G. received a grant from the Dr Mildred Scheel Stiftung für Krebsforschung, Bonn, Germany. I.O. held an NCIC Postdoctoral Fellowship. C.J.E. was a Terry Fox Cancer Research Scientist of the NCIC.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Connie J. Eaves, Terry Fox Laboratory, 601 W 10th Ave, Vancouver, BC V5Z 1L3, Canada; e-mail: ceaves@bccancer.bc.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal