Abstract
Pentraxins are acute-phase proteins produced in vivo during inflammatory reactions. Classical short pentraxins, C-reactive protein, and serum amyloid P component are generated in the liver in response to interleukin (IL)–6. The long pentraxin PTX3 is produced in tissues under the control of primary proinflammatory signals, such as lipopolysaccharide, IL-1β, and tumor necrosis factor-α, which also promote maturation of dendritic cells (DCs). Cell death commonly occurs during inflammatory reactions. In this study, it is shown that PTX3 specifically binds to dying cells. The binding was dose dependent and saturable. Recognition was restricted to extranuclear membrane domains and to a chronological window after UV irradiation or after CD95 cross-linking–induced or spontaneous cell death in vitro. PTX3 bound to necrotic cells to a lesser extent. Human DCs failed to internalize dying cells in the presence of PTX3, while they took up normally soluble or inert particulate substrates. These results suggest that PTX3 sequesters cell remnants from antigen-presenting cells, possibly contributing to preventing the onset of autoimmune reactions in inflamed tissues.
Introduction
Dying cells are removed from living tissues. Errors in the clearance of cell corpses may cause autoimmunity.1,2 Specific proteases (caspases) cleave many autoantigens and determine the generation of nucleosomes, a major antigen in the prototypic systemic autoimmune disease, systemic lupus erythematosus (SLE).3 Key autoantigens cluster into apoptotic blebs, and cleavage by granzyme B, which leads cytotoxic T-cell–induced apoptosis, selectively modifies autoantigens.4 Conversely, the processing of the internalized dying cells yields T-cell epitopes.5
Scavenger phagocytes, which are devoid of the ability to initiate immune responses, perform most of the clearance of dying cells in vivo.1 However, the most potent antigen-presenting cells (APCs), dendritic cells (DCs),6,7 also internalize dying cells and present epitopes derived from their processing to major histocompatibility complex (MHC) class I– and class II–restricted T lymphocytes.8-15
DCs originate from bone marrow CD34+progenitors.16 DC precursors enter the blood and reach peripheral organs, where they develop to immature DCs. Immature DCs capture soluble antigens via macropinocytosis7,16-18 and particulates through phagocytosis.19-21 Primary proinflammatory signals, such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), promote DC maturation and migration to draining lymph nodes, where they perform the antigen presentation function.6,7,22 The rearrangement of the actin-based cytoskeleton20 allows migration to T-cell areas of lymphoid organs following a well-regulated pathway of chemotactic signals23-25 where, upon interaction with T cells, they complete their maturation process.
The outcome of presentation of antigens from dying cells by DCs is tightly regulated. Cells (by definition, reservoirs of “self-antigens”) die continuously during development and normal tissue turnover, and DCs constitutively shuttle the material derived from their intracellular processing to lymphoid organs.26Normal cell death via apoptosis occurs in the absence of inflammatory signals causing DC maturation. Besides, apoptotic cell clearance by macrophages triggers the release of anti-inflammatory cytokines,27,28 including IL-10 and transforming growth factor-β, that prevent DC maturation.7,29Antigen presentation by immature DCs may induce tolerance in T cells reactive toward peripheral antigens.30
In contrast, during inflammation, cells die in a context in which proinflammatory cytokines promote DC maturation and T-cell priming and immune responses start toward the initiating noxa(pathogens, etc). Immature DCs challenged with cells undergoing postapoptotic necrosis9,21 or directly killed by necrosis11,13 mature and cross-present antigens to autoreactive T cells. Therefore, dying cells and maturing DCs coexist, threatening tolerance toward peripheral antigens.12Autoimmunity, however, does not as a rule associate with inflammation. Factors in the milieu, possibly recruited by the inflammatory signals themselves, must prevent phagocytosis of dying cell by DCs.
Pentraxins are acute-phase proteins, usually characterized by cyclic pentameric structure, that are conserved during phylogenesis.31-33 Short pentraxins are produced in the liver in response to inflammatory mediators.34,35 They recognize several ligands, including substrates targeted during systemic autoimmunity such as chromatin or small nuclear ribonucleoproteins.36-40 The function of pentraxins includes amplification of innate resistance against microbes and regulation of the scavenging of DNA released from dying cells.32
PTX3 is the prototypic long pentraxin, structurally related to, but distinct from, C-reactive protein (CRP) and serum amyloid P component (SAP). Signals, including lipopolysaccharide (LPS), IL-1β, and TNF-α, induce the release of PTX3 by endothelial cells and mononuclear phagocytes41-45 (also in P.R. et al, unpublished data, April 1999). Signals that favor DC maturation determine the production of PTX3 in situ, before the generation of short pentraxins starts in the liver. PTX3 is therefore an intriguing candidate for interaction with cells dying at inflammatory sites.
In this study, we report that PTX3 binds to dying but not to living cells and that recognition is restricted to a phase in which nuclear antigens segregate to the cell membrane. The binding to apoptotic cells during inflammatory reactions and the regulation of clearance by DCs may contribute to limit autoimmune phenomena.
Materials and methods
Cells
Human leukemia Jurkat T cells were purchased from ATCC (Rockville, MD) and grown in RPMI (Gibco BRL, Grand Island, NY) containing 100 U/mL penicillin, 0.1 mg/mL streptomycin, 2 mmol/L L-glutamine, and 10% fetal calf serum (Hyclone, Logan, UT) (tissue culture medium [TCM]) or 1% Nutridoma-SP (Boehringer Mannheim, Germany) (TCM-nut). Human DCs were derived by monocytes cultured for 1 week in TCM supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 as described.17,18 Polymorphonuclear leukocytes were isolated and characterized as described.46
Apoptosis induction and detection
Jurkat cells were committed to apoptosis with anti-CD95 monoclonal antibody (CH-11, 100 ng/106 cells) in TCM-nut at 37°C or irradiated under a UV source for 20 seconds and incubated in TCM-nut at 37°C. Apoptosis was verified by flow cytometry and by morphological features, as described.47The exposure of phosphatidylserine (PS) was assessed by flow cytometry and confocal microscopy (see below) after staining with fluorescein isothiocyanate (FITC)–labeled annexin V (Bender MedSystems, Vienna, Austria) (0.5 μg/mL) for 10 minutes at room temperature in phosphate-buffered saline (PBS) containing 0.1 mmol/L MgCl2 and 0.1 mmol/L CaCl2 (PBS++). DNA content was detected by flow cytometry. Cells were permeabilized with saponin (500 μg/mL), committed to apoptosis by UV irradiation followed by 16 or 72 hours at 72°C, or by different pharmacological treatment (5 μmol/L 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl) ester, 10 μg/mL cycloheximide, 20 μmol/L staurosporin, 100 μmol/L and 50 μmol/L pyrrolidine dithiocarbamate, and 100 nmol/L dexamethasone). Necrosis was achieved either by heating cells (95°C for 5 minutes), freezing and thawing them or by hyperosmotic shock (10 minutes in a buffer of 150 mmol/L NaP, pH 7.5, and 1.4 M NaCl).
Proteins
Human PTX3 was purified from Chinese hamster ovary (CHO) cells stably and constitutively expressing the protein, as described.45 Purified human CRP, SAP, and bovine serum albumin (BSA) were purchased from Sigma (St Louis, MO). Biotinylation was performed as described.45 Biotinylated PTX3 was analyzed in the native state in 5% to 10% gradient polyacrylamide gel electrophoresis (PAGE) as described.45 Gels were stained with silver nitrate. Molecular weight markers for native gels (Pharmacia Biotech Europe, Brussels, Belgium) are as follows: thyroglobulin, 669 000; ferritin, 440 000; catalase, 232 000; lactate dehydrogenase, 140 000; BSA, 67 000. Human β2–glycoprotein I (β2-GPI) was purified from normal human serum by sequential perchloric acid precipitation, heparin-sepharose (HiTrap Heparin, Pharmacia) affinity chromatography and cationic exchange chromatography (Resource S, Pharmacia), as described.48
Binding of soluble factors to apoptotic cells
Cells were challenged with biotinylated PTX3 (range tested 0.1 to 500 μg/mL) for 30 minutes at room temperature. Cell-bound cofactors were revealed by flow cytometry after addition of FITC-conjugated streptavidin (Pierce, Rockford, IL). The fluorescence background was calculated with FITC-conjugated streptavidin only. The binding of purified β2-GPI (10 μg/mL) was revealed by means of rabbit polyclonal anti–β2-GPI antibodies and FITC-labeled antirabbit antibodies (Sigma).48 Propidium iodide (PI) (10 μg/mL) was added immediately before flow cytometry. When indicated, assays were performed in the absence of divalent cations. The specificity of the binding was verified before incubating apoptotic cells with PTX3, SAP, CRP, and BSA (500 μg/mL) and before addition of biotinylated PTX3 (10 μg/mL) and FITC streptavidin, and fluorescence-activated cell sorter (FACS) analysis. The inhibition percentage was calculated as follows: 100 − (mean fluorescence intensity in the presence of biotinylated PTX3 and FITC-streptavidin / mean fluorescence intensity in the presence of FITC-streptavidin alone) × 100.
Phagocytosis
Jurkat cells were committed to apoptosis by UV irradiation and labeled with the green fluorescent aliphatic dye PKH2-GL (Sigma).5,49 After washing, cells were incubated or not with PTX3 (100 μg/mL for 30 minutes at room temperature) and co-incubated with DCs for 90 minutes at 37°C or 4°C. Both immature DCs or DCs that matured after treatment with TNF-α20 were used. As a control, the internalization of FITC-labeled ovalbumin (Sigma) or of green fluorescent latex beads (2 μm diameter) (Polysciences, Warrington, PA) by immature and mature DCs was assessed in the presence or absence of PTX3. After phagocytosis, DCs were incubated in PBS containing EDTA and trypsin for 15 minutes at 37°C and analyzed by flow cytometry (FACS Scan, Becton Dickinson, San Josè, CA). When indicated, trypsin was omitted and the percentages of cells that bound or internalized apoptotic cells were compared in independent samples.
DC maturation
Jurkat cells were committed to apoptosis by UV irradiation or killed by primary necrosis (5 cycles of freezing and thawing). After washing, cells were incubated or not with PTX3, as described above, and coincubated with immature DCs for 24 hours at 37°C. DCs were also incubated in the presence of PTX3 alone. As a positive control, DCs were committed to maturation with the use of LPS (10 ng/mL). DCs were then retrieved by centrifugation over a Percol density gradient (50% to 30% interface). DCs were then analyzed by flow cytometry (FACS Scan) for MHC class II, CD40, CD80, and CD86 expression by means of appropriately diluted FITC- or phycoerythrin-labeled monoclonal antibodies (Caltag, Burlingame, CA).
Confocal microscopy
Stained cells were seeded on polylysine-coated coverslip for 20 minutes at room temperature, fixed for 15 minutes in 4% (wt/vol) paraformaldehyde, and mounted in Mowiol medium. Confocal laser scanning microscopy was performed with a Leica TCS-NT (Leica Microsystems, Heidelberg, Germany) confocal laser scanning microscope, equipped with laser Argon/Krypton, 75-mW multiline. Focal series of horizontal planes of section were simultaneously monitored for FITC and PI by means of the 488- and 568-nm laser lines, a FITC band pass 590/30 filter, and a long pass 590 filter for PI.
Results
PTX3 binds to dying cells
Jurkat cells were propagated in TCM-nut to avoid interference by uncharacterized serum cofactors and were committed to apoptosis by CD95 cross-linking or UV irradiation. After both treatments, most Jurkat T cells (more than 95%) underwent apoptosis, as verified by morphological and cytometric criteria.47 PTX3 was purified from CHO cells stably and constitutively expressing the protein and the binding to living or apoptotic cells revealed by flow cytometry. Biotinylated PTX3 did not bind to living Jurkat cells (Figure1A). In contrast, it bound efficiently after induction of apoptosis (Figure 1B; Table1). The procedure of biotinylation does not influence the structural or functional integrity of the molecule.45 Accordingly, purified biotinylated PTX3, PTX3 in the conditioned medium of transfected CHO cells, and purified PTX3 all migrated as 440-kd apparent molecular mass species, possibly corresponding to a decamer, when gel electrophoresis was performed in nondenaturing, nonreducing conditions (Figure 1C and Bottazzi et al45). Linear increase of the binding was observed when increasing concentrations of biotinylated PTX3 were used; it reached a plateau at around 100 μg/mL (Figure 1D). The preincubation of apoptotic cells with the native molecule inhibited the binding (Figure 1E), demonstrating its specificity. The preincubation with an excess of unlabeled CRP or SAP also inhibited PTX3 binding to apoptotic cells (Figure 1E); the preincubation with the unrelated plasma protein BSA did not exert any effect (Figure 1E). PTX3 does not have a specific site for Ca++ and does not bind classical Ca++-dependent ligands of pentraxins.45Accordingly, the binding of PTX3 to dying cells did not require the presence of extracellular divalent cations (Figure 1F), which were necessary for binding of the unrelated plasma factor annexin V (Figure 1F).
The long pentraxin PTX3 specifically binds to Jurkat cells after apoptosis induction.
Jurkat leukemia cells, either untreated (A) or after CD95 cross-linking (6 hours) (B), were incubated with biotinylated PTX3 (50 μg/mL). Bound PTX3 was revealed by addition of FITC-streptavidin (solid profiles). The background fluorescence in the absence of biotinylated PTX3 is also reported (light profiles). (C) Biotinylated PTX3 was separated on a 5% to 10% native PAGE and stained with silver nitrate. Lanes are as follows: (1) biotinylated PTX3, (2) conditioned medium from cells transfected with PTX3, (3) purified PTX3. Molecular weight markers are indicated on the left. (D) Jurkat cells undergoing CD95-triggered apoptosis were treated with increasing concentrations (x-axis) of biotinylated PTX3, and the extent of the binding was assessed by flow cytometry after addition of FITC-streptavidin. Results are expressed as relative fluorescence intensity (RFI) (y-axis), calculated by dividing the mean fluorescence intensity in the presence of PTX3 and FITC-streptavidin with that obtained in the presence of FITC-streptavidin alone. (E) Jurkat cells were preincubated with unlabeled PTX3, CRP, SAP, or BSA (500 μg/mL) before addition of biotinylated PTX3 and FITC-streptavidin and analysis by flow cytometry. Results are expressed as inhibition percentage, calculated as described in “Materials and methods.” (F) Binding of PTX3 (50 μg/mL) and FITC–annexin V (5 μg/mL) to apoptotic Jurkat cells was performed in the presence or absence of divalent cations. Results are expressed as mean fluorescence intensity (MFI).
The long pentraxin PTX3 specifically binds to Jurkat cells after apoptosis induction.
Jurkat leukemia cells, either untreated (A) or after CD95 cross-linking (6 hours) (B), were incubated with biotinylated PTX3 (50 μg/mL). Bound PTX3 was revealed by addition of FITC-streptavidin (solid profiles). The background fluorescence in the absence of biotinylated PTX3 is also reported (light profiles). (C) Biotinylated PTX3 was separated on a 5% to 10% native PAGE and stained with silver nitrate. Lanes are as follows: (1) biotinylated PTX3, (2) conditioned medium from cells transfected with PTX3, (3) purified PTX3. Molecular weight markers are indicated on the left. (D) Jurkat cells undergoing CD95-triggered apoptosis were treated with increasing concentrations (x-axis) of biotinylated PTX3, and the extent of the binding was assessed by flow cytometry after addition of FITC-streptavidin. Results are expressed as relative fluorescence intensity (RFI) (y-axis), calculated by dividing the mean fluorescence intensity in the presence of PTX3 and FITC-streptavidin with that obtained in the presence of FITC-streptavidin alone. (E) Jurkat cells were preincubated with unlabeled PTX3, CRP, SAP, or BSA (500 μg/mL) before addition of biotinylated PTX3 and FITC-streptavidin and analysis by flow cytometry. Results are expressed as inhibition percentage, calculated as described in “Materials and methods.” (F) Binding of PTX3 (50 μg/mL) and FITC–annexin V (5 μg/mL) to apoptotic Jurkat cells was performed in the presence or absence of divalent cations. Results are expressed as mean fluorescence intensity (MFI).
PTX3 recognition requires cell death and does not depend on the stimulus triggering apoptosis
| Cells . | Treatment . | Relative fluorescence intensity . |
|---|---|---|
| Alive | none | 1 |
| Permeabilized | saponin | 1.2 |
| Apoptotic | cycloheximide | 10 |
| staurosporin | 19.8 | |
| PDTC | 12.3 | |
| BAPTA | 11.7 | |
| dexamethasone | 17.9 | |
| UV irradiation (16 h) | 22.4 | |
| Postapoptotic | UV irradiation (48 h) | 2.8 |
| Necrotic | freezing | 7.3 |
| boiling | 2.4 | |
| hyperosmotic shock | 5.6 |
| Cells . | Treatment . | Relative fluorescence intensity . |
|---|---|---|
| Alive | none | 1 |
| Permeabilized | saponin | 1.2 |
| Apoptotic | cycloheximide | 10 |
| staurosporin | 19.8 | |
| PDTC | 12.3 | |
| BAPTA | 11.7 | |
| dexamethasone | 17.9 | |
| UV irradiation (16 h) | 22.4 | |
| Postapoptotic | UV irradiation (48 h) | 2.8 |
| Necrotic | freezing | 7.3 |
| boiling | 2.4 | |
| hyperosmotic shock | 5.6 |
Jurkat cells untreated, permeabilized, and killed by apoptosis or necrosis were challenged with biotinylated PTX3 (10 μg/mL) and fluorescein isothiocyanate (FITC)–streptavidin. The binding was then assessed by flow cytometry, as described in “Materials and methods.” Results are expressed as relative fluorescence intensity calculated by dividing the mean fluorescence intensity in the presence of PTX3 and FITC-streptavidin and in the presence of FITC-streptavidin alone. Results are representative of 4 independent experiments.
PDTC indicates pyrrolidine dithiocarbamate; BAPTA, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl) ester.
PTX3 binds to membrane domains of apoptotic cells
The binding of PTX3 to dying cells was characterized by confocal imaging. Jurkat cells undergoing CD95-triggered apoptosis were easily identified on the basis of nuclear characteristics (Figure2B,C,F, red color), such as chromatin margination, condensation, and fragmentation. Biotinylated PTX3 bound to discrete membrane domains of apoptotic cells (Figure 2D,F, green color), as revealed with FITC-labeled streptavidin. The binding of streptavidin alone to Jurkat cells was negligible (Figure 2A,C, green color). PTX3 did not bind to living cells, whose nuclei displayed normal morphology (Figure 2, upper left quadrant of each panel).
PTX3 recognizes membrane domains of cells undergoing apoptosis.
Jurkat cells, either alive (upper left quadrant of each panel) or committed to apoptosis by CD95 cross-linking (see “Materials and methods”) were fixed, permeabilized with saponin, and stained with biotinylated PTX3 (50 μg/mL) (panels D and F). Bound molecules were revealed by addition of FITC-streptavidin (panel D and green color in panel F). The fluorescence background obtained in the presence of streptavidin only is shown in panel A and as green color in panel C. Characteristics of the nuclei were revealed by addition of PI (panels B and E and red color in panels C and F).
PTX3 recognizes membrane domains of cells undergoing apoptosis.
Jurkat cells, either alive (upper left quadrant of each panel) or committed to apoptosis by CD95 cross-linking (see “Materials and methods”) were fixed, permeabilized with saponin, and stained with biotinylated PTX3 (50 μg/mL) (panels D and F). Bound molecules were revealed by addition of FITC-streptavidin (panel D and green color in panel F). The fluorescence background obtained in the presence of streptavidin only is shown in panel A and as green color in panel C. Characteristics of the nuclei were revealed by addition of PI (panels B and E and red color in panels C and F).
PTX3 recognition is restricted to a window during the apoptotic program
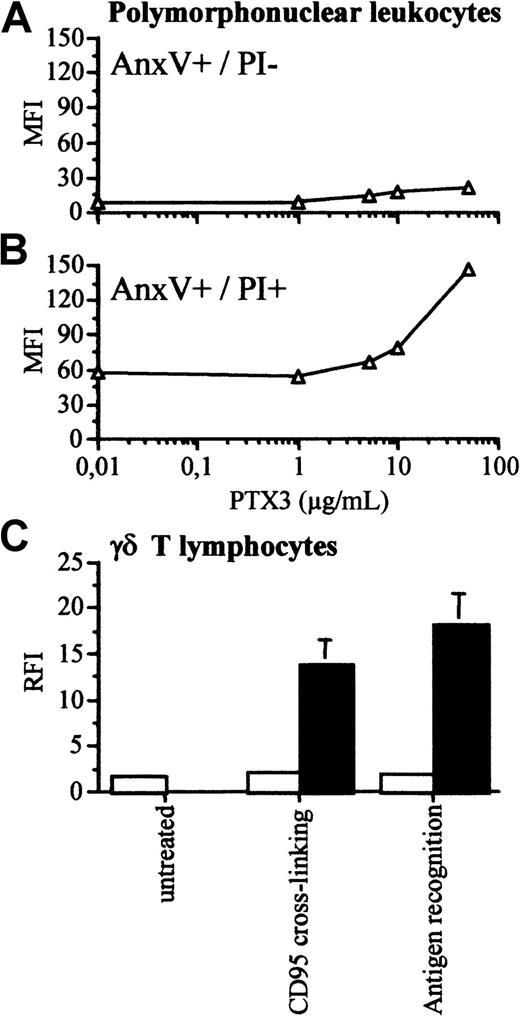
We analyzed whether the phase of apoptosis influenced PTX3 binding. Figure 3 shows that PTX3 did not bind 2 hours after the cross-linking of the CD95 receptor (panel A). At this time point, the PS-binding proteins annexin V and β2-GPI bound to most cells (Figure 3B-C). PTX3 acquired the ability to bind to cells undergoing later apoptosis: most cells that were recognized by PTX3 after 4 hours of treatment failed to exclude PI (Figure 3E). On the contrary, β2-GPI and annexin V bound to both PI+ and PI− dying cells (Figure 3F-G). PTX3 did not bind to polymorphonuclear leukocytes undergoing early phases of apoptosis (annexin V+ and PI−) (Figure4A). They acquired this ability only at later time points (72 hours) (Figure 4B). PTX3 also recognized normal γδ T cells after cross-linking of the CD95 receptor or antigen recognition by the T-cell receptor50 during established apoptosis only (Figure 4C).
Pentraxin binding depends on the phase of apoptosis.
Jurkat cells were committed to undergo apoptosis by CD95 cross-linking. The percentage of cells that bound to biotinylated PTX3 (A), to β2-GPI (B), to FITC–annexin V (C), and to PI (D) was assessed by flow cytometry (see “Materials and methods”) at different times after apoptosis induction (panels A-D, x-axis). The ability of cells that bound to PTX3 (green fluorescence, panel E, x-axis), to the PS-binding plasma cofactors β2-GPI (green fluorescence, panel F, x-axis), or to annexin V (green fluorescence, panel G, x-axis) to exclude PI (panels E-G, red fluorescence, y-axis) was assessed by double parametric flow cytometry after 5 hours of treatment with the anti-CD95 monoclonal antibody.
Pentraxin binding depends on the phase of apoptosis.
Jurkat cells were committed to undergo apoptosis by CD95 cross-linking. The percentage of cells that bound to biotinylated PTX3 (A), to β2-GPI (B), to FITC–annexin V (C), and to PI (D) was assessed by flow cytometry (see “Materials and methods”) at different times after apoptosis induction (panels A-D, x-axis). The ability of cells that bound to PTX3 (green fluorescence, panel E, x-axis), to the PS-binding plasma cofactors β2-GPI (green fluorescence, panel F, x-axis), or to annexin V (green fluorescence, panel G, x-axis) to exclude PI (panels E-G, red fluorescence, y-axis) was assessed by double parametric flow cytometry after 5 hours of treatment with the anti-CD95 monoclonal antibody.
Polymorphonuclear leukocytes and γδ T lymphocytes acquire the ability to bind to biotinylated PTX3 only when undergoing late apoptosis in vitro.
Human polymorphonuclear leukocytes were purified from peripheral blood and allowed to undergo apoptosis in vitro. (A) The early apoptotic cell population, identified after 16 hours as annexin V+ cells that excluded PI did not bind to biotinylated PTX 3 (y-axis, mean fluorescence intensity). (B) Upon prolonged in vitro culture (72 hours), polymorphonuclear leukocytes lost the ability to exclude PI and acquired the ability to bind to biotinylated PTX3. (C) The ability of nontransformed γδ T cells to bind to PTX3 was assessed by flow cytometry after apoptosis induced by CD95 cross-linking or recognition of the tubercular antigen isopentenylpyrophosphate. Results of the binding of cells undergoing early apoptosis (PI−, □) or late apoptosis (PI+, ■) are reported as RFI (y-axis), calculated by dividing the mean fluorescence intensity in the presence of biotinylated PTX3 and FITC-streptavidin with that obtained in the presence of FITC-streptavidin alone.
Polymorphonuclear leukocytes and γδ T lymphocytes acquire the ability to bind to biotinylated PTX3 only when undergoing late apoptosis in vitro.
Human polymorphonuclear leukocytes were purified from peripheral blood and allowed to undergo apoptosis in vitro. (A) The early apoptotic cell population, identified after 16 hours as annexin V+ cells that excluded PI did not bind to biotinylated PTX 3 (y-axis, mean fluorescence intensity). (B) Upon prolonged in vitro culture (72 hours), polymorphonuclear leukocytes lost the ability to exclude PI and acquired the ability to bind to biotinylated PTX3. (C) The ability of nontransformed γδ T cells to bind to PTX3 was assessed by flow cytometry after apoptosis induced by CD95 cross-linking or recognition of the tubercular antigen isopentenylpyrophosphate. Results of the binding of cells undergoing early apoptosis (PI−, □) or late apoptosis (PI+, ■) are reported as RFI (y-axis), calculated by dividing the mean fluorescence intensity in the presence of biotinylated PTX3 and FITC-streptavidin with that obtained in the presence of FITC-streptavidin alone.
PTX3 lost the ability to bind to cells evolving toward a postapoptotic phase (Table 1). Table 1 also shows that recognition by PTX3 does not depend on the initiating stimuli used to trigger apoptosis. Some binding to cells killed in vitro by necrosis was also observed. The mere entry of PTX3 into cells after permeabilization with saponin was not sufficient for binding, suggesting that cell death is necessary to make proper ligand binding sites available (Table 1).
Apoptotic cells bound by PTX3 escape internalization by DCs
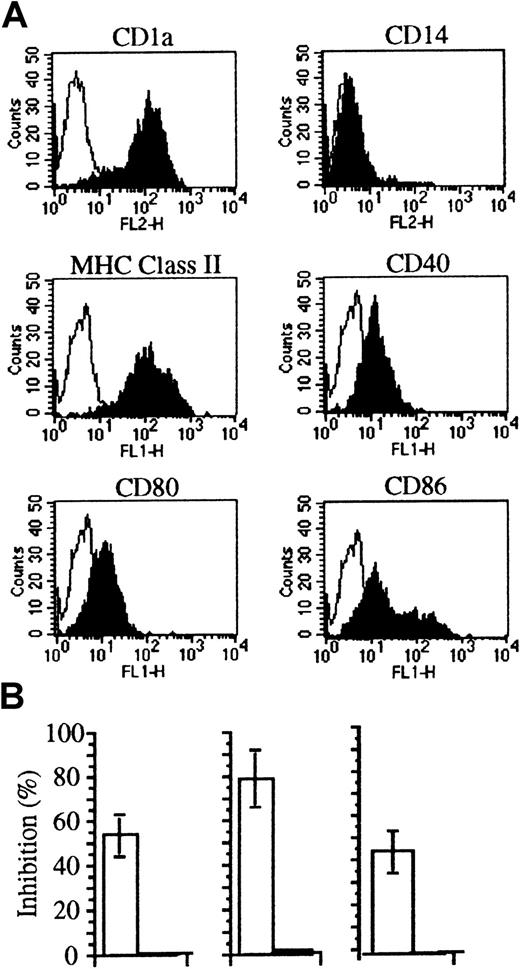
Soluble factors bound to apoptotic cells may provide new ligands or mask signals for phagocytes (discussed in Ren and Savill1). We verified the rate of internalization of PTX3-bound apoptotic cells by immature human DCs, derived from circulating monocytes cultured in the presence of recombinant GM-CSF and IL-4.17 18 This treatment allowed the generation of homogenous populations of immature DCs (Figure5A). Figure 5B shows that internalization of apoptotic cells abated as a consequence of PTX3 binding. This was not due to a toxic effect on DCs, since the molecule did not influence the uptake of fluorescent ovalbumin by macropinocytosis or of inert particulate substrates such as latex beads (Figure 5; Table2). Furthermore, PTX3 did not influence the binding of apoptotic cells to immature DCs (Table 2). As expected, the ability to bind and to internalize apoptotic cells was down-regulated during DC maturation–ensuing treatment with TNF-α (Table 2). Treatment with PTX3 did not influence the extent of the phenomenon (Table 2). Furthermore, treatment with PTX3 did not affect the maturation state of DCs challenged with either apoptotic or necrotic cells (not shown).
The binding of pentraxins to apoptotic cells inhibits their phagocytosis by immature DCs.
(A) DCs derived from monocytes by culture with recombinant cytokines expressed CD1, MHC class II molecules, as well as CD40, CD80, and CD86 molecules as assessed by flow cytometry after staining with specific monoclonal antibodies (see “Materials and methods”). (B) DCs from different donors internalize fewer apoptotic cells (□) in the presence of PTX3 (50 μg/mL). On the contrary, no inhibition was observed in their ability to take up soluble FITC-ovalbumin (■). Uptake of apoptotic cells and antigens was assessed by flow cytometry (see “Materials and methods”). Results are expressed as percentage of inhibition calculated as follows: 100 − (% DCs that internalized pentraxin bound apoptotic cells/% DCs that internalized apoptotic cells) × 100.
The binding of pentraxins to apoptotic cells inhibits their phagocytosis by immature DCs.
(A) DCs derived from monocytes by culture with recombinant cytokines expressed CD1, MHC class II molecules, as well as CD40, CD80, and CD86 molecules as assessed by flow cytometry after staining with specific monoclonal antibodies (see “Materials and methods”). (B) DCs from different donors internalize fewer apoptotic cells (□) in the presence of PTX3 (50 μg/mL). On the contrary, no inhibition was observed in their ability to take up soluble FITC-ovalbumin (■). Uptake of apoptotic cells and antigens was assessed by flow cytometry (see “Materials and methods”). Results are expressed as percentage of inhibition calculated as follows: 100 − (% DCs that internalized pentraxin bound apoptotic cells/% DCs that internalized apoptotic cells) × 100.
PTX3 does not influence the phagocytosis of inert substrates by immature dendritic cells
| Substrate . | Immature DCs . | TNF-α treated DCs . | ||
|---|---|---|---|---|
| − . | +PTX3 . | − . | +PTX3 . | |
| Apoptotic cells | 56 ± 8 | 21 ± 6 | 6 ± 5 | 8 ± 6 |
| Latex beads | 30 ± 9 | 28 ± 12 | 11 ± 5 | 11 ± 2 |
| Substrate . | Immature DCs . | TNF-α treated DCs . | ||
|---|---|---|---|---|
| − . | +PTX3 . | − . | +PTX3 . | |
| Apoptotic cells | 56 ± 8 | 21 ± 6 | 6 ± 5 | 8 ± 6 |
| Latex beads | 30 ± 9 | 28 ± 12 | 11 ± 5 | 11 ± 2 |
DC indicates dendritic cell; TNF-α, tumor necrosis factor-α.
The percentage of DCs, either immature or treated with TNF-α for 24 hours, that internalized particulate substrates (green fluorescent apoptotic cells or latex beads) in the absence or in the presence of PTX3 (50 μg/mL) was assessed by flow cytometry, as described in “Materials and methods.” Results represent the mean ± SEM of 3 separate experiments.
Discussion
The general objective of this study was to assess whether the long pentraxin PTX3 binds to dying cells and whether it regulates the clearance by professional APCs. We found that spontaneous (polymorphonuclear neutrophils) or induced (normal and transformed T lymphocytes) apoptosis is associated with recognition by PTX3. Expression of PTX3 binding sites occurs late in the apoptotic process, subsequent to exposure of PS recognized by annexin V and β2-GPI. PTX3 binding inhibits recognition of apoptotic cells by DCs.
PTX3 binds also to necrotic cells, although less efficiently. This suggests that caspase activation is dispensable. Accordingly, interference with enzyme activity by means of diffusible peptide caspase inhibitors does not prevent PTX3 binding to dying cells (P.R. et al, unpublished data, March 1999). Suitable candidates may become available for pentraxin recognition–ensuing plasma membrane disruption. Besides classical ligands, such as phosphoethanolamine and phosphocoline, CRP binds to small nuclear ribonucleoproteins and SAP to chromatin/nucleolar components.36,38,51 These intracellular moieties abandon their original location and redistribute to the plasma membrane during late apoptosis.3,4 They are therefore present in the districts and during the time frame associated with PTX3 recognition. This preferential redistribution could also explain why PTX3 recognizes apoptotic cells with higher efficiency than necrotic cells. Alternatively, alteration in the glycosylation pathways, which occurs during cell death, may contribute to their recognition by pentraxins.40 Further studies are under way to define a possible role for these molecules as binding sites for PTX3 on the membrane of dying cells.
The DC system of APCs is the initiator and modulator of normal immune responses.6,7 Immature DCs internalize dying cells, process them, and present antigens to T cells.7 This apoptosis-dependent antigen presentation pathway contributes to immune tolerance: DCs from noninflamed tissues phagocytose cells dying by apoptosis during normal tissue turnover, move to the T-cell areas of lymph nodes, and, in the absence of interaction with T cells, die. Resident DCs in the lymph node phagocytose the dying migratory DCs and induce tolerance in, or regulate, autoreactive T cells.10 30
During inflammation, the balance is skewed toward the initiation of immune responses. DCs mature, express costimulatory signals, and, after arrival at the lymph node, stimulate T cells that in turn maintain their viability.10 This event is crucial for cross-priming of T cells specific for pathogens.10 14
Efficient censorship mechanisms must prevent DC activation of autoreactive T cells after internalization of dying cells during inflammation. This is particularly so since DCs do not mature when challenged with low numbers of apoptotic cells,9representative of apoptotic cells they meet in vivo during normal tissue turnover or development. In contrast, in the presence of an excess of dying cells that evolve toward postapoptotic necrosis, or of cells killed by primary necrosis, DCs mature, up-regulate costimulatory signal expression, and enhance their ability to cross-present antigens and initiate immune responses. Inflamed tissues—where dying cells, antigen-capturing immature DCs, and maturative stimuli for DCs coexist—would be at risk for generation of autoimmunity.
The results described here suggest that the first cloned long pentraxin PTX3 controls the interaction between maturing DCs and dying cells. PTX3 is expressed by diverse cell types, most prominently mononuclear phagocytes and endothelial cells, in response to primary proinflammatory signals such as LPS, IL-1β, or TNF-α.41-45 Accordingly, elevation of PTX3 blood levels precedes that of CRP in sepsis and myocardial infarction (G.P. et al, unpublished data). Rapid induction of PTX341-44 by primary proinflammatory signals in tissues would function as a negative feedback mechanism, censoring apoptotic cell internalization by maturing DCs and the subsequent induction of autoimmunity.
Classical short pentraxins, produced in the liver under the control of IL-6, may serve the same function systemically. SAP, the major acute-phase reactant in the mouse, binds to keratin bodies.52 After immunization with native chromatin, mice with targeted deletion of the SAP gene develop a syndrome that resembles SLE, a systemic inflammatory autoimmune disease characterized by the generation of high-affinity T-cell–dependent autoantibodies.53 Regulation of antigen degradation by SAP may be involved in these findings.54 The results presented here suggest the alternative complementary explanation that pentraxins represent a safeguard mechanism against autoimmunity by preventing apoptotic cell internalization by APCs. Development of PTX3gene–targeted mice, currently under way, will be useful to dissect the relative role of different pentraxins and to put the “PTX3 censorship” model to a test.
Acknowledgments
G. Balestrieri and A. Tincani for the gift of β2–glycoprotein I, and M. Ferrarini for enjoyable discussions and for providing human γδ T cells.
Supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC); the MURST “Cofinanziamento 1998” and “Cofinanziamento 1999”; and the Ministero della Sanità (Progetto Finalizzato Biotecnologie and Ricerca Finalizzata 1999).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Angelo Manfredi, H S Raffaele, via Olgettina 60, 20132 Milano, Italy; e-mail: a.manfredi@hsr.it.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal