Abstract
Mice lacking both the gene encoding the shared receptor for granulocyte macrophage–colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), and IL-5 common β-chain (Bc) and the gene for the IL-3 specific receptor (BIL3) were generated. This was achieved by targeting the Bclocus in embryonic stem cells that were heterozygous for a null mutation of BIL3. Cells from mice generated with the doubly targeted embryonic stem cells were unresponsive to all 3 cytokines. Considerable previous data suggested a role for common beta-chain (βc) in modulating signaling of cytokines including erythropoietin (EPO), G-CSF, and stem cell factor (SCF). However, bone marrow cells from mice lacking βc and βIL3 showed normal responsiveness to these cytokines. Thus, there was no evidence for a biologically significant interaction between signaling via βc or βIL3 and signaling by EPO, G-CSF, or SCF. Previously documented biochemical phenomena, including receptor transmodulation, receptor transphosphorylation, and even direct physical interaction, involving the βc/βIL-3 receptor systems do not reflect genuine interactions of physiological significance in primary hematopoietic cells. This study provided results that challenge conclusions previously established using a variety of biochemical assays.
Introduction
Interleukin (IL)-3 has numerous effects on hematopoietic cells including actions on precursors and mature cells.1-4 The receptor for IL-3 consists of a unique specific α-chain, IL-3Rα, which binds IL-3 with low affinity,5,6 and the common β-chain (βc) which is also used by granulocyte macrophage–colony-stimulating factor (GM-CSF) and IL-5. Following the binding of IL-3 to IL-3Rα, βc converts the interaction to one of high affinity.7 In mouse cells, but not human cells, an additional IL-3–specific β-chain (βIL3) is used in preference to βc for signaling by IL-38 and, unlike βc, uses low affinity to directly bind IL-3.
Mice lacking βc (βcnull mice), have an eosinopenia and, like mice deficient in GM-CSF, develop lung disease reminiscent of human pulmonary alveolar proteinosis.9-12 Cells from these mice lack high-affinity binding for GM-CSF and IL-5. Mice that lack βIL-3(βIL-3 null mice)8,10 show decreased biological responsiveness of cells to IL-3 (via intact βcsignaling).8 Ablation of βIL-3 explained the conflicting results observed for hierarchical receptor interactions in mouse cells versus human cells. Although GM-CSF was not able to “transmodulate” IL-3 receptors and alter IL-3 binding in wild-type murine cells (because of the availability to IL-3 of βIL-3), it was able to trans-down-modulate IL-3 binding in βIL-3 null cells, as a result of competition between GM-CSF and IL-3 for binding to βc chains.8
Accumulated evidence suggests a role for βc in modulating signaling of other hematopoietic cytokines including G-CSF, erythropoietin (EPO), and stem cell factor (SCF). Both GM-CSF and IL-3 transmodulated binding of G-CSF and M-CSF in normal cells.13 IL-3 also showed this effect in both βc null and in βIL3null cells.8 However, transmodulation by GM-CSF required a functional βc receptor. Based on these results we proposed that βc and βIL-3 interacted with the G-CSF receptor (G-CSFR) and M-CSFR and/or that the GM-CSF or IL-3 activation of cellular signaling pathways modified G-CSFR and M-CSFR, perhaps resulting in their internalization. However, this phenomenon remains unexplained.8
Interaction between βc and the EPO receptor (EPOR) has been demonstrated. EPO stimulated tyrosine phosphorylation of βc in the UT-7 erythroleukemia cell line,14 although neither GM-CSF14 nor IL-315 stimulated tyrosine phosphorylation of EPOR. It was therefore suggested that EPO might activate the GM-CSF signaling pathway by phosphorylating βc.14 Functional and physical interactions between βc and EPOR were demonstrated using the murine IL-3–dependent cell line (Ba/F3), which expresses IL-3Rα, βc, and βIL-3. The Ba/F3 cells transfected with murine EPOR acquired responsiveness to EPO, and increased expression of murine βc resulting in heightened responsiveness to EPO. Conversely, inhibition of murine βc function in Ba/F3/EPOR cells inhibited both IL-3–dependent and EPO-dependent cell growth. Moreover, an EPO-independent physical interaction between βc and EPOR was demonstrated by coimmunoprecipitation.16 We sought to address interactions involving the βc/βIL-3R system using cells from βc/βIL-3null mice.
Study design
Generation of Bc/BIL3 null mice
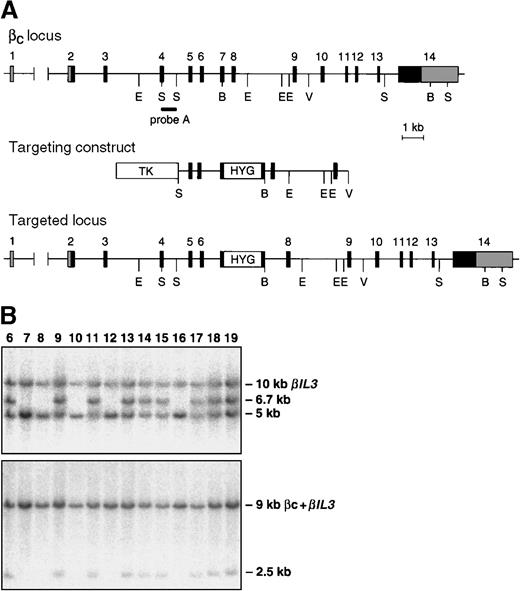
The Bc and βIL-3loci are closely linked on mouse chromosome 15.17 To generate mice with a mutation in both loci, the embryonic stem (ES) cell line 3.15, which contains a targeted mutation of one allele of theBIL3 locus, was electroporated with a linearized targeting construct for the Bc locus. This construct was as previously described,9 except that a cassette containing the hygromycin-resistance gene18 was inserted in exon 7, and a thymidine kinase cassette19 was ligated to the 5′-end of the construct. Selection and screening of hygromycin and FIAU-resistant ES cell clones were performed as previously described. To detect homologous recombinants, BamHI-digested DNA was hybridized with probe A (Figure1) and a 3′-probe. Correctly targeted clones were further analyzed by Southern blot analysis for the presence of the BIL3 mutation.8 We used 3 ES cell lines to create chimeric mice, which were screened for cosegregation of theBIL3 and Bc mutations. Control mice were C57BL/6 or 129/Sv, and the experiments were conducted on mice 6 to 14 weeks of age.
Targeting the Bc and βIL-3 loci.
(A) Partial map of the Bc locus, targeting construct, and predicted alteration of the Bclocus after homologous recombination. Coding exons are numbered and shown as black boxes. Noncoding exons are shaded gray. The position of probe A is indicated. This probe was used to identify homologous recombinants and to genotype mice by Southern blotting. Restriction enzyme sites are shown, where B indicates BamHI; E,EcoRI; S, SacI; V,EcoRV. (B) Southern blot analysis of tail DNA from offspring of a chimera generated by injection of ES cells containing targeted mutations of the Bc and BIL3 loci. DNA was digested with BamHI. Blots were probed with probe A (top panel) or a probe that detects the targeted mutation of theBIL3 locus (bottom panel).8 In the top panel, the targeted Bc allele is a 6.7-kb (kilobase) band, and the wild type allele is a 5-kb band. The probe cross-hybridizes with the BIL3 locus, which is seen as a 10-kb band. In the bottom panel, the 9-kb band represents hybridization of the probe to the Bcand BIL3 wild type alleles. The targeted BIL3 allele is seen as a 2.5-kb band. In the ES cell line used to generate these mice, the targeted mutations in the Bc and BIL3 loci are always observed in the same offspring, indicating that the homologous recombination events in the 2 loci lie on the same chromosome.
Targeting the Bc and βIL-3 loci.
(A) Partial map of the Bc locus, targeting construct, and predicted alteration of the Bclocus after homologous recombination. Coding exons are numbered and shown as black boxes. Noncoding exons are shaded gray. The position of probe A is indicated. This probe was used to identify homologous recombinants and to genotype mice by Southern blotting. Restriction enzyme sites are shown, where B indicates BamHI; E,EcoRI; S, SacI; V,EcoRV. (B) Southern blot analysis of tail DNA from offspring of a chimera generated by injection of ES cells containing targeted mutations of the Bc and BIL3 loci. DNA was digested with BamHI. Blots were probed with probe A (top panel) or a probe that detects the targeted mutation of theBIL3 locus (bottom panel).8 In the top panel, the targeted Bc allele is a 6.7-kb (kilobase) band, and the wild type allele is a 5-kb band. The probe cross-hybridizes with the BIL3 locus, which is seen as a 10-kb band. In the bottom panel, the 9-kb band represents hybridization of the probe to the Bcand BIL3 wild type alleles. The targeted BIL3 allele is seen as a 2.5-kb band. In the ES cell line used to generate these mice, the targeted mutations in the Bc and BIL3 loci are always observed in the same offspring, indicating that the homologous recombination events in the 2 loci lie on the same chromosome.
Progenitor cell assays
Bone marrow (BM) progenitor cells were assayed in clonal culture as previously described.9 Semisolid 1-mL agar cultures containing 5 × 104 BM cells or 105 spleen cells in 0.3% agar in Dulbecco's modified Eagle's medium (DMEM) with 20% newborn calf serum were plated in triplicate and stimulated by multiple combinations of purified recombinant growth factors. To determine cytokine responsiveness, BM cells fromβc/βIL3 null mice and control mice were cultured in agar using serial dilutions of G-CSF (initial G-CSF concentration, 500 U/mL) or SCF (initial SCF concentration, 100 ng/mL) for 7 days of incubation at 37°C in a fully humidified atmosphere of 10% carbon dioxide (CO2) in air. The colonies were enumerated using a dissection microscope. For colony-forming unit–E (CFU-E) assays, bone marrow cells were cultured using serial dilutions of EPO (initial EPO concentration, 4 U/mL) in methylcellulose cultures incubated for 2 days in 5% CO2 in air. The colonies were enumerated using an inverted microscope. Cultures were scored by an investigator blinded to the genotype of the cells.
Results and discussion
Baseline hematopoiesis in βc/βIL-3 null mice was no different than that seen in βc null mice (C.L.S., L.R., and C. G. B., unpublished observations). This was in keeping with previous reports of mice lacking eitherβc,9,10βIL-3,8,10 or the combination of βc and βIL-3.20 21Thus, in mouse cells and in spite of the presence of an additional βc specific for IL-3, which might imply an important function, IL-3 did not have an essential role in steady-state hematopoiesis.
Hematopoietic progenitor cells fromβc/βIL-3 null mice were examined. The lack of responsiveness to IL-3 and GM-CSF was confirmed. There was no proliferation when BM cells fromβc/βIL3 null mice were stimulated by either cytokine. In contrast, colony formation in response to stimulation with other hematopoietic cytokines, including SCF and the combination of SCF, G-CSF, and IL-6, was normal. Analysis of erythroid colonies in methylcellulose cultures revealed that the number of erythroid progenitor cells (both BFU-E and CFU-E) was also normal (C.L.S., L.R., and C.G.B., unpublished observations).
We have previously demonstrated that IL-3 was able to transmodulate both G-CSFR and M-CSFR in the absence of eitherβc null or βIL3 null cells.13 However, transmodulation of G-CSFR and M-CSFR by GM-CSF required the presence of βc.8 These results predict that a biochemical analysis of transmodulation of G-CSFR and M-CSFR by IL-3 in the absence of both β chains would show a lack of transmodulation capacity by IL-3. Indeed, in keeping with the lack of high-affinity IL-3 binding that results from generating βc/βIL-3 null cells, we did not see transmodulation of G-CSF receptors by IL-3 on BM cells from βc/βIL-3 null mice, even at doses of 100-nmol/L IL-3. This was in contrast to the transmodulation of G-CSF receptors by IL-3 that was observed on normal BM cells in the same experiments (N.A.N., unpublished observations).
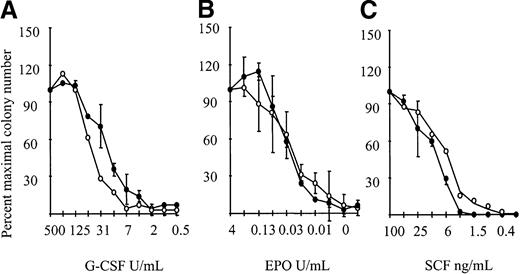
The corollary of the G-CSFR transmodulation results predicted that the response to G-CSF may, in part, be mediated by βc or βIL-3, although neither receptor was directly engaged by G-CSF. This issue was addressed usingβc/βIL3 null cells. We would have predicted that the absence of Bcrendered cells less sensitive to stimulation with G-CSF. However, as shown in Figure 2,βc/βIL3 null cells showed normal responsiveness to G-CSF. This indicated that biological responsiveness to G-CSF was not dependent on βcor βIL3, implying that the biochemical phenomenon of transmodulation was of no genuine biological significance.
Cytokine responsiveness of theβc null andβc/βIL3 null mice.
Responsiveness to (A) 500 U/mL G-CSF (initial concentration), (B) 4 U/mL EPO, and (C) 100 ng/mL SCF with serial 2-fold dilutions. Results are the colony number (the mean plus or minus SD) at each cytokine dilution expressed as a percentage of maximal colony number, using 1-2 mice per genotype. Similar results were seen in 3 independent experiments. A minimum of 3-4 mice were examined per genotype. Wild type is indicated by open circles and βc/βil-3 null by closed circles.
Cytokine responsiveness of theβc null andβc/βIL3 null mice.
Responsiveness to (A) 500 U/mL G-CSF (initial concentration), (B) 4 U/mL EPO, and (C) 100 ng/mL SCF with serial 2-fold dilutions. Results are the colony number (the mean plus or minus SD) at each cytokine dilution expressed as a percentage of maximal colony number, using 1-2 mice per genotype. Similar results were seen in 3 independent experiments. A minimum of 3-4 mice were examined per genotype. Wild type is indicated by open circles and βc/βil-3 null by closed circles.
Several different observations have suggested a role for βc in signaling by EPO. In addition to the phosphorylation and physical interaction data described above, IL-3 and GM-CSF are known to cooperate with EPO in erythropoiesis in vitro,22,23 and common signal transduction pathways involving STAT5 are used by their receptors.24 In addition, βc has been implicated in signaling by SCF via its receptor, c-kit, and SCF is able to induce serine/threonine phosphorylation of βc.25
To determine the physiological significance of these observations, responsiveness of βc/βIL-3 null BM cells to EPO and SCF was examined. However, there was no observed difference in responsiveness to EPO (Figure 2). Nor was there a difference in responsiveness to SCF for BM cells cultured from βc/βIL-3 null mice compared to wild-type mice (Figure 2).
We therefore conclude that previously documented biochemical phenomena, including receptor transmodulation and receptor transphosphorylation, are not physiologically relevant in the context of hematopoietic cell growth responses to individual cytokines. Moreover, even the demonstration of direct physical interaction involving the βc/βIL-3 receptor systems in cell lines did not extrapolate to an interaction of physiological significance in primary hematopoietic cells. This result is important for interpreting the significance of biochemical interactions between receptor molecules, particularly in studies in which cell lines are employed.
Acknowledgment
The authors thank Louise Barnett for her work in generating the ES cell line.
Supported in part by the Anti-Cancer Council of Victoria and the Cooperative Research Centre for Cellular Growth Factors, Victoria, Australia; the Bone Marrow Donor Institute; the Sylvia and Charles Viertel Charitable Foundation; grant HL62275 from the National Institutes of Health, Bethesda, MD; and the National Health and Medical Research Council of Australia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
C. L. Scott, The Walter and Eliza Hall Institute of Medical Research, PO Royal Melbourne Hospital, Victoria 3050, Australia; email:scottc@wehi.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal