Abstract
Platelets from Gαq knockout mice are unable to aggregate in response to physiological agonists like adenosine 5′-diphosphate (ADP), thromboxane A2, thrombin, or collagen, although shape change still occurs in response to all of these agonists except ADP. ADP-induced platelet aggregation results from simultaneous activation of the purinergic P2Y1receptor coupled to calcium mobilization and shape change and of a distinct P2 receptor, P2cyc, coupled through Gi to adenylyl cyclase inhibition, which is responsible for completion and amplification of the response. P2cyc could be the molecular target of the antithrombotic drug clopidogrel and the adenosine triphosphate (ATP) analogs AR-C69931MX, AR-C67085, and AR-C66096. The aim of the present study was to determine whether externally added ADP could still act through the Gi pathway in Gαq-deficient mouse platelets and thereby amplify the residual responses to agonists such as thrombin or collagen. It was found that (1) ADP and adrenaline still inhibited cyclic AMP accumulation in Gαq-deficient platelets; (2) both agonists restored collagen- but not thrombin-induced aggregation in these platelets; (3) the effects of ADP were selectively inhibited in vitro by the ATP analog AR-C69931MX and ex vivo by clopidogrel and hence were apparently mediated by the P2cyc receptor; and (4) high concentrations of ADP (100 μmol/L) induced aggregation without shape change in Gαq-deficient platelets through activation of P2cyc. Since adrenaline was not able to induce platelet aggregation even at high concentrations, we conclude that the effects of ADP mediated by P2cyc are not restricted to the inhibition of adenylyl cyclase through Gi2.
Introduction
Platelet aggregation can be induced through G-protein–coupled receptors responsive to agonists such as adenosine 5′-diphosphate (ADP), thrombin, adrenaline, platelet activating factor (PAF) or thromboxane A2 (TXA2). These receptors are coupled to different heterotrimeric G-proteins, such as Gq, Gi, G12, and G13.1,2 The crucial role of the Gαq/PLCβ pathway has been highlighted recently by the finding that platelets from Gαq-deficient mice are unable to fully aggregate or secrete the contents of storage granules in response to any physiological agent, including the strong agonists thrombin and collagen.3 These platelets are nevertheless still able to undergo shape change when exposed to TXA2, thrombin, or collagen, and this has been shown to be due to G12/13activation and to involve the Rho/Rho-kinase pathway leading to phosphorylation of the myosin light chain.4 Platelet shape change is regulated by a dichotomous pathway involving independently Ca++-calmodulin and Rho-kinase.4-6 The ADP-induced shape change of human platelets is insensitive to the Rho-kinase inhibitor Y-27632, suggesting that ADP promotes platelet shape change through the Ca++-dependent pathway alone.5 In fact, it has been reported that ADP does not induce shape change in Gαq-deficient mouse platelets.3
ADP plays a key role in hemostasis as it stimulates platelet aggregation and, when secreted from platelet-dense granules, potentiates the aggregation response induced by other agents.7,8 ADP-induced platelet activation involves at least 2 receptors.9-13 The purinergic P2Y1receptor, which is coupled to Gαq and intracellular calcium mobilization, mediates platelet shape change and initiates aggregation, while another, as yet unidentified, P2 receptor, presumably coupled to Gi2 and adenylyl cyclase inhibition,14 is responsible for completion and amplification of the response. This latter receptor, termed P2cyc, P2TAC or P2YADP,15 could be the molecular target of the antithrombotic drug clopidogrel16,17 and of adenosine triphosphate (ATP) analogs of the AR-C series.13,18,19 It might also be defective in patients with a selective congenital impairment of ADP-induced platelet aggregation.13,20 21 Thus, the P2cyc-mediated activation of Gi by released ADP would appear to be of major importance in platelet aggregation.
The aim of the present study was to assess the consequences of Gαq deficiency for ADP-induced platelet activation. In Gαq-deficient mouse platelets, the Gi signaling pathway remained intact. ADP added at intermediate concentrations (1 to 10 μmol/L) did not induce aggregation by itself but was able to restore full aggregation in response to collagen, but not thrombin, through activation of the P2cyc receptor. Moreover, in contrast to adrenaline, which also activates Gi, high concentrations of ADP (100 μmol/L) promoted integrin αIIbβ3–dependent platelet aggregation, without shape change, an effect that was inhibited by the selective antagonist AR-C69931MX and by clopidogrel.
Materials and methods
Materials
The ATP analog AR-C69931MX was a generous gift from Astra Charnwood (Loughborough, Leicestershire, England) while clopidogrel was kindly provided by Sanofi Recherche (Toulouse, France). ADP, ATP, thrombin, epinephrine, prostaglandin E1 (PGE1), bovine collagen type I, and essentially-fatty-acid–free human serum albumin were from Sigma (Saint Quentin-Fallavier, France). Human fibrinogen was from Kabi (Stockholm, Sweden), and fura-2/acetoxymethyl ester (fura-2/am) from Calbiochem (Meudon, France). The cyclic adenosine 3′-5′-monophosphate (cAMP) assay kit was purchased from Amersham (Les Ulis, France). Apyrase was purified from potatoes as previously described22 while the monoclonal rat antimouse integrin αIIbβ3 antibody (RAM.2)23 24 was a gift from F. Lanza. Spleen cells from rats immunized against whole-platelet proteins were fused with P3.X63 mouse myeloma cells, and positive clones were selected by fluorescence-activated cell sorter analysis of mouse platelets and further characterized by Western blotting, immunoprecipitation, and functional studies.
Animals
Mutant mice deficient in Gαq were produced as described,3 and both wild-type (WT) and mutant mice were of 129/Sv × C57BL/6 genetic background.
Platelet aggregation and secretion
Washed mouse platelets were prepared from blood (9 vol) drawn from the abdominal aorta of anesthetized mice into a plastic syringe containing acid citrate dextrose (1 vol) and centrifuged at 175g for 15 minutes at 37°C. Platelet-rich plasma was removed and centrifuged at 1570g for 15 minutes at 37°C. The platelet pellet was washed twice in Tyrode's buffer (137 mmol/L NaCl, 2 mmol/L KCl, 12 mmol/L NaHCO3, 0.3 mmol/L NaH2PO4, 2 mmol/L CaCl2, 1 mmol/L MgCl2, 5.5 mmol/L glucose, 5 mmol/L Hepes, pH 7.3) containing 0.35% human serum albumin and finally resuspended at a density of 2 × 105 platelets per microliter in the same buffer in the presence of 0.02 U/mL of the ADP scavenger apyrase (adenosine 5′-triphosphate diphosphohydrolase, EC 3.6.1.5), a concentration sufficient to prevent desensitization of platelet ADP receptors during storage. Platelets were kept at 37°C throughout all experiments.
Aggregation was measured at 37°C by a turbidimetric method in a dual-channel Payton aggregometer (Payton Associates, Scarborough, Ontario, Canada). A 450-μL aliquot of platelet suspension was stirred at 1100 rpm and activated by addition of different agonists, with or without antagonists, in the presence of human fibrinogen (0.07 mg/mL), in a final volume of 500 μL. The extent of aggregation was estimated quantitatively by measuring the maximum curve height above baseline level. Secretion was determined as previously described25after loading the platelets with [3H]5HT.
[Ca++]i measurements
After centrifugation of platelet-rich plasma at 1570gfor 15 minutes at 37°C, the platelet pellet was resuspended in Tyrode's buffer containing no calcium, at a density of 7.5 × 105 platelets per microliter, in the presence of 0.02 U/mL apyrase. Platelets were loaded with 15 μmol/L fura-2/am for 45 minutes at 37°C in the dark, washed in Tyrode's buffer containing 0.35% human serum albumin, and finally resuspended at 20°C, at a density of 105 platelets per microliter, in Tyrode's buffer containing apyrase and 0.1% essentially-fatty-acid–free human serum albumin. Aliquots of fura-2/am–loaded platelets were transferred to a 10 × 10 mm quartz cuvette and prewarmed to 37°C for 2 minutes, and fluorescence measurements were performed under continuous stirring, with the use of a PTI Deltascan spectrofluorimeter (Photon Technology International Inc, South Brunswick, NJ). The excitation wavelength was alternately fixed at 340 and 380 nm, and fluorescence emission was determined at 510 nm.
Measurement of cAMP in intact platelets
A 450-μL aliquot of washed platelets was stirred at 1100 rpm in an aggregometer cuvette. and reagents were added at 30-second intervals. At 1 minute after addition of ADP, the reaction was stopped by the addition of 50 μL of ice-cold 6.6 N perchloric acid. Cyclic AMP was isolated from the supernatants26 with the use of a mixture of trioctylamine and freon (28/22, vol/vol). The upper aqueous phase was lyophilized and the dry residue dissolved in the buffer provided with the commercial cAMP radioimmunoassay kit.
Electron microscopy
A 450-μL aliquot of platelet suspension was fixed in the aggregometer cuvette by addition of an equal volume of fixative solution (2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer containing 2% sucrose, 305 mOsm/L, pH 7.3) previously warmed to 37°C. After 5 minutes at 37°C, the platelets were centrifuged at 700g for 20 seconds, and the pellet was resuspended in 0.1 mol/L sodium cacodylate buffer. Samples were prepared for scanning electron microscopy (SEM) by allowing the fixed platelets to adhere for 45 minutes to coverslips preincubated with 10 μg/mL poly-L-lysine. The coverslips were then washed 3 times with 0.9% NaCl, and the platelets dehydrated in graded ethanol solutions. After replacement of ethanol by hexadimethyldisilazane, the samples were air-dried, sputtered with gold, and examined under a Hitachi (Tokyo, Japan) scanning electron microscope (5 kV). Platelets were prepared for transmission electron microscopy (TEM) by further fixation for 45 minutes with 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer. The cells were then rinsed, postfixed for 1 hour at 4 °C with 1% osmium tetroxide in cacodylate buffer, washed in the same buffer, dehydrated in graded ethanol solutions, and embedded in epon. The resin was allowed to polymerize at 50°C for 2 days. Ultrathin sections (100 nm) were stained with lead citrate and uranyl acetate and examined under a Philips CM 120 BioTwin (Eindhoven, The Netherlands) transmission electron microscope (120 kV).
Results
ADP restores full aggregation in response to collagen but not thrombin in Gαq-deficient mouse platelets
In different experiments, collagen (5 μg/mL) induced shape change only or shape change plus a weak aggregation response in Gαq-deficient platelets (Figure 1A, lower panel). Secretion in response to collagen was also less in Gαq-deficient (29%) than in WT platelets (61%). In contrast, when ADP (10 μmol/L) and collagen were added together, strong and irreversible aggregation occurred while secretion increased slightly from 29% to 40% (Figure 1B). Similar results were obtained with adrenaline (Figure 1C). In contrast, ADP did not restore aggregation in response to thrombin (data not shown).
ADP and adrenaline restored collagen-induced platelet aggregation.
(A) Responses of wild-type (WT) (upper panel) and Gαq-deficient platelets (lower panel) to 5 μg/mL collagen. Tritiated serotonin secretion is indicated in brackets. (B) ADP (10 μmol/L) restored aggregation in Gαq-deficient platelets and slightly enhanced secretion. (C) Adrenaline (10 μmol/L) similarly restored the collagen-induced aggregation of Gαq-deficient mouse platelets.
ADP and adrenaline restored collagen-induced platelet aggregation.
(A) Responses of wild-type (WT) (upper panel) and Gαq-deficient platelets (lower panel) to 5 μg/mL collagen. Tritiated serotonin secretion is indicated in brackets. (B) ADP (10 μmol/L) restored aggregation in Gαq-deficient platelets and slightly enhanced secretion. (C) Adrenaline (10 μmol/L) similarly restored the collagen-induced aggregation of Gαq-deficient mouse platelets.
ADP acts through the P2cyc receptor
As expected from previous studies,3 by itself ADP (10 μmol/L) induced platelet aggregation and a rise in intracellular calcium in WT but not in Gαq-deficient mouse platelets (data not shown). However, ADP still inhibited cAMP production to a similar extent in Gαq-deficient and WT platelets (Figure2C). This effect of ADP was inhibited in vitro by the selective antagonist AR-C69931MX and ex vivo by treatment of the mice with 100 mg/kg clopidogrel (Figure 2C, hatched and black bars). Thus, the P2cyc receptor was functional in platelets from the Gαq-deficient mice. Oral administration of 100 mg/kg clopidogrel to Gαq-deficient mice or addition of 10 μmol/L AR-C69931MX to the aggregometer cuvette resulted in strong inhibition of the potentiating effect of ADP on collagen-induced platelet aggregation (Figure 2A). Conversely, the potentiating effect of adrenaline was not affected by clopidogrel or AR-C69931MX, showing that ADP was acting through the P2cyc receptor (Figure 2B). Consistent with these observations, the effect of ADP on adenylyl cyclase was blocked by clopidogrel or AR-C69931MX (Figure 2D), while adrenaline still inhibited cAMP accumulation (Figure 2E). Collagen alone had no effect on adenylyl cyclase in Gαq-deficient mice, while in control mice, it was found to be entirely due to released ADP as could be demonstrated by the use of AR-C69931MX as well as clopidogrel, both of which blocked collagen-induced inhibition of cAMP formation (data not shown).
ADP acts through the P2cyc receptor.
(A) The selective P2cyc receptor antagonist AR-C69931MX (10 μmol/L) and clopidogrel (100 mg/kg) inhibited the potentiation by ADP of collagen-induced aggregation of Gαq-deficient mouse platelets. (B) In contrast, the effect of adrenaline was not inhibited by the P2cyc-selective compounds. (C) By itself, ADP (10 μmol/L) still inhibited cAMP production after PGE1 (10 μmol/L)–induced stimulation in Gαq-deficient mouse platelets, an effect that was blocked by AR-C69931MX (10 μmol/L) and by clopidogrel (100 mg/kg). (D) Blockade of the ADP-induced inhibition of adenylyl cyclase even in the presence of collagen by AR-C69931MX and by clopidogrel. (E) No inhibition of adrenaline-induced inhibition of adenylyl cyclase by AR-C69931MX or by clopidogrel in the presence of collagen.
ADP acts through the P2cyc receptor.
(A) The selective P2cyc receptor antagonist AR-C69931MX (10 μmol/L) and clopidogrel (100 mg/kg) inhibited the potentiation by ADP of collagen-induced aggregation of Gαq-deficient mouse platelets. (B) In contrast, the effect of adrenaline was not inhibited by the P2cyc-selective compounds. (C) By itself, ADP (10 μmol/L) still inhibited cAMP production after PGE1 (10 μmol/L)–induced stimulation in Gαq-deficient mouse platelets, an effect that was blocked by AR-C69931MX (10 μmol/L) and by clopidogrel (100 mg/kg). (D) Blockade of the ADP-induced inhibition of adenylyl cyclase even in the presence of collagen by AR-C69931MX and by clopidogrel. (E) No inhibition of adrenaline-induced inhibition of adenylyl cyclase by AR-C69931MX or by clopidogrel in the presence of collagen.
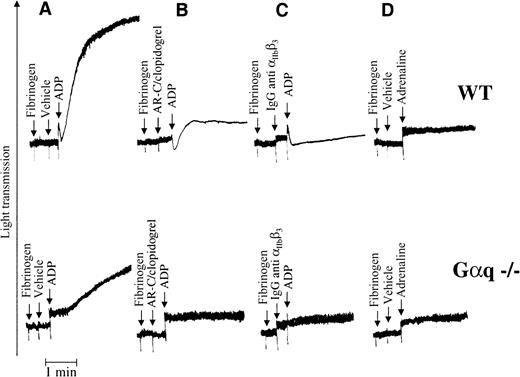
Activation of the P2cyc receptor induces platelet aggregation
In an attempt to highlight the consequences of strong activation of the Gi pathway in platelets, we added high concentrations (100 μmol/L) of ADP and adrenaline to Gαq-deficient mouse platelets. ADP induced a gradual increase in light transmission (Figure3A, lower panel) owing to the formation of small aggregates that were observed optically and further characterized by SEM and TEM (see below). This effect of ADP was inhibited by clopidogrel treatment of the mice or the addition of AR-C69931MX to the platelet suspension (Figure 3B), indicating that it resulted from activation of the P2cyc receptor. The P2cyc-mediated aggregation was also integrin dependent, since it was inhibited by a monoclonal antimouse integrin αIIbβ3antibody (Figure 3C). However, a high concentration of adrenaline had no such effect on platelets (3D), suggesting that ADP was not acting solely through the Gi pathway.
Strong activation of P2cyc results in aggregation of Gαq-deficient mouse platelets.
(A) Addition of ADP (100 μmol/L) to a stirred suspension of Gαq-deficient washed platelets resulted in a gradual increase in light transmission owing to the formation of optically visible small aggregates. (B) This effect of ADP was blocked by AR-C69931MX (10 μmol/L) or clopidogrel (100 mg/kg), suggesting that it is mediated by the P2cyc receptor. (C) The P2cyc-induced platelet aggregation was integrin dependent since it was blocked by an antimouse integrin αIIbβ3 antibody. (D) Adrenaline (100 μmol/L) was unable to induce the same effect as ADP.
Strong activation of P2cyc results in aggregation of Gαq-deficient mouse platelets.
(A) Addition of ADP (100 μmol/L) to a stirred suspension of Gαq-deficient washed platelets resulted in a gradual increase in light transmission owing to the formation of optically visible small aggregates. (B) This effect of ADP was blocked by AR-C69931MX (10 μmol/L) or clopidogrel (100 mg/kg), suggesting that it is mediated by the P2cyc receptor. (C) The P2cyc-induced platelet aggregation was integrin dependent since it was blocked by an antimouse integrin αIIbβ3 antibody. (D) Adrenaline (100 μmol/L) was unable to induce the same effect as ADP.
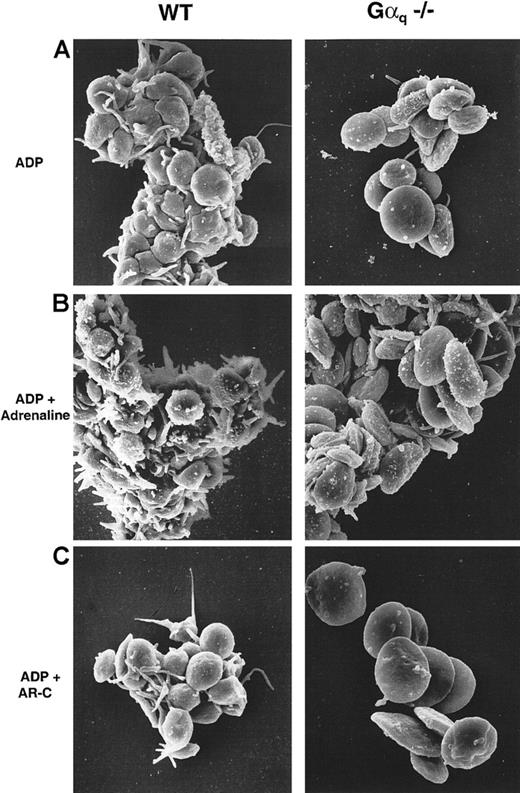
P2cyc-receptor–mediated platelet aggregation occurs without shape change or secretion
The platelet aggregates induced by ADP stimulation were examined by SEM and TEM. Gαq-deficient mouse platelets stimulated with 100 μmol/L ADP in the presence of fibrinogen formed small aggregates of 10 to 20 cells that had not changed shape as compared with WT platelets (Figure 4A). Since this response was potentiated by adrenaline in both WT and Gαq-deficient platelets, adrenaline and ADP were apparently activating separate pathways. This potentiation also occurred without modification of the discoid shape of the Gαq-deficient platelets (Figure 4B). The P2cyc antagonist AR-C69931MX completely inhibited aggregation in platelets from both WT and Gαq-deficient mice (Figure 4C) but not shape change in the WT platelets. Finally, neither WT nor Gαq-deficient platelets released their granule contents upon stimulation with 100 μmol/L ADP alone (Figure 5A) or in combination with adrenaline (Figure 5B).
P2cyc-induced aggregation occurs without shape change in Gαq-deficient platelets.
SEM of WT (left panels) and Gαq-deficient mouse platelets (right panels). (A) 100 μmol/L ADP induced shape change and aggregation in WT platelets, whereas Gαq-deficient platelets did not change shape and formed comparatively smaller aggregates. (B) Addition of adrenaline (10 μmol/L) increased the size of these aggregates without modifying the platelet shape. (C) The P2cyc antagonist AR-C69931MX (10 μmol/L) inhibited aggregation in both WT and Gαq-deficient mouse platelets although not the shape change of WT platelets.
P2cyc-induced aggregation occurs without shape change in Gαq-deficient platelets.
SEM of WT (left panels) and Gαq-deficient mouse platelets (right panels). (A) 100 μmol/L ADP induced shape change and aggregation in WT platelets, whereas Gαq-deficient platelets did not change shape and formed comparatively smaller aggregates. (B) Addition of adrenaline (10 μmol/L) increased the size of these aggregates without modifying the platelet shape. (C) The P2cyc antagonist AR-C69931MX (10 μmol/L) inhibited aggregation in both WT and Gαq-deficient mouse platelets although not the shape change of WT platelets.
P2cyc-induced aggregation occurs without degranulation in Gαq-deficient platelets.
TEM of WT (left panels) and Gαq-deficient mouse platelets (right panels) stimulated with 100 μmol/L ADP in the presence (panel B) or absence (panel A) of adrenaline (10 μmol/L). Secretion did not occur in WT or Gαq-deficient platelets, while shape change was blocked only in Gαq-deficient mouse platelets.
P2cyc-induced aggregation occurs without degranulation in Gαq-deficient platelets.
TEM of WT (left panels) and Gαq-deficient mouse platelets (right panels) stimulated with 100 μmol/L ADP in the presence (panel B) or absence (panel A) of adrenaline (10 μmol/L). Secretion did not occur in WT or Gαq-deficient platelets, while shape change was blocked only in Gαq-deficient mouse platelets.
Discussion
In Gαq-deficient mouse platelets, ADP inhibited adenylyl cyclase in the absence of turbidimetrically measurable platelet shape change or aggregation or detectable calcium mobilization. This suggests that the inhibition of adenylyl cylase induced by ADP is independent of the P2Y1 receptor–triggered Gq pathway leading to shape change and the initiation of platelet aggregation.10,27 Moreover, selective P2Y1 receptor antagonists, such as A2P5P,28 were without effect on Gαq-deficient mouse platelets (data not shown). Similar findings have been reported from studies of P2Y1 receptor knockout mice,11,12which demonstrated that platelets possess a distinct P2 receptor coupled to adenylyl cyclase inhibition and independent of the presence of P2Y1. This receptor, termed P2cyc, P2TAC, or P2YADP, is the target of the antithrombotic drug clopidogrel and of ATP analogs of the AR-C series.15 Since both clopidogrel and AR-C69931MX inhibited the effect of ADP on cAMP accumulation in Gαq-deficient platelets (Figure 2C), this effect can most probably be attributed to the P2cyc receptor.
Numerous pharmacological and genetic studies have emphasized the importance of the P2cyc receptor not only in normal hemostasis but also in thrombosis, owing to its role in amplifying platelet responses to ADP and to strong agonists like thrombin or collagen that induce the release reaction.15 Thrombin and low concentrations of collagen fail to induce aggregation or granule secretion in Gαq-deficient platelets, but are able to trigger shape change.3 It was therefore of interest to determine whether externally added ADP could modify the residual responses of Gαq-deficient mouse platelets to thrombin and collagen. ADP was found to restore full aggregation of these platelets in response to collagen but not thrombin; this is consistent with triggering of distinct signal transduction pathways by the 2 agonists. Collagen-induced platelet activation is a complex process involving binding of collagen to at least 2 membrane proteins, the integrin α2β1 and GPVI; tyrosine phosphorylation of cytosolic substrates; PLCγ2 activation; and, finally, TXA2 generation and ADP release.29,30 In contrast, thrombin-induced platelet activation does not involve PLCγ2, and there is no intracellular calcium rise in response to thrombin in Gαq-deficient platelets. Interestingly, adrenaline restored the aggregation of collagen-stimulated Gαq-deficient platelets in the same way as ADP (Figure 1C), suggesting that this potentiating effect is in fact due to Gi activation. Although Gi-induced inhibition of adenylyl cyclase is not itself sufficient to trigger platelet aggregation,25,31 it appears to be able to potentiate the weak activation linked to small increases in intracellular calcium. In the case of collagen, this weak stimulation of Gαq-deficient platelets is due to PLCγ2 activation, and hence there is cross talk between tyrosine kinase/PLCγ2 and Gi-triggered pathways. Recent studies32 33 have established the interplay between PLCγ2 activation and phosphatidylinositol (PI)–3 kinase activation and the critical role of PI 3,4,5-trisphosphate for PLCγ2 activation. Moreover, the requirement for the Gi pathway (activated by ADP or by adrenaline) for an efficient synthesis of PI 3,4,5-trisphosphate has recently been demonstrated (Gratacap et al, unpublished data; and personal communication from B. Payrastre, April 2000). All together, these results emphasize the role of the Gi pathway and of ADP as a cofactor of tyrosine kinase/PLCγ2–dependent platelet activation.
Since the potentiating effects of ADP were inhibited in vitro by the ATP analog AR-C69931MX and ex vivo by the thienopyridine compound clopidogrel, they were probably due to activation of the P2cyc receptor. Previously, we showed that high concentrations of ADP (100 μmol/L) could induce aggregation of P2Y1-deficient mouse platelets through activation of P2cyc.12 The same phenomenon was observed in the present Gαq-deficient mouse platelets (Figures 3, 4, and 5), where 100 μmol/L ADP, unlike similar concentrations of adrenaline, caused partial platelet aggregation. This P2cyc-mediated platelet aggregation occurred in the absence of shape change or granule secretion, but was integrin dependent as it was completely blocked by an antimouse integrin αIIbβ3 antibody. The biochemical mechanisms that could be involved in such a process are not yet clear. There was no change in intracellular calcium in response to 100 μmol/L ADP (data not shown), while activation of the Gi pathway alone would appear to be insufficient since adrenaline, acting on α2Aadrenergic receptors, did not promote the same aggregation, even at high concentrations (Figure 3). Although adrenaline was less potent than ADP in inhibiting the cAMP accumulation induced by PGE1 (40% vs 75% in Figure 2 and data not shown), this probably does not explain the unique ability of ADP to promote the partial aggregation of Gαq-deficient mouse platelets. Thus, direct inhibitors of adenylyl cyclase, such as SQ22536, which inhibit cAMP formation as potently as ADP, are unable to induce aggregation of these platelets or to potentiate their aggregation in response to agonists like vasopressin, arachidonate, or collagen.31 In addition, since SQ22536 does not reverse the anti-aggregatory effects of the P2cyc inhibitor clopidogrel, the properties of ADP mediated by the P2cyc receptor cannot be restricted to activation of Gi2 and inhibition of adenylyl cyclase.34 On the other hand, the fact that 10 μmol/L adrenaline could potentiate the aggregation of Gαq-deficient platelets induced by 100 μmol/L ADP favors the triggering of separate signaling pathways by these 2 agonists. Hence it would appear that ADP, among all physiological agonists, has the unique property of inducing platelet aggregation in the absence of a detectable increase in free cytosolic calcium. Recently generated P2Y1-deficient mice should help in defining the molecular and biochemical bases of this pathway.11 12 Indeed, using P2Y1-deficient mouse platelets, we could demonstrate that this effect is PI-3 kinase dependent since it was inhibited by PI-3 kinase inhibitors (unpublished observation).
Partial aggregation was observed in studies of human platelets incubated with DTT,35 and it has been shown that this results from exposure of the fibrinogen-binding sites on the integrin αIIbβ3.36 On the other hand, ADP is known to stabilize platelet aggregates37through a mechanism that must be distinct from its activation of the Gi pathway since adrenaline does not mimic ADP in this respect. Whether intracellular or extracellular signaling events are involved remains an open question. Stabilization of platelet aggregates by ADP has been found to be related to late activation of phosphoinositide 3 kinase through the P2cyc receptor.38 Several signaling events occurring in response to ADP, including Syk activation and cortactin phosphorylation, are absent in a patient with a congenital defect of ADP-induced platelet aggregation that could be due to P2cyc deficiency39 and results in unstable platelet aggregates.37 These defects might arise from impaired inside-out or outside-in mechanisms related to P2cyc. Striking are the selective blockade of ADP-induced platelet aggregation and inhibition of adenylyl cyclase by several nonpenetrating thiol reagents.40,41 Whether coincidence or further evidence, the active metabolite of clopidogrel is a thiol reagent thought to form a disulfide bridge between its reactive thiol group and that of a cysteine residue of the ADP receptor.42
In conclusion, the present work provides insight into the role of the P2cyc receptor in the unique platelet aggregatory properties of the physiological autocrine agonist ADP. Further studies in knockout animals lacking P2Y1, other platelet receptors, or transduction proteins should help in future characterization of the still elusive P2cyc receptor.
Acknowledgments
Dominique Cassel, Catherine Schwartz, Michèle Finck, and Ursula Brandt for expert technical assistance and Juliette Mulvihill for reviewing the English of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Christian Gachet, INSERM U.311, Etablissement Français du Sang-Alsace, 10, rue Spielmann, B.P. No 36, 67065 Strasbourg Cédex, France; e-mail:christian.gachet@etss.u-strasbg.fr.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal