Abstract
Relapsed B-cell lymphomas are incurable with conventional chemotherapy and radiation therapy, although a fraction of patients can be cured with high-dose chemoradiotherapy and autologous stem-cell transplantation (ASCT). We conducted a phase I/II trial to estimate the maximum tolerated dose (MTD) of iodine 131 (131I)–tositumomab (anti-CD20 antibody) that could be combined with etoposide and cyclophosphamide followed by ASCT in patients with relapsed B-cell lymphomas. Fifty-two patients received a trace-labeled infusion of 1.7 mg/kg 131I-tositumomab (185-370 MBq) followed by serial quantitative gamma-camera imaging and estimation of absorbed doses of radiation to tumor sites and normal organs. Ten days later, patients received a therapeutic infusion of 1.7 mg/kg tositumomab labeled with an amount of131I calculated to deliver the target dose of radiation (20-27 Gy) to critical normal organs (liver, kidneys, and lungs). Patients were maintained in radiation isolation until their total-body radioactivity was less than 0.07 mSv/h at 1 m. They were then given etoposide and cyclophosphamide followed by ASCT. The MTD of131I-tositumomab that could be safely combined with 60 mg/kg etoposide and 100 mg/kg cyclophosphamide delivered 25 Gy to critical normal organs. The estimated overall survival (OS) and progression-free survival (PFS) of all treated patients at 2 years was 83% and 68%, respectively. These findings compare favorably with those in a nonrandomized control group of patients who underwent transplantation, external-beam total-body irradiation, and etoposide and cyclophosphamide therapy during the same period (OS of 53% and PFS of 36% at 2 years), even after adjustment for confounding variables in a multivariable analysis.
Introduction
Conventional chemotherapy regimens cure fewer than 40% of patients with aggressive non-Hodgkin lymphomas (NHL)1,2 and fewer than 5% of patients with indolent lymphomas.3 Patients with persistent or recurrent NHL of any histologic type cannot be cured with standard therapies, although 15% to 50% of such patients can achieve long-term disease-free survival when treated with high-dose chemoradiotherapy and autologous stem cell transplantation (ASCT).4 Because lymphomas are exquisitely radiosensitive, external-beam total-body irradiation (TBI) is an effective component of many transplant conditioning regimens for patients with NHL. However, standard TBI delivers as much radiation to normal organs as it does to tumor sites, thus limiting the doses of TBI that can be administered safely to 12 to 15.75 Gy.5Several years ago, we hypothesized that targeted radiotherapy using radiolabeled monoclonal anti–B-cell antibodies would be superior to external-beam TBI, since radioimmunotherapy could deliver higher doses to the tumor and lower doses to normal organs than could TBI. We previously documented the feasibility6,7 and potential efficacy6-9 of this approach in phase I and phase II trials using iodine-131 (131I)–labeled anti–B-cell antibodies with ASCT. Although there is no consensus on the optimal radioisotope for radioimmunotherapy, we selected 131I because it is readily accessible and relatively inexpensive, it emits β particles with favorable characteristics for killing malignant cells, it is easily conjugated to antibodies by a variety of techniques, it can be used for both imaging and therapy, and it has a long, successful history in the treatment of thyroid carcinoma.
In the single-agent trials, substantial patient-to-patient variability was observed in the biodistribution, metabolism, and circulating half-life of 131I-labeled anti-CD20 antibodies (tositumomab), leading us to administer individualized amounts of radioisotopes chosen on the basis of patient-specific dosimetric findings. We estimated 27 Gy to be the maximum tolerated dose (MTD) of this low-dose-rate radioactivity that could be delivered safely to critical normal organs (eg, liver, kidneys, and lungs) when used as a single agent with stem-cell rescue. The toxic effects of this therapy were moderate compared with those of traditional transplant regimens, with reversible cardiopulmonary toxicity being dose limiting. Mucositis and alopecia occurred in ≤ 20% of patients and were mild when they did occur. Veno-occlusive disease of the liver was not observed in patients treated with single-agent 131I-tositumomab. The response rates were encouraging, especially for phase I and II trials: 86% of patients had objective remissions and 79% of these were complete responses (CRs). Many of these responses have been durable, with 11 of 29 patients (38%) currently remaining continuously disease free without further therapy for 5 to 10 years. Although these results are encouraging, more than half of the patients treated with131I-tositumomab relapsed within 5 years of treatment, indicating that there is considerable room for improvement. Because most curative therapies for disseminated neoplasms require combinations of agents, we subsequently initiated a trial examining the feasibility of treating patients with 131I-tositumomab in conjunction with high-dose etoposide and cyclophosphamide, 2 drugs commonly employed in standard transplant regimens with external-beam TBI. In this report, we describe the results of this phase I/II dose-escalation trial.
Patients, materials, and methods
Selection of patients
Adults with relapsed NHL expressing the CD20 antigen were eligible if previous chemotherapy had failed and they had evaluable disease, normal renal and hepatic function, no systemic cytotoxic therapy for 4 weeks, and less than 25% involvement of their bone marrow (BM) with lymphoma. Patients with a life expectancy of less than 2 months or a serious coexistent medical condition, acquired immunodeficiency syndrome, chronic lymphocytic leukemia, central nervous system lymphoma, or human antimouse antibodies (HAMAs) were excluded. Patients with splenomegaly or tumor burdens greater than 500 cm3 on computed tomographic (CT) volumetric assessment were eligible only if they underwent tumor cytoreduction with chemotherapy or splenectomy before transplantation. All patients had autologous hematopoietic stem cells harvested and cryopreserved before study entry. Fifteen patients entered into the study before November 1997 had autologous BM harvested and purged with a combination of anti–B-cell antibodies (eg, anti-CD9, CD10, CD19, and CD20) and complement before cryopreservation, as described previously.6 Thirty-seven patients (including all patients who entered the study after November 1997) had peripheral blood stem cells (PBSC) harvested by apheresis. Patients undergoing PBSC collection who had evidence of circulating malignant cells on flow cytometry or polymerase chain reaction (PCR), had the leukopheresis product purged by using immunoabsorption columns (Ceprate; Cellpro, Bothell, WA, or Isolex; Nexell, Irvine, CA) with CD34-positive selection and CD19-plus-CD20–negative selection. All patients gave informed written consent to participation in the protocol, which was approved by the institutional review boards and radiation safety committees of the Fred Hutchinson Cancer Research Center and the University of Washington.
Antibody biodistribution studies
The murine monoclonal anti-CD20 antibody (tositumomab, anti-B1, IgG2a; Coulter Pharmaceuticals Inc, South San Francisco, CA) was radioiodinated with sodium 131I (specific activity, 296 GBq/mg; New England Nuclear, Boston, MA) by the chloramine T method, purified, and tested as described previously.6-10Patients initially received an infusion of 1.7 mg/kg anti-CD20 antibody trace labeled with 131I (185-370 MBq) to assess antibody biodistribution and perform dosimetry estimates for treatment planning. Saturated potassium iodide was administered orally (5 drops daily), beginning 24 hours before the antibody infusion and continuing for at least 30 days. Immediately after each infusion and at 48, 120, and 144 hours afterward, quantitative gamma-camera imaging was performed. Absorbed radiation doses to tumor sites, normal organs, and the whole body were estimated by using methods consistent with those recommended by the Medical Internal Radiation Dose Committee of the Society of Nuclear Medicine.11 Time-activity curves for tumor sites, major source organs, and the whole body were constructed from the serial imaging data, as described previously.9,10 12Absorbed doses were corrected to account for actual patient weight and the masses of major organs and tumor tissues, as determined for each patient from evaluations of CT images.
Therapeutic infusions
Approximately 10 days after the trace-labeled biodistribution infusion, patients were given single therapeutic intravenous infusions of 131I-labeled tositumomab containing 1.7 mg/kg protein (median, 197.5 mg; range, 108-347 mg) labeled with an amount of131I (median, 17 945 MBq; range, 10 064-31 080 MBq) calculated to deliver the target dose to the critical normal organ (liver, lungs, or kidneys) that would receive the highest radiation dose (Table 1). The primary objective of the study was to estimate the MTD of 131I-labeled tositumomab that could be administered with 60 mg/kg etoposide and 100 mg/kg cyclophosphamide. The first cohort of patients was treated with a dose of 131I-tositumomab calculated to deliver 20 Gy to dose-limiting normal organs, followed by 100 mg/kg cyclophosphamide and, 48 hours later, by ASCT (by which time all patients had total-body radioactivity levels below 0.02 mSv/h at 1 m). Patients treated at the next dose level received the same dose of131I-tositumomab, followed by etoposide (60 mg/kg), cyclophosphamide (100 mg/kg), and ASCT. Subsequent dose levels escalated the amount of 131I-tositumomab administered (23, 25, and 27 Gy) while maintaining the same doses of chemotherapy. Cohorts of 4 patients each were studied according to dose escalation and de-escalation rules designed to target a true toxicity rate of 25%. Specifically, the dose level would be increased to the next higher level if none of the 4 patients in a cohort had a serious grade 3 or 4 nonhematopoietic toxic effect, graded according to a modified Bearman transplant toxicity scale.13 If 1 of 4 patients in a cohort had a grade 3 or 4 toxic effect, another cohort of 4 patients was treated at the same dose level; if 2 or more patients in a cohort had such an effect, then the next 4 patients were treated at 1 lower dose level. The MTD was defined after 20 patients had been treated at a particular dose, and this level was considered to be the MTD, provided that the lower limit of the 80% one-sided confidence interval (CI) associated with the observed toxicity rate did not exceed the 25% target toxicity rate. A fifth patient was treated at dose level 1 (after we consulted with the institutional review board) because one patient in this group died of disseminated varicella zoster 2 months after treatment and could not be evaluated for late nonhematopoietic toxic effects.
Dose levels for phase I/II protocol of iodine 131–anti-CD20 (tositumomab), etoposide, and cyclophosphamide
| Dose level . | No. of patients . | 131I–anti-CD20 dose* (Gy) . | Etoposide dose (mg/kg) . | CTX dose (mg/kg) . |
|---|---|---|---|---|
| 0 (starting) | 5† | 20 | 0 | 100 |
| 1 | 12 | 20 | 60 | 100 |
| 2 | 4 | 23 | 60 | 100 |
| 3 | 23 | 25 | 60 | 100 |
| 4 | 8 | 27 | 60 | 100 |
| Dose level . | No. of patients . | 131I–anti-CD20 dose* (Gy) . | Etoposide dose (mg/kg) . | CTX dose (mg/kg) . |
|---|---|---|---|---|
| 0 (starting) | 5† | 20 | 0 | 100 |
| 1 | 12 | 20 | 60 | 100 |
| 2 | 4 | 23 | 60 | 100 |
| 3 | 23 | 25 | 60 | 100 |
| 4 | 8 | 27 | 60 | 100 |
131I indicates iodine-131; CTX, cyclophosphamide.
The 131I–anti-CD20 dose is expressed as the radiation-absorbed dose delivered to the normal organ receiving the greatest dose of radiation (usually the lungs). The actual dose of131I administered varied from patient to patient within a cohort according to antibody biodistribution and time-activity curves.
One patient in group 0 could not be evaluated for late nonhematologic toxic effects, so 5 patients were treated in the first cohort before the escalation to dose level 1.
As is customary at our center, 7 patients over the age of 55 were given reduced doses of etoposide (30 mg/kg) and cyclophosphamide (60 mg/kg). Because of the potentially confounding effects of these dose reductions on interpretation of study results, additional patients under the age of 55 were added at the end of the study to replace these older patients with patients treated at the MTD with full doses of cyclophosphamide and etoposide.
Patients were treated in lead-lined isolation rooms. The radiolabeled antibodies were infused through an automatic pump from a lead-shielded reservoir. Patients remained in radiation isolation until their total-body radioactivity decreased to a dose rate of less than 0.07 mSv/h at 1 m (median time, 11 days). Etoposide was administered intravenously over 4 hours within 24 hours of release from radiation isolation. Cyclophosphamide was given intravenously over 1 hour beginning 48 hours after the etoposide was given. Purged autologous BM or PBSC were infused 36 to 48 hours after the administration of cyclophosphamide, provided that the total-body activity of131I was less than 0.02 mSv/h at 1 m. Filgrastim (5 μg/kg per day) was administered subcutaneously each day to patients receiving BM transplants until the neutrophil count rose to more than 1.0 × 109/L, but growth factors were generally not used in patients receiving PBSC, because studies at our institution indicated minimal enhancement of engraftment with such cells. A CR was defined as complete disappearance of all tumor for at least 1 month; a partial response (PR), 50% to 99% regression; and a minor response, 25% to 50% regression. Patients meeting the Cotswolds definition of “complete response (unconfirmed)”14 (CRu) were included in the category of complete responders.
At study entry, all patients had routine blood chemistry analyses; complete blood counts with differential assessments; quantitative immunoglobulin (Ig) evaluations; thyroid function testing; HAMA testing; CT studies of the chest, abdomen, and pelvis; pulmonary function testing; measurement of the cardiac ejection fraction by gated blood-pool scan; BM aspiration and biopsy with flow cytometric studies; cytogenetic and PCR testing; and flow cytometric analysis of blood for lymphocyte Ig light-chain analysis and B-cell surface markers. Blood counts and blood chemistry analyses were not performed while patients were in radiation isolation but were measured daily thereafter until hematopoietic reconstitution was complete.
Evaluation of toxicity
Toxic effects were assessed by using a grading scale devised by Bearman to assess BM transplant conditioning regimens.6-8 13 Originally, this scale assessed only nonhematopoietic toxic effects. For this study, the scale was modified to include assessment of infections (grade I, minor; grade II, serious [eg, bacteremia]; grade III, life-threatening [eg, septic shock and need for intensive care]; and grade IV, fatal) and thyroid function (grade I, asymptomatic abnormalities on thyroid function tests; grade II, symptomatic hypothyroidism; grade III, myxedema coma, and grade IV, fatal).
Follow-up
Patients were formally assessed 1, 3, 6, and 12 months after transplantation and at least annually thereafter and whenever symptoms or signs of disease progression occurred by means of blood counts, blood chemistry analyses, thyroid function tests, HAMA testing, serum Ig measurements, and CT scans. Blood and BM samples were assessed morphologically and by flow cytometry, cytogenetic studies (BM only), and PCR 1 and 12 months after transplantation and at least annually thereafter. B-cell clonality testing was performed by using primers specific for the CDRIII of the Ig heavy chain, including a VH and JH consensus primer, as described previously.15,16 Three primer sets were used to screen for presence of the t(14;18) translocation, including one for the major breakpoint region and 2 for the minor cluster region (sensitivity, 1 neoplastic cell in 100 000 normal cells).17-19 The t(11;14) translocation was detected by using a heminested PCR method using one primer set that detects the major t(11;14) breakpoint (sensitivity, approximately 1 neoplastic cell in 100 000 cells, but only 50% of mantle cell lymphomas are detected because of variability in the breakpoints20). When possible, original diagnostic tissue was used to determine whether the clonal Ig gene rearrangement or translocation was detectable.
Statistical analysis
One of the major objectives of this study was to estimate the MTD of 131I-tositumomab when combined with 60 mg/kg etoposide and 100 mg/kg cyclophosphamide in the setting of ASCT. The MTD was defined as the dose associated with a targeted true toxicity rate of 25% according to the dose-finding scheme described above. Secondary objectives included the examination of potential efficacy within the confines of a dose-finding study. For these purposes, overall survival (OS) and progression-free survival (PFS) were estimated by using the method of Kaplan and Meier, and all patients who received the planned therapy were considered together, regardless of the dose of 131I-tositumomab received. These outcomes were also compared with those observed in a nonrandomized control group of patients to provide tentative information on the potential efficacy of the current approach compared with a conventional regimen. The control group consisted of 105 patients under the age of 60 who were treated at our institution between 1990 and 1999 with a conventional regimen of TBI (1.5 Gy twice a day for 4 days), etoposide (60 mg/kg), and cyclophosphamide (100 mg/kg). As noted above, patients over the age of 55 received attenuated doses of etoposide (30 mg/kg) and cyclophosphamide (60 mg/kg) in both the experimental and conventional protocols. The control patients were not enrolled in the clinical trial containing 131I -tositumomab because of either patient refusal or failure to obtain insurance approval. Patients with T-cell lymphoma, Burkitt lymphoma, or small lymphocytic lymphoma were excluded from the control group because no patients with these histologic types of disease were entered into the experimental protocol.
Proportional hazards regression was used to compare the hazard of failure appropriate to OS (death) and PFS (death or progression of disease) in the treated and control groups. Patient files were reviewed and the following explanatory variables extracted: patient age, sex, histologic type, stage, tumor bulk, sensitivity to conventional chemotherapy at time of transplantation, number of previous chemotherapy regimens, previous treatment with anthracycline-containing and platinum-containing regimens, and presence or absence of splenomegaly. Outcome measures extracted were time to lymphoma progression, time to death, and date of last contact. The hazards of failure appropriate to the outcomes OS and PFS in patients in the control group and those in the investigational group were compared after adjustment for the explanatory variables. Reported 2-sidedP values were derived from the Wald test.
Results
Patient information
Fifty-five patients were registered and 52 received the planned therapy. Three patients were excluded after registration, one because of a symptomatic exacerbation of chronic hepatitis C, one because no evidence of lymphoma was detected at the time of planned therapy, and one because of marked splenomegaly rendering him ineligible according to the protocol entry criteria unless he underwent splenectomy, which he declined to do. The characteristics of the 52 treated patients are summarized in Table 2. Thirty-eight patients (72%) had follicular lymphomas and 14 (28%) had aggressive or transformed lymphomas. Eight patients had primary refractory lymphoma unresponsive to front-line chemotherapy, 2 patients had chemotherapy-resistant relapses, and 42 patients had chemosensitive relapses (defined by at least a PR to the last chemotherapy regimen administered before transplantation). The median tumor burden after cytoreductive and mobilization chemotherapy was 43 cm3(range, 2-444 cm3) on CT volumetric analysis in 32 patients with measurable disease; 20 patients had evaluable but nonmeasurable disease after cytoreductive and mobilization chemotherapy to harvest PBSC. The median spleen size was 370 cm3 (excluding 12 patients who had undergone splenectomy). Two patients (4%) were at low risk, 31 (60%) were at low-intermediate risk, and 19 (37%) were at high-intermediate risk, according to the age-adjusted international prognostic index score.22 Patients had been heavily pretreated, with a median of 3.0 previous chemotherapy regimens; 42% had B symptoms; and 36% had an elevated level of lactate dehydrogenase. Seven patients had been rejected by other transplant centers as being at too high a risk to undergo transplantation, either because of primary chemotherapy-refractory lymphoma or because their tumors could not be cytoreduced to minimal disease as required by other centers.
Characteristics of 52 patients with relapsed B-cell lymphomas
| Characteristic . | Value . |
|---|---|
| Median (range) age, y | 47 (34-58) |
| Stage | |
| II | 2 (4) |
| III | 7 (13) |
| IV | 43 (83) |
| Histologic type | |
| Follicular (grade I) | 22 (42) |
| Follicular (grade II) | 12 (23) |
| Follicular (grade III) | 4 (8) |
| Mantle cell lymphoma | 6 (12) |
| Transformed diffuse large cell lymphoma | 6 (12) |
| De novo diffuse large cell lymphoma | 2 (4) |
| B symptoms | 22 (42) |
| Elevated lactase dehydrogenase | 19 (36) |
| International prognostic index score21 | |
| Low risk | 2 (4) |
| Low-intermediate risk | 31 (60) |
| High-intermediate risk | 19 (37) |
| Characteristic . | Value . |
|---|---|
| Median (range) age, y | 47 (34-58) |
| Stage | |
| II | 2 (4) |
| III | 7 (13) |
| IV | 43 (83) |
| Histologic type | |
| Follicular (grade I) | 22 (42) |
| Follicular (grade II) | 12 (23) |
| Follicular (grade III) | 4 (8) |
| Mantle cell lymphoma | 6 (12) |
| Transformed diffuse large cell lymphoma | 6 (12) |
| De novo diffuse large cell lymphoma | 2 (4) |
| B symptoms | 22 (42) |
| Elevated lactase dehydrogenase | 19 (36) |
| International prognostic index score21 | |
| Low risk | 2 (4) |
| Low-intermediate risk | 31 (60) |
| High-intermediate risk | 19 (37) |
Values are numbers (percentages) of patients unless otherwise indicated.
Hematopoietic toxicity
All patients had severe myelosuppression (neutrophils < 0.5 × 109/L and platelets < 20 × 109/L) after the treatment regimen, but all evaluated patients achieved hematopoietic engraftment of transplanted autologous stem cells. The median time to neutrophil engraftment (neutrophils > 0.5 × 109/L) was 10 days after transplantation in patients receiving PBSC and 13.5 days in patients receiving BM transplants. The median time to platelet engraftment was 13 days in patients receiving PBSC and 23 days in patients receiving BM transplants. Patients given PBSC received a median of 4 red cell transfusions and 7 platelet transfusions, whereas patients given BM required a median of 8 red cell transfusions and 13 platelet transfusions. Neither purging nor CD34 selection had any apparent effect on engraftment (data not shown).
Estimation of MTD
Table 1 shows the doses of agents used and the number of patients treated at each dose level. Table 3 shows the serious (grade III-IV) adverse events observed, stratified according to dose level and severity of toxicity. Doses were escalated according to protocol-specified guidelines from dose level 0 to dose level 4, and then de-escalated to dose level 3 when the highest dose level was found to be excessively toxic (2 consecutively treated patients at dose level 4 had 3 life-threatening toxic effects, 1 of which was fatal). An additional 19 patients were then treated at dose level 3 according to the rules specified in the protocol, and 3 additional grade III to IV toxic effects were observed. Dose level 3 (25 Gy delivered by 131I–anti-CD20, 60 mg/kg etoposide, and 100 mg/kg cyclophosphamide) was considered the estimate of the MTD for this regimen as defined prospectively by the rules of the protocol.
Grade III to IV toxic effects of 131I-labeled anti-CD20 (tositumomab) antibody, etoposide, and CTX followed by autologous stem-cell transplantation in patients with relapsed B-cell lymphomas, according to dose level of therapy
| Toxic effect . | Dose levels 0-2 (n = 21; 40% of total) . | Dose level 3 (n = 23; 44% of total) . | Dose level 4 (n = 8; 15% of total) . |
|---|---|---|---|
| Infections3-150 | 1 (5) | 2 (9) | 1 (12) |
| Hepatic | 1 (5) | 0 | 0 |
| Mucositis | 1 (5) | 0 | 0 |
| Thyroid | 0 | 0 | 0 |
| GI | 0 | 1 (4) | 1 (12) |
| Renal3-150 | 0 | 1 (4) | 0 |
| Cardiac3-150 | 0 | 0 | 0 |
| Pulmonary | 0 | 3 (13) | 0 |
| CNS | 0 | 0 | 1 (12) |
| Any grade III-IV toxic effect | 2 (9.5) | 4 (17) | 2 (25) |
| Toxic effect . | Dose levels 0-2 (n = 21; 40% of total) . | Dose level 3 (n = 23; 44% of total) . | Dose level 4 (n = 8; 15% of total) . |
|---|---|---|---|
| Infections3-150 | 1 (5) | 2 (9) | 1 (12) |
| Hepatic | 1 (5) | 0 | 0 |
| Mucositis | 1 (5) | 0 | 0 |
| Thyroid | 0 | 0 | 0 |
| GI | 0 | 1 (4) | 1 (12) |
| Renal3-150 | 0 | 1 (4) | 0 |
| Cardiac3-150 | 0 | 0 | 0 |
| Pulmonary | 0 | 3 (13) | 0 |
| CNS | 0 | 0 | 1 (12) |
| Any grade III-IV toxic effect | 2 (9.5) | 4 (17) | 2 (25) |
Values are numbers (percentages) of patients unless otherwise indicated. Toxic effects are graded according to the scale described by Bearman et al,13 with slight modifications.
CTX indicates cyclophosphamide; GI, gastrointestinal toxicity (anorexia, nausea, vomiting, or diarrhea); CNS, central nervous system toxicity (eg, confusion or somnolence).
Patients who died of sepsis syndromes with secondary multiple-organ failure were considered to have grade IV infections, but individual-organ toxicity is not included in the table.
Toxic effects
Mucositis (92%), nausea (63%), and infections (63%) occurred in most patients treated with this regimen, but these conditions were usually mild to moderate in severity (grades I-II). In 42 patients (81%), abnormalities on liver function tests developed after therapy, due to either high-dose chemotherapy, viral infections, or veno-occlusive disease. In all cases except 2 (4%), these abnormalities were mild and transient. The exceptions were one case of grade III veno-occlusive disease and one case of disseminated varicella zoster. In 25 patients (48%), creatinine levels became elevated, but this was nearly always a small increase (< 177 μmol/L), reversible, and attributable to prophylactic administration of amphotericin. Forty-six patients could be evaluated for the effect of high-dose chemoradioimmunotherapy on thyroid status (3 patients died too early for assessment, 2 were receiving thyroid replacement therapy before study entry, and no data were available for 1 patient). In 26 of the 46 patients evaluated (56%), levels of thyroid-stimulating hormone became elevated and thyroid hormone replacement therapy was given. In 4 patients, mild to moderate idiopathic pneumonitis developed 2 to 8 months after they received this regimen. In all cases of delayed pneumonitis, the pulmonary symptoms responded to outpatient corticosteroid therapy.
Eight patients (15%) had 13 grade III to IV toxic effects (Table 3), including 3 patients with adult respiratory distress syndrome requiring mechanical ventilation (2 after sepsis, both of whom died), 3 patients with grade III mucositis or gastrointestinal toxicity (all of whom had resolution), 1 with reversible grade III veno-occlusive disease, and 4 with a fatal infection (disseminated aspergillosis with a brain abscess, streptococcal sepsis, staphylococcal sepsis, and disseminated varicella zoster). In 1 of the 7 patients between 55 and 60 years of age who received modified doses of cyclophosphamide and etoposide, a grade III pulmonary toxic effect developed, but none of the patients in this age group has died. Our conclusions regarding the potential efficacy, toxicity, and estimation of the MTD in this study were not affected by inclusion or exclusion of these 7 older patients, so results in all patients are reported together.
Serial HAMA testing detected at least one positive result in 10 of 47 patients who could be evaluated (21%), but in only 7 of the 45 evaluated cases (15%) did HAMA test results remain positive on subsequent assays. The median time to development of HAMA was 3 months after transplantation (range, 1 month to 2 years).
Response to therapy
Thirty-one patients could be fully evaluated for response to the conditioning regimen; 18 patients had minimal residual (nonmeasurable) disease after mobilization chemotherapy and 3 patients died of toxic effects soon after transplantation and could not be evaluated for response. Of the 31 patients who were evaluated, 24 (77%) had a CR, 3 (10%) had a PR, 2 (6%) had stable disease, and 1 had progressive disease (3%). Six of the patients with CR had some residual areas of radiographic stranding in areas of previous lymphadenopathy that were not considered pathologic (CRu). Overall, 27 of 31 patients evaluated (87%) had an objective remission (PR, CRu, or CR). If the entire treatment program is considered (including cytoreductive and mobilization chemotherapy and the transplant conditioning regimen), 42 of 49 patients evaluated (86%) achieved a disease-free state. Sixteen patients had progression of lymphoma, including 11 of 21 treated with dose levels 0 to 2, 4 of 23 treated with dose level 3, and 1 of 8 treated with dose level 4.
OS and PFS
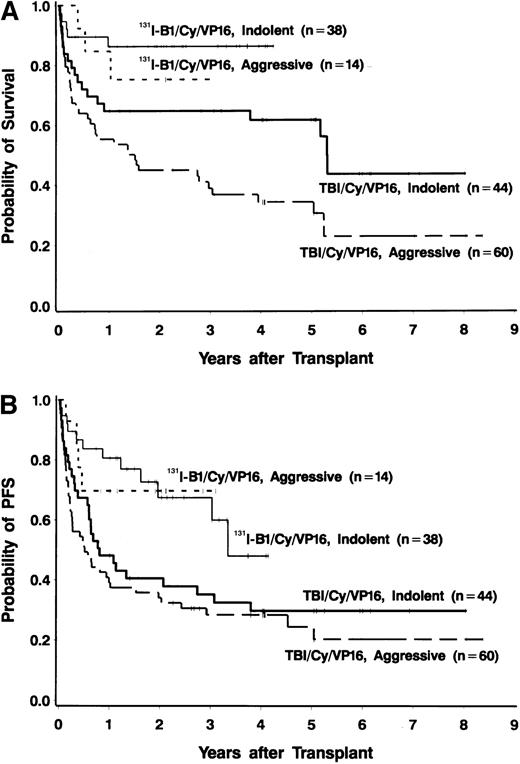
Forty-four of the 52 patients (85%) were alive at the time of data analysis (September 28, 1999), yielding an estimated 2-year survival of 83% (95% CI, 72%-94%). Four patients died of progressive disease and 4 patients died of infections. PFS at 2 years was estimated to be 68% (95% CI, 53%-83%). PFS and OS both appeared to be superior to those in a nonrandomized control group of 105 patients treated with conventional TBI, etoposide, and cyclophosphamide (Figures 1 and2), even though many of the patients treated in the radioimmunotherapy group were given suboptimal doses in this phase I/II dose-escalation study. The doses of etoposide and cyclophosphamide (and the dose reductions for patients aged 55 to 60 years) were the same for the patients receiving TBI and those treated with 131I-tositumomab. Table4 shows a comparison of important prognostic features of the 131I-tositumomab and the TBI groups. The 2 groups were similar in many respects (eg, age, history of splenomegaly, and previous exposure to anthracyclines). However, patients receiving 131I-tositumomab had higher-stage disease (83% with stage IV disease versus 67% in patients receiving TBI) and had been more heavily pretreated (median of 3 previous chemotherapy regimens versus 2 previous regimens in TBI patients). On the other hand, more patients in the TBI group had aggressive histologic disease types (58% versus 27%) and tumor bulk greater than 5 cm (26% versus 6%). Because the disparity in histologic subtypes is particularly noteworthy and might explain the apparent differences in OS and PFS between the 2 groups, we analyzed the outcomes in the indolent-disease and aggressive-disease subsets of patients treated with each regimen. As shown in Figure 3, patients with both indolent and aggressive lymphomas appeared to benefit from transplantation using the radiolabeled antibody.
Overall survival of patients with relapsed B-cell lymphomas with 2 treatments.
Fifty-two patients were treated with 131I-tositumomab, etoposide, cyclophosphamide, and autologous stem-cell transplantation (ASCT) (thin line), and 105 patients were treated with external-beam total-body irradiation (TBI) (1.5 Gy twice a day for 4 days), etoposide (60 mg/kg), cyclophosphamide (100 mg/kg), and ASCT (thick line).
Overall survival of patients with relapsed B-cell lymphomas with 2 treatments.
Fifty-two patients were treated with 131I-tositumomab, etoposide, cyclophosphamide, and autologous stem-cell transplantation (ASCT) (thin line), and 105 patients were treated with external-beam total-body irradiation (TBI) (1.5 Gy twice a day for 4 days), etoposide (60 mg/kg), cyclophosphamide (100 mg/kg), and ASCT (thick line).
Progression-free survival in patients with relapsed B-cell lymphomas.
Fifty-two patients were treated with 131I-tositumomab, etoposide, cyclophosphamide, and ASCT (thin line), and 105 patients were treated with external-beam TBI (1.5 Gy twice a day for 4 days), etoposide (60 mg/kg), cyclophosphamide (100 mg/kg), and ASCT (thick line).
Progression-free survival in patients with relapsed B-cell lymphomas.
Fifty-two patients were treated with 131I-tositumomab, etoposide, cyclophosphamide, and ASCT (thin line), and 105 patients were treated with external-beam TBI (1.5 Gy twice a day for 4 days), etoposide (60 mg/kg), cyclophosphamide (100 mg/kg), and ASCT (thick line).
Prognostic features of the study group (treated with131I-tositumomab, etoposide, and CTX) and the nonrandomized control group (treated with total-body irradiation, etoposide, and CTX)
| Characteristic . | Study group (n = 52) . | Control group (n = 105)4-150 . |
|---|---|---|
| Median (range) age, y | 47.1 (34-58) | 47.4 (17.4-59.5) |
| Median (range) no. previous regimens | 3 (1-7) | 2 (1-7) |
| Stage4-151 | ||
| I-II | 2 (4) | 14 (14) |
| III | 7 (13) | 20 (19) |
| IV | 43 (83) | 69 (67) |
| Unknown | 0 | 2 |
| Tumor bulk4-151 | ||
| < 5 cm | 48 (94) | 70 (74) |
| > 5 cm | 4 (6) | 25 (26) |
| Unknown | 0 | 10 |
| Splenomegaly | 12 (23)4-151 | 22 (25) |
| No splenomegaly | 40 (77) | 66 (75) |
| Unknown spleen size | 0 | 17 |
| Histologic type | ||
| Indolent | 38 (73) | 44 (42) |
| Aggressive | 14 (27) | 60 (58) |
| Unknown | 0 | 1 |
| Sensitivity to chemotherapy | ||
| Resistant | 10 (19)‡ | 19 (22) |
| Sensitive | 42 (81) | 68 (78) |
| Unknown | 0 | 18 |
| Previous anthracycline therapy | 44 (85%) | 99 (94%) |
| Characteristic . | Study group (n = 52) . | Control group (n = 105)4-150 . |
|---|---|---|
| Median (range) age, y | 47.1 (34-58) | 47.4 (17.4-59.5) |
| Median (range) no. previous regimens | 3 (1-7) | 2 (1-7) |
| Stage4-151 | ||
| I-II | 2 (4) | 14 (14) |
| III | 7 (13) | 20 (19) |
| IV | 43 (83) | 69 (67) |
| Unknown | 0 | 2 |
| Tumor bulk4-151 | ||
| < 5 cm | 48 (94) | 70 (74) |
| > 5 cm | 4 (6) | 25 (26) |
| Unknown | 0 | 10 |
| Splenomegaly | 12 (23)4-151 | 22 (25) |
| No splenomegaly | 40 (77) | 66 (75) |
| Unknown spleen size | 0 | 17 |
| Histologic type | ||
| Indolent | 38 (73) | 44 (42) |
| Aggressive | 14 (27) | 60 (58) |
| Unknown | 0 | 1 |
| Sensitivity to chemotherapy | ||
| Resistant | 10 (19)‡ | 19 (22) |
| Sensitive | 42 (81) | 68 (78) |
| Unknown | 0 | 18 |
| Previous anthracycline therapy | 44 (85%) | 99 (94%) |
Values are numbers (percentages) of patients unless otherwise indicated. All patients entered in both transplant protocols had a good performance status (0-1) according to criteria described by Shipp.21
CTX indicates cyclophosphamide.
Unknown cases are excluded from calculations of percentages.
The patients in the study group with splenomegaly underwent splenectomy before radioimmunotherapy, as required by the study protocol.
Eight patients had primary refractory lymphoma that did not respond to front-line chemotherapy and 2 had chemotherapy-resistant relapses.
Survival analyses according to type of lymphoma.
(A) Overall survival in 38 patients with relapsed indolent lymphomas (thin solid line) and 14 patients with relapsed aggressive lymphomas (short dashes) treated with 131I-tositumomab, etoposide, cyclophosphamide, and ASCT and in 44 patients with relapsed indolent lymphomas (thick solid line) and 60 patients with relapsed aggressive lymphomas (long thick dashes) treated with external-beam TBI (1.5 Gy twice a day for 4 days), etoposide (60 mg/kg), cyclophosphamide (100 mg/kg), and ASCT. (B) Progression-free survival in 38 patients with relapsed indolent lymphomas (thin solid line) and 14 patients with relapsed aggressive lymphomas (short dashes) treated with131I-tositumomab, etoposide, cyclophosphamide, and ASCT and in 44 patients with relapsed indolent lymphomas (thick solid line) and 60 patients with relapsed aggressive lymphomas (long thick dashes) treated with external-beam TBI (1.5 Gy twice a day for 4 days), etoposide (60 mg/kg), cyclophosphamide, (100 mg/kg) and ASCT.
Survival analyses according to type of lymphoma.
(A) Overall survival in 38 patients with relapsed indolent lymphomas (thin solid line) and 14 patients with relapsed aggressive lymphomas (short dashes) treated with 131I-tositumomab, etoposide, cyclophosphamide, and ASCT and in 44 patients with relapsed indolent lymphomas (thick solid line) and 60 patients with relapsed aggressive lymphomas (long thick dashes) treated with external-beam TBI (1.5 Gy twice a day for 4 days), etoposide (60 mg/kg), cyclophosphamide (100 mg/kg), and ASCT. (B) Progression-free survival in 38 patients with relapsed indolent lymphomas (thin solid line) and 14 patients with relapsed aggressive lymphomas (short dashes) treated with131I-tositumomab, etoposide, cyclophosphamide, and ASCT and in 44 patients with relapsed indolent lymphomas (thick solid line) and 60 patients with relapsed aggressive lymphomas (long thick dashes) treated with external-beam TBI (1.5 Gy twice a day for 4 days), etoposide (60 mg/kg), cyclophosphamide, (100 mg/kg) and ASCT.
Multivariable analysis
To compensate for potential selection biases and the discrepant patient characteristics noted above, we performed a multivariable regression analysis to compare PFS and OS in the experimental131I-tositumomab group with PFS and OS in the nonrandomized control TBI group. Variables included in the multivariable regression model for both OS and PFS were grade of disease (indolent versus aggressive), chemosensitivity at the time of transplantation, the number of previous regimens, and tumor bulk. The number of observed events (deaths and relapses) was too small to include all variables in the multivariable regression analyses, so only the variables found to be significant in a univariate analysis were included in the multivariable analysis. The results of the multivariable analysis suggested that the antibody-containing regimens had significant advantages with respect to both OS (P < 0.004) and PFS (P < 0.002; Table 5).
Univariate and multivariable hazard ratios for overall survival and progression-free survival in patients with relapsed non-Hodgkin lymphoma treated with either 131I-tositumomab, etoposide, and CTX or total-body irradiation, etoposide, and CTX
| Survival results/treatment . | Univariate hazard ratio (95% CI) . | Pvalue . | Multivariable hazard ratio5-150 (95% CI) . | P value . |
|---|---|---|---|---|
| OS | ||||
| TBI, etoposide, CTX | 1.0 | — | 1.0 | — |
| 131I-tositumomab, etoposide, CTX | 0.3 (0.1-0.6) | .0007 | 0.3 (0.1-0.7) | .004 |
| PFS | ||||
| TBI, etoposide, CTX | 1.0 | — | 1.0 | — |
| 131I-tositumomab, etoposide, CTX | 0.4 (0.2-0.6) | .0003 | 0.4 (0.2-0.7) | .002 |
| Survival results/treatment . | Univariate hazard ratio (95% CI) . | Pvalue . | Multivariable hazard ratio5-150 (95% CI) . | P value . |
|---|---|---|---|---|
| OS | ||||
| TBI, etoposide, CTX | 1.0 | — | 1.0 | — |
| 131I-tositumomab, etoposide, CTX | 0.3 (0.1-0.6) | .0007 | 0.3 (0.1-0.7) | .004 |
| PFS | ||||
| TBI, etoposide, CTX | 1.0 | — | 1.0 | — |
| 131I-tositumomab, etoposide, CTX | 0.4 (0.2-0.6) | .0003 | 0.4 (0.2-0.7) | .002 |
OS indicates overall survival; PFS, progression-free survival; CTX, cyclophosphamide; TBI, total-body irradiation.
Adjusted for histologic type (aggressive versus indolent NHL), number of previous chemotherapy regimens, sensitivity to chemotherapy at time of transplantation (chemosensitive versus chemoresistant), and tumor bulk.
Molecular monitoring
For 46 of the 52 patients in the study, serial BM specimens were available to assess the presence of clonal B-cell populations by PCR for clonal IgH CDR3 rearrangements and for t(14;18), and t(11;14) translocations. Twenty of the 46 patients evaluated (43%) had baseline clonal abnormalities in their BM, and 12 of the 20 (60%) had at least temporary conversion to negative PCR results after therapy (Figure4A). Similarly, 13 of 33 patients (39%) who underwent serial PCR testing of the blood had circulating clonal abnormalities, and 9 of the 13 (69%) had conversion to negative PCR results after transplantation (Figure 4B). Currently, there are no significant differences in OS or PFS between patients who had molecular remissions and patients who did not (data not shown).
Landmark analyses of bone marrow and blood.
Solid symbols indicate presence of a clonal marker indicative of lymphoma; open symbols indicate absence of clonal markers. Only informative markers are shown. (A) Landmark analysis of serial bone marrow samples from 20 patients with informative markers monitored by polymerase chain reaction (PCR) for clonal immunoglobulin heavy-chain rearrangements (circles), the t(14:18) translocation (squares), and the t(11;14) translocation (triangles). (B) Landmark analysis of serial peripheral blood samples from 13 patients with informative markers monitored by PCR for clonal immunoglobulin heavy-chain rearrangements (circles), the t(14:18) translocation (squares), and the t(11;14) translocation (triangles).
Landmark analyses of bone marrow and blood.
Solid symbols indicate presence of a clonal marker indicative of lymphoma; open symbols indicate absence of clonal markers. Only informative markers are shown. (A) Landmark analysis of serial bone marrow samples from 20 patients with informative markers monitored by polymerase chain reaction (PCR) for clonal immunoglobulin heavy-chain rearrangements (circles), the t(14:18) translocation (squares), and the t(11;14) translocation (triangles). (B) Landmark analysis of serial peripheral blood samples from 13 patients with informative markers monitored by PCR for clonal immunoglobulin heavy-chain rearrangements (circles), the t(14:18) translocation (squares), and the t(11;14) translocation (triangles).
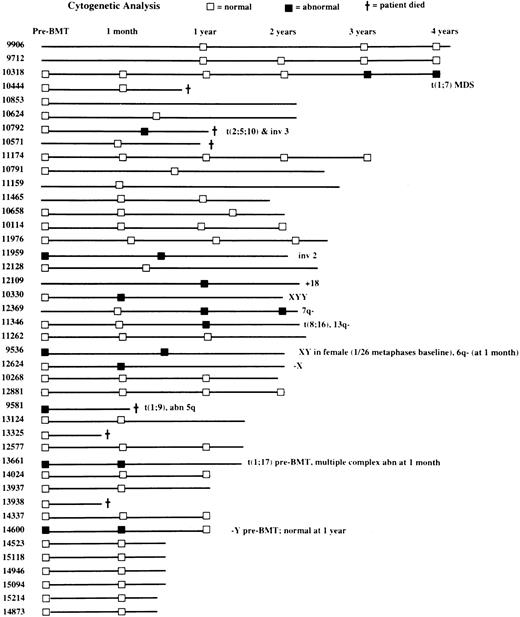
Cytogenetic studies
Baseline (before ASCT) BM cytogenetic findings were available for 35 patients, 30 of whom (86%) had normal results on those assessments (Figure 5). Five patients (14%) had baseline cytogenetic abnormalities in hematopoietic cells, including one patient with both t(1;9) and an abnormality of the long arm of chromosome 5 and one patient each with t(1;17), inv(2), −Y, and XY in a woman (but in only 1 of 26 metaphases). Posttransplantation cytogenetic results were available for 38 patients and were normal in 27 (71%) and abnormal in 11 (29%; Figure 5). Abnormal findings after transplantation included both t(2;5;10) and inv(3) (one patient), both t(8;16) and del(13q) (one patient), and t(1;7), inv(2), 6q−, 7q−, +18, −X, −Y, and XYY (one patient each). One patient in whom t(1;7) developed after transplantation has moderate thrombocytopenia (platelets, 78 ×109/L) with macrocytic red cells 3 years after transplantation and is considered to have myelodysplastic syndrome (MDS), although he is asymptomatic. Another patient had a progressive increase in the percentage of BM cells bearing the 7q− translocation on serial cytogenetic examinations, but his condition does not meet criteria for MDS because peripheral blood cell counts and BM morphologic evaluations have yielded normal results.
Landmark analyses in the 42 patients with serial cytogenetic assessments of bone marrow samples.
Normal cytogenetic findings, ■; abnormal results, ▪.
Landmark analyses in the 42 patients with serial cytogenetic assessments of bone marrow samples.
Normal cytogenetic findings, ■; abnormal results, ▪.
Discussion
Radiolabeled monoclonal antibodies have recently emerged as an effective new treatment for patients with relapsed B-cell lymphomas.6-9,23-33 Studies at several institutions found response rates of 60% to 80% for nonmyeloablative doses of131I- and yttrium- 90–labeled anti-CD20 antibodies, including CR rates of 25% to 40%.23,26,32 33 The median duration of response in these trials ranged from 6 to 18 months, although some CRs lasted more than 5 years. We focused on the use of myeloablative doses of 131I–anti-CD20 antibodies in an attempt to increase the overall and CR rates and the duration of remissions. This study demonstrates the feasibility of administering high-dose 131I-tositumomab with etoposide and cyclophosphamide followed by ASCT. The study also establishes 25 Gy as the MTD of 131I-tositumomab that can be given safely to dose-limiting normal organs (especially the heart and lungs) with 60 mg/kg etoposide and 100 mg/kg cyclophosphamide. Opportunistic infections were the most serious complications in this protocol and were responsible for all 4 treatment-related deaths, often in association with multiple-organ failure and cardiopulmonary compromise.
Although previous studies showed that single-agent131I-tositumomab alone rarely causes alopecia, mucositis, or veno-occlusive disease of the liver, even when given at myeloablative doses,6-8 these complications were frequent, though rarely serious, with our combination regimen containing high-dose cyclophosphamide and etoposide. As expected, fever, nausea, and elevated levels of thyrotropin were observed in most patients. Overall, the types of toxic effects observed in this study appear to be similar to those previously occurring with TBI, cyclophosphamide, and etoposide at our institution,34 although more deaths were observed with the TBI-based regimen (17% [33] versus 7.6% with 131I-tositumomab). A prospective, randomized comparison will be required to compare the toxic effects of these 2 approaches definitively.
Previous studies of high-dose chemoradiotherapy with ASCT for relapsed NHL using conventional TBI, chemotherapy, or both found a substantial risk of MDS and acute myelogenous leukemia (AML), with observed incidence rates of 4% to 8% and cumulative incidence projections of 12% to 20% at 20 years after transplantation.34-40 This complication was speculated to be more common with TBI-containing regimens in some38 but not all40 studies. It is currently unclear whether MDS and AML in patients with NHL undergoing ASCT result from previous chemotherapy with alkylating agents and topoisomerase inhibitors, previous external radiotherapy, exposure to high doses of chemoradiotherapy in the transplant conditioning regimen, enforced oligoclonal proliferation of the limited numbers of stem cells administered after ASCT, damage to the hematopoietic microenvironment from chemoradiotherapy, or a combination of these factors. We observed one case of MDS in our 52 patients, who were followed for a median of 2 years, and several others have cytogenetic abnormalities, half of which were present before transplantation (Figure 5). Fortunately, none of the patients have become symptomatic, required transfusions, or had onset of AML or other secondary malignant disease. Careful surveillance and serial hematologic and cytogenetic monitoring will be necessary to ascertain the risks of MDS and AML with this regimen.
Although an assessment of efficacy was not a primary objective of this dose-finding study, the preliminary results are encouraging. Twenty-seven of the 31 patients (87%) who had measurable disease at the time of transplantation had an objective response and 24 (77%) had a CR. The PFS and OS of patients treated in this investigational protocol appear to be superior to those in a nonrandomized control group treated with a conventional transplant regimen using the same doses of etoposide and cyclophosphamide but employing external TBI rather than targeted 131I-tositumomab (Figures 1-4). This apparent superiority was evident even though many of the patients treated in this dose-finding protocol received doses significantly below the MTD. Furthermore, 60% of patients with informative markers achieved molecular remissions in the BM and 69% achieved molecular remissions in the bloodstream after treatment, as assessed by serial PCR monitoring after treatment with this novel regimen. The importance of molecular remissions in this setting remains unclear, however, since patients who had such remissions have not yet shown significantly greater PFS or OS than those who did not, although the number of evaluated patients is small and the follow-up time short (data not shown).
Despite the encouraging findings of this trial, the type of nonrandomized comparison used has many limitations and potential selection biases, especially because the 2 groups that were compared differed in some important prognostic variables (eg, histologic composition). Unfortunately, the number of cases available did not permit a formal case-matched analysis. However, it is reassuring that both the subgroup with indolent disease and the subgroup with aggressive disease had a superior OS and PFS with the131I-tositumomab regimen and that the superiority of the investigational regimen persisted after we performed a multivariable analysis to control for confounding variables. However, such comparisons are not definitive and must be considered exploratory in nature. Consequently, a prospective, randomized controlled clinical trial comparing 131I-tositumomab, etoposide, and cyclophosphamide with a standard regimen of TBI, etoposide, and cyclophosphamide will be required. Nonetheless, the current study provides evidence that targeted radioimmunotherapy may be preferable to nontargeted external-beam TBI and suggests that additional investigations using this approach are warranted.
Acknowledgments
We gratefully acknowledge the technical support provided by Caroline Thosteson, Minna Zheng, Carol Dean, and Bhavesha Patel; the secretarial assistance by Lynn Planet; and the administrative support by Kara McBroom. We also thank George Tidmarsh, Robert Stagg, Virginia Langmuir, and Anish Bhatnagar, Coulter Pharmaceuticals, who provided the tositumomab antibody for this trial.
Supported by National Institutes of Health grant P01CA44991 and a gift from the Hext Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Oliver Press, University of Washington Cancer Center, Box 356043, Seattle, WA 98195.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal