Abstract
A cell-adhesive protein of the human serum, 90K binds galactin-3, β1-integrins, collagens, and fibronectin, and it is of importance in cell-cell and cell–extracellular matrix adhesion. Serum 90K levels in 137 patients with lymphoma were measured by enzyme-linked immunosorbent assay. Compared with healthy controls, pretreatment serum 90K levels in patients with lymphoma were elevated (P < .001). Of 97 patients who showed objective response to treatment, 20 (21%) had pretreatment 90K levels above the normal cutoff compared with 17 (53%) of 32 patients who did not respond (P = .002). When used as a plastic-immobilized substrate, 90K caused a significant reduction in chemotherapy-induced apoptosis of Jurkat T lymphoma cells. This finding could explain the lack of response in lymphoma patients with high 90K serum levels.
Introduction
Serum tumor products have become routine tools in clinical oncologic practice, mostly as prognostic markers or for predicting response to treatment. In lymphoma, clinical status, computed tomography scans, and bone marrow biopsies are commonly used to assess the response to treatment, and reliable serum markers for this purpose have not been available so far. The commonly used lymphoma markers, serum lactate dehydrogenase and thymidine kinase, are useful as prognostic markers when measured at the time of diagnosis. However, these markers cannot be reliably used to predict response to treatment because their serum levels are influenced by several factors, including cytotoxic drugs, hematopoietic growth factors, and concurrent disease.1
A large oligomeric protein composed of approximately 90-kd subunits was originally identified as a tumor-secreted antigen in the culture fluid of human breast cancer cells.2 This protein was named 90K. The sequence of 90K was elucidated after cDNA cloning and shown to contain a signal peptide and a number of cysteines and N-glycosylation sites.3 90K was identified independently as a ligand of the lactose-specific S-type lectin, galactin-3 (formerly known as Mac-2), and was named Mac-2 BP.4 The functions of 90K are not yet well defined but may include enhancement of killer cell activity and cytokine production,3,5endotoxin-Lipopolysaccharide–dependent binding to CD14,6and tumor growth suppression.7 Other possible functions of 90K have been described, most notable of which are related to its role in cell-cell and cell–extracellular matrix interactions. For example, promotion of homotypic cell adhesion through cross-linking of surface-bound galectin-3 has been documented in human melanoma cells.8 Moreover, binding studies in vitro demonstrated β1 integrin–mediated cell adhesion for 90K and for interactions with some collagen-type and fibronectin, properties that are consistent with an extracellular matrix localization.9 90K is present in the μg/mL range in serum and other fluids, where it may exert some of its functions.4,10 In previous studies, we showed that a high 90K serum level is associated with a poor outcome in several types of cancer.11-13 We show here that patients with lymphoma who have high 90K serum levels at the time of diagnosis displayed significantly lower responses to chemotherapy than did patients with low 90K levels. Furthermore, preliminary evidence is provided that the adhesion of lymphoma cells to 90K confers resistance to chemotherapy-induced apoptosis, thereby accounting for the in vivo drug resistance seen in patients with high levels of circulating 90K.
Patients, materials, and methods
Patients
One hundred thirty-seven patients with lymphoma diagnosed and treated at the Department of Hematology and Oncology of the University of Modena Medical School between 1992 and 1997 were included in this retrospective study. Of them, 116 patients had non-Hodgkin lymphoma (NHL) and 21 had Hodgkin disease. Sixty-seven (49%) of the patients were male, and the median age was 54 years (range, 14-89 years). The histologic subtypes of NHL according to the revised European-American classification of lymphoid neoplasms14 were as follows: diffuse large-cell lymphoma (DLCL), 59 patients; follicular lymphoma (FL), 26 patients; immunocytoma (IM), 6 patients; mantle-cell lymphoma (MCL), 5 patients; T-cell lymphoblastic lymphoma (LL), 9 patients; anaplastic large-cell Ki-1–positive T-cell lymphoma, 9 patients; and other peripheral T-cell lymphomas, 7 patients. Clinical staging was done according to the Ann Arbor classification system.15
Patients with early-stage indolent lymphoma (follicular lymphoma, extranodal marginal zone B-cell lymphoma) were treated with megavoltage radiotherapy alone. These patients usually received single-agent chlorambucil or cyclophosphamide if they had symptoms. Patients with early-stage aggressive lymphoma (large B-cell lymphoma, peripheral T-cell lymphoma) received a brief course of chemotherapy before radiotherapy. Patients with aggressive advanced-stage disease and those with mantle-cell lymphoma were treated with an anthracycline-containing combination chemotherapy, usually ProMACE-CytaBOM or MACOP-B. The initial staging workup included a complete physical examination, routine blood chemistry analyses, chest and abdominal computed tomography imaging, bilateral posterior iliac crest bone marrow needle biopsy, and laparoscopy with spleen and liver biopsy when clinically indicated. The disease was considered bulky if the patient had a measurable mass of at least 10 cm at its largest diameter. A complete response was defined as the complete disappearance of all symptoms and signs of initially documented disease for a period more than 4 weeks and the absence of new lesions as documented by negative findings on pathology restaging procedures. Partial response was a reduction of at least 50% in the sum of the products of the perpendicular diameters of all measurable tumor masses for a minimum of 4 weeks without the appearance of new lesions. Patients not included in these categories and those who died early were considered nonresponders.
A serum sample taken at the time of diagnosis was available for each patient. Serum samples were also obtained from 50 (30 women, 20 men) blood donors with a median age of 42 years (range, 21 to 65 years).
Measurement of 90K
The levels of 90K in serum were determined with a commercially available enzyme-linked immunosorbent assay kit (Diesse, Siena, Italy). Values of intra-assay and interassay variation were within the ranges given by the manufacturer (6%-12% and 8%-17%, respectively).
Human recombinant 90K
Human recombinant 90K (hr90K) was obtained as previously described and affinity purified on an SP-2 Sepharose matrix.16
Cell culture
The human Jurkat T-lymphoma cell line17 was routinely cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mmol/L glutamine, and 100 μg/mL streptomycin at 37°C in an atmosphere of 5% CO2.
Chemosensitivity assay
First, 96-well plates were coated with 100 μL of 20 μg/mL hr90K overnight at 4°C and were blocked with 1 mg/mL bovine serum albumin (BSA; 2 hours at room temperature). Then cells (0.5-1.0 × 105) in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum and antibiotics were seeded into the wells. After 1 hour of incubation at 37°C, the cytotoxic agents doxorubicin (Sigma, St Louis, MO) and cyclophosphamide (Asta Pharmaceuticals, Frankfurt, Germany) were added to each well. Where indicated, function-blocking β1 antibody 4B4 (20 μg/mL; Coulter, Hialeah, Florida) was added at the time of cell seeding. After 48 hours, the cells were collected and the percentage of apoptotic cells was assessed.
Assessment of apoptosis
To assess apoptosis, 2 μL of a propidium iodide (100 μg/mL)/acridine orange (100 μg/mL) (1:1 vol/vol) mixture was added to 200 μL Jurkat T cells, and the percentage of cells undergoing apoptosis was determined by fluorescence microscopy, as described.18 Apoptosis was also assessed by staining cytospin preparations using May-Grünwald-Giemsa stain.
Statistics
Differences between patient groups were tested with the Mann-Whitney U test or the Kruskal-Wallis test.
Results and discussion
Circulating levels of 90K in healthy control subjects varied between 1.4 μg/mL and 16.1 μg/mL (median, 4.7 μg/mL). There was no significant difference in 90K serum concentration levels between males and females, nor was there a correlation between serum 90K levels and age or blood group. Cutoff level of serum 90K was determined to be 13.5 μg/mL, which was the mean concentration ± 2 SD. Serum 90K levels in patients with NHL (median, 14.8 μg/mL; range, 2.1-64.4 μg/mL) were significantly higher than in healthy blood donors (P < .001), and they did not statistically differ from 90K levels in patients with Hodgkin disease (P = .2).
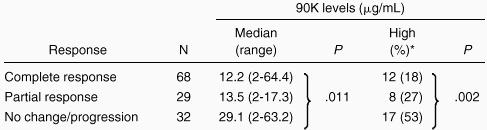
In Table 1 the percentage of patients with serum 90K levels above the cutoff level (13.5 μg/mL) is listed for the various groups, divided by patient characteristics. Serum level of 90K was not associated with median age at diagnosis, gender, median serum lactate dehydrogenase level, histologic classification, Ann Arbor clinical stage, or bulky disease (at least 10 cm in diameter). Patients with lymphoma who had bone marrow involvement tended to have higher serum 90K concentrations (P = .06), and this association was significant (P = .04) among patients with NHL.
Relation between 90K and initial patient characteristics
| Variable . | N* . | 90K levels . | |
|---|---|---|---|
| High (%)† . | P . | ||
| All patients | 137 | 39 (28) | |
| Age (years) | |||
| ≤54 | 71 | 22 (31) | |
| >54 | 66 | 17 (26) | .24 |
| Gender | |||
| Male | 77 | 20 (26) | |
| Female | 60 | 19 (32) | .72 |
| Lactate dehydrogenase (U/l) | |||
| ≤284 | 62 | 14 (22) | |
| >284 | 68 | 22 (32) | .45 |
| Histologic subtype | |||
| B cell | |||
| DLCL | 59 | 17 (29) | |
| FL | 26 | 6 (23) | |
| IM | 6 | 3 (50) | |
| MCL | 5 | 2 (40) | |
| T cell | |||
| LL | 9 | 4 (44) | .13 |
| Peripheral T-cell lymphoma | 7 | 2 (28) | |
| Anaplastic T-cell lymphoma | 4 | 2 (50) | |
| Ann Arbor stage | |||
| I | 18 | 7 (39) | |
| II | 21 | 5 (24) | |
| III | 33 | 9 (27) | .28 |
| IV | 56 | 17 (30) | |
| Bone marrow involvement | |||
| Yes | 35 | 14 (40) | |
| No | 85 | 22 (26) | .06 |
| Bulky disease | |||
| Yes | 28 | 6 (21) | |
| No | 100 | 32 (32) | .14 |
| Variable . | N* . | 90K levels . | |
|---|---|---|---|
| High (%)† . | P . | ||
| All patients | 137 | 39 (28) | |
| Age (years) | |||
| ≤54 | 71 | 22 (31) | |
| >54 | 66 | 17 (26) | .24 |
| Gender | |||
| Male | 77 | 20 (26) | |
| Female | 60 | 19 (32) | .72 |
| Lactate dehydrogenase (U/l) | |||
| ≤284 | 62 | 14 (22) | |
| >284 | 68 | 22 (32) | .45 |
| Histologic subtype | |||
| B cell | |||
| DLCL | 59 | 17 (29) | |
| FL | 26 | 6 (23) | |
| IM | 6 | 3 (50) | |
| MCL | 5 | 2 (40) | |
| T cell | |||
| LL | 9 | 4 (44) | .13 |
| Peripheral T-cell lymphoma | 7 | 2 (28) | |
| Anaplastic T-cell lymphoma | 4 | 2 (50) | |
| Ann Arbor stage | |||
| I | 18 | 7 (39) | |
| II | 21 | 5 (24) | |
| III | 33 | 9 (27) | .28 |
| IV | 56 | 17 (30) | |
| Bone marrow involvement | |||
| Yes | 35 | 14 (40) | |
| No | 85 | 22 (26) | .06 |
| Bulky disease | |||
| Yes | 28 | 6 (21) | |
| No | 100 | 32 (32) | .14 |
DLCL indicates diffuse large-cell lymphoma, 59 patients; FL, follicular lymphoma, 26 patients; IM, immunocytoma; 6 patients; MCL, mantle-cell lymphoma, 5 patients; LL, lymphoblastic lymphoma, 9 patients.
Information on all variables was not always available.
Number (percentage) of patients with 90K levels above the cutoff value of 13.5 μg/mL.
Of 129 patients evaluable for response to treatment, 97 showed objective response, indicating an overall response rate of 75% (68 complete responses, 29 partial responses). Pretreatment 90K levels above the normal cutoff were detected in 17 (53%) of 32 nonresponders compared with 20 (21%) of 97 responders (P = .002; Table2). Moreover, 90K levels were significantly higher in nonresponders than in responders (P = .011).
90K and response to therapy
Number (percentage) of patients with 90K levels above the cutoff value of 13.5 μg/mL.
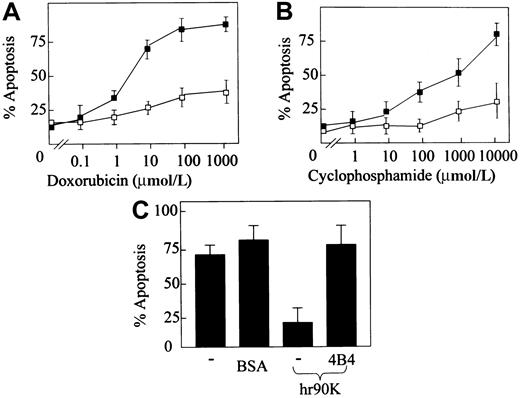
Some forms of chemotherapy, such as those used for the treatment of lymphoma, exert their cytotoxic effects mainly by inducing apoptosis.19,20 Regulation of apoptosis in tumor cells remains poorly understood. However, an increased level of adhesion to extracellular matrix may help tumor cells to evade the proapoptotic effects of anticancer drugs. A link between integrin-mediated cell adhesion to matricellular proteins and protection from chemotherapy-induced apoptosis has been reported.21,22Because 90K is able to mediate the adhesion of tumor cells at comparable strength with laminin,9 we evaluated whether adhesion of lymphoma cells to 90K resulted in resistance to chemotherapy-induced apoptosis. The addition of doxorubicin and cyclophosphamide induced a concentration-dependent increase in Jurkat T–lymphoma cell apoptosis, as judged by acridine orange–propidium iodide staining and morphology. Adhesion of lymphoma cells to hr90K substantially protected the cells against the apoptosis induced by these chemotherapeutic agents, reducing the percentage of apoptotic cells (Figure 1A-B). For example, in response to 10 μmol/L doxorubicin, hr90K reduced apoptosis from 70% to 25% (similar results were obtained with cyclophosphamide). Nonspecific adhesion of lymphoma cells to BSA did not protect cells from doxorubicin-induced apoptosis. Furthermore, the addition of the β1 function-blocking antibody 4B4 abolished the hr90K-mediated protection from doxorubicin-induced apoptosis (Figure 1C).
β1-Integrin–mediated adhesion to hr90K protects lymphoma cells from chemotherapy-induced apoptosis.
Jurkat T-lymphoma cells were seeded in the absence (▪) or in the presence (■) of precoated hr90K. Then increasing concentrations of doxorubicin (A) or cyclophosphamide (B) were added. Apoptosis was determined by propidium iodide–macridine orange or Giemsa staining. Each point represents the mean ± SEM of 3 independent experiments. (C) The effect of doxorubicin (10 μmol/L) on Jurkat T-cell apoptosis in the absence of coating protein (−) and the BSA or hr90K in the presence or absence of the function-blocking antibody 4B4. Each bar represents the mean ± SEM.
β1-Integrin–mediated adhesion to hr90K protects lymphoma cells from chemotherapy-induced apoptosis.
Jurkat T-lymphoma cells were seeded in the absence (▪) or in the presence (■) of precoated hr90K. Then increasing concentrations of doxorubicin (A) or cyclophosphamide (B) were added. Apoptosis was determined by propidium iodide–macridine orange or Giemsa staining. Each point represents the mean ± SEM of 3 independent experiments. (C) The effect of doxorubicin (10 μmol/L) on Jurkat T-cell apoptosis in the absence of coating protein (−) and the BSA or hr90K in the presence or absence of the function-blocking antibody 4B4. Each bar represents the mean ± SEM.
In conclusion, we show here that patients with lymphoma who have high serum 90K levels fail to respond to treatment more often than patients with low 90K levels. Our data also provide strong preliminary evidence that adhesion to 90K is important in lymphoma cell resistance to chemotherapy. This occurs as a result of β1-integrin–dependent process. Clinically, elevated 90K levels in the serum of patients with lymphoma may be an indicator of higher production and deposition of the protein in the extracellular matrix, leading to an environment that protects tumor cells against the cytotoxic effects of anticancer drugs. Once their role in lymphoma is firmly established, specific 90K/β1-integrin signal transduction elements they use may prove to be promising therapeutic targets.
Acknowledgment
We thank Mrs A. Sirotti for excellent technical assistance.
Supported in part by grants from the Associazione Italiana per la Ricerca sul Cancro 1999 and MURST Cofin ex-40% 1998.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stefano Iacobelli, Department of Oncology and Neurosciences, University G. D'Annunzio Medical School, Via dei Vestini, 5, 66100 Chieti, Italy; e-mail:iacobell@unich.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal