Abstract
Canine hemophilia A closely mimics the human disease and has been used previously in the development of factor VIII (FVIII) protein replacement products. FVIII-deficient dogs were studied to evaluate an in vivo gene therapy approach using an E1/E2a/E3-deficient adenoviral vector encoding canine FVIII. Results demonstrated a high level of expression of the canine protein and complete phenotypic correction of the coagulation defect in all 4 treated animals. However, FVIII expression was short-term, lasting 5 to 10 days following vector infusion. All 4 dogs displayed a biphasic liver toxicity, a transient drop in platelets, and development of anticanine FVIII antibody. Canine FVIII inhibitor development was transient in 2 of the 4 treated animals. These data demonstrate that systemic delivery of attenuated adenoviral vectors resulted in liver toxicity and hematologic changes. Therefore, the development of further attenuated adenoviral vectors encoding canine FVIII will be required to improve vector safety and reduce the risk of immunologic sequelae, and may allow achievement of sustained phenotypic correction of canine hemophilia A.
Introduction
Hemophilia A is a severe, X-linked bleeding disorder caused by a deficiency of blood coagulation factor VIII (FVIII). Hemophilia A has an incidence approaching 1 in 4000 males in all populations,1 and in its severe form, is a life-threatening, crippling disease. Infusion of plasma-derived or recombinant FVIII protein in response to bleeding crises is currently the most widely accepted therapy1 and has dramatically increased the life expectancy and quality of life for many patients with hemophilia. However, the high cost and short supply of FVIII replacement products has resulted in their availability being limited to less than 10% of the world's hemophilic population.
Gene therapy for hemophilia A would provide prophylactic expression of FVIII and correction of the coagulation defect. Considerable progress has been made recently in the development of adenoviral vector-mediated gene therapy for hemophilia A.2,3 Potent adenoviral vectors encoding a human FVIII complementary DNA (cDNA) have been developed that mediated expression of physiologic levels of FVIII in mice,4-7monkeys,8 and dogs,9 and sustained human FVIII expression in normal5 and hemophilic mice.7 Treatment of hemophilic mice and dogs resulted in human FVIII expression and complete phenotypic correction, verifying the feasibility of adenoviral vector administration for the treatment of hemophilia A.7,9-11 Expression in the hemophilic mice was sustained for at least 1 year,7,11 whereas the duration of expression in the hemophilic dogs was short-term, limited by a rapid antibody response to the human FVIII protein.9
Canine hemophilia A was first described 50 years ago,12,13and FVIII-deficient dogs have been used to support the development of FVIII pharmaceutical products.14-19 However, human FVIII is highly immunogenic in dogs when the protein is delivered intravenously20 or via gene therapy.9 In contrast, canine FVIII is significantly less immunogenic than the human protein in hemophilic dogs and most animals can be repeatedly treated with canine plasma without developing inhibitory antibodies.21 The establishment of sustained phenotypic correction in hemophilic dogs may require the development of adenoviral vectors that encode the canine cDNA.
Recently, we isolated the canine FVIII cDNA22and constructed a third generation, E1/E2a/E3-deleted23adenoviral vector encoding canine FVIII.11 Comparison of the FVIII expression level of the canine vector to that of an analogous vector encoding a human FVIII cDNA in hemophilic mice demonstrated expression of the canine protein at levels at least 10-fold higher than that of human FVIII.11 Canine FVIII expression was sustained for over 1 year in the hemophilic mice.11
In this work, we treated 4 FVIII-deficient hemophilic dogs with the canine FVIII adenoviral vector. Two dogs received a higher vector dose (3 × 1012 particles/kg) and the other 2 dogs received a 5-fold lower vector dose (6 × 1011particles/kg). We measured whole blood clotting time (WBCT) and cuticle bleeding time (CBT), canine FVIII plasma activity levels, liver enzymes, platelet and fibrinogen levels, and the development of canine FVIII inhibitory antibodies.
Materials and methods
Construction of recombinant adenoviruses
Cloning of the canine FVIII cDNA and 3′ untranslated region,22 construction of the B-domain–deleted canine FVIII cDNA, and generation of the canine FVIII-encoding recombinant adenovirus, Av3H8401, were described previously.11 The analogous, recombinant adenovirus encoding human FVIII, Av3H8101, has been described.7 Vector concentrations were determined by spectrophotometric analysis.24 Titers are stated as particles per milliliter. The vector preparations were checked for the presence of replication-competent adenovirus contamination by polymerase chain reaction (PCR) directed at E1a sequences,25 and all vector preparations contained fewer than 10 particles of E1a-containing vector per 108particles.
Hemophilic dog procedures and adenoviral vector administration
The experimental animals used in this study were mixed-breed dogs from the hemophilia A colony housed at Queen's University.13 Phlebotomy was performed from the cephalic vein. The adenoviral vector was diluted in Hanks' buffered saline solution (Life Technologies, Gaithersburg, MD) and was administered through an in-dwelling cephalic vein catheter by slow infusion at a rate of 3 mL/min. All animals were housed in facilities accredited by the Canadian Council for Animal Care and experimental procedures were approved by both the Genetic Therapy, Inc, and Queen's University Animal Care Committees.
Coagulation and FVIII-specific assays
The WBCT was measured following standard procedures. CBT was measured as described.13,15 21 Briefly, dogs were lightly anesthetized and placed in a supine position, and the fur around the nail was clipped. The nail was then severed directly proximal to the dorsal nail groove and the time in minutes until clot formation was recorded. The one-stage FVIII coagulant analyses (based on the activated partial thromboplastin time, APTT) were performed on the dog plasma samples using the Organon Teknika Automated APTT reagent (Durham, NC) and an automated coagulometer (General Diagnostics Coag-A-Mate, Toronto, ON, Canada) following the manufacturer's instructions. Both human reference plasma (Normal Reference Plasma, Precision Biologicals, Dartmouth, NS, Canada) and canine pooled plasma were used as the controls. The limit of sensitivity of the one-stage coagulant assay using canine plasma was less than 300 mU/mL.
Biologically active canine FVIII was also measured in the dog plasma using the Coatest chromogenic bioassay (DiPharma, West Chester, OH). Coatest measures the FVIII-dependent generation of factor Xa from factor X, with 1 U defined as the amount of FVIII activity in 1 mL of pooled human plasma, 100 to 200 ng/mL.26 Pooled human plasma (George King Bio-Medical, Overland Park, KS) was used as the FVIII activity standard to generate a standard curve. When compared to human plasma, normal canine plasma FVIII levels were 5- to 10-fold higher.9,27 28 The Coatest assay displayed a limit of sensitivity of 7 mU/mL in the presence of canine plasma.
Anticanine FVIII inhibitory antibodies were measured using the Bethesda assay.29 Various dilutions of test plasmas were mixed 1:1 with a normal canine plasma pool and incubated at 37°C for 2 hours and the residual FVIII coagulant activity determined with a conventional one-stage FVIII:C assay. The dilution with residual activity closest to 50% was used to calculate the inhibitor titer, in which 50% residual FVIII activity equals 1 Bethesda unit (BU) per milliliter.
Hematology assays
Fibrinogen assays and platelet counts were performed at Queen's University, using standard procedures. Fibrin split products were measured using the semiquantitative D-dimer ACCUCLOT kit (Sigma Diagnostics, St Louis, MO). To assay liver toxicity, serum was collected before and at the indicated times following vector administration, centrifuged, aliquoted, and frozen. A total of 60 μL of each serum sample was submitted to Ani-Lytics, Inc (Gaithersburg, MD) and analyzed for the presence of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (AP), and total bilirubin levels.
Results
High-level canine FVIII expression and phenotypic correction of canine hemophilia A
The recombinant adenoviral vector, Av3H8401,11contains the mouse albumin promoter, an intron from the humanapolipoprotein A1 gene, a canine B-domain deleted (BDD) FVIII cDNA, and 1.5 kb of the canine 3′ untranslated region (UTR). The vector backbone was derived from adenovirus serotype 5 (Ad5) and is devoid of the E1, E2a, and E3 regions.23 Evaluation of Av3H8401 in hemophiliac mice demonstrated expression of canine FVIII sustained for at least 1 year at levels 10-fold higher than human FVIII expression from an analogous vector.11
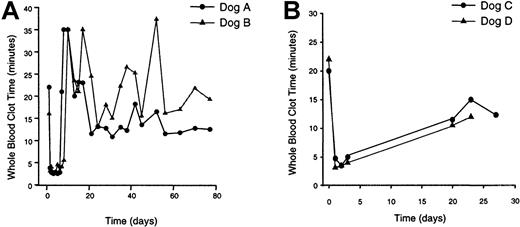
Av3H8401 was administered to 4 dogs by cephalic vein infusion (Table1). Dogs A and B received a vector dose of 3 × 1012 particles/kg; dogs C and D received a 5-fold lower vector dose (6 × 1011 particles/kg). WBCT was measured before vector infusion and at the indicated times following treatment (Figure 1). Prior to treatment all 4 dogs displayed an abnormal WBCT of more than 15 minutes. At 1 day, the WBCT was normalized to less than 5 minutes in all 4 animals. The WBCT remained corrected for 3 to 5 days after which the WBCT returned to abnormal levels.
Treatment summary of FVIII-deficient dogs
| Animal . | Gender . | Age (mo) . | Weight (kg) . | Vector dose (part/kg) . | Peak FVIII plasma activity (mU/mL) . | FVIII antibody titer (BU) . | ||
|---|---|---|---|---|---|---|---|---|
| APTT . | Coatest . | Peak . | Pre/Post reinfusion . | |||||
| Dog A (Riley) | Male | 10 | 11.8 | 3 × 1012 | 18 700 | 58 680 | 1210 | 41/51 |
| Dog B (JJ) | Male | 10 | 10.0 | 3 × 1012 | 19 900 | 43 591 | 37 | 1.7/2.5 |
| Dog C (Cocoa) | Female | 12 | 12.0 | 6 × 1011 | 1347 | 1335 | 1.9 | 0/0 |
| Dog D (Java) | Male | 12 | 18.5 | 6 × 1011 | 1427 | 1725 | 1.4 | 0/0 |
| Animal . | Gender . | Age (mo) . | Weight (kg) . | Vector dose (part/kg) . | Peak FVIII plasma activity (mU/mL) . | FVIII antibody titer (BU) . | ||
|---|---|---|---|---|---|---|---|---|
| APTT . | Coatest . | Peak . | Pre/Post reinfusion . | |||||
| Dog A (Riley) | Male | 10 | 11.8 | 3 × 1012 | 18 700 | 58 680 | 1210 | 41/51 |
| Dog B (JJ) | Male | 10 | 10.0 | 3 × 1012 | 19 900 | 43 591 | 37 | 1.7/2.5 |
| Dog C (Cocoa) | Female | 12 | 12.0 | 6 × 1011 | 1347 | 1335 | 1.9 | 0/0 |
| Dog D (Java) | Male | 12 | 18.5 | 6 × 1011 | 1427 | 1725 | 1.4 | 0/0 |
Four hemophilic dogs were treated with the canine FVIII-encoding adenoviral vector, Av3H8401, via cephalic vein infusion. Age and weight of the animals directly prior to vector administration is displayed. Plasma samples were analyzed for FVIII biologic activity using the one-stage FVIII coagulant assay (APTT) and the Coatest chromogenic bioassay. Peak FVIII plasma levels were obtained 2 days after vector treatment in all 4 dogs. Plasma samples collected prior to and following vector treatment were analyzed for the presence of anticanine FVIII inhibitory antibodies using the Bethesda assay. All animals displayed a Bethesda titer of 0 prior to vector treatment. Peak FVIII antibody titers were obtained 12 days after vector treatment in dogs A and B and 14 days after vector treatment in dogs C and D. Canine cryoprecipitate (20 FVIII Unit/kg) was delivered by IV administration to dogs A and B at 26 months and dogs C and D at 20 months after vector treatment. Directly prior to and 10 days following FVIII protein infusion, antibody levels were measured. Dog A received 2 infusions of cryoprecipitate (20 FVIII U/kg). Following the first infusion, antibody levels were followed for 4 weeks with no change detected. The data displayed are antibody levels following the second protein infusion.
WBCT of vector-treated hemophilic dogs.
Blood was collected from the dogs at the indicated times prior to and following vector treatment and assessed for WBCT. (A) Dogs treated with Av3H8401 at a dose of 3 × 1012 particles/kg. (B) Dogs treated with Av3H8401 at a dose of 6 × 1011particles/kg. Pretreatment WBCT for dog A was 22 minutes; at 22 months, WBCT was 16 minutes. Pretreatment WBCT for dog B was 16 minutes; at 22 months, WBCT was 20 minutes. Pretreatment WBCT for dog C was 20 minutes; at 16 months, WBCT was 16 minutes 45 seconds. Pretreatment WBCT for dog D was 22 minutes; at 16 months, WBCT was 15 minutes 45 seconds.
WBCT of vector-treated hemophilic dogs.
Blood was collected from the dogs at the indicated times prior to and following vector treatment and assessed for WBCT. (A) Dogs treated with Av3H8401 at a dose of 3 × 1012 particles/kg. (B) Dogs treated with Av3H8401 at a dose of 6 × 1011particles/kg. Pretreatment WBCT for dog A was 22 minutes; at 22 months, WBCT was 16 minutes. Pretreatment WBCT for dog B was 16 minutes; at 22 months, WBCT was 20 minutes. Pretreatment WBCT for dog C was 20 minutes; at 16 months, WBCT was 16 minutes 45 seconds. Pretreatment WBCT for dog D was 22 minutes; at 16 months, WBCT was 15 minutes 45 seconds.
The CBT, an in vivo test sensitive to discrete coagulation factor deficiencies,13 was performed before vector treatment and after vector administration. The normal range for clot formation in dogs following cuticle severing is 2 to 8 minutes.13 For dogs C and D, CBT before vector treatment was 13 and 14 minutes, respectively, indicative of the hemophiliac phenotype. At 2 days, CBT was completely corrected at 4 minutes for both animals. CBT for dogs A and B before treatment was 11 and 12 minutes, respectively. At 6 days, dog A's CBT was abnormal at 12 minutes, and dog B displayed a CBT of 9 minutes. CBT was not performed on dogs A and B at 2 days. Normalization of the WBCT and CBT coagulation parameters demonstrated complete, albeit transient, phenotypic correction of canine hemophilia A.
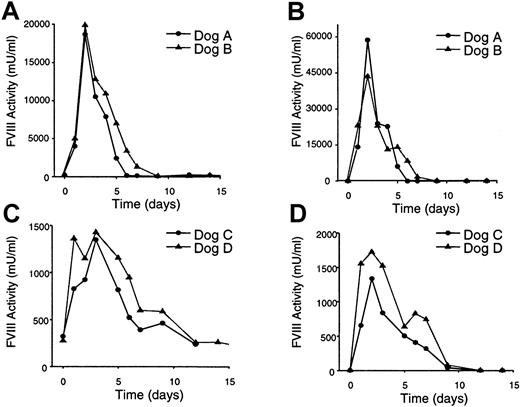
Canine FVIII biologic activity was determined by analysis of plasma samples collected before and after vector administration, using the one-stage FVIII coagulant assay (based on the APTT), and the FVIII chromogenic bioassay, Coatest (Figure 2). Prior to treatment, FVIII biologic activity detected by APTT was less than 300 mU/mL, and no FVIII was detected in the plasma using the Coatest assay. Peak FVIII expression in all 4 treated dogs was detected at 2 days with both the APTT and Coatest assays. Dogs A and B displayed extremely high plasma levels of FVIII, up to 58 times normal human FVIII plasma levels26 (Table 1). Dogs C and D displayed peak FVIII plasma levels of 1 to 2 U/mL, equivalent to normal human physiologic levels26 (Table 1). FVIII expression persisted in dog A for 5 days, whereas dogs B, C, and D displayed FVIII expression out to 9 days (Figure 2). The FVIII expression values obtained using the APTT and Coatest assays were in good agreement; however, Coatest activity values at high FVIII expression levels were routinely 2- to 3-fold higher than those detected by the APTT assay (Figure 2). This discrepancy between assays had been noted previously when using a recombinant B domain-deleted FVIII concentrate and appears to be phospholipid dependent.30
Plasma FVIII expression levels in treated hemophilic dogs.
Plasma was collected from the dogs at the indicated times prior to and following vector treatment and assessed for FVIII biologic activity. (A) One-stage FVIII coagulant assay (APTT) plasma analyses from dogs treated with the higher vector dose (3 × 1012particles/kg). (B) Coatest bioassay plasma analyses from dogs treated with higher vector dose (3 × 1012 particles/kg). (C) One-stage FVIII coagulant assay (APTT) plasma analyses from dogs treated with the lower vector dose (6 × 1011particles/kg). (D) Coatest bioassay plasma analyses from dogs treated with the lower vector dose (6 × 1011particles/kg).
Plasma FVIII expression levels in treated hemophilic dogs.
Plasma was collected from the dogs at the indicated times prior to and following vector treatment and assessed for FVIII biologic activity. (A) One-stage FVIII coagulant assay (APTT) plasma analyses from dogs treated with the higher vector dose (3 × 1012particles/kg). (B) Coatest bioassay plasma analyses from dogs treated with higher vector dose (3 × 1012 particles/kg). (C) One-stage FVIII coagulant assay (APTT) plasma analyses from dogs treated with the lower vector dose (6 × 1011particles/kg). (D) Coatest bioassay plasma analyses from dogs treated with the lower vector dose (6 × 1011particles/kg).
Liver toxicity and inhibitor development in treated dogs
Peripheral vein administration of adenoviral vectors results in efficient liver transduction in mice,31dogs,9 and monkeys.8 We have shown previously that vector-induced hepatotoxicity resulted in short-term FVIII expression caused by the loss of vector DNA from the transduced mouse livers.9 Therefore, the persistence of FVIII expression in the treated dogs may have been limited by the direct toxicity of the vector,9 a cellular immune response against expressed adenoviral genes or the FVIII transgene,32-35 or the development of FVIII-specific inhibitory antibodies.9
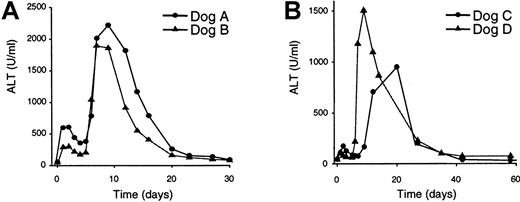
To measure hepatotoxicity in the treated dogs, serum collected before and after vector administration was analyzed for the presence of several liver enzymes: ALT (Figure 3), AST, AP, and bilirubin (data not shown). Elevation of ALT and AST indicates hepatocellular necrosis, whereas increased levels of AP and bilirubin correlate with biliary obstruction.36 All 4 treated dogs displayed a significant increase in the ALT, AST, and AP levels. Interestingly, both animals that received the higher vector dose (dogs A and B; Figure 3A) and the lower vector dose (dogs C and D; Figure 3B) showed a similar pattern of ALT accumulation in the serum. Following an initial elevation in ALT levels 1 to 3 days after vector infusion, the ALT levels began to resolve at days 4 to 5, and then displayed a dramatic increase at days 7 to 12. However, the initial elevation in ALT levels was attenuated in the animals that received the lower vector dose, whereas the secondary elevation was similar in both groups of dogs (Figure 3). AST and AP displayed a similar accumulation pattern (data not shown). Bilirubin was detected in the serum of dog B 9 days after vector infusion at a level 2- to 3-fold higher than normal, and levels returned to normal by day 12. No accumulation of bilirubin was detected in the other 3 treated dogs. These data demonstrate that vector administration to the hemophilic dogs resulted in a biphasic hepatotoxicity that resolved over the course of the study (16-22 months).
Plasma liver enzyme analyses.
Plasma was collected from the dogs at the indicated times prior to and following vector treatment and assessed for the presence of the liver enzyme ALT. (A) ALT analyses of dogs that received the higher vector dose (3 × 1012 particles/kg). (B) ALT analyses of dogs that received the lower vector dose (6 × 1011particles/kg). Pretreatment ALT value for dog A was 54 U/mL; at 22 months, ALT was 69 U/mL. Pretreatment ALT value for dog B was 47 U/mL; at 22 months, ALT was 55 U/mL. Pretreatment ALT value for dog C was 46 U/mL; at 16 months, ALT was 33 U/mL. Pretreatment ALT value for dog D was 44 U/mL; at 16 months, ALT was 47 U/mL.
Plasma liver enzyme analyses.
Plasma was collected from the dogs at the indicated times prior to and following vector treatment and assessed for the presence of the liver enzyme ALT. (A) ALT analyses of dogs that received the higher vector dose (3 × 1012 particles/kg). (B) ALT analyses of dogs that received the lower vector dose (6 × 1011particles/kg). Pretreatment ALT value for dog A was 54 U/mL; at 22 months, ALT was 69 U/mL. Pretreatment ALT value for dog B was 47 U/mL; at 22 months, ALT was 55 U/mL. Pretreatment ALT value for dog C was 46 U/mL; at 16 months, ALT was 33 U/mL. Pretreatment ALT value for dog D was 44 U/mL; at 16 months, ALT was 47 U/mL.
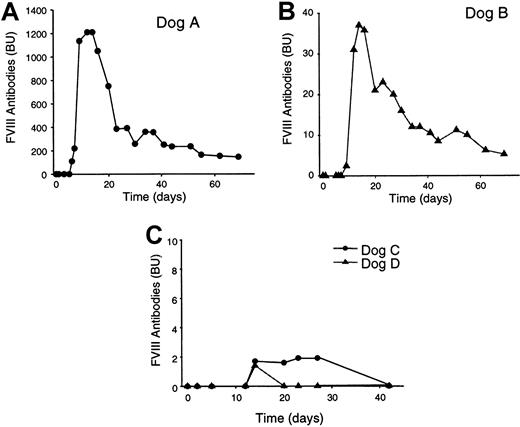
To determine if the loss of FVIII expression was due to the development of canine FVIII-specific inhibitory antibodies, Bethesda assays29 were used to measure FVIII inhibitors in plasma samples before and after treatment. All 4 dogs displayed no detectable Bethesda titer before vector treatment (Table 1, Figure4). Dog A developed a high-level anticanine FVIII antibody titer (109 BU) by day 6 following vector treatment, which increased rapidly to a peak of 1210 BU at days 12 and 14 (Figure 4). The inhibitor titer decreased rapidly, however, and by day 69 was reduced to 145 BU (Figure5). At 22 months, dog A had an anticanine FVIII titer of 46 BU. The occurrence of anticanine FVIII antibodies in dog A was not unexpected because this animal was from the line of hemophilic dogs within the Queen's University colony, genetically predisposed to the development of canine FVIII-specific antibodies.21 In contrast, dogs B, C, and D were from the hemophilic dog line that does not develop antibodies even after repeated treatment with normal canine plasma or cryoprecipitate.21 Dog B developed an anticanine FVIII inhibitor titer of 2.4 BU by day 9, which increased to a peak of 37 BU by day 14 (Table 1, Figure 4). This inhibitor titer also decreased rapidly and by day 69 was 5.2 BU and at 22 months was 2.3 BU (Table 1, Figure 4). In contrast, the dogs that received the lower vector dose, dogs C and D, developed low-level, transient, inhibitors (1-2 BU) lasting 1 (dog D) to 4 (dog C) weeks (Figure 4). By 16 months, both dogs C and D remained inhibitor-free (Figure 4).
Anticanine FVIII inhibitory antibody development in treated dogs.
Plasma was collected from the dogs at the indicated times prior to and following vector treatment and tested for the presence of FVIII inhibitory antibodies by Bethesda assay. (A) Bethesda titer of dog A. (B) Bethesda titer of dog B. (C) Bethesda titer of dogs C and D. All animals displayed a Bethesda titer of 0 prior to vector treatment. Final titer for dog A was 46.0 BU at 22 months; final titer for dog B was 2.3 BU at 22 months; final titer for dog C was 0 BU at 16 months; and final titer for dog D was 0 BU at 16 months.
Anticanine FVIII inhibitory antibody development in treated dogs.
Plasma was collected from the dogs at the indicated times prior to and following vector treatment and tested for the presence of FVIII inhibitory antibodies by Bethesda assay. (A) Bethesda titer of dog A. (B) Bethesda titer of dog B. (C) Bethesda titer of dogs C and D. All animals displayed a Bethesda titer of 0 prior to vector treatment. Final titer for dog A was 46.0 BU at 22 months; final titer for dog B was 2.3 BU at 22 months; final titer for dog C was 0 BU at 16 months; and final titer for dog D was 0 BU at 16 months.
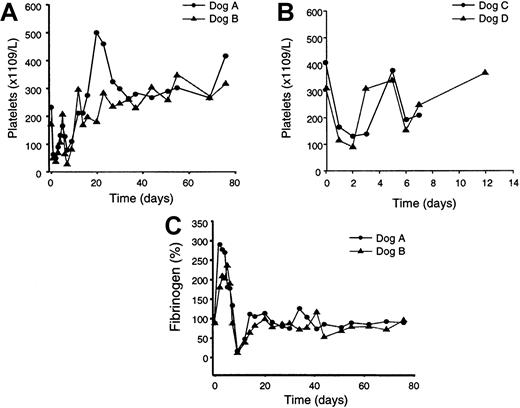
Platelet counts and fibrinogen levels of treated dogs.
Blood was collected from the dogs at the indicated times prior to and following vector treatment and platelet and fibrinogen levels were measured. (A) Platelet counts in the dogs treated with the higher vector dose (3 × 1012 particles/kg). (B) Platelet counts in the dogs treated with the lower vector dose (6 × 1011particles/kg). (C) Fibrinogen levels in the dogs treated with the higher vector dose. Pretreatment platelet counts for dog A were 231 (× 1109/L); 22-month counts were 276 (× 1109/L). Pretreatment platelet counts for dog B were 169 (× 1109/L); 22-month counts were 240 (× 1109/L). Pretreatment platelet counts for dog C were 405 (× 1109/L); 16-month counts were 185 (× 1109/L). Pretreatment platelet counts for dog D were 312 (× 1109/L); 16-month counts were 168 (× 1109/L).
Platelet counts and fibrinogen levels of treated dogs.
Blood was collected from the dogs at the indicated times prior to and following vector treatment and platelet and fibrinogen levels were measured. (A) Platelet counts in the dogs treated with the higher vector dose (3 × 1012 particles/kg). (B) Platelet counts in the dogs treated with the lower vector dose (6 × 1011particles/kg). (C) Fibrinogen levels in the dogs treated with the higher vector dose. Pretreatment platelet counts for dog A were 231 (× 1109/L); 22-month counts were 276 (× 1109/L). Pretreatment platelet counts for dog B were 169 (× 1109/L); 22-month counts were 240 (× 1109/L). Pretreatment platelet counts for dog C were 405 (× 1109/L); 16-month counts were 185 (× 1109/L). Pretreatment platelet counts for dog D were 312 (× 1109/L); 16-month counts were 168 (× 1109/L).
The effect of infusion of canine FVIII protein on the titer of FVIII inhibitory antibodies in the vector-treated dogs was evaluated. FVIII cryoprecipitate (20 U/kg) was delivered through intravenous administration to dogs A and B at 26 months and to dogs C and D at 20 months following initial vector exposure. Inhibitory antibody levels were measured directly before and 10 days after protein administration (Table 1). No significant increase in inhibitory antibody titer was detected in any animal. Furthermore, dog A received 2 infusions of FVIII cryoprecipitate (20 U/kg). Following the first infusion, antibody levels were followed for 4 weeks with no change detected (data not shown). The second protein administration was performed simultaneously with the other 3 dogs. Following the second infusion, again, no increases in antibody levels were detected (Table 1).
Platelet counts, fibrinogen levels, and fibrin split products in treated dogs
Platelet and fibrinogen levels in the 2 dogs treated with the higher vector dose, dogs A and B, were followed for 76 days after vector administration (Figure 5A,C). Two days after vector treatment, the platelet levels in both dogs dropped approximately 80%. Levels remained low until day 12, after which the platelet levels increased to levels up to 2-fold higher than the pretreatment values. In contrast, fibrinogen levels increased 1.5- to 3-fold 2 days after vector treatment and remained elevated until day 7 (Figure 5C), likely representing an acute phase response to the vector delivery and initial hepatotoxicity. At day 9, however, fibrinogen levels dropped sharply coincident with the second phase of hepatotoxicity (Figure 3A). By days 14 to 16, fibrinogen levels were completely normalized to pretreatment values and remained normal to 22 months. Analysis of the day 9 plasma from dogs A and B revealed the presence of fibrin split products, suggesting the occurrence of disseminated intravascular coagulation (DIC). Platelet counts in the 2 dogs receiving the lower vector dose, dogs C and D, also showed an initial 70% decline 1 to 2 days following vector treatment, which resolved by 12 days (Figure 5B). Fibrinogen measurements were not performed with dogs C and D. Fibrin split products were not detected in day 5 plasma samples from dogs C and D.
Discussion
This work represents the first report of in vivo gene therapy of FVIII-deficient dogs using an adenoviral vector-encoding canine FVIII. Expression of the canine protein in hemophilic dogs would be analogous to treating humans with hemophilia with vectors encoding the human protein. Previous work demonstrated that expression of human FVIII in hemophilic dogs resulted in the development of a strong, antihuman FVIII antibody response and short-term FVIII expression.9 Because canine FVIII is significantly less immunogenic in hemophilic dogs than the human protein,20 21 we reasoned that sustained phenotypic correction of canine hemophilia A would require the expression of canine FVIII. In this work, we have achieved an extremely high level of expression of canine FVIII in the hemophilic dogs and complete phenotypic correction of the bleeding disorder. However, FVIII expression was short-term, lasting less than 2 weeks. All treated animals developed severe liver toxicity that resolved over the course of the study. All 4 dogs developed canine FVIII inhibitory antibodies, which in one animal, genetically predisposed to inhibitor development, reached a peak of 1210 BU; the 2 animals treated with the lower vector dose developed low-level, transient inhibitory antibodies lasting 1 to 4 weeks.
The hepatotoxicity observed in the treated dogs displayed a biphasic pattern. The first phase was dose-dependent, whereas the second phase was dose-independent. Dogs that received the higher vector dose initially showed a 6- to 11-fold increase in ALT levels, whereas dogs that received the 5-fold lower vector dose initially showed a 2- to 4-fold increase in ALT levels. In all animals, the ALT levels began to normalize 3 days after treatment but by 7 days had increased sharply to extremely high levels. The initial, dose-dependent hepatotoxicity may have been caused by the direct toxicity of the vector to the transduced hepatocytes, similar to what had been observed previously in FVIII adenoviral vector-treated mice.5
The secondary, dose-independent rise in liver enzyme levels at 7 days may be best explained by the development of a cellular immune response directed against transduced hepatocytes expressing adenoviral backbone genes or the canine FVIII transgene or both. Previous studies, using mouse models, have demonstrated that adenoviral vectors or immunogenic transgenes may be associated with cytotoxic T lymphocyte-mediated elimination of transduced cells and, thereby, the loss of gene expression.32,33,35,37-39 In many cases, the response to the transgene or viral backbone gene expression was influenced by the mouse strain used in the study.38,40 Therefore, the use of a large, out-bred animal model such as the hemophilic dogs may complicate the dissection of the immune components involved in adenoviral vector-mediated gene therapy of canine hemophilia A. However, the further attenuation of viral backbone gene expression, by the generation of gutless adenoviral vectors from which all viral backbone genes have been removed,41 will reduce the response to viral gene products and may reduce vector toxicity.42-45
Alternatively, the hepatotoxicity observed in the treated dogs may have been caused by the expression of extremely high levels of canine FVIII. A high level of FVIII protein expression is known to be toxic to cultured rodent cells in vitro (unpublished observations). However, this possibility seems unlikely because dogs C and D, animals that received the lower vector dose and displayed significant liver toxicity, expressed canine FVIII at low levels, 1 to 2 U/mL, which is 5- to 10-fold lower than the FVIII plasma levels measured in normal dogs (5-10 U/mL).9,27 28
Sustained canine FVIII expression in the vector-treated dogs was also limited by the development of canine FVIII inhibitory antibodies. The 2 dogs that received the higher vector dose displayed the higher initial elevation in liver enzyme levels and rapidly developed high titers of canine FVIII inhibitor. The occurrence of canine FVIII inhibitory antibodies in dog A was not unexpected because this animal was derived from the line of hemophiliac dogs within the colony predisposed to the development of canine FVIII-specific antibodies.21This propensity to inhibitor development is genetically based and independent of the FVIII mutation.21 However, the mechanism of inhibitor development remains uncertain and is the subject of continued research in the laboratory. In contrast, dogs B, C, and D, derived from the hemophiliac dog line that does not routinely develop canine FVIII-specific antibodies,21 also developed inhibitors. Of these animals, dog B, who received the higher vector dose, showed the highest inhibitor level (peak of 37 BU), whereas dogs C and D showed low (1-2 BU) antibody levels persisting for less than 1 month. These observations suggest that a higher vector dose and, perhaps, more severe vector-induced hepatotoxicity were responsible for inhibitor development in these animals. It is possible, therefore, that vector-mediated liver toxicity resulted in the hyperreactivity of the immune system to canine FVIII, potentially caused by the incomplete or incorrect processing of the canine FVIII protein in the transduced hepatocytes. This hypothesis suggests that elimination of the liver toxicity may also limit the development of canine FVIII-specific inhibitory antibodies in hemophilic dogs that are not genetically prone to inhibitor generation. Indeed, FVIII-deficient mice treated with an analogous vector encoding human FVIII display minimal hepatotoxicity7,11 and do not develop an immune response directed toward human FVIII.7 In contrast, a recent report has demonstrated sustained expression of human FVIII in some hemophilic mice treated with an adenoviral vector lacking all viral genes.43 Although no hepatotoxicity was observed with this attenuated vector, 13 of 16 treated mice developed antihuman FVIII antibodies, which resulted in short-term expression of the human protein.43 It will be interesting to test a similar, highly attenuated vector in the FVIII-deficient canine model.
Interestingly, infusion of canine FVIII cryoprecipitate in the vector-treated dogs had no effect on the titer of inhibitory antibodies. Furthermore, dog A, the animal predisposed to the development of canine FVIII inhibitory antibodies and the animal with the highest inhibitor titer, was treated with canine cryoprecipate twice following vector delivery. No change in inhibitor titer was detected at any time point evaluated. This observation was unexpected because infusion of canine FVIII cryoprecipitate in animals with existing inhibitor titers induced by protein infusion consistently resulted in an anamnestic response.21 These data suggest a difference between vector-induced inhibitory antibody development and FVIII protein-induced antibody development. Further studies investigating the immunogenicity of gene therapy versus protein replacement treatment are in progress.
We found a treatment-dependent, transient decrease in mean platelet counts in all 4 treated dogs. Additionally, transient fluctuations in fibrinogen levels, peaking 3-fold higher than baseline, and then dropping to 20% of pretreatment levels, were also observed. A similar pattern of fibrinogen fluctuation was observed in monkeys treated with a first-generation adenoviral vector encoding human factor IX.46 However, none of the dogs developed symptomatic bleeding. The 2 animals that received the higher vector dose showed an 80% to 90% decrease in platelets, whereas the 2 animals that received the lower vector dose showed a 70% decrease in platelets. Transient thrombocytopenia caused by systemic delivery of adenoviral vectors has been described previously in mice,47rabbits,47 and monkeys,8 although its cause remains elusive. The first and most worrying hypothesis is that the drop in platelets was due to DIC. This mechanism is consistent with the transient decrease in fibrinogen levels observed on days 9 to 12 in the higher-dosed dogs and the presence of fibrin split products in the day 9 plasma samples. However, fibrin split products were not detected in the lower-dosed dogs, and none of the dogs developed symptomatic evidence of a consumptive coagulopathy. A second explanation for this transient hypofibrinogenemia may have been causally related to the second phase of hepatotoxicity. Additionally, there was no evidence of DIC in the rabbit47 and nonhuman primate8models of vector-mediated thrombocytopenia. Another explanation for the decrease in platelet levels is that the vector bound to platelets resulting in sequestration in the spleen or destruction via complement activation. However, in vitro evaluation in the rabbit model yielded no evidence to support complement-mediated platelet destruction.47 Furthermore, vector exposure did not cause platelet aggregation, loss of reactivity, or changes in morphology.47 Two other possibilities remain largely unexplored. First, adenoviral vectors could activate endothelial cells resulting in platelet adsorption. Indeed, Channon and coworkers48 have demonstrated that vector infusion activates endothelial cells in a rabbit artery model. Finally, vector infusion could result in a transient decrease in platelet production by suppressing megakaryocyte maturation. Megakaryocyte dysfunction has been associated with a variety of viral infections.49 A better understanding of the mechanism of platelet loss will be necessary to determine the suitability of adenoviral vectors for the treatment of hemophilia as well as other diseases.
In this work, effective, albeit transient, correction of canine hemophilia A was achieved by systemic delivery of an adenoviral vector encoding the canine FVIII cDNA. Furthermore, important hepatotoxic and hematologic consequences to systemic delivery of adenoviral vectors were observed. The development of further attenuated vectors designed to eliminate vector-mediated toxicity and reduce the immune response to transduced cells41 may be necessary to enable long-term FVIII expression. The achievement of sustained phenotypic correction of the coagulation defect in a clinically relevant large animal model will provide a crucial step in the verification of the feasibility of adenoviral vector-mediated gene therapy for hemophilia A.
Acknowledgment
The authors thank Dr Ying Huang for critical review of the manuscript.
Supported in part by grant MT-10912 from the Medical Research Council of Canada. D.L. is supported by a Career Investigator Award from the Heart and Stroke Foundation of Ontario.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sheila Connelly, Genetic Therapy, Inc, 9 West Watkins Mill Rd, Gaithersburg, MD; e-mail:sheila.connelly@pharma.novartis.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal