The chemokine, stromal cell–derived factor-1 (SDF1), is produced in the bone marrow and has been shown to modulate the homing of stem cells to this site by mediating chemokinesis and chemotaxis. Therefore, it was hypothesized that elevation of SDF1 level in the peripheral circulation would result in mobilization of primitive hematopoietic stem and progenitor cells. SDF1 plasma level was increased by intravenous injection of an adenoviral vector expressing SDF1α (AdSDF1) into severe combined immunodeficient mice. This resulted in a 10-fold increase in leukocyte count, a 3-fold increase in platelets, and mobilization of progenitors, including colony-forming units–granulocyte-macrophage to the peripheral circulation. In addition, AdSDF1 induced mobilization of cells with stem cell potential, including colony-forming units in spleen and long-term reconstituting cells. These data demonstrate that overexpression of SDF1 in the peripheral circulation results in the mobilization of hematopoietic cells with repopulating capacity, progenitor cells, and precursor cells. These studies lay the foundation for using SDF1 to induce mobilization of hematopoietic stem and progenitor cells in in vivo studies.
Introduction
In adulthood, hematopoiesis is restricted to the extravascular compartment of the bone marrow (BM) separated by a single layer of BM endothelial cells (BMECs). Thus, hematopoietic stem cells (HSCs) arriving at the BM must first be recognized by the luminal surface of the BMEC.1-5 Molecules that mediate adhesion of HSCs to BMECs are likely to play a pivotal role in the phenomenon of HSC homing.1-3,6-8 Similar to leukocyte trafficking,9 10 homing of HSCs from the peripheral circulation to the BM is a multistep process and involves sequential interaction of CD34+ cells with adhesion molecules expressed on BMECs, and specific chemokine(s) expressed within the BM. Chemokines orchestrate this process by providing directional cues for CD34+ cells to migrate into the BM microenvironment.
The chemokine, stromal cell–derived factor-1 (SDF1), which is produced by marrow stromal cells, has been shown to play a key role in CD34+ trafficking.11-14 Targeted gene knockout of either SDF115 or its receptor CXCR416,17resulted in a defect in BM hematopoiesis, whereas fetal liver hematopoietic activity remained intact. Recently, it was shown that transendothelial migration of primitive hematopoietic precursors, long-term BM culture-initiating cells, and cobblestone-area–forming cells are CXCR4 dependent and essential for homing and engraftment of nonobese diabetic (NOD)/severe combined immunodeficiency (SCID) mice engrafting cells.13 18
On the basis of these results, we hypothesized that elevation of SDF1 in the peripheral circulation may result in significant mobilization of HSCs and progenitor cells. In this regard, to explore the potential of SDF1 to induce mobilization of hematopoietic cells, adenoviral (Ad) vector expressing SDF1 (AdSDF1) was injected intravenously (IV) into mice. Ad vectors are ideal vectors to deliver factors with chemokinetic potential, such as SDF1, because they allow for sufficient plasma elevation of a given chemokine for durations long enough to exert their physiologic effect. The IV injection of Ad vectors expressing a given chemokine results primarily in the localization of the Ad vectors to the liver of mice facilitating constitutive release of chemokine to the peripheral circulation.19 Duration of chemokine expression is dependent on the rate of clearance of the Ad vector by the immune response. Indeed, we have shown that IV injection of Ad vector expressing thrombopoietin results in thrombocytosis for 10 days in immunocompetent BALB/c mice, and it can last as long as 30 days in immunodeficient SCID mice without any apparent toxicities.20
In this report we demonstrate that IV injection of AdSDF1 into mice resulted in profound mobilization of progenitors and cells with repopulating capacity. In addition, AdSDF1 induced a remarkable increase in white blood cell (WBC) and platelet counts without any apparent toxicity. On the basis of these results, we conclude that SDF1 may provide a useful means of mobilizing hematopoietic stem, progenitor, and precursor cells.
Materials and methods
Animals
All BALB/c background SCID mice matched for age (8 weeks), weight (more than 20 g), and sex were purchased from the Jackson Laboratory (Bar Harbor, ME) and maintained in germ-free conditions. All mice received AdSDF1, the adenoviral vector expressing macrophage inflammatory protein-3α (AdMIP3α), and no transgene (AdNull) (1 × 109 plaque-forming unit (pfu) in a volume of 100 μL, single IV administration on day 0). Each study contained 6 animals. Age-matched female BALB/c mice were used in colony-forming unit-spleen (CFU-S) assay and syngeneic transplantation as recipients. Female C57BL/6 mice were used in allogeneic peripheral blood cell transplantation as recipients.
Ad vectors
AdSDF1 is an Ad5-derived E1a-, E3-deficient (E1a−E3−E4+) vector with an expression cassette in the E1a region containing the human SDF1α complementary DNA (cDNA) and driven by the cytomegalovirus (CMV) major immediate/early promoter/enhancer. AdMIP3α is also an Ad5-derived E1a−E3−E4+ vector with an expression cassette in the E1a region containing the human MIP3α cDNA and driven by the CMV major immediate/early promoter/enhancer. The control vector, AdNull, is similar in design, except that it contains no transgene in the expression cassette. All vectors were amplified, purified, and titered as previously described.21
Immunoassays of SDF1
Human SDF1 in plasma and BM samples of Ad-treated mice were quantitated by SDF1 time-resolved fluoro-immuno assay (TRFIA)22 by using 2 anti-SDF1 antibodies (established by K.T. et al; Kyoto University, Kyoto, Japan). Each level of SDF1 shows the total of the levels of human SDF1α and human SDF1β. To obtain murine BM samples for TRFIA, 5 mice in each group were humanely killed by cervical dislocation 3, 5, and 14 days after vector administration. BM was collected from these animals by aspiration of the BM from femur and tibia.23
Peripheral blood analysis
Initially, every 2 to 3 days and later on a weekly basis, retro-orbital blood was collected with capillary pipettes (Unopette; Fisher Scientific, Springfield, MA). Platelets, total WBCs, and granulocytes (polymorphonuclear leukocytes) were counted using a Neubauer hematocytometer (Fisher Scientific). Differential leukocyte counts were obtained by examination of blood smears from each mouse stained with Wright-Giemsa stain (200 cells counted/smear). The plasma was collected, stored at −80°C, and assessed later by immunoassay for human SDF1.
Flow cytometry
A total of 1 × 104 to 1 × 105cells were incubated for 30 minutes at 4°C with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies (MoAbs); CD3-FITC (145-2C-11; Pharmingen, San Diego, CA), CD11b-FITC (M1/70; Pharmingen), Gr-1-PE (RB6-8C5; Pharmingen), Sca-1-PE (D7; Pharmingen), CD34-FITC (RAM34; Pharmingen), H-2Kb-FITC (AF6-88.5; Pharmingen), and H-2Kd-FITC (SF-1-1.1; Pharmingen). The cells were analyzed by 2-color flow cytometry using a Coulter Elite flow cytometer (Coulter, Hialeah, FL).
Progenitor assay
Three animals in each group were humanely killed 7 days following vector administration. One femur per animal was flushed with cold (4°C) Iscoves modified Dulbecco medium (IMDM)/20% fetal calf serum (FCS), and the total yield of BM and spleen mononuclear cells (MNCs) was determined by counting an aliquot in 0.36% acetic acid using a Neubauer hematocytometer. Peripheral blood MNCs (PBMNCs) were isolated after centrifugation over a discontinuous gradient using Lympholyte-M (Cederlane, Ontario, Canada). MNCs (105) were plated in triplicate in 1 mL 0.8% methylcellulose containing 30% FCS, 1% L-glutamine, 2.5% hemin, 0.05 mM 5-ME, recombinant mouse interleukin 3 (rm IL-3; 50 ng/mL; R&D Systems, Minneapolis, MN), rm c-kit ligand (20 ng/mL; Immunex, Seattle, WA), and recombinant human erythropoietin (2 U/mL; Sandoz, Basel, Switzerland) in 35-mm suspension culture dishes (Nunc, Naperville, IL). Cultures were incubated at 37°C in 100% humidity and 5% CO2 for 7 days. Scoring was performed with an inverted microscope with ×40 magnification on day 7.
CFU-S assay
For each data point, 3 recipient mice were irradiated with 9 Gy from a 137Cs γ-ray source at a dose rate of approximately 0.90 Gy per minute to prevent the production of endogenous spleen colonies. Irradiated BALB/c mice (3 mice in each group) were injected intravenously through the tail vein with 1 × 105 PBMNCs within several hours after the completion of irradiation. The mice were humanely killed by cervical dislocation 12 days later, and their spleens were removed and fixed in Bouin solution. The number of macroscopic spleen colonies was then scored.
Allogeneic peripheral blood cell transplantation
After the vector administration, the peripheral blood from SCID mice (4 mice in each group) was collected on day 5 using Lympholyte-M (Cederlane) to remove erythrocytes. The recipient C57BL/6 mice (n = 8) were lethally irradiated (9.5 Gy) and intravenously injected with 1 × 106 PBMNCs of AdSDF1-treated SCID mice after irradiation on day 0. As a control, PBMNCs (1 × 106) of AdNull-treated SCID mice were transferred into irradiated C57BL/6 mice (n = 6). The chimeric mice were maintained in a laminar flow isolator. Chimerism was determined by flow-activated cell sorter (FACS) analyses (donor SCID mice, H-2Kd; recipient C57BL/6 mice, H-2Kb). The chimeric mice (at least 3) were humanely killed 90 days, 120 days, and 150 days after transplantation at each time point.
Syngeneic peripheral blood cell transplantation
After the vector administration, the peripheral blood from SCID mice (4 mice in each group) was collected on day 5 using Lympholyte-M (Cederlane) to remove erythrocytes. The recipient BALB/c mice (6 mice in each group) were lethally irradiated (9.5 Gy) and intravenously injected with serial cell doses (1 × 104, 1 × 105, and 1 × 106) PBMNCs after irradiation on day 0. Survival was monitored every day beyond day 150.
Spleen and BM analyses
BM was obtained by flushing both femoral bones with 3 mL cold IMDM containing 20% FCS. Manual leukocyte differentials were performed on Wright-Giemsa–stained cytospin preparations of BM cells and splenocytes.
Histopathology
Tissues were fixed in 10% buffered formalin and paraffin embedded. Sections were stained with hematoxylin and eosin and examined under microscopy.
Statistical evaluation
The results were expressed as mean ± SEM. Statistical analyses were performed using the unpaired 2-tailed Studentt test. Survival rates were compared between the 2 groups by the log-rank test.
Results
AdSDF1 promotes mobilization of leukocytes to the peripheral circulation
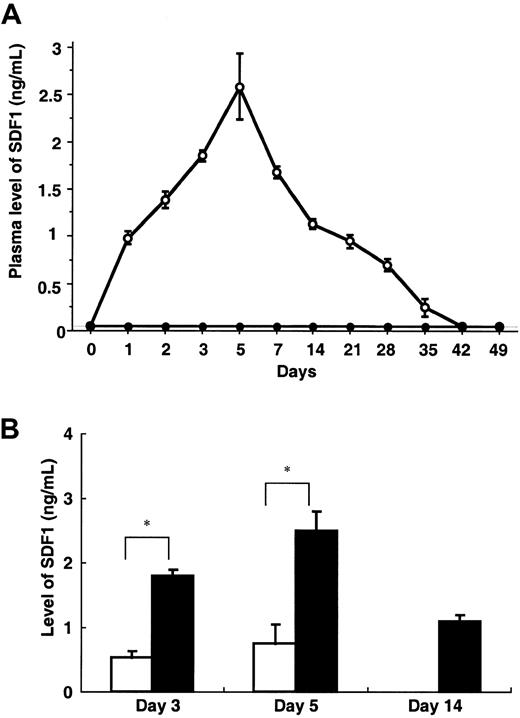
Intravenous administration of AdSDF1 into SCID mice resulted in the elevation of SDF1 plasma levels, peaking at day 5 (2.5 ± 0.3 ng/mL) and returning to pretreatment level at day 35 (Figure1A). However, IV administration of AdSDF1 resulted in only a small elevation of SDF1 levels (0.8 ± 0.1 ng/mL) in BM compared with plasma (*P < .005; Figure 1B). Plasma SDF1 was not detectable in the AdNull-treated mice.
Plasma level of SDF1.
(A) In SCID mice, the concentration of human SDF1 was measured throughout the study in the plasma of AdSDF1-treated (○) or AdNull-treated mice (●). All data are expressed as the mean (n = 4) ± SEM. (B) In SCID mice, the concentration of human SDF1 was measured in the plasma (▪) and BM (■) of AdSDF1-treated mice 3 days and 5 days after vector administration. All data are expressed as the mean (n = 5) ± SEM. The result from SDF1 level of BM compared to level of plasma achieves statistical significance (*P < .005).
Plasma level of SDF1.
(A) In SCID mice, the concentration of human SDF1 was measured throughout the study in the plasma of AdSDF1-treated (○) or AdNull-treated mice (●). All data are expressed as the mean (n = 4) ± SEM. (B) In SCID mice, the concentration of human SDF1 was measured in the plasma (▪) and BM (■) of AdSDF1-treated mice 3 days and 5 days after vector administration. All data are expressed as the mean (n = 5) ± SEM. The result from SDF1 level of BM compared to level of plasma achieves statistical significance (*P < .005).
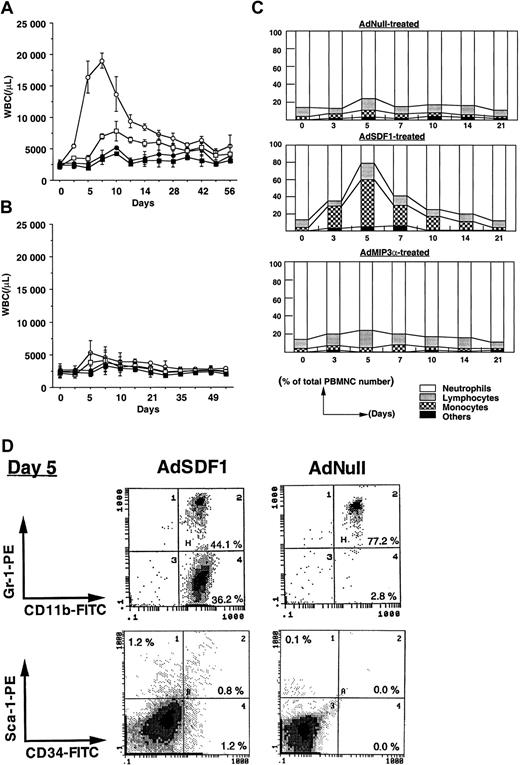
To examine the effect of SDF1 in the peripheral blood in mobilization of hematopoietic cells, we injected SCID mice intravenously with 109 pfu AdSDF1 on day 0 and monitored the change in leukocyte number and hematopoietic progenitor frequency in BM, spleen, and peripheral blood. As controls we used AdMIP3α or AdNull. The AdSDF1-treated SCID mice had peak leukocyte levels 10-fold above baseline at day 7, returning to the level of AdNull-treated control mice by 5 weeks after injection (Figure2A). The administration of AdMIP3α to SCID mice resulted in a slight increase of leukocyte levels, peaking at day 7 and returning to the control level at day 21 (Figure 2B). In general, increase in the neutrophil counts correlated with the level of WBCs in each AdSDF1-treated group; however, there was a significant difference in the leukocyte frequencies in AdSDF1-treated SCID mice. The percentage of monocytes in WBCs had increased dramatically at day 3 to 10 and almost returned to baseline after 3 weeks in AdSDF1-treated SCID mice (Figure 2C). Using flow-cytometric analysis, we demonstrated a rapid increase in CD11b+/Gr-1− monocytes in the peripheral blood of AdSDF1-treated SCID mice at day 5 (36.1% ± 5.0%) (Figure 2D). Moreover, there was an increase in the relative frequency of Sca-1+ (1.8% ± 0.4%) and CD34+cells (1.6% ± 0.4%) in peripheral blood of AdSDF1-treated SCID mice at day 5 (Figure 2D). These results are representative of 4 mice in each group. Each analysis was done twice. There were no significant changes in the leukocyte frequencies in AdMIP3α-treated mice (Figure2B).
Effect of AdSDF1 on peripheral blood cell counts.
(A) Some SCID mice were treated with 1 × 109 pfu AdSDF1 or AdNull IV on day 0. Total white blood cells (○, AdSDF1; ●, AdNull) and granulocytes (■, AdSDF1; ▪, AdNull) were counted using a Neubauer hematocytometer on crystal violet stained. (B) Some SCID mice were treated with 1 × 109 pfu AdMIP3α or AdNull IV on day 0. Total white blood cells (○, AdMIP3α; ●, AdNull) and granulocytes (■, AdMIP3α; ▪, AdNull). All data are expressed as mean (n = 4 to 6) ± SEM. (C) Effect of AdSDF1 on differential leukocyte counts. Two strains of mice were treated with 1 × 109 pfu AdNull or AdSDF1 or AdMIP3α IV on day 0. Manual leukocyte differentials were performed on Wright-Giemsa–stained smears of peripheral blood. All data are expressed as mean percentages (n = 4 to 6). (D) Phenotypic characterization of peripheral blood mononuclear cells. Peripheral blood MNCs were prepared from AdSDF1-treated or AdNull-treated SCID mice on day 5 and stained with FITC-conjugated anti-CD11b and PE-conjugated anti–Gr-1, or FITC-conjugated anti-CD34 and PE-conjugated anti–Sca-1 MoAbs. Cells (1 × 104) were analyzed on a Coulter Elite flow cytometer. The representative percentages of positive populations in PBMNCs are shown.
Effect of AdSDF1 on peripheral blood cell counts.
(A) Some SCID mice were treated with 1 × 109 pfu AdSDF1 or AdNull IV on day 0. Total white blood cells (○, AdSDF1; ●, AdNull) and granulocytes (■, AdSDF1; ▪, AdNull) were counted using a Neubauer hematocytometer on crystal violet stained. (B) Some SCID mice were treated with 1 × 109 pfu AdMIP3α or AdNull IV on day 0. Total white blood cells (○, AdMIP3α; ●, AdNull) and granulocytes (■, AdMIP3α; ▪, AdNull). All data are expressed as mean (n = 4 to 6) ± SEM. (C) Effect of AdSDF1 on differential leukocyte counts. Two strains of mice were treated with 1 × 109 pfu AdNull or AdSDF1 or AdMIP3α IV on day 0. Manual leukocyte differentials were performed on Wright-Giemsa–stained smears of peripheral blood. All data are expressed as mean percentages (n = 4 to 6). (D) Phenotypic characterization of peripheral blood mononuclear cells. Peripheral blood MNCs were prepared from AdSDF1-treated or AdNull-treated SCID mice on day 5 and stained with FITC-conjugated anti-CD11b and PE-conjugated anti–Gr-1, or FITC-conjugated anti-CD34 and PE-conjugated anti–Sca-1 MoAbs. Cells (1 × 104) were analyzed on a Coulter Elite flow cytometer. The representative percentages of positive populations in PBMNCs are shown.
AdSDF1 induces profound increase in platelet count
We also monitored the change in platelet number in peripheral blood. Intravenous injection of AdSDF1 into SCID mice resulted in an increase in platelet count 3-fold above baseline 10 days after AdSDF1 administration (Figure 3A). Subsequently, platelets dropped to baseline levels by 6 weeks after injection. No significant increase in platelet levels was observed with administration of either AdMIP3α vector or AdNull (Figure3B).
Effect of AdSDF1 on platelet counts.
Some SCID mice were treated with 1 × 109 pfu AdSDF1 or AdMIP3α or AdNull IV on day 0. The number of platelets (○, AdSDF1 [A]; ○, AdMIP3α [B]; ●, AdNull [A, B]) were counted using a Neubauer hematocytometer. All data are expressed as the mean (n = 4 to 6) ± SEM.
Effect of AdSDF1 on platelet counts.
Some SCID mice were treated with 1 × 109 pfu AdSDF1 or AdMIP3α or AdNull IV on day 0. The number of platelets (○, AdSDF1 [A]; ○, AdMIP3α [B]; ●, AdNull [A, B]) were counted using a Neubauer hematocytometer. All data are expressed as the mean (n = 4 to 6) ± SEM.
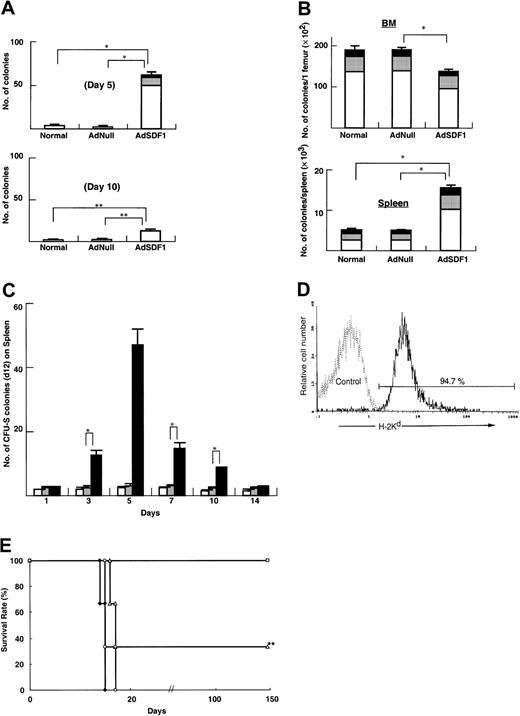
AdSDF1 promotes mobilization of progenitors (colony-forming unit-culture [CFU-C]) and cells with the potential of CFU-S. AdSDF1 caused significant mobilization of CFU-C to the peripheral blood, predominantly colony-forming unit–granulocyte-macrophage (CFU-GM), as compared to AdNull and normal control mice at day 5 (*P < .005), with the numbers of CFU-C returning to control levels at day 10 (**P < .05; Figure4A). CFU-Cs increased 15-fold in SCID mice in peripheral circulation for 5 days.
Effects of AdSDF1 on progenitor and stem cells.
(A) Peripheral blood was obtained from each group on day 5 and day 10 after administration of vector. Cells from BM, spleen, and peripheral blood in these mice were seeded in the colony assays and 3 CFU types were scored: CFU-GM (■), BFU-E (░), and CFU-GEMM (▪). (B) Seven days after adenovirus vector administration, 3 mice in each group were humanely killed, and progenitor content was quantified by methylcellulose-based colony assays. (A) and (B) are expressed as the mean (n = 3) ± SEM. Points that differ significantly from data for AdNull administration on the same day are marked: *P < .005, **P < .05. (C) Effects of AdSDF1 on CFU-S in peripheral blood. Peripheral blood was collected from the orbital plexus and pooled before and 1, 3, 5, 7, 10, and 14 days after the onset of treatment. After elimination of erythrocytes, 1 × 105 MNCs from AdSDF1 (▪) or AdNull (░) or normal (■) SCID were injected to irradiated recipient mice. The spleen of the recipients was obtained 12 days later for spleen colony counting. All data are expressed as the mean ± SEM of 3 to 4 experiments. Asterisk (*) indicates results that differ significantly from data for AdNull administration on the same day: *P < .005. (D) Flow cytometry analyses were done in bone marrow MNCs (BMMNCs) 150 days after allogeneic peripheral blood cell transplantation. BMMNCs were stained with H-2Kd–FITC (donor type). The representative percentages of positive populations in BMMNCs are shown. The data from age-matched normal C57BL/6 mice are also represented as a control. (E) Limiting dilution analysis of PBMNCs after administration of adenovirus vector. Lethally irradiated BALB/c mice were transplanted with serial doses of PBMNCs from AdNull- or AdSDF1-treated SCID mice 5 days after vector administration (■, 1 × 106 PBMNCs derived from the peripheral circulation of AdSDF1-treated mice; ▵, 1 × 105 PBMNCs from ADSDF1-treated mice; ♦, 1 × 104 PBMNCs from AdSDF1-treated mice; ○, 1 × 106 PBMNCs from AdNull-treated mice). Survival rate was monitored until 150 days. We used 6 mice in each group. The result from the 1 × 105 injected group compared with AdNull control group achieves statistical significance (**P < .05).
Effects of AdSDF1 on progenitor and stem cells.
(A) Peripheral blood was obtained from each group on day 5 and day 10 after administration of vector. Cells from BM, spleen, and peripheral blood in these mice were seeded in the colony assays and 3 CFU types were scored: CFU-GM (■), BFU-E (░), and CFU-GEMM (▪). (B) Seven days after adenovirus vector administration, 3 mice in each group were humanely killed, and progenitor content was quantified by methylcellulose-based colony assays. (A) and (B) are expressed as the mean (n = 3) ± SEM. Points that differ significantly from data for AdNull administration on the same day are marked: *P < .005, **P < .05. (C) Effects of AdSDF1 on CFU-S in peripheral blood. Peripheral blood was collected from the orbital plexus and pooled before and 1, 3, 5, 7, 10, and 14 days after the onset of treatment. After elimination of erythrocytes, 1 × 105 MNCs from AdSDF1 (▪) or AdNull (░) or normal (■) SCID were injected to irradiated recipient mice. The spleen of the recipients was obtained 12 days later for spleen colony counting. All data are expressed as the mean ± SEM of 3 to 4 experiments. Asterisk (*) indicates results that differ significantly from data for AdNull administration on the same day: *P < .005. (D) Flow cytometry analyses were done in bone marrow MNCs (BMMNCs) 150 days after allogeneic peripheral blood cell transplantation. BMMNCs were stained with H-2Kd–FITC (donor type). The representative percentages of positive populations in BMMNCs are shown. The data from age-matched normal C57BL/6 mice are also represented as a control. (E) Limiting dilution analysis of PBMNCs after administration of adenovirus vector. Lethally irradiated BALB/c mice were transplanted with serial doses of PBMNCs from AdNull- or AdSDF1-treated SCID mice 5 days after vector administration (■, 1 × 106 PBMNCs derived from the peripheral circulation of AdSDF1-treated mice; ▵, 1 × 105 PBMNCs from ADSDF1-treated mice; ♦, 1 × 104 PBMNCs from AdSDF1-treated mice; ○, 1 × 106 PBMNCs from AdNull-treated mice). Survival rate was monitored until 150 days. We used 6 mice in each group. The result from the 1 × 105 injected group compared with AdNull control group achieves statistical significance (**P < .05).
The mobilization of progenitors was associated with a parallel decrease in progenitors within the BM. In fact, BM-derived CFU-C per femur significantly decreased in AdSDF1-treated SCID mice, compared with AdNull-treated SCID at day 7 (Figure 4B) (*P < .005). In contrast, in AdSDF1-treated mice, total numbers of CFU-C per spleen at day 7 were significantly increased when compared with AdNull-treated mice or age-matched normal mice. This was mainly due to an increase of CFU-GM (Figure 4B) (*P < .005).
The formation of spleen colonies in the irradiated mice injected with SDF1-mobilized cells was also measured. Administration of AdSDF1 for 5 days caused a 24-fold increase in the peripheral blood cells of SCID mice CFU-S (day 12) (Figure 4C) (*P < .005). Administration of AdNull did not mobilize CFU-S (day 12) into the blood.
Allogeneic peripheral blood cell transplantation
Long-term reconstitutive ability was assessed using PBMNCs of adenovirus-treated SCID mice. After collecting the peripheral blood from SCID mice on day 5 after AdSDF1 and AdNull administration, PBMNCs (1 × 106) were transferred into lethally irradiated (9.5 Gy) C57BL/6 mice. After 150 days, H-2 typing showed that cells in the BM of chimeric mice that were transplanted with PBMNCs mobilized by AdSDF1 were more than 90% of donor H-2Kd–type origin (Figure 4D). In contrast, all chimeric mice died within 21 days after AdNull-treated PBMNC transplantation.
Syngeneic peripheral blood cell transplantation
Long-term reconstitutive ability was assessed using PBMNCs of AdNull- or AdSDF1-treated SCID mice (BALB/c background) in the syngeneic peripheral blood cell transplantation system. After collecting the peripheral blood from AdSDF1- and AdNull-treated SCID mice on day 5, different cell doses of PBMNCs (1 × 104, 1 × 105, and 1 × 106) were transferred into lethally irradiated (9 Gy) BALB/c mice. All mice were injected with various numbers of PBMNCs from AdNull-treated mice. Mice injected with 1 × 104 PBMNCs of AdSDF1-treated mice died within 17 days (Figure 4E). Two mice injected with 1 × 105 PBMNCs from AdSDF1-treated mice survived beyond 150 days. All of the mice transplanted with 1 × 106PBMNCs from AdSDF1-treated mice survived beyond 150 days.
Effect of AdSDF1 on spleen and BM
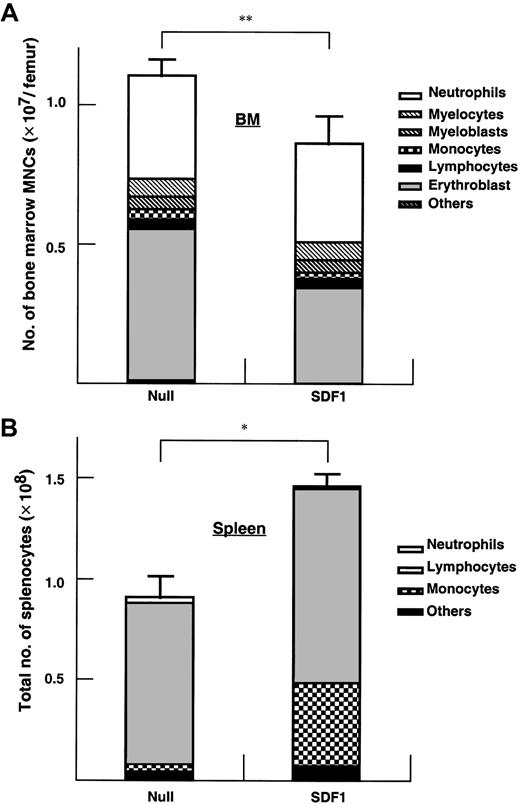
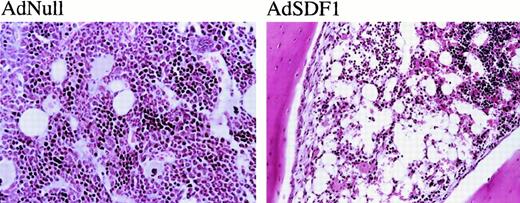
The BM cellularity in AdSDF1-treated SCID mice was lower than AdNull-treated mice (Figure 5A) (**P < .05). Splenic cellularity increased at day 7 in the AdSDF1-treated SCID mice compared to AdNull-treated mice (Figure5B) (*P < .005). The percentage of monocytes in the splenocytes of AdSDF1-treated mice was increased at day 7 compared to AdNull-treated mice. Histologically, there were regional decreases in cellularity in the BM of AdSDF1-treated mice at day 7 (Figure6). However, at day 60, BM cellularity was normalized in AdSDF1-treated mice (not shown).
Effect of AdSDF1 on BM and spleen mononuclear cells.
Some SCID mice were treated with 1 × 109 pfu AdSDF1 or AdNull IV on day 0. On day 7, 3 mice in each group were humanely killed, and both femora and the spleen were dissected. Manual leukocyte differentials were performed on Wright-Giemsa–stained cytospin preparations of the BM (A) and the spleen (B) mononuclear cells. All data of cellularity are expressed as the mean (n = 4) ± SEM. Points that differ significantly from data for AdNull administration on the same day are marked: *P < .005, **P < .05.
Effect of AdSDF1 on BM and spleen mononuclear cells.
Some SCID mice were treated with 1 × 109 pfu AdSDF1 or AdNull IV on day 0. On day 7, 3 mice in each group were humanely killed, and both femora and the spleen were dissected. Manual leukocyte differentials were performed on Wright-Giemsa–stained cytospin preparations of the BM (A) and the spleen (B) mononuclear cells. All data of cellularity are expressed as the mean (n = 4) ± SEM. Points that differ significantly from data for AdNull administration on the same day are marked: *P < .005, **P < .05.
Histopathologic examination.
The administration of AdNull or AdSDF1 was performed as described in Figure 1. On day 7, 3 SCID mice in each group were humanely killed. Paraffin sections of the BM were stained by hematoxylin and eosin. Original magnification, ×100.
Histopathologic examination.
The administration of AdNull or AdSDF1 was performed as described in Figure 1. On day 7, 3 SCID mice in each group were humanely killed. Paraffin sections of the BM were stained by hematoxylin and eosin. Original magnification, ×100.
Discussion
Among the known chemokines, SDF1 has been shown to induce chemotaxis of hematopoietic stem and progenitor cells in vitro.16-18 23 SDF1 is produced primarily by the BM stromal cells and seems to play a critical role in facilitating homing of HSCs and progenitors from fetal liver to the BM and establishing hematopoiesis. SDF1 also seems to be a key factor in maintaining HSCs in the BM during postnatal hematopoiesis. Therefore, we hypothesized that the SDF1-induced chemokinesis in conjunction with elevation in the extravascular space would result in mobilization of HSCs to the peripheral circulation. In this report, we demonstrate that elevation of SDF1 levels in the peripheral circulation results in mobilization of progenitors and cells with repopulating potential (CFU-S) into the peripheral circulation. SDF1 also induces an increase in platelets and WBCs. These data underscore the significance of SDF-1 in maintaining hematopoietic progenitors and precursors within the BM. Furthermore, these results suggest a role for SDF1 as an effective mobilization agent. Moreover, the time course of cell mobilization and changes in cell number paralleled SDF1 plasma levels suggest that SDF1-induced cell mobilization is controlled by a chemokine concentration gradient.
Trafficking of HSCs is mediated through a combination of adhesion molecules and chemokines.24,25 At the present time the exact mechanism whereby HSCs home to the BM is not known. Studies have shown that interaction of HSCs with E-selectin and vascular cell adhesion molecule-1 seems to play a critical role in selective homing to the BM.26-28 Chemokines such as SDF1 orchestrate this process by providing directional cues for the HSCs to lodge within the BM microenvironment. Emerging data strongly suggest that CXCR4, the natural ligand for SDF1, is also expressed on HSCs as well as on mature monocytes, lymphocytes, neutrophils, megakaryocytes, and hematopoietic progenitors. Therefore, given the relatively large amounts of SDF1 released by the BM microenvironment, it is believed SDF1 plays a role in maintaining these cells within the marrow.
According to studies, cytokines involved in stem cell mobilization, like SCF and IL-6, can up-regulate CXCR4 expression on CD34+ cells.29 Overnight incubation of CD34+ cells on a plastic surface could significantly increase the expression of CXCR4 at the cell surface. SDF1 itself increased colony formation by peripheral blood CD34+ cells in synergy with different cytokines.30 These data suggested that the SDF1/CXCR4 signal may play an important role in the stem cell engraftment and homing process, which involves not only chemokine and cytokine-induced mobilization and migration but also adhesion and proliferation of stem and progenitor cells.
By using adenoviral vectors, we were able to increase the circulating levels of SDF1 to 2.5 ng/mL. This level was sufficient to reverse the gradient across the BM barrier, forcing CXCR4-expressing cells to exit the BM. As a control chemokine, we overexpressed MIP3α using adenoviral vectors. AdNull or AdMIP3α was ineffective in mobilization of a substantial number of HSCs or progenitors, suggesting that mobilization induced by AdSDF1 is not due to adenoviral infection. We used SCID mice to induce long-term expression of the transgene without induction of immune response and premature clearance of AdSDF1. This allowed examination of the long-term effect of SDF1 overexpression for stem cell mobilization.
We demonstrated that AdSDF1 induced migration of significant numbers of CFU-S. Among the known chemokines only IL-8 has been shown to effectively induce mobilization of CFU-S.31 In addition, injection of a limiting number of mobilized PBMNCs secondary to AdSDF1, but not AdNull, resulted in reconstitution of hematopoiesis in the recipient mice. Injection of as low as 1 × 105 PBMNCs mobilized by AdSDF1 resulted in reconstitution of hematopoiesis, strongly suggesting that subsets of SDF1-mobilized cells have stem cell potential. Therefore, given that mobilization of HSCs with repopulating capacity is critical for adequate peripheral stem cell collection and BM transplantation, SDF1 may provide a useful chemokine that could be incorporated into mobilization regimens.
AdSDF1 induced a significant increase in the number of WBCs, including monocytes, neutrophils (Figure 2A,C), and immature myelomonocytic cells in both BALB/c and SCID mice. CXCR4 has been shown that it is expressed by all of these myeloid cells, suggesting that the primary mechanism resulting in their mobilization is mediated through SDF1.
The increase in the platelet count was detected at day 5 after injection of the AdSDF1. We and other researchers have shown that CXCR4 is expressed on the mature polyploid megakaryocytes and platelets.32-34 SDF1 induces transendothelial migration of megakaryocytes and enhances platelet formations, possibly in extramedullary sites such as lung or spleen. Therefore, overexpression of SDF1 may also enhance migration of megakaryocytes and augment platelet release.
In our studies presented here we needed to use adenoviral vectors to overexpress SDF1 to induce mobilization of progenitor cells. In fact, a single injection of recombinant SDF1 was ineffective in mobilizing progenitors (data not shown). These data suggest that constitutive production of high titers of SDF1 is essential to generate the reverse gradient and is essential for mobilization of the hematopoietic progenitor and stem cells. The CXCR4 expression by platelets as well as endothelial cells produces a large sink for circulating SDF1, thus increasing the requirement for constitutive production of high titers of SDF1 to induce elevated plasma levels.
On the basis of the data presented, systemic overexpression of SDF1 provides a novel mechanism to induce multilineage mobilization of hematopoietic progenitor and precursor cells, mature monocytes, and platelets. This effect may not only be used clinically to recover HSCs for peripheral stem cell transplantation but also to improve clinical disorders in which there is profound multilineage pancytopenia. The use of Ad vectors to elevate the plasma levels of chemokine provides an efficient mechanism to induce transient increase in circulating progenitor and repopulating cells. These studies lay the foundation for examining the potential of SDF1 for mobilization of hematopoietic stem cells for gene therapy and cell transplantation.
We thank Harry G. Satterwhite, Maureen Sullivan, and Koji Shido for helpful assistance, Kate deBeer for preparing the manuscript, and Dr Masaya Ikegawa and Dr Kazuko Matsumoto for performing TRFIA studies. K.H. is the recipient of a fellowship from the Uehara Memorial Foundation (Tokyo, Japan). B.H. is the recipient of a fellowship from the Dr. Mildred Scheel Stiftung für Krebsforschung (Bonn, Germany).
Supported by National Institutes of Health (NIH) grant R01 HL-61401 (M.A.S.M.); the Gar Reichman fund of Cancer Research Institute. (M.A.S.M.); National Heart, Lung and Blood Institute (NHLBl) grants R01 HL-58707 (S.R.), R01 HL-61849 (S.R.); Program Project HL-66952 (Project 2) (S.R.); Pilot Project P01 HL-59312 (S.R.); the Dorothy Rodbell Foundation for Sarcoma Research (S.R.); the Rich Foundation (S.R.); R01 HL-57318 U01 (R.G.C.), U01 HL-66952-01 (R.G.C.); the Will Rogers Memorial Fund (R.G.C.); and GenVec, Gaithersburg, MD (R.G.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Malcolm A. S. Moore, James Ewing Laboratory of Developmental Hematopoiesis, Sloan-Kettering Institute for Cancer Research, 1275 York Ave, Mailbox 101, Rm 717, RRL, New York, NY 10021-6007; e-mail: m-moore@ski.mskcc.org.

![Fig. 3. Effect of AdSDF1 on platelet counts. / Some SCID mice were treated with 1 × 109 pfu AdSDF1 or AdMIP3α or AdNull IV on day 0. The number of platelets (○, AdSDF1 [A]; ○, AdMIP3α [B]; ●, AdNull [A, B]) were counted using a Neubauer hematocytometer. All data are expressed as the mean (n = 4 to 6) ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/11/10.1182_blood.v97.11.3354/6/m_h81111133003.jpeg?Expires=1765007746&Signature=v~OM5koPICWZ4mENbUGaOdo04RxqlohMzOpRPlA5brCJ0UGcgHkFP5C4uf7kvVWFGdJ0Y3~IS1C4e1oxhM9SS8MqDJ27naxsvYsXZvgFZ0wZkccrMAgV1-wIX4AhVFGcu1eGvOwLK67kQXjZhX82b~66BXnCk2scrPhYfMFsMWSOG9PqbH8hNhYZR~5CNbAHW8~nIDakcEBYkudnZ3mGswFb7Tja2wU4Q-WsbA7iL0HNF-6jr8oY7YChwQp4GPAknw9per6cxRBuhogKY66~IsEiptpxf~i2DxRtUm3NkYF1NU0utbyxYxQ2NI8~oWFmVkan2Xz0ICVHSRsY4b1tZA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal