Interleukin-12 (IL-12) plays a critical role in modulating the function of T lymphocytes and natural killer cells. IL-12 has potent antitumor effects in animal models, mediated primarily by its ability to enhance cytolytic activity and secretion of interferon-γ (IFN-γ). Unfortunately, the antitumor effect of IL-12 has not been demonstrated in clinical trials. Repeated administration of IL-12 in humans results in decreasing levels of IFN-γ secretion. To understand the mechanism underlying this loss of responsiveness, the effect of IL-12 on its own signaling in activated human T cells was examined. These experiments demonstrate that the level of the signal transducer and activator of transcription 4 (STAT4) protein, a critical IL-12 signaling component, is dramatically decreased 24 hours after IL-12 stimulation, whereas levels of STAT4 messenger RNA are not affected. The decrease of STAT4 protein appears to be due to specific degradation of phospho-STAT4, possibly through the proteasome degradation pathway. Decreased levels of STAT4 protein lead to decreased STAT4 DNA-binding activity and reduced proliferation and secretion of IFN-γ. This down-regulation of STAT4 is specific for IL-12 signaling, presumably owing to the prolonged activation of STAT4 induced by IL-12. IFN-α stimulation, which leads to transient phosphorylation of STAT4, does not reduce the level of STAT4 protein. These findings provide new insights into the regulation of IL-12 signaling in human T cells, where IL-12 promotes TH1 responses, but persistent IL-12 stimulation may also limit this response. The cellular depletion of STAT4 following prolonged IL-12 stimulation may also explain the loss of responsiveness following the repeated administration of IL-12 in clinical trials.
Introduction
Interleukin-12 (IL-12) is a heterodimeric cytokine produced primarily by antigen-presenting cells.1,2 IL-12 has a variety of immunoregulatory effects on both T cells and natural killer (NK) cells, including the induction of interferon-γ (IFN-γ) production and enhancement of cytolytic activity. IL-12 also enhances the proliferation of activated T cells and promotes TH1-type immune responses.3 IL-12 mediates its biological effects by signaling through specific cell surface receptors, which activate the Jak–signal transducer and activator of transcription (STAT) pathways. The functional IL-12 receptor is a heterodimeric receptor consisting of 2 subunits, termed IL-12Rβ1 and IL-12Rβ2.4,5 The intracellular signaling pathway of IL-12 involves 2 members of the Janus kinase family, Jak2 and Tyk2, and several members of the STAT transcription activator family, including STAT1, STAT4, and STAT5.6-8 Among the STATs that are involved in IL-12 signaling, STAT4 plays a critical role. STAT4 knockout mice exhibit abrogation of all major IL-12–induced functions in both T cells and NK cells, including loss of IFN-γ production and impaired TH1 responses.9 10
Previous studies have demonstrated that a variety of other cytokines modulate the response of cells to IL-12. These effects occur through modulation of different components of the IL-12 signaling pathway. For example, the expression of IL-12Rβ2 appears to be highly regulated in different cell types. IL-2, IL-12, and IFN-γ enhance the expression of IL-12Rβ2 in NK cells and T cells, and IL-4 inhibits the expression of this receptor component in T cells.11-14 Recent studies have shown that different cytokines can also modulate STAT expression. In NK cells, the expression of STAT1 is enhanced by IL-2 and IL-12 and the expression of STAT4 is enhanced by IL-2.11
The ability of IL-12 to enhance the cytotoxicity of NK cells and cytotoxic T lymphocytes (CTLs) probably plays a role in the therapeutic potential of IL-12 in patients with cancer. Preclinical studies in animal models demonstrated that administration of IL-12 resulted in regression of established tumors and activation of antitumor immunity.15-18 It was also demonstrated that treatment with a single dose of IL-12 augmented the cytolytic activity and proliferation of lymphocytes in cancer patients.19However, significant antitumor effects were not observed in patients treated with IL-12.20,21 The production of IFN-γ induced by administration of IL-12 in these patients was dramatically decreased following continued treatment with IL-12, indicating a loss of responsiveness to IL-12 during treatment.20 This loss of responsiveness may contribute to the ineffectiveness of IL-12 in clinical trials. To understand the mechanism underlying the loss of responsiveness, we investigated the effect of IL-12 treatment in vitro on IL-12 signaling in human T cells. We found that IL-12 treatment significantly affected the expression of several components of its own signaling pathway. The most functionally significant effect appeared to be reduced cellular levels of STAT4 protein. These findings provide insights into the mechanism for regulating IL-12 function in vivo and may explain the loss of responsiveness observed in IL-12 clinical trials.
Materials and methods
Cytokines, antibodies, and reagents
Recombinant human IL-2 was provided by Amgen (Thousand Oaks, CA). Human recombinant IL-12 was provided by the Genetics Institute (Cambridge, MA). Human IFN-α was obtained from Schering-Plough (Madison, NJ). Purified unconjugated mouse anti-CD3 (immunoglobulin G2a [IgG2a]) and isotype control MsIgG1 were provided by Beckman Coulter (Hialeah, FL). Phycoerythrin (PE)–conjugated goat antimouse IgG1 was purchased from Southern Biotechnology (Birmingham, AL). The generation of mouse monoclonal anti–IL-12Rβ1 and β2 subunits, β44 and 5A7, was described previously.11,13 Antibodies specific for STAT1, STAT5, STAT4, Tyk2, and Jak2 and phosphotyrosine antibody PY99 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Proteasome inhibitor MG-132 was purchased from Calbiochem (San Diego, CA). The anti–phosphotyrosine-STAT1, which also cross-reacts with phosphotyrosine-STAT5, was described previously.22
Enrichment and culture of human T cells
Enriched white blood cells were obtained from healthy donors undergoing platelet pheresis at the Dana-Farber Cancer Institute Blood Donor Center, Boston, MA. Peripheral blood mononuclear cells were isolated by Ficoll-Hypaque (Pharmacia LKB, Piscataway, NJ) gradient centrifugation and nylon wool column depletion. The remaining cell population contains more than 90% T cells. Cells were then cultured at 2 × 106 cells/mL with RPMI 1640 medium containing 15% fetal calf serum for 3 days in a 175-cm2flask coated with 20 μg anti-CD3 to induce the expression of IL-12Rβ2. Activated T cells were transferred into a 25-cm2 flask coated with 2 μg anti-CD3 and cultured at 2 × 106 cells/mL with or without cytokines for various times. All cytokines were used at 100 U/mL, if not otherwise specified.
Immunofluorescence analysis of IL-12R expression
CD3-activated T cells were cultured with or without indicated cytokines for 3 days. Cells were labeled with IgG1 isotype control, anti–IL-12Rβ1 antibody (12Rβ44) or anti–IL-12Rβ2 antibody (5A7). Cells were then labeled with secondary antibody PE-conjugated goat antimouse IgG1. The expression of IL-12Rβ1 and β2 was analyzed on a Coulter EPICS XL flow cytometer.
Western blotting and Northern blot analysis of STAT4 messenger RNA
Whole-cell extracts were prepared and Western blot was performed as described previously.23 Total RNA was prepared from activated T cells that were cultured with or without cytokines for 24 hours. We used 20 μg total RNA in Northern blot analysis. The probe used for Northern blotting was generated by reverse transcriptase–polymerase chain reaction (RT-PCR) with total RNA from activated T cells and 2 STAT4-specific primers designed according to the STAT4 complementary DNA (cDNA) sequence in GenBank (AccessionL78440). Reverse transcription and PCR were performed as described previously.24
Immunoprecipitation of STAT4 protein
Whole-cell extracts from 20 × 106 untreated or treated cells were prepared, and the STAT4 protein was immunoprecipitated with 10 μg STAT4 antibody as described previously.23
Nuclear extracts and electrophoretic mobility shift assay
Activated T cells were cultured with or without cytokines in anti-CD3–coated flasks for 24 hours. Cells were then treated with IL-12 for 20 minutes. Nuclear extracts were prepared and the electrophoretic mobility shift assay (EMSA) was performed as previously described.23 The STAT-binding oligonucleotides used, 5′-GAGCCTGATTTCCCCGAAATGATGAGC-3′ and its complement, are derived from the IFN-γ–responsive factor 1 gene promoter.25
IFN-γ enzyme-linked immunosorbent assay
CD3-activated T cells were cultured with or without cytokines in anti-CD3–coated flasks for 2 days. Cells were then washed and plated at 50 000 cells per well in duplicate into 96-well U-bottom plates and cultured with or without IL-12 for 2 days. The supernatants were collected, and the concentration of IFN-γ in all supernatants was determined by IFN-γ enzyme-linked immunosorbent assay (ELISA) (Endogen, Boston, MA).
Proliferation assay
CD3-activated T cells were cultured with or without cytokines in anti-CD3–coated flasks for 2 days. Cells were then washed and plated at 30 000 cells per well in triplicate into 96-well U-bottom plates and cultured with or without IL-12. After 24 hours, cells were pulsed with 1 μCi per well of [3H]-thymidine (TdR) for 16 hours and harvested (EG&G Wallac, Terku, Finland). The incorporation of [3H]TdR was measured with a liquid scintillation counter (1205 Betaplate, Pharmacia LKB).
Results
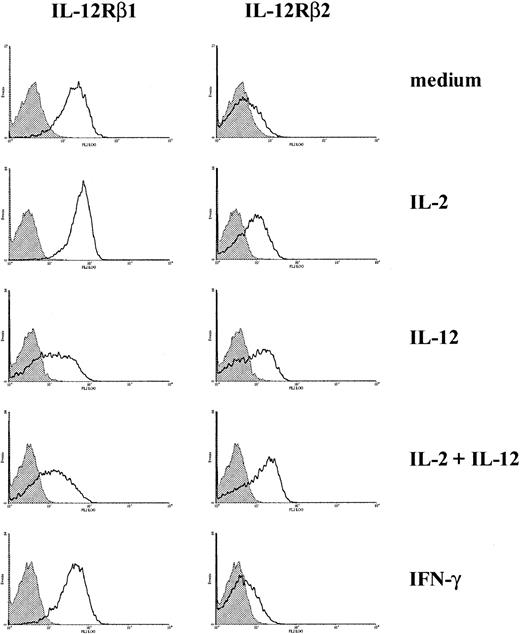
IL-12 up-regulates the expression of IL-12Rβ2 and down-regulates the expression of IL-12Rβ1 in CD3-activated T cells
Modulation of the expression of IL-12 receptor chains has been previously shown to play an important role in regulating the response of T cells to IL-12. Using monoclonal antibodies we developed against IL-12Rβ1 and β2 subunits, we investigated the effect of IL-12 on the expression of β1 and β2 on activated T cells. CD3-activated T cells were incubated with or without cytokines for 3 days, and the expression of IL-12 receptor subunits was examined by flow cytometry. As shown in Figure 1, the expression of IL-12Rβ2 was upregulated by IL-2 or IL-12 and by IL-2 plus IL-12. IFN-γ, which was used as a control cytokine, did not affect the expression of IL-12Rβ2. In contrast, the expression of IL-12Rβ1 was significantly decreased in T cells treated with IL-12 or with IL-2 plus IL-12 (Figure 1). The up-regulation of IL-12Rβ2 by IL-2 and IL-12 and the down-regulation of IL-12Rβ1 by IL-12 in activated T cells are similar to what we observed previously in NK cells.11These results indicate that in both cell populations the expression of IL-12Rβ1 and IL-12Rβ2 is regulated through different mechanisms. However, IL-12–treated T cells still express significant levels of IL-12Rβ1, and the lower levels of IL-12Rβ1 expression induced by IL-12 may not have any functional consequence in these cells.
Expression of IL-12Rβ1 and IL-12Rβ2 on activated T cells.
CD3-activated T cells were cultured with or without cytokines, as indicated. Cell surface expression of IL-12Rβ1 and IL-12Rβ2 was measured by flow cytometry. Representative histograms from 3 experiments are presented.
Expression of IL-12Rβ1 and IL-12Rβ2 on activated T cells.
CD3-activated T cells were cultured with or without cytokines, as indicated. Cell surface expression of IL-12Rβ1 and IL-12Rβ2 was measured by flow cytometry. Representative histograms from 3 experiments are presented.
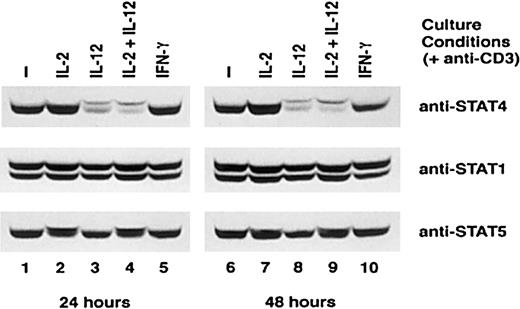
IL-12 down-regulates the expression of STAT4 in activated T cells
STAT4 plays a major role in IL-12 signaling in both T cells and NK cells. Our previous studies demonstrated that IL-12 did not promote the expression of STAT4 in NK cells. Instead, IL-12 inhibits IL-2–induced STAT4 expression in this cell population.11 We therefore examined whether IL-12 activation affected the expression of various STAT proteins, especially STAT4, in activated T cells. Activated T cells were cultured with or without IL-2, IL-12, IL-2 plus IL-12, or IFN-γ for 4 days. The expression of STAT1, STAT4, and STAT5 at the end of each day was examined by Western blot. Both STAT1 and STAT5 expression remained at the same level in T cells cultured with and without cytokines (Figure 2, middle, lower panels, and data not shown). The appearance of a shift of the STAT5 band is due to the phosphorylation of STAT5 induced by IL-2 (Figure 2, lower panel, lanes 2, 4, 7, and 9). Treatment with IL-12 also induced a shifted STAT4 band, which consists of tyrosine-phosphorylated STAT4 (Figure 2, upper panel, lanes 3, 4, 8, and 9).11,23 However, in contrast to STAT1 and STAT5, the level of total STAT4 was significantly decreased in IL-12 or IL-2 plus IL-12–treated T cells within 24 hours, and low levels of STAT4 protein persisted for the 4 days we examined this phenomenon (Figure 2, upper panel, lanes 3, 4, 8, and 9, and data not shown). IFN-γ did not influence the expression of any of the STAT proteins examined. Similarly to what we previously observed in NK cells, the expression of STAT4 in these activated T cells was enhanced by IL-2 (Figure 2, top panel).11 The effect of IL-12 on the expression of the 2 kinases involved in IL-12 signaling, Jak2 and Tyk2, was also examined, and neither kinase was decreased at the protein level after IL-12 treatment (data not shown). This result indicated that among the kinases and STATs that are involved in IL-12 signaling, the expression of STAT4 protein was specifically down-regulated by IL-12. Because STAT4 is the major STAT involved in IL-12 signaling, the down-regulation of STAT4 expression by IL-12 may have functional consequences on the subsequent T-cell responsiveness to IL-12.
Expression of STAT proteins in T cells cultured with cytokines.
CD3-activated T cells were cultured with or without cytokines, as indicated. Cells were harvested at the indicated times, whole-cell extracts were prepared, and Western blots were performed with anti-STAT4 (top panel). The membranes were reprobed with anti-STAT1 (middle panel) and anti-STAT5 (bottom panel). Representative data from 3 separate experiments are shown.
Expression of STAT proteins in T cells cultured with cytokines.
CD3-activated T cells were cultured with or without cytokines, as indicated. Cells were harvested at the indicated times, whole-cell extracts were prepared, and Western blots were performed with anti-STAT4 (top panel). The membranes were reprobed with anti-STAT1 (middle panel) and anti-STAT5 (bottom panel). Representative data from 3 separate experiments are shown.
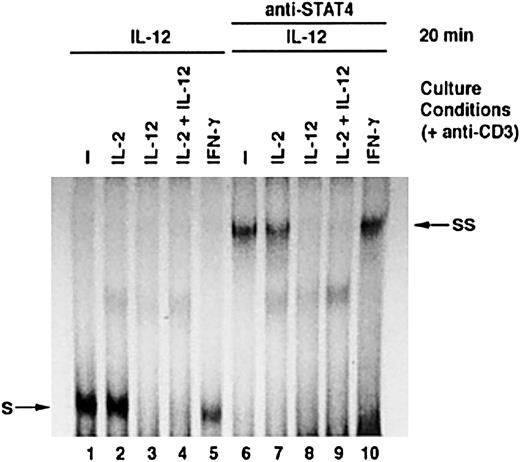
Functional impact of IL-12–induced STAT4 degradation
STAT4 plays a critical role in IL-12 signaling. To investigate the impact of IL-12–induced STAT4 down-regulation on T-cell functional responses to IL-12, we examined STAT4 DNA-binding activity in response to IL-12. Activated T cells were cultured with or without additional cytokines for 24 hours and then treated with IL-12. As shown in Figure4, IL-12 induced significant STAT4 DNA-binding activity in T cells cultured with medium alone, IL-2, or IFN-γ, as indicated by the formation of STAT4 and DNA complex (complex S). The addition of STAT4 antibody resulted in a new shifted complex, SS, formed by STAT4, DNA, and anti-STAT4 antibody (Figure 3, lanes 6, 7, and 10). This confirmed that complex S represented the STAT4-DNA complex. However, no visible level of complex S was induced by IL-12 in T cells cultured with IL-12 or IL-2 plus IL-12 (Figure 3, lanes 3 and 4). These results indicate that T cells treated with IL-12 have much less STAT4 DNA-binding activity in response to further IL-12 stimulation. We further examined other IL-12–induced functions in activated T cells, including IFN-γ production and proliferation. In response to IL-12 restimulation, T cells that were previously cultured with IL-12, either alone or in combination with IL-2, displayed significantly reduced IFN-γ production compared with T cells cultured without cytokine or with IFN-γ. Similarly to what we observed in NK cells, T cells treated with IL-2 showed enhanced IFN-γ production in response to IL-12 (Figure4A).11 Moreover, T cells that were pretreated with IL-12, either alone or in combination with IL-2, also showed significantly reduced proliferative response to IL-12 compared with T cells cultured in other conditions (Figure 4B). These data indicate that treatment of activated T cells with IL-12 results in significantly reduced functional responses to further IL-12 stimulation.
IL-12–induced STAT4 DNA-binding activity in activated T cells cultured with cytokines.
Activated T cells were cultured with or without cytokines, as indicated, for 24 hours. All cells were then treated with IL-12 for 20 minutes. Nuclear extracts were prepared, and an EMSA was performed in the absence or presence of antibody to STAT4 as indicated. S: STAT4 and DNA complex. SS: Complex formed by STAT4, DNA, and anti-STAT4 antibody.
IL-12–induced STAT4 DNA-binding activity in activated T cells cultured with cytokines.
Activated T cells were cultured with or without cytokines, as indicated, for 24 hours. All cells were then treated with IL-12 for 20 minutes. Nuclear extracts were prepared, and an EMSA was performed in the absence or presence of antibody to STAT4 as indicated. S: STAT4 and DNA complex. SS: Complex formed by STAT4, DNA, and anti-STAT4 antibody.
Functional responses after IL-12 stimulation of cytokine-primed T cells.
Activated T cells were primed with or without cytokines as indicated for 2 days. These cells were then washed and treated with or without IL-12 for 2 days. IFN-γ produced by activated cells was quantified by ELISA (A). All values represent net IFN-γ production measured by the difference between levels produced in the presence and absence of IL-12. T-cell proliferation (B) was quantified by measuring the incorporation of [3H]TdR. The fold proliferation compares values in T cells treated with IL-12 versus T cells without additional IL-12.
Functional responses after IL-12 stimulation of cytokine-primed T cells.
Activated T cells were primed with or without cytokines as indicated for 2 days. These cells were then washed and treated with or without IL-12 for 2 days. IFN-γ produced by activated cells was quantified by ELISA (A). All values represent net IFN-γ production measured by the difference between levels produced in the presence and absence of IL-12. T-cell proliferation (B) was quantified by measuring the incorporation of [3H]TdR. The fold proliferation compares values in T cells treated with IL-12 versus T cells without additional IL-12.
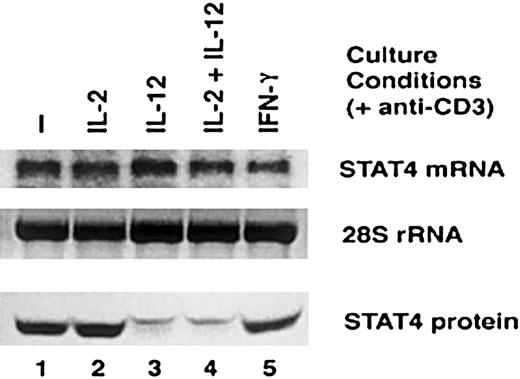
The effect of IL-12 treatment on the expression of STAT4 messenger RNA
Having shown that IL-12 significantly reduces the expression of STAT4 protein in activated T cells, we undertook further experiments to examine the mechanism whereby STAT4 expression was reduced. First, we examined the expression of STAT4 messenger RNA (mRNA) in activated T cells by Northern blot. Although STAT4 protein is markedly reduced in T cells treated with IL-12 or IL-2 plus IL-12 (Figure 5, bottom panel), there was no significant reduction in the level of STAT4 mRNA in these cells compared with untreated T cells or T cells treated with IL-2 or IFN-γ (Figure 5, top panel). These results suggest that IL-12 does not affect the expression of STAT4 at the level of gene transcription.
Comparison of STAT4 mRNA and STAT4 protein expression after IL-12 activation.
Activated T cells were cultured with cytokines indicated in the presence of anti-CD3 for 24 hours. Whole-cell extracts and total RNA were prepared. Northern blot was probed with RT-PCR–amplified STAT4 cDNA fragment (top panel), and the 28S ribosomal RNA in the RNA gel was used as quantity control (middle panel). Western blots were performed with whole-cell extracts and probed with anti-STAT4 (bottom panel).
Comparison of STAT4 mRNA and STAT4 protein expression after IL-12 activation.
Activated T cells were cultured with cytokines indicated in the presence of anti-CD3 for 24 hours. Whole-cell extracts and total RNA were prepared. Northern blot was probed with RT-PCR–amplified STAT4 cDNA fragment (top panel), and the 28S ribosomal RNA in the RNA gel was used as quantity control (middle panel). Western blots were performed with whole-cell extracts and probed with anti-STAT4 (bottom panel).
Inhibition of proteasome-pathway degradation blocks IL-12–induced down-regulation of STAT4 protein
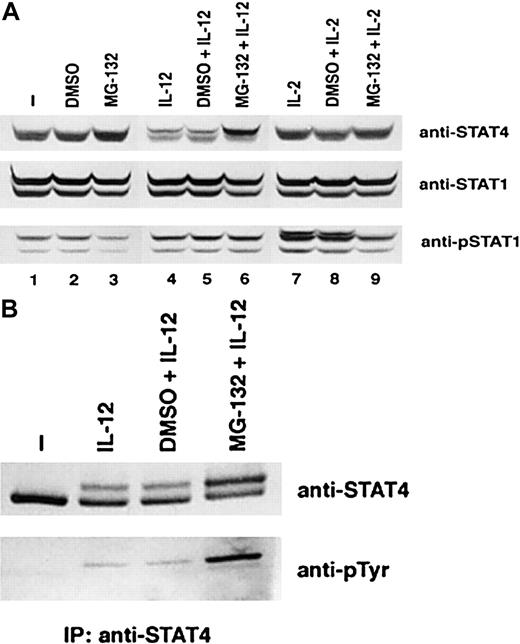
The proteasome pathway has been shown to be involved in the turnover of tyrosine phospho-STATs activated in several signaling pathways.26-28 To examine whether the proteasome pathway is also involved in the down-regulation of STAT4, we examined the effect of a specific inhibitor of this pathway, MG-132, on the down-regulation of STAT4 in IL-12–treated T cells. As noted previously, STAT4 protein was decreased in IL-12–treated T cells compared with untreated or IL-2–treated T cells (Figure6A, top panel). Pretreatment with DMSO had no effect on the decrease of STAT4 induced by IL-12. Preincubation with MG-132 did not alter the expression of STAT4 in control T cells or IL-2–treated T cells. However, MG-132 inhibited the reduction of STAT4 protein induced by IL-12 (Figure 6A, top panel). More importantly, there appeared to be an accumulation of the slowly migrating phosphorylated STAT4 band in these T cells. The more rapidly migrating band, which represents unphosphorylated STAT4, was not affected in comparison with IL-12–treated T cells without the inhibitor MG-132 (Figure 6A, top panel, lanes 4 to 6). To confirm that the STAT4 protein that was degraded through the proteasome pathway was indeed the tyrosine-phosphorylated STAT4, we immunoprecipitated STAT4 protein from untreated cells or IL-12–treated cells in the absence or presence of DMSO or MG-132 (Figure 6B). Similarly to what we observed in Figure 6A, treatment with IL-12 induced a reduction of STAT4 protein in the absence or presence of DMSO. The presence of MG-132 in IL-12–treated cells led to an accumulation of the slowly migrating STAT4 band (Figure6B, top panel). Reprobing with phospho-tyrosine–specific antibody revealed that this slowly migrating STAT4 band represents the tyrosine-phosphorylated STAT4 protein (Figure 6B, bottom panel). These results suggest that the proteasome pathway is involved in the IL-12–induced STAT4 down-regulation. More importantly, the degradation appears to involve primarily the phosphorylated form of STAT4. Although previous studies have demonstrated that IL-12 also activates STAT1,23 MG-132 does not lead to any increase in phospho-STAT1α or phospho-STAT1β in IL-12–treated cells. MG-132 also did not result in accumulation of phospho-STAT1 induced by IL-2 (Figure 6A, bottom panel). These observations suggest that degradation through the proteasome pathway specifically targets phospho-STAT4. The delayed activation of this degradation process suggests that phosphorylation of STAT4 alone may not be sufficient for the degradation of STAT4. Other IL-12–induced factors may also be involved in this process.
Effect of proteasome pathway inhibitor MG-132 on the degradation of phospho-STAT4 protein.
Activated T cells were cultured with the inhibitor MG-132 (10 nM) or the solvent dimethyl sulfoxide (DMSO) for 1 hour and then cultured with or without cytokines as indicated for 6 hours. Whole-cell extracts were prepared. (A) Western blots were probed with anti-STAT4 (top panel). The membrane was reprobed with anti-STAT1 (middle panel) and anti–phospho-STAT1 (bottom panel). (B) STAT4 protein was immunoprecipitated with anti-STAT4 antibody. The Western blot was probed with anti-STAT4 (top panel) and reprobed with antiphosphotyrosine antibody (bottom panel).
Effect of proteasome pathway inhibitor MG-132 on the degradation of phospho-STAT4 protein.
Activated T cells were cultured with the inhibitor MG-132 (10 nM) or the solvent dimethyl sulfoxide (DMSO) for 1 hour and then cultured with or without cytokines as indicated for 6 hours. Whole-cell extracts were prepared. (A) Western blots were probed with anti-STAT4 (top panel). The membrane was reprobed with anti-STAT1 (middle panel) and anti–phospho-STAT1 (bottom panel). (B) STAT4 protein was immunoprecipitated with anti-STAT4 antibody. The Western blot was probed with anti-STAT4 (top panel) and reprobed with antiphosphotyrosine antibody (bottom panel).
Down-regulation of STAT4 through the proteasome pathway is specific for IL-12 signaling
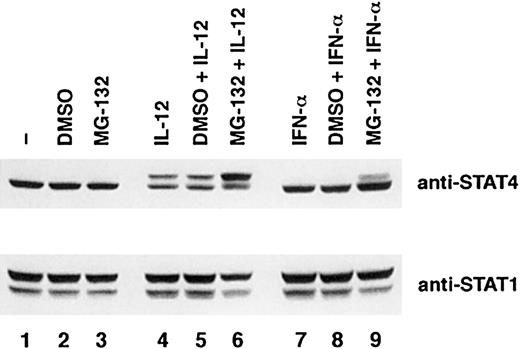
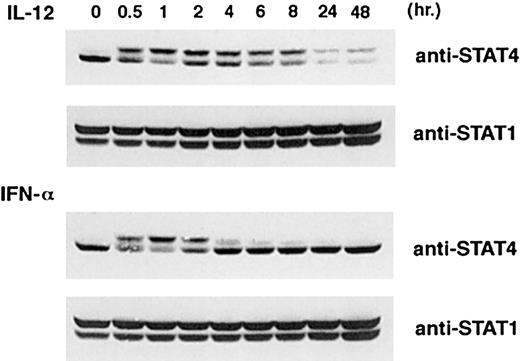
IFN-α is also known to induce phosphorylation of STAT4.29 To investigate whether the down-regulation of STAT4 is specific for IL-12, we examined the effect of MG-132 on IFN-α–induced activation of STAT4. As shown in Figure7, activated T cells were stimulated for 6 hours with IFN-α, and results were compared with similar treatment with IL-12. In contrast to IL-12–treated T cells, there was no visible phospho-STAT4 band in the IFN-α–treated T cells (Figure 7, lanes 7 and 8), and there is a low level of phospho-STAT4 accumulation after MG-132 treatment (Figure 7, lane 9). The unphosphorylated STAT4 continues to represent the predominant STAT4 band in cells treated with MG-132 (Figure 7, lane 9). To understand the mechanism that leads to the different effects of MG-132 on phospho-STAT4 induced by these 2 cytokines, we compared the kinetics of STAT4 activation. As shown in Figure 8, IL-12–treated T cells exhibited persistent activation of STAT4 as indicated by the appearance of a shifted STAT4 band throughout the time we tested. This was associated with a marked decrease of total STAT4 protein by 24 hours (Figure 8, top panel). However, IFN-α induced only a transient phosphorylation of STAT4, as indicated by the disappearance of the shifted STAT4 band by 6 hours (Figure 8, panel 3). In contrast to IL-12–treated T cells, the level of STAT4 protein in IFN-α–treated T cells was maintained at a similar level throughout the time we observed. These observations suggest that although both IL-12 and IFN-α induce activation of STAT4, the degradation of phospho-STAT4 was specific for the IL-12 signaling pathway. This may be attributed to the persistent activation of STAT4 induced by IL-12 rather than the transient activation of STAT4 induced by IFN-α.
Differential effects of MG-132 on STAT4 protein in IL-12 and INF-α signaling pathways.
Activated T cells were cultured with the proteasome inhibitor MG-132 (10 nM) or DMSO for 1 hour and then cultured with or without cytokines as indicated for 6 hours. Whole-cell extracts were prepared, and Western blots were probed with anti-STAT4 (top panel). The membrane was reprobed with anti-STAT1 as a quantity control (bottom panel).
Differential effects of MG-132 on STAT4 protein in IL-12 and INF-α signaling pathways.
Activated T cells were cultured with the proteasome inhibitor MG-132 (10 nM) or DMSO for 1 hour and then cultured with or without cytokines as indicated for 6 hours. Whole-cell extracts were prepared, and Western blots were probed with anti-STAT4 (top panel). The membrane was reprobed with anti-STAT1 as a quantity control (bottom panel).
Kinetics of STAT4 activation induced by IL-12 and INF-α.
Activated T cells were treated with IL-12 or INF-α for the time indicated. Whole-cell extracts were prepared, and Western blots were probed with anti-STAT4 (top and third panels). The membrane was reprobed with anti-STAT1 as a quantity control (second and bottom panels).
Kinetics of STAT4 activation induced by IL-12 and INF-α.
Activated T cells were treated with IL-12 or INF-α for the time indicated. Whole-cell extracts were prepared, and Western blots were probed with anti-STAT4 (top and third panels). The membrane was reprobed with anti-STAT1 as a quantity control (second and bottom panels).
Discussion
In previous studies, it has been demonstrated that expression of the IL-12 receptor can be modulated by other cytokines, such as IL-2 and IL-4, and that this provides an important mechanism for the regulation of IL-12 responsiveness. IL-12 also modulates further responsiveness to IL-12, but the mechanism whereby IL-12 autoregulation occurs has not been defined. In the present study, we examined various components of the IL-12 signaling pathway in activated human T cells and confirmed that once these cells have been activated by IL-12, they become resistant to further IL-12 stimulation. This specific down-regulation of IL-12 signaling appears to be mediated primarily through reduced levels of STAT4 protein, a critical component of the IL-12 signaling pathway. Decreased levels of STAT4 following IL-12 activation do not appear to result from decreased STAT4 gene transcription but from increased degradation of STAT4 protein. Initial experiments suggest that this increased degradation specifically targets the tyrosine-phosphorylated STAT4 protein that results from IL-12–induced activation. Other STAT proteins and STAT4 activation following IFN-α signaling are not regulated in this fashion, and this therefore appears to represent a unique regulatory mechanism whereby IL-12 down-regulates the responsiveness of activated T cells to further IL-12 stimulation.
Cytokine signaling through the Jak-STAT pathways involves the phosphorylation of STAT proteins by Jak kinases, the translocation of phospho-STATs to the nucleus, the dephosphorylation of STATs by tyrosine phosphatase in the nucleus, and the return of the dephosphorylated STAT protein to the cytoplasm.27 Several mechanisms have previously been shown to regulate this signaling pathway. One mechanism is inhibition of STAT phosphorylation by members of the suppressor of cytokine signaling (SOCS) family. SOCS proteins are induced following cytokine stimulation, bind to Jak kinases, and inhibit activated Jak kinases from further phosphorylation of STAT proteins.30,31 This mechanism has been shown to down-regulate IFN-γ, IL-3, IL-6, and IFN-α signaling.32,33 As a result of inhibition by SOCS proteins, STAT signaling becomes a transient response, and levels of phosphorylated STAT proteins decrease within hours of activation. However, the SOCS feedback mechanism does not result in reduced levels of STAT proteins. Another mechanism known to down-regulate STAT signaling involves tyrosine phosphatases, such as SHP-1 and SHP-2. These tyrosine phosphatases have been shown to down-regulate the activity of STAT1, STAT3, or STAT5 following activation by IFN-γ, leukemia inhibitory factor, or IL-2 either by dephosphorylating STAT proteins directly or through dephosphorylation of Jak kinases.34-36 Like the SOCS feedback mechanism, the down-regulation through tyrosine phosphatases also results in transient activation of STAT proteins and does not lead to reduced levels of STAT proteins. The transient activation of STAT4 by IFN-α may reflect the involvement of SOCS proteins or other mechanisms in down-regulating the IFN-α signaling. However, the persistence of phospho-STAT4 and loss of STAT4 protein in IL-12–treated T cells suggest that it is unlikely that either of these mechanisms contribute to the down-regulation of IL-12 signaling following IL-12 activation.
Several studies have demonstrated that the proteasome pathway can be involved in the turnover of phosphorylated STAT proteins. In previous studies, addition of the proteasome-specific inhibitor MG-132 was shown to prolong the tyrosine phosphorylation of STAT1, STAT4, STAT5, and STAT6 induced by IFN-γ, IL-2, IL-3, IL-4, or IL-12.11,26-28 This suggests that the proteasome pathway may also contribute to the transient activation of STAT proteins following cytokine signaling. However, no down-regulation of total STAT proteins were observed in these systems. In particular, Wang et al11 demonstrated that MG-132 prolonged the tyrosine phosphorylation of STAT4 in the Kit225 cell line, but the activation of STAT4 by IL-12 in their system appeared to be transient and no loss of total STAT4 was noted. The persistent phosphorylation of STAT4 and the loss of STAT4 protein after IL-12 stimulation appear to represent a unique example of autoregulation of cytokine signaling. MG-132 inhibition of the down-regulation of STAT4 by IL-12 in our experiments suggests that the proteasome pathway may play an important role in the modulation of cytokine signaling. Following IFN-α signaling, the transient activation of STAT4 suggests that other negative feedback mechanisms are activated, and the proteasome pathway does not appear to play a major role in the down-regulation of this signaling pathway. However, following IL-12 stimulation, STAT4 is persistently activated, and the proteasome pathway appears to play a major role in the down-regulation of this signaling pathway. These observations suggest that degradation of phospho-STATs through the proteasome pathway, which leads to the depletion of cellular STAT protein, represents an important alternative mechanism for down-regulating a signaling pathway where other negative feedback mechanisms are not activated.
The depletion of cellular STAT4 in activated T cells markedly affects the function of these cells and specifically reduces their ability to secrete IFN-γ and proliferate in response to further IL-12 stimulation. These deficiencies are similar to those observed in STAT4 knockout mice, which have markedly impaired ability to develop TH1 responses.9 10 Although IL-12 is known to be a primary stimulating cytokine for initiation of TH1 responses, these observations suggest that persistent stimulation with IL-12 will also inhibit the TH1 response in vivo. Thus, IL-12 appears to be able to both initiate a TH1 response and subsequently limit further TH1 responses if the production of IL-12 persists for prolonged periods. These opposing functional effects appear to be mediated through initial activation of STAT4 followed by the depletion of STAT4 protein after prolonged IL-12 activation.
Although IL-12 has been shown to activate STAT1 in addition to STAT4, our results demonstrated that neither STAT1 nor phospho-STAT1 was depleted after IL-12 activation. This finding suggests that the degradation process activated by IL-12 is specific for STAT4 and does not affect other STAT proteins activated in the same pathway. In addition, STAT4 was not depleted after IFN-α stimulation. These results suggest that STAT4 degradation may include an active recruiting process that involves one or more factors capable of recognizing and targeting phosphorylated STAT4 to the degradation pathway. The delayed onset of the degradation process and the specificity for STAT4 suggest that the activation of these factors may also require the persistent activation of STAT4 itself. The identity of these factors and the mechanism by which they recruit phospho-STAT4 and down-regulate the IL-12 signaling pathway require further investigation.
The negative regulation of IL-12 signaling through down-regulation of STAT4 expression may contribute to the ineffectiveness of IL-12 in clinical trials in patients with cancer. In these studies, single doses of IL-12 augment the cytolytic activity, secretion of INF-γ, and proliferation of lymphocytes in vivo.19 However, these immunologic effects were not sustained with continued treatment. Unlike many other cytokines, IL-12 is a relatively large heterodimeric molecule that is not rapidly degraded or excreted.21 The relatively long half-life of infused recombinant IL-12 leads to the persistence of physiologic levels of active cytokine for prolonged periods after each administration, and this further prolongs the cellular activation mediated by exogenous IL-12. Our studies suggest that the prolonged half-life of IL-12 may therefore also contribute to the selective depletion of STAT4 following IL-12 treatment and loss of responsiveness in vivo. The effectiveness of IL-12 as a therapeutic agent for cancer treatment may depend on the design of alternative treatment regimens or the development of IL-12 agonists with shorter half-lives. Our results suggest that the immunologic effects of IL-12 on T cells would be enhanced by intermittent administration of relatively low doses of IL-12. This schedule would allow cellular levels of STAT4 to recover after each treatment. Intermittent administration of IL-12 alternating with doses of IL-2 would also be expected to enhance recovery of STAT4 and enhance IL-12 responsiveness.11 In contrast, continuous exposure to IL-12, either with or without IL-2, would be expected to suppress cellular STAT4 for prolonged periods and reduce responsiveness to IL-12. These types of schedules can be evaluated in future clinical trials and may provide a mechanism for effectively enhancing the cytolytic activity of NK cells and CTLs in vivo.
Supported by National Institutes of Health grant CA41619. E.Z. is supported by the Lawrence and Susan Marx Immunotherapy Fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jerome Ritz, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: jerome_ritz@dfci.harvard.edu.

![Fig. 4. Functional responses after IL-12 stimulation of cytokine-primed T cells. / Activated T cells were primed with or without cytokines as indicated for 2 days. These cells were then washed and treated with or without IL-12 for 2 days. IFN-γ produced by activated cells was quantified by ELISA (A). All values represent net IFN-γ production measured by the difference between levels produced in the presence and absence of IL-12. T-cell proliferation (B) was quantified by measuring the incorporation of [3H]TdR. The fold proliferation compares values in T cells treated with IL-12 versus T cells without additional IL-12.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/12/10.1182_blood.v97.12.3860/6/m_h81211192004.jpeg?Expires=1763500198&Signature=V1ifjeW4AvUI7ySJ2rjmKZSh2vxQGJo5T6qFP-Z2XLiDymOTacFTeYHfeFfzAqGYpUq~aSM31yCk~dIPsTmOrBDCy-OfLeJEiUsirrBaKTl2EuqeDEPzklNhOR8CpzEHg7pWwaieSANB77KGQ9ZAYyyNM5iZ9-AwzPaEZNzYeciMWbO6cDz-bVOubFG28TU5tPGhOn02L6xe1cu8pWwGEo-kNspkd9dQP6N8TG9CBaRDXhw7OxCHJSH9xvITsp0rZFBiW0zkoAzbtesj5eBZBpXQbPvpvoZCafaLuXQJqdpP7B5yxlUy1gSE8z2JwHensoIxVj9fMti4-aF5ZeqnuA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal