The activation of caspase-8, a crucial upstream mediator of death receptor signaling, was investigated in epirubicin- and Taxol-induced apoptosis of B-lymphoma cells. This study was performed because the CD95/Fas receptor-ligand interaction, recruitment of the Fas-associated death domain (FADD) adaptor protein, and subsequent activation of procaspase-8 have been implicated in the execution of drug-induced apoptosis in other cell types. Indeed, active caspase-8 was readily detected after treatment of mature and immature B-lymphoid cells with epirubicin or Taxol. However, neither constitutive nor drug-induced expression of the CD95/Fas ligand was detectable in B-lymphoma cells. Furthermore, overexpression of a dominant-negative FADD mutant (FADDdn) did not block caspase-8 processing and subsequent DNA fragmentation, indicating that drug-induced caspase-8 activation was mediated by a CD95/Fas-independent mechanism. Instead, caspase-8 cleavage was slightly preceded by activation of caspase-3, suggesting that drug-induced caspase-8 activation in B-lymphoma cells is a downstream event mediated by other caspases. This assumption was confirmed in 2 experimental systems—zDEVD-fmk, a cell-permeable inhibitor of caspase-3–like activity, blocked drug-induced caspase-8 cleavage, and depletion of caspase-3 from cell extracts impaired caspase-8 cleavage after in vitro activation with dATP and cytochrome c. Thus, these data indicate that drug-induced caspase-8 activation in B-lymphoma cells is independent of death receptor signaling and is mediated by postmitochondrial caspase-3 activation.
Introduction
Apoptosis, a morphologically and biochemically defined form of cell death,1 plays a role in a wide variety of biologic systems, including tissue homeostasis and regulation of the immune system.2,3 The process is a highly orchestrated cellular pathway leading to activation of the downstream death machinery. The central mediator and executioner of the death machinery is a proteolytic system involving a family of cysteinyl proteases, called caspases (for review, see Thornberry and Lazebnik4). Triggering of the apoptotic cascade by different death stimuli, such as ionizing radiation5 and chemotherapeutic drugs,6 culminates in caspase-dependent cleavage of a set of regulatory proteins, degradation of cellular DNA, and complete disassembly of the cell. Thus far, 14 caspase family members have been identified, and some of them, such as caspase-8, mediate apoptotic signals after the activation of death receptors.7,8 Others, such as caspase-9, are part of the apoptosome and play a role in signal transduction after mitochondrial damage.9 Recently, an endoplasmic–reticulum-specific pathway of apoptosis has been described that is mediated by caspase-12.10
Previous data suggest that cytotoxic drugs induce cell death through CD95/Fas–CD95 ligand interaction,11 and the relevance of this particular death pathway has been shown, for instance, in doxorubicin-induced apoptosis of leukemic T cells.12However, other groups were unable to confirm these findings and showed CD95/Fas-independent induction of apoptosis by chemotherapeutic drugs—for example, doxorubicin and etoposide—in T-lymphoma cells.13,14 The finding that CD95/Fas-signaling and DNA damage induce different apoptosis-signaling pathways has also been shown in a murine and a human B-lymphoma cell line.15Furthermore, experimental evidence has been provided that neither FADD16 nor caspase-817 is required for drug-induced apoptosis.
In B cells, the induction of apoptosis plays important roles in humoral immunity. In this context, it has been shown that the antigen receptor BCR and the CD95/Fas receptor transduce pro-apoptotic signals in mature B cells (for review, see Tsubata18). We previously showed that activation-induced apoptosis of normal and malignant B lymphocytes upon antigen-receptor ligation is independent of CD95/Fas and CD95/Fas ligand.19 This has been confirmed by another study demonstrating that B-cell receptor-mediated apoptosis, in contrast to activation-induced T-cell apoptosis, is not mediated through known death receptor systems, nor does it involve the initial activation of caspase-8.20 We nevertheless observed that drug treatment leads to processing and activation of procaspase-8. We therefore focused our interest on the specific mechanisms leading to apoptotic death after the treatment of immature and mature B-lymphoma cells with cytotoxic drugs. By Northern blot analysis for the detection of CD95 ligand, by blocking of CD95 ligand/CD95 receptor interaction, and by overexpression of dominant-negative FADD (FADDdn), we show here that epirubicin-induced apoptosis in B cells is independent of a functional CD95 ligand–CD95 death-inducing signaling complex. This CD95-independent mechanism was also found after the treatment of B cells with Taxol, a chemotherapeutic agent that perturbs microtubule dynamics.21 Furthermore, experimental evidence is provided that the activation of caspase-8 in B cells occurs downstream of the mitochondrial apoptosome and caspase-3. Thus, in contrast to the death receptor-mediated activation of apoptosis, caspase-8 appears to function as an amplifying executioner caspase in drug-induced B cell apoptosis.
Materials and methods
Materials
Polyclonal rabbit antihuman caspase-3 (developed against human recombinant protein), monoclonal anti-PARP (clone C2-10), and polyclonal rabbit antihuman Rho-GDI/D4-GDI (developed against full-length recombinant human Rho-GDI-1) antibodies were from Pharmingen (Hamburg, Germany). Monoclonal anti-FADD antibody was from Transduction Laboratories (Lexington, KY). Monoclonal antihuman caspase-8 antibody directed against the active p18 subunit of caspase-8 has been described previously.22 Agonistic, monoclonal anti-CD95 antibody13 was diluted into growth medium to give a final concentration of 1 μg/mL. Secondary antimouse horseradish peroxidase-conjugated antibodies and secondary antirabbit horseradish peroxidase-conjugated antibodies were from Promega (Mannheim, Germany). The caspase-3 substrate (Ac-DEVD-pNA, in whichpNA stands for P-nitroanilide) and the caspase-8 substrate (Ac-IETD-pNA) were from Calbiochem-Novabiochem GmbH (Bad Soden, Germany). The caspase-3 inhibitor zDEVD-fmk (in which z stands for benzyloxycarbonyl and fmk for fluoromethyl ketone) was from Syrinx Diagnostica (Frankfurt, Germany) and was dissolved in dimethyl sulfoxide to give a 20 mM stock solution. According to the manufacturer, this inhibitor was synthesized as a methyl ester to enhance cell permeability. RNase A was from Roth (Karlsruhe, Germany). Epirubicin was purchased from Pharmacia Upjohn (Erlangen, Germany), and Taxol (paclitaxel) was purchased from Bristol Arzneimittel GmbH (Munich, Germany). The dominant-negative FADD construct (FADDdn) was a kind gift from A. M. Chinnaiyan and V. M. Dixit (Ann Arbor, MI).
Cell culture
Control vector- (pcDNA3-mock-transfected), pcDNA3-FADDdn-transfected BJAB cells, which were stably transfected with a dominant-negative FADD mutant lacking the N-terminal death effector domain,22 Jurkat T-cells, NALM-6, and REH B-lymphoid cells were grown in RPMI 1640 medium supplemented with 10% fetal calf serum, 0.56 g/L L-glutamine, 100 000 U/L penicillin, and 0.1 g/L streptomycin. Media and culture reagents were from Life Technologies GmbH (Karlsruhe, Germany). Cells were subcultured every 3 to 4 days by dilution of the cells to a concentration of 1 × 105 cells/mL.
Additionally, bone marrow material from patients with childhood acute lymphoblastic leukemia (ALL) was obtained by bone marrow aspiration. Lymphoblasts and mononuclear cells were separated by centrifugation over Ficoll. The percentage of leukemic lymphoblasts was greater than 90%, which is in accordance with findings of a former study.23 The diagnosis was established by immunophenotyping of leukemia cells according to Béné et al.24 After separation, cells were immediately seeded at a density of 1 × 105 cells/mL in RPMI 1640 complete cell culture medium and treated with epirubicin. Leukemic lymphoblasts were also treated with Taxol. However, because of the missing cell proliferation in this experimental setting, Taxol did not induce significant apoptosis or caspase activation in primary lymphoblasts (data not shown).
CD95/Fas-mediated induction of apoptosis
Anti-CD95 (anti-APO-1 IgG3) or FII23c F(ab)2fragments were produced as described25 and were added to the cultures before exposure to the cytostatic drugs. As a positive control for the ability of the F(ab)2 fragments to block CD95/Fas receptor ligand interaction, we used Jurkat T cells induced for apoptosis by immobilized OKT3 anti-CD3 monoclonal antibody.19
Measurement of cell death
For the determination of cell death, 3 × 104cells per well were seeded in microtiter plates and treated for different time periods with 1 μg/mL anti-CD95 or the chemotherapeutic agents. Cell death was assessed by the uptake of propidium iodide (2 μg/mL) in phosphate-buffered saline (PBS) into nonfixed cells and by subsequent flow cytometric analysis using the FSC–FL2 profile. Analyses were performed on a FACScalibur (Becton Dickinson; Heidelberg, Germany) using CellQuest analysis software. Additionally, cell death was determined by trypan blue exclusion as described.26Both methods virtually gave the same results.
Measurement of DNA fragmentation
DNA fragmentation was measured essentially as described.27 Briefly, cells were seeded at a density of 1 × 105 cells/mL and treated with different concentrations of the respective cytotoxic drugs. After 24, 48, and 72 hours, cells were collected by centrifugation at 300g for 5 minutes, washed with PBS at 4°C, and fixed in PBS/2% (vol/vol) formaldehyde on ice for 30 minutes. After fixation, cells were incubated with ethanol/PBS (2:1, vol/vol) for 15 minutes, pelleted, and resuspended in PBS containing 40 μg/mL RNase A. After incubation for 30 minutes at 37°C, cells were pelleted again and finally resuspended in PBS containing 50 μg/mL propidium iodide. Nuclear DNA fragmentation was then quantified by flow cytometric determination of hypodiploid DNA. Data were collected and analyzed using a FACScan (Becton Dickinson) equipped with the CELLQuest software. Data are given in percentage hypoploidy (subG1), which reflects the number of apoptotic cells. Specific apoptosis was calculated by subtracting background apoptosis observed in control cells from total apoptosis observed in drug-treated cells. Background apoptosis was approximately 5%, depending on the cell line and the experimental settings.
Northern blotting
Northern blotting and hybridization were performed as previously described.19 CD95 ligand expression (1.3 kB mRNA) was detected using a 500-bp polymerase chain reaction-cloned fragment, and β-actin was detected with a cloned and sequenced 246-bp fragment obtained by reverse transcriptase polymerase chain reaction as described.19
Immunoblotting
After incubation with the respective cytostatic drugs, cells were washed twice with PBS and lysed in buffer containing 10 mM Tris/HCl, pH 7.5, 300 mM NaCl, 1% Triton X-100, 2 mM MgCl2, 5 μM EDTA, 1 μM pepstatin, 1 μM leupeptin, and 0.1 mM phenylmethylsulfonyl fluoride. Protein concentration was determined using the bicinchoninic acid assay28 from Pierce (Rockford, IL), and equal amounts of protein (usually 20 μg per lane) were separated by SDS-PAGE.29 Then, immunoblotting was performed as described.30 Membranes (Schleicher & Schuell, Dassel, Germany) were swollen in CAPS-buffer (10 mM 3-[cyclohexylamino]propane-1-sulfonic acid, pH 11, 10% MeOH) for several minutes, and blotting was performed at 1 mA/cm2 for 1 hour in a transblot SD cell (Bio-Rad, Munich, Germany). The membrane was blocked for 1 hour in PBST (PBS, 0.05% Tween-20) containing 3% nonfat dry milk and incubated with primary antibody for 1 hour. After the membrane had been washed 3 times in PBST, secondary antibody in PBST was applied for 1 hour. Finally, the membrane was washed in PBST again, and protein bands were detected using the enhanced chemiluminescence system (Amersham Buchler, Braunschweig, Germany).
Preparation of cytoplasmic cell extracts and induction of the cell-free apoptotic system
Cell extracts were prepared as described.27Briefly, BJAB cells were washed twice with PBS, resuspended in buffer A containing 20 mM HEPES, pH 7.4, 10 mM KCl, 2 mM MgCl2, and 1 mM EDTA and incubated on ice for 15 minutes. Phenylmethylsulfonyl fluoride was added to give a final concentration of 0.1 mM. Cells were then disrupted by 15 passages through a 21-gauge × 1 1/2 needle (0.80 mm × 40 mm; Braun, Melsungen, Germany). The homogenates were cleared by centrifugation at 16 000g, 4°C for 15 minutes. The centrifugation step was repeated, and the clear supernatants were used for in vitro caspase activation, immunoprecipitation of caspase-3, or measurement of caspase activities. For initiating in vitro caspase activation, 1 mM dithiothreitol, 10 μM horse heart cytochrome c (Sigma; Munich, Germany), and 1 mM dATP were added, and the extracts were incubated at 30°C for 5 minutes followed by incubation at 37°C for different time periods. Control extracts were similarly incubated in the absence of cytochrome c and dATP.
Measurement of caspase-3–like and caspase-8–like activities
Caspase-3–like activity was measured in cell extracts from BJAB cells as described31 with some modifications. To this end, 10 μL extract, 90 μL buffer B containing 50 mM HEPES, pH 7.4, 100 mM NaCl, 1 mM EDTA, 0.1% 3-[(3-cholamidopropyl)dimethyl-ammonio]-1-propanesulfonate, 10% saccharose, 5 mM dithiothreitol, and 2 μL colorimetric substrate (10 mM Ac-DEVD-pNA in dimethyl sulfoxide) were mixed. Samples were incubated at 37°C, and the absorbance at 405 nm was measured in an enzyme-linked immunosorbent assay reader every 5 minutes. One unit enzyme activity is defined as μmol Ac-DEVD-pNA cleaved per minute. Caspase-8–like activity was measured in BJAB cells using Ac-IETD-pNA as substrate.
Immunoprecipitation of caspase-3
Caspase-3 was immunoprecipitated from cellular extracts of BJAB cells essentially as described elsewhere.32 Briefly, 100 μL cellular extract was incubated with 0.5 mg protein A–Sepharose and 20 μL antihuman caspase-3 antibody at 4°C for 4 hours and moderately shaken. The protein A–Sepharose immune complex was sedimented by centrifugation at 300g, 4°C for 5 minutes, and the supernatant was used for in vitro activation by the addition of dATP and cytochrome c as described above. In addition, 100 μL control extract was incubated in the presence of protein A–Sepharose and the absence of antihuman caspase-3 antibody.
Measurement of the mitochondrial permeability transition
After incubation with the respective cytostatic drugs, cells were collected by centrifugation at 300g, 4°C for 5 minutes. Mitochondrial permeability transition was then determined by staining the cells with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolylcarbocyanin iodide (JC-1; Molecular Probes, Leiden, The Netherlands) as described.33 34 1 × 105 cells were resuspended in 500 μL phenol red-free RPMI 1640 without supplements, and JC-1 was added to give a final concentration of 2.5 μg/mL. The cells were incubated for 30 minutes at 37°C and moderately shaken. Control cells were incubated in the absence of JC-1 dye. The cells were harvested by centrifugation at 300g, 4°C for 5 minutes, washed with ice-cold PBS, and resuspended in 200 μL PBS at 4°C. Mitochondrial permeability transition was then quantified by flow cytometric determination of cells with decreased fluorescence—that is, with mitochondria displaying a lower membrane potential. Data were collected and analyzed using a FACScan (Becton Dickinson) equipped with the CELLQuest software. Data are given in percentage cells with low ΔΨm, which reflects the number of apoptotic cells.
Results
Anticancer drugs induce apoptosis and caspase-8 activation in CD95/Fas-sensitive and -resistant B-lymphoid cells
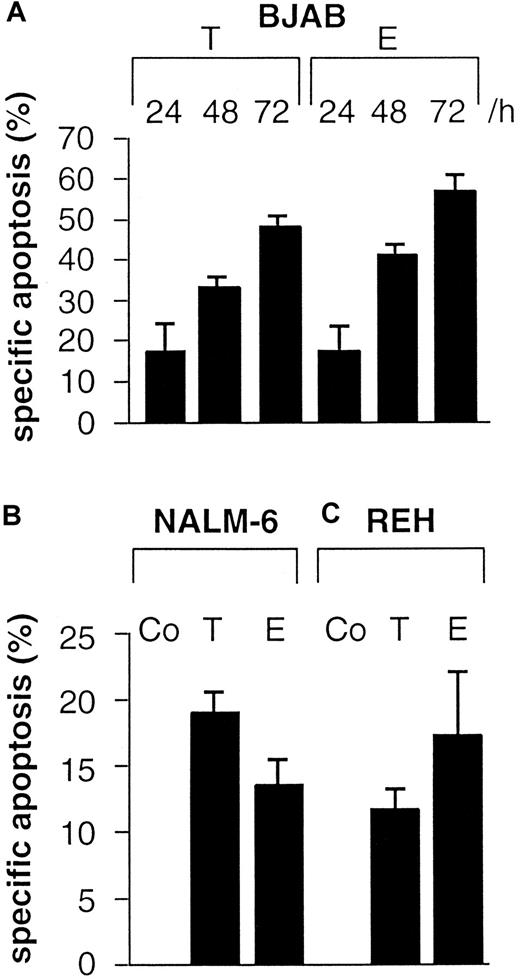
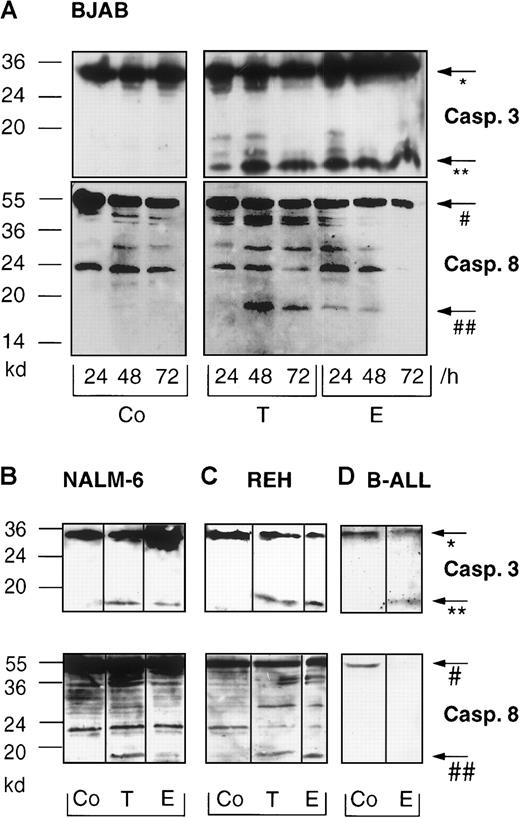
To investigate B cell apoptosis induced by anticancer drugs, BJAB cells were treated with 0.1 μg/mL Taxol or 1.0 μg/mL epirubicin for 24 hours, 48 hours, and 72 hours. Apoptosis was determined by flow cytometry using a modified cell cycle assay and measurement of hypodiploid DNA. These experiments revealed that not only BJAB cells (Figure 1A) but also immature B cells, namely NALM-6 (Figure 1B) and REH cells (Figure 1C), and primary B-ALL cells (Table 1) displayed significant DNA fragmentation after treatment with the cytotoxic drugs. To verify that the cells underwent apoptotic cell death, the cleavage of 2 known caspase substrates, poly-(ADP-ribose)polymerase and GDP dissociation inhibitor (D4-GDI),27 was investigated by Western blot analysis. Cleavage of these proteins occurred in a time-dependent manner corresponding to the results obtained by flow cytometric analysis. Cleavage was detected as early as 24 hours after treatment and reached a maximum after 48 hours (data not shown). To further examine apoptosis signaling pathways in B cells, we determined drug-induced processing and activation of caspase-3 in our experimental systems. As shown in Figure 2A, the immunoreactive 17-kd active subunit of caspase-3 was detected in BJAB cells as early as 24 hours after treatment and persisted for 72 hours. Furthermore, the appearance of the 17-kd subunit of caspase-3 was observed in drug-treated NALM-6 (Figure 2B, upper panel), REH (Figure 2C, upper panel), and primary B ALL cells (Figure 2D, upper panel) after 48 hours.
Taxol and epirubicin time-dependently induce DNA fragmentation in B-lymphoid cells.
Cells were treated with control medium (Co), 0.1 μg/mL Taxol (T), or 1 μg/mL epirubicin (E). DNA fragmentation was measured in drug-treated BJAB cells (A) after the indicated time, in NALM-6 cells (B) after 48 hours, and in REH cells (C) after 48 hours as described in “Materials and methods.” Values are given as percentage of specific apoptosis ± SD (n = 3).
Taxol and epirubicin time-dependently induce DNA fragmentation in B-lymphoid cells.
Cells were treated with control medium (Co), 0.1 μg/mL Taxol (T), or 1 μg/mL epirubicin (E). DNA fragmentation was measured in drug-treated BJAB cells (A) after the indicated time, in NALM-6 cells (B) after 48 hours, and in REH cells (C) after 48 hours as described in “Materials and methods.” Values are given as percentage of specific apoptosis ± SD (n = 3).
Blocking experiments with anti-CD95-Fas-F(ab)2fragments in primary B-lineage ALL cells
| Patient . | B-lineage ALL (% apoptosis) . | ||||
|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | |
| Medium control | 35.1 ± 5.8 | 54.8 ± 5.3 | 23.9 ± 3.1 | 44.2 ± 3.7 | 38.5 ± 4.2 |
| Epirubicin | 99.1 ± 9.9 | 98.1 ± 9.8 | 92.2 ± 7.2 | 97.5 ± 4.1 | 62.3 ± 6.9 |
| Epirubicin + control F(ab)2 | 99.1 ± 2.9 | 99.2 ± 6.2 | 95.6 ± 6.9 | 98.7 ± 4.7 | 68.4 ± 7.4 |
| Epirubicin + anti-CD95 F(ab)2 | 99.3 ± 5.7 | 95.4 ± 7.9 | 98.6 ± 8.1 | 94.7 ± 3 | 65.4 ± 4.1 |
| Patient . | B-lineage ALL (% apoptosis) . | ||||
|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | |
| Medium control | 35.1 ± 5.8 | 54.8 ± 5.3 | 23.9 ± 3.1 | 44.2 ± 3.7 | 38.5 ± 4.2 |
| Epirubicin | 99.1 ± 9.9 | 98.1 ± 9.8 | 92.2 ± 7.2 | 97.5 ± 4.1 | 62.3 ± 6.9 |
| Epirubicin + control F(ab)2 | 99.1 ± 2.9 | 99.2 ± 6.2 | 95.6 ± 6.9 | 98.7 ± 4.7 | 68.4 ± 7.4 |
| Epirubicin + anti-CD95 F(ab)2 | 99.3 ± 5.7 | 95.4 ± 7.9 | 98.6 ± 8.1 | 94.7 ± 3 | 65.4 ± 4.1 |
B-lineage ALL cells were cultured for 48 hours in the presence or absence of epirubicin (0.1 μg/mL). F(ab)2 fragments were added at 1 μg/mL. Apoptosis was measured on the single-cell level by assessing the nuclear DNA content. Each experiment was performed in triplicate. Mean values for the percentage of apoptotic cells ± SD (n = 3) are shown for 5 patients with childhood B-lineage ALL.
Taxol and epirubicin induce processing of caspase-3 and caspase-8 in different B-lymphoid cell lines and in primary B-lineage ALL cells.
BJAB cells (A) were cultured in control medium (Co), 0.1 μg/mL Taxol (T), or 1 μg/mL epirubicin (E) for different time intervals as indicated. NALM-6 (B), REH (C), and primary B-ALL cells (D) were treated with control medium (Co), 0.1 μg/mL Taxol (T), or 1 μg/mL epirubicin (E) for 48 hours. Western blot analyses were performed as described in “Materials and methods” with antihuman caspase-3 and antihuman caspase-8 antibodies. Positions of molecular mass markers are indicated at the left. Arrows indicate the positions of procaspase-3 (*), the 17-kd active subunit of caspase-3 (**), procaspase-8 (#), and the 18-kd active subunit of caspase-8 (##). Experiments were repeated twice and yielded similar results.
Taxol and epirubicin induce processing of caspase-3 and caspase-8 in different B-lymphoid cell lines and in primary B-lineage ALL cells.
BJAB cells (A) were cultured in control medium (Co), 0.1 μg/mL Taxol (T), or 1 μg/mL epirubicin (E) for different time intervals as indicated. NALM-6 (B), REH (C), and primary B-ALL cells (D) were treated with control medium (Co), 0.1 μg/mL Taxol (T), or 1 μg/mL epirubicin (E) for 48 hours. Western blot analyses were performed as described in “Materials and methods” with antihuman caspase-3 and antihuman caspase-8 antibodies. Positions of molecular mass markers are indicated at the left. Arrows indicate the positions of procaspase-3 (*), the 17-kd active subunit of caspase-3 (**), procaspase-8 (#), and the 18-kd active subunit of caspase-8 (##). Experiments were repeated twice and yielded similar results.
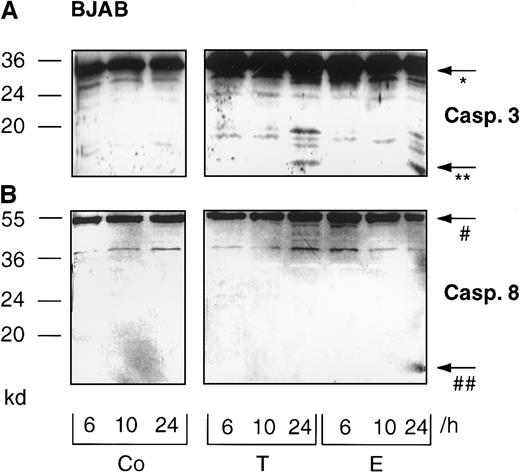
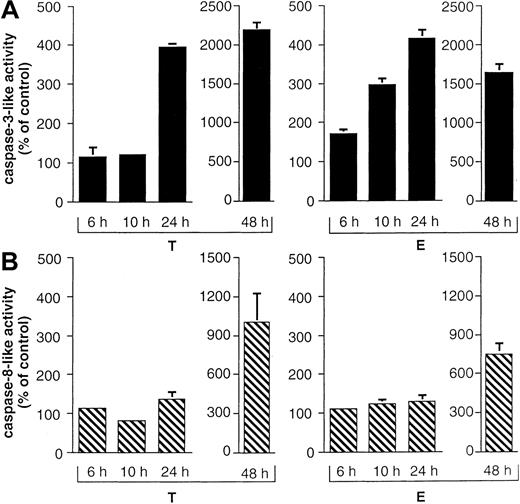
We also detected caspase-8 processing in all B-lineage cells tested in this study (Figure 2A-C, lower panel). Interestingly, prolonged incubation of primary childhood B-lineage ALL blasts for 48 hours in the presence of epirubicin led to complete processing of procaspase-8, as evidenced by the disappearance of the proenzyme (Figure 2D, lower panel). However, more detailed time-course experiments in BJAB cells revealed that caspase-8 cleavage was delayed compared with that of caspase-3. Whereas the 17-kd active subunit of caspase-3 was already observed after 24 hours of Taxol and epirubicin treatment (Figure3A), caspase-8 processing and detection of the 18-kd active subunit of caspase-8 was only marginal at this time point (Figure 3B). After 6 hours and 10 hours of drug treatment, we could not detect any processing of caspase-3 (Figure 3A) or caspase-8 (Figure 3B). To further substantiate these data, caspase-3–like and caspase-8–like activities were measured in Taxol- and epirubicin-treated BJAB cells. Again, Taxol- and epirubicin-induced caspase-3–like activity preceded caspase-8–like activity. After 24 hours of treatment with both drugs, caspase-3–like activity was enhanced 4 times compared with control cells (Figure4A). On the other hand, no significant increase of caspase-8 activity was observed at time points up to 24 hours (Figure 4B). At a later time point, however, the processing of procaspase-8 and the appearance of the 18-kd active subunit of caspase-8 coincided with an elevation of caspase-8–like enzyme activity in drug-treated BJAB cells. After 48 hours of incubation, the measurement of Ac-IETD-pNA cleavage showed 7.9-fold and 10.2-fold increases in caspase-8–like activity in epirubicin- and Taxol-treated BJAB cells, respectively (Figure 4B). A significant increase in caspase-8–like activity was also measured after the treatment of NALM-6 and REH cells with epirubicin and Taxol (data not shown).
Time-course of Taxol- and epirubicin-induced processing of caspase-3 and caspase-8 in BJAB cells.
BJAB cells were cultured in control medium (Co), 0.1 μg/mL Taxol (T), or 1 μg/mL epirubicin (E) for different time intervals as indicated. Western blot analyses were performed as described in “Materials and methods” with antihuman caspase-3 (A) and antihuman caspase-8 antibodies (B). Positions of the molecular mass markers are indicated at the left. Arrows indicate the positions of procaspase-3 (*), the 17-kd active subunit of caspase-3 (**), procaspase-8 (#), and the 18-kd active subunit of caspase-8 (##). Experiments were repeated twice and yielded similar results.
Time-course of Taxol- and epirubicin-induced processing of caspase-3 and caspase-8 in BJAB cells.
BJAB cells were cultured in control medium (Co), 0.1 μg/mL Taxol (T), or 1 μg/mL epirubicin (E) for different time intervals as indicated. Western blot analyses were performed as described in “Materials and methods” with antihuman caspase-3 (A) and antihuman caspase-8 antibodies (B). Positions of the molecular mass markers are indicated at the left. Arrows indicate the positions of procaspase-3 (*), the 17-kd active subunit of caspase-3 (**), procaspase-8 (#), and the 18-kd active subunit of caspase-8 (##). Experiments were repeated twice and yielded similar results.
Time-course of Taxol- and epirubicin-induced caspase-3– and caspase-8–like activity in BJAB cells.
BJAB cells were cultured in control medium, 0.1 μg/mL Taxol (T), or 1 μg/mL epirubicin (E) for different time intervals as indicated. Caspase-3–like activity was determined as described in “Materials and methods” using Ac-DEVD-pNA (A). Caspase-8–like activity was assessed in parallel using Ac-IETD-pNA (B). Caspase-3– and caspase-8–like activities are given as percentage of control ± SD (n = 3).
Time-course of Taxol- and epirubicin-induced caspase-3– and caspase-8–like activity in BJAB cells.
BJAB cells were cultured in control medium, 0.1 μg/mL Taxol (T), or 1 μg/mL epirubicin (E) for different time intervals as indicated. Caspase-3–like activity was determined as described in “Materials and methods” using Ac-DEVD-pNA (A). Caspase-8–like activity was assessed in parallel using Ac-IETD-pNA (B). Caspase-3– and caspase-8–like activities are given as percentage of control ± SD (n = 3).
Drug-induced apoptosis in B-lineage leukemia is independent of CD95 ligand and CD95 death-inducing signaling complex
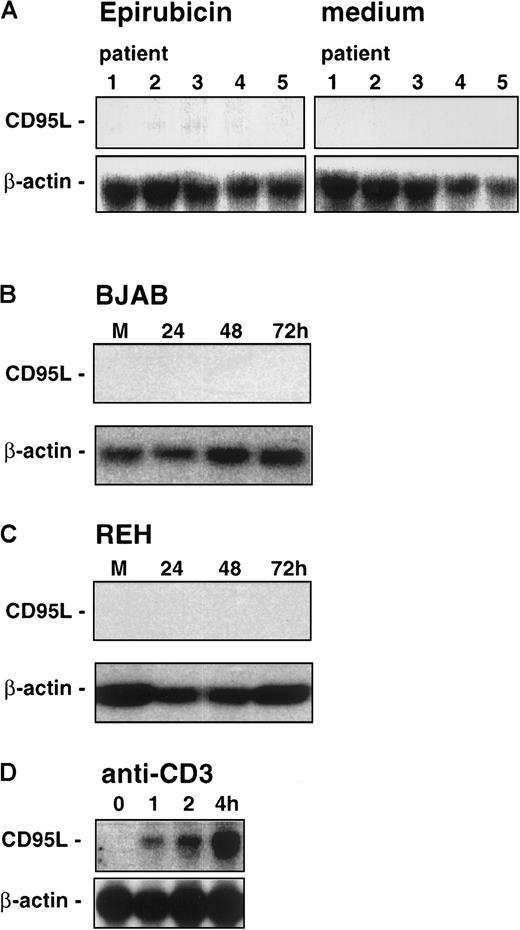
In previous studies, it has been demonstrated that cytotoxic drugs strongly stimulate CD95 ligand messenger RNA expression in human leukemia T-cell lines.11 To test whether CD95 ligand expression in B-lineage leukemia cells after challenge with epirubicin correlates with the activation of caspase-8, we performed Northern blot analyses for CD95 ligand expression and blocking experiments to assess the requirement of CD95/Fas–CD95 ligand interaction in our system. As already shown above, the onset of the apoptotic program after treatment with epirubicin takes at least 24 hours, and no evidence for induction of the initiator caspase-8 at earlier time points could be found. Nevertheless, we performed control experiments to check the time frame of epirubicin-induced CD95 ligand expression, and significant levels of CD95 ligand mRNA were detected in primary leukemic T-lineage ALL cells after 24 hours of incubation with this drug (data not shown). In clear contrast, Northern blot analyses revealed that neither primary leukemic cell samples of patients with childhood B-lineage ALL (Figure5A) nor BJAB (Figure 5B) and REH cells (Figure 5C) expressed significant levels of CD95 ligand mRNA after drug exposure. However, using cross-linking of Jurkat T cells by anti-CD3 as an additional control, we could clearly demonstrate CD95 ligand mRNA expression (Figure 5D).
Northern blot analysis of CD95/Fas ligand expression in epirubicin-induced cells.
Leukemic cell samples of 5 patients (the same as in Table 1) with childhood B-lineage ALL (A) were induced for 24 hours in the presence of epirubicin (0.1 μg/mL) or medium alone. BJAB (B) or REH cells (C) were induced for 24 to 72 hours in the presence of epirubicin. M, medium control at 72 hours. (D) As a positive control for the induction of CD95/Fas ligand expression, Jurkat T cells were cultured in the presence of plastic-immobilized anti-CD3 monoclonal antibody OKT3 for 0, 1, 2, or 4 hours. To control for equal amounts and the integrity of the RNA, the blots were rehybridized with a cDNA probe for β-actin.
Northern blot analysis of CD95/Fas ligand expression in epirubicin-induced cells.
Leukemic cell samples of 5 patients (the same as in Table 1) with childhood B-lineage ALL (A) were induced for 24 hours in the presence of epirubicin (0.1 μg/mL) or medium alone. BJAB (B) or REH cells (C) were induced for 24 to 72 hours in the presence of epirubicin. M, medium control at 72 hours. (D) As a positive control for the induction of CD95/Fas ligand expression, Jurkat T cells were cultured in the presence of plastic-immobilized anti-CD3 monoclonal antibody OKT3 for 0, 1, 2, or 4 hours. To control for equal amounts and the integrity of the RNA, the blots were rehybridized with a cDNA probe for β-actin.
In another set of experiments, we used anti-CD95/Fas F(ab)2fragments to block CD95 ligand–CD95 receptor interaction. In accordance with the lack of CD95 ligand expression, epirubicin-induced apoptosis of BJAB (Figure 6A) and REH cells (Figure 6B) was not influenced by neutralizing anti-CD95 antibody F(ab)2 fragments. The same was true for primary B-lineage ALL cells, which also showed epirubicin-induced DNA fragmentation that could not be inhibited by neutralizing anti-CD95 antibody F(ab)2 fragments (Table 1). As a positive control for these blocking experiments, again Jurkat T cells were treated with immobilized anti-CD3 monoclonal antibody OKT3 to trigger activation-induced cell death (see also the control experiment shown in Figure 5D). In this experimental setup, anti-CD3–triggered cell death was inhibited in a concentration-dependent manner by the addition of anti-CD95 F(ab)2 fragments (Figure 6C), thereby confirming that this approach allows discrimination between CD95-dependent and CD95-independent signaling pathways. Thus, epirubicin-induced apoptosis in B lymphoid cells is not functionally linked to CD95 ligand–CD95 receptor interaction.
Effects of blocking of CD95 ligand–CD95 receptor interaction.
BJAB (A) or REH (B) B cells were induced for apoptosis by epirubicin (0.1 μg/mL) in the presence or absence of neutralizing anti-CD95/Fas or control F(ab)2 fragments. Open circles, medium control; open triangles, epirubicin; open squares, epirubicin plus control F(ab)2; filled squares, epirubicin plus anti-CD95/Fas F(ab)2 fragments. Jurkat T-cells (C) were induced for apoptosis for 24 hours in the presence (squares) or absence (circles) of immobilized anti-CD3 as described.19 Control (open circles, open squares) or anti-CD95/Fas F(ab)2 fragments (filled circles, filled squares) were present in the cultures at 1 μg/mL. Apoptosis was measured at the single cell level by assessing the nuclear DNA content as described in “Materials and methods.” Mean values for the percentage of apoptotic cells ± SD (n = 3) are shown.
Effects of blocking of CD95 ligand–CD95 receptor interaction.
BJAB (A) or REH (B) B cells were induced for apoptosis by epirubicin (0.1 μg/mL) in the presence or absence of neutralizing anti-CD95/Fas or control F(ab)2 fragments. Open circles, medium control; open triangles, epirubicin; open squares, epirubicin plus control F(ab)2; filled squares, epirubicin plus anti-CD95/Fas F(ab)2 fragments. Jurkat T-cells (C) were induced for apoptosis for 24 hours in the presence (squares) or absence (circles) of immobilized anti-CD3 as described.19 Control (open circles, open squares) or anti-CD95/Fas F(ab)2 fragments (filled circles, filled squares) were present in the cultures at 1 μg/mL. Apoptosis was measured at the single cell level by assessing the nuclear DNA content as described in “Materials and methods.” Mean values for the percentage of apoptotic cells ± SD (n = 3) are shown.
Processing of caspase-8 occurs independently of CD95 signaling in BJAB cells
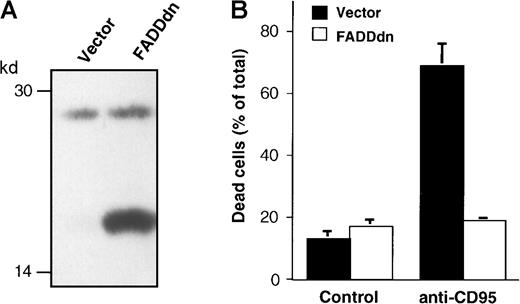
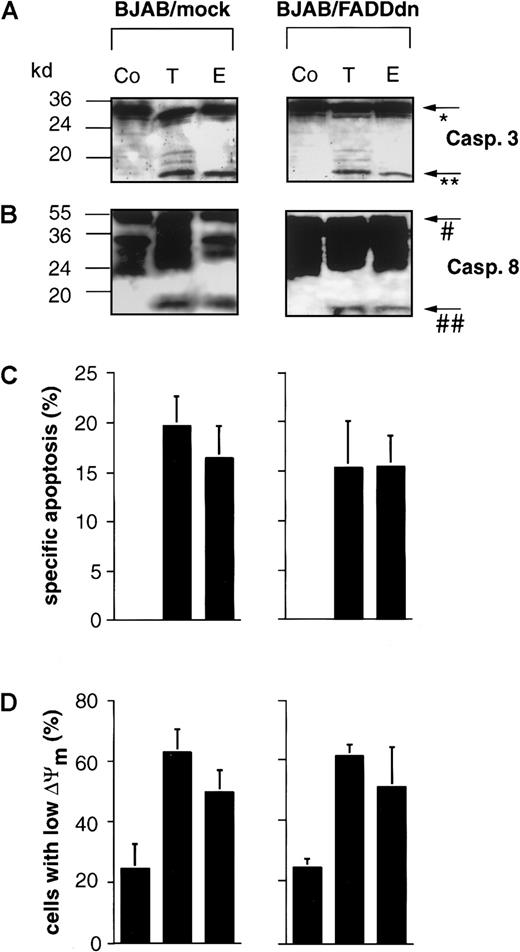
As shown above, drug-induced apoptosis in B cells was not accompanied by the expression of CD95 ligand mRNA and occurred independently of CD95 ligand–CD95 receptor interaction. Nevertheless, the induction of apoptosis in these cells also led to the cleavage of caspase-8. To further exclude an involvement of the CD95 or other death receptor systems, we used BJAB cells overexpressing a dominant-negative FADD mutant (FADDdn). Both mock and FADDdn transfectants express endogenous FADD (27 kd), whereas the FADDdn transfectants also show a lower band representing the truncated FADDdn (see Figure7A). This FADD mutant blocks CD95-induced apoptosis22 and completely prevents caspase-3 activation upon triggering of the BJAB transfectants with agonistic anti-CD95 antibodies.35 In our hands, BJAB/FADDdn cells were completely resistant to treatment with 1 μg/mL agonistic anti-CD95 antibody, whereas approximately 70% of the control vector-transfected BJAB/mock cells were killed under the same conditions (Figure 7B), thereby demonstrating the efficacy of FADDdn in this cell line. However, in accordance with the hypothesis that drug-induced caspase-8 processing in BJAB cells is independent of CD95 signaling, the active 18-kd subunit of caspase-8 was detected in BJAB/FADDdn cells after challenge with Taxol and epirubicin (Figure8B). The cleavage of caspase-8 in these cells coincided with the appearance of the 17-kd active subunit of caspase-3 (Figure 8A). CD95-independent apoptosis was further supported by the assessment of drug-induced DNA fragmentation in BJAB/FADDdn cells as compared with control vector-transfected BJAB/mock cells. As shown in Figure 8C, Taxol- and epirubicin-induced apoptosis was not significantly influenced by the overexpression of FADDdn. In recent publications, the critical role of mitochondrial damage for drug-induced apoptosis36,37 and CD95-dependent signaling to mitochondria through caspase-8–mediated cleavage of Bid has been demonstrated.38 We thus measured mitochondrial activation after treatment with Taxol and epirubicin in our experimental system. Treatment of BJAB/mock cells with both agents led to significant mitochondrial permeability transition and to an approximately 2.5-fold increase of cells with low ΔΨm after 48 hours (Figure 8D). This drug-induced mitochondrial permeability transition was not influenced by the overexpression of FADDdn (Figure 8D), thereby excluding the possibility that the mitochondrial activation in BJAB cells was mediated by a CD95-dependent mechanism. Time-course experiments in BJAB/mock, BJAB/FADDdn, NALM-6, and REH cells revealed that the mitochondrial permeability transition already occurred 24 hours after the addition of epirubicin and Taxol to the culture medium and thus preceded, or at least coincided with, the processing of procaspase-8 (data not shown).
BJAB cells overexpressing a dominant-negative FADD mutant (FADDdn) are resistant to CD95-mediated apoptosis.
(A) Expression of FADDdn. Lysates of vector-transfected BJAB cells and BJAB cells stably expressing FADDdn were subjected to immunoblotting using a monoclonal anti-FADD antibody. The positions of molecular mass markers are indicated at the left. (B) Apoptosis induction. BJAB vector control or FADDdn-expressing cells were either left untreated (Control) or were stimulated for 30 hours with 1 μg/mL anti-CD95 antibody. Then cell death was assessed by the uptake of propidium iodide and flow cytometry as described in “Materials and methods.” Values are given as percentage of dead cells ± SD (n = 3).
BJAB cells overexpressing a dominant-negative FADD mutant (FADDdn) are resistant to CD95-mediated apoptosis.
(A) Expression of FADDdn. Lysates of vector-transfected BJAB cells and BJAB cells stably expressing FADDdn were subjected to immunoblotting using a monoclonal anti-FADD antibody. The positions of molecular mass markers are indicated at the left. (B) Apoptosis induction. BJAB vector control or FADDdn-expressing cells were either left untreated (Control) or were stimulated for 30 hours with 1 μg/mL anti-CD95 antibody. Then cell death was assessed by the uptake of propidium iodide and flow cytometry as described in “Materials and methods.” Values are given as percentage of dead cells ± SD (n = 3).
Overexpression of dominant-negative FADD in BJAB cells does not impair drug-induced processing of caspase-3, caspase-8, DNA fragmentation, and mitochondrial activation.
Mock- or dominant-negative FADD (FADDdn)-transfected BJAB cells were either left untreated (Co) or were incubated with 0.1 μg/mL Taxol (T) or 1 μg/mL epirubicin (E) for 48 hours. Then Western blot analyses were performed with antihuman caspase-3 (A) and antihuman caspase-8 antibodies (B). Positions of molecular mass markers are indicated at the left. Arrows indicate the positions of procaspase-3 (*), the 17-kd active subunit of caspase-3 (**), procaspase-8 (#), and the 18-kd active subunit of caspase-8 (##). The experiments were repeated and yielded similar results. Additionally, DNA fragmentation (C) and mitochondrial permeability transition (D) in drug-treated BJAB cells were measured as described in “Materials and methods.” Values are given as percentage of specific apoptosis ± SD (n = 3) and as percentage of cells with low ΔΨm ± SD (n = 3), respectively.
Overexpression of dominant-negative FADD in BJAB cells does not impair drug-induced processing of caspase-3, caspase-8, DNA fragmentation, and mitochondrial activation.
Mock- or dominant-negative FADD (FADDdn)-transfected BJAB cells were either left untreated (Co) or were incubated with 0.1 μg/mL Taxol (T) or 1 μg/mL epirubicin (E) for 48 hours. Then Western blot analyses were performed with antihuman caspase-3 (A) and antihuman caspase-8 antibodies (B). Positions of molecular mass markers are indicated at the left. Arrows indicate the positions of procaspase-3 (*), the 17-kd active subunit of caspase-3 (**), procaspase-8 (#), and the 18-kd active subunit of caspase-8 (##). The experiments were repeated and yielded similar results. Additionally, DNA fragmentation (C) and mitochondrial permeability transition (D) in drug-treated BJAB cells were measured as described in “Materials and methods.” Values are given as percentage of specific apoptosis ± SD (n = 3) and as percentage of cells with low ΔΨm ± SD (n = 3), respectively.
Caspase-3 triggers activation of caspase-8 in vivo and in vitro
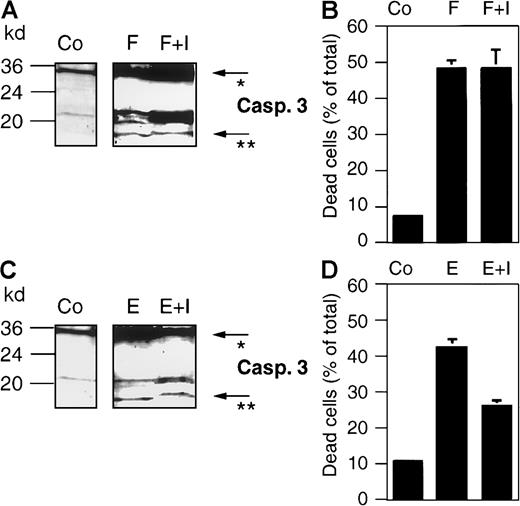
Different death stimuli activate mitochondria, which in turn release cytochrome c. The latter triggers an apoptotic cascade through Apaf-1–caspase-9 complex formation,9 leading to the subsequent activation of the main executioner of the apoptotic machinery, caspase-3. From the results described above, we reasoned that the processing of procaspase-8 to the active caspase-8 occurs downstream of caspase-3. To verify this hypothesis, we used zDEVD-fmk, a cell-permeable peptide-inhibitor that specifically inhibits caspase-3–like enzyme activities by irreversibly binding to the active site of caspase-3. In a control experiment, we first excluded that zDEVD-fmk interferes with upstream initiator caspases, for example, caspase-8. Indeed, this inhibitor did not inhibit CD95- or epirubicin-induced cleavage of caspase-3 (Figure9A and C, respectively). Addition of the inhibitor to anti-CD95 antibody- and drug-treated cells led to a clear shift of the 17-kd active subunit of caspase-3 to a higher apparent molecular weight of approximately 18 kd (Figures 9A and C, 10A), indicating that the inhibitor had irreversibly bound to the enzyme. Interestingly, zDEVD-fmk only inhibited drug-induced cell-death (Figures 9D, 10C), whereas CD95-induced cell death was not influenced (Figure 9B). This might be explained by the recent finding that caspase-3 is involved in a feedback loop for the amplification of drug-induced mitochondrial cytochrome c release.39 Furthermore, the inhibition of caspase-3–like activity by zDEVD-fmk in this experimental setting was confirmed by Western blot analysis of the cleavage of the caspase-3 substrate D4-GDI, which was completely inhibited when drug-treated cells were incubated in the presence of the peptide inhibitor (data not shown). Thus, zDEVD-fmk represents a suitable tool to investigate apoptosis-associated events located downstream of caspase-3. As shown in Figure 10B, the treatment of BJAB cells with zDEVD-fmk completely blocked Taxol- and epirubicin-induced processing of procaspase-8, and its 18-kd active cleavage product was no longer detected. This again provided evidence that drug-induced activation of caspase-8 occurred downstream of caspase-3. To further investigate the mechanism of caspase-8 processing in vitro, a cell-free system based on the activation of BJAB cytosol with cytochrome c and dATP40 was established. As shown in Figure 11A, the addition of cytochrome c and dATP to cellular extracts led to the rapid cleavage of procaspase-3 and to the appearance of the 17-kd active cleavage product of caspase-3 after 10 minutes. Interestingly, processing of procaspase-8 was delayed compared with that of caspase-3, and the 18-kd active subunit of caspase-8 was detected after 120 minutes of incubation (Figure 11A, lower panel). This was verified by the measurement of caspase-3– and caspase-8–like enzyme activities using the colorimetric peptide substrates Ac-DEVD-pNA and Ac-IETD-pNA, respectively. Again, the activation of caspase-3 preceded the activation of caspase-8 (Figure 11B). This was reflected by a steady increase of the caspase-8–caspase-3 ratio from 0.156 after 10 minutes to 0.215 after 70 minutes and to 0.290 after 130 minutes of incubation with cytochrome c and dATP.
The caspase-3 inhibitor zDEVD-fmk does not interfere with CD95- or epirubicin-induced processing of caspase-3 in BJAB cells.
BJAB cells were either incubated in control medium (Co) or stimulated with 1 μg/mL anti-CD95/Fas antibody (F) or 1 μg/mL epirubicin (E). Some cultures were preincubated with 10 μM of the caspase-3 inhibitor zDEVD-fmk (I) 2 hours before anti-CD95 or drug treatment. After 24 hours of incubation, Western blot analyses were performed with antihuman caspase-3 (A, C). The positions of molecular mass markers are indicated at the left. Arrows indicate the positions of procaspase-3 (*) and the 17-kd active subunit of caspase-3 (**). The experiments were repeated and yielded similar results. Additionally, cell death was determined in the cultures after 24 hours of anti-CD95 or epirubicin treatment by trypan blue exclusion (B, D). Values are given as percentage of dead cells ± SD (n = 3).
The caspase-3 inhibitor zDEVD-fmk does not interfere with CD95- or epirubicin-induced processing of caspase-3 in BJAB cells.
BJAB cells were either incubated in control medium (Co) or stimulated with 1 μg/mL anti-CD95/Fas antibody (F) or 1 μg/mL epirubicin (E). Some cultures were preincubated with 10 μM of the caspase-3 inhibitor zDEVD-fmk (I) 2 hours before anti-CD95 or drug treatment. After 24 hours of incubation, Western blot analyses were performed with antihuman caspase-3 (A, C). The positions of molecular mass markers are indicated at the left. Arrows indicate the positions of procaspase-3 (*) and the 17-kd active subunit of caspase-3 (**). The experiments were repeated and yielded similar results. Additionally, cell death was determined in the cultures after 24 hours of anti-CD95 or epirubicin treatment by trypan blue exclusion (B, D). Values are given as percentage of dead cells ± SD (n = 3).
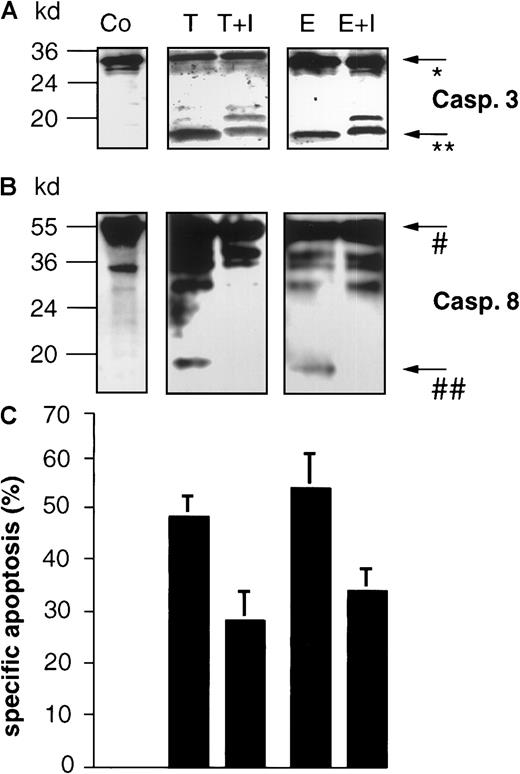
The caspase-3 inhibitor zDEVD-fmk blocks drug-induced processing of caspase-8 and inhibits DNA fragmentation in BJAB cells.
BJAB cells were either incubated in control medium (Co) or stimulated with 0.1 μg/mL Taxol (T) or 1 μg/mL epirubicin (E). Some cultures were preincubated with 10 μM caspase-3 inhibitor zDEVD-fmk (I) 2 hours before drug treatment. After 48 hours of incubation, Western blot analyses were performed with antihuman caspase-3 (A) and antihuman caspase-8 antibodies (B). Positions of molecular mass markers are indicated at the left. Arrows indicate the positions of procaspase-3 (*), the 17-kd active subunit of caspase-3 (**), procaspase-8 (#), and the 18-kd active subunit of caspase-8 (##). The experiments were repeated and yielded similar results. Additionally, DNA fragmentation (C) was measured in the cultures after 48 hours of drug treatment. Values are given as percentage of specific apoptosis ± SD (n = 3).
The caspase-3 inhibitor zDEVD-fmk blocks drug-induced processing of caspase-8 and inhibits DNA fragmentation in BJAB cells.
BJAB cells were either incubated in control medium (Co) or stimulated with 0.1 μg/mL Taxol (T) or 1 μg/mL epirubicin (E). Some cultures were preincubated with 10 μM caspase-3 inhibitor zDEVD-fmk (I) 2 hours before drug treatment. After 48 hours of incubation, Western blot analyses were performed with antihuman caspase-3 (A) and antihuman caspase-8 antibodies (B). Positions of molecular mass markers are indicated at the left. Arrows indicate the positions of procaspase-3 (*), the 17-kd active subunit of caspase-3 (**), procaspase-8 (#), and the 18-kd active subunit of caspase-8 (##). The experiments were repeated and yielded similar results. Additionally, DNA fragmentation (C) was measured in the cultures after 48 hours of drug treatment. Values are given as percentage of specific apoptosis ± SD (n = 3).
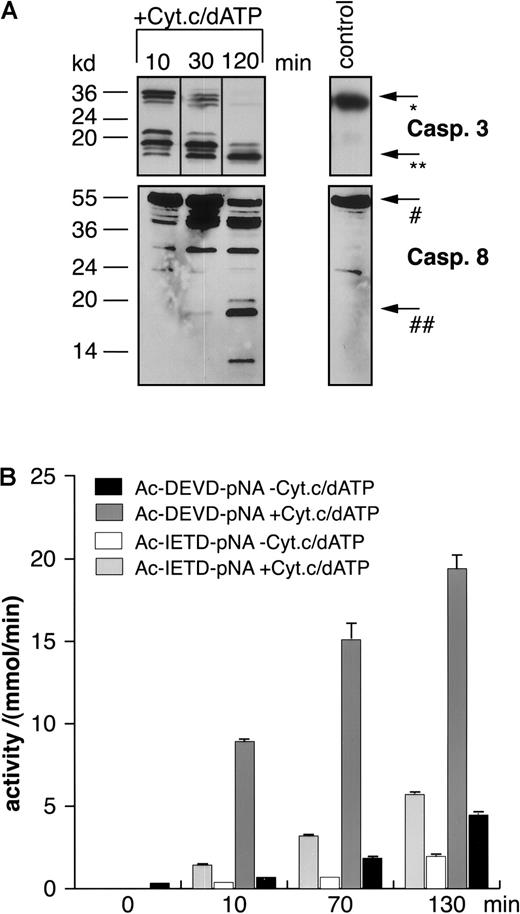
Time-dependent in vitro activation of caspase-8 by cytochrome c and dATP in extracts of BJAB cells.
Extracts from BJAB cells were incubated for different time periods in the presence or absence of 10 μM cytochrome c and 1 mM dATP. Then Western blot analyses were performed by the use of antihuman caspase-3 (A, upper panel) or antihuman caspase-8 antibodies (A, lower panel). Positions of molecular mass markers are indicated at the left. Arrows indicate the positions of procaspase-3 (*), the 17-kd active subunit of caspase-3 (**), procaspase-8 (#), and the 18-kd active subunit of caspase-8 (##). The experiments were repeated twice and yielded similar results. Additionally, caspase-3–like or caspase-8–like activities were measured in a colorimetric assay using Ac-DEVD-pNA or Ac-IETD-pNA, respectively (B). Caspase activities are given as mmol substrate cleaved per minute ± SD (n = 3).
Time-dependent in vitro activation of caspase-8 by cytochrome c and dATP in extracts of BJAB cells.
Extracts from BJAB cells were incubated for different time periods in the presence or absence of 10 μM cytochrome c and 1 mM dATP. Then Western blot analyses were performed by the use of antihuman caspase-3 (A, upper panel) or antihuman caspase-8 antibodies (A, lower panel). Positions of molecular mass markers are indicated at the left. Arrows indicate the positions of procaspase-3 (*), the 17-kd active subunit of caspase-3 (**), procaspase-8 (#), and the 18-kd active subunit of caspase-8 (##). The experiments were repeated twice and yielded similar results. Additionally, caspase-3–like or caspase-8–like activities were measured in a colorimetric assay using Ac-DEVD-pNA or Ac-IETD-pNA, respectively (B). Caspase activities are given as mmol substrate cleaved per minute ± SD (n = 3).
To order the processing of procaspase-8 within the cytochrome c–initiated caspase cascade, we immunoprecipitated caspase-3 from BJAB cell extracts. Measurement of caspase-3–like enzyme activity in extracts that had been treated with anti–caspase-3 antibodies, followed by sedimentation of the immune complex using protein A–Sepharose, showed that 90% of the total caspase-3 activity was successfully precipitated (Figure 12A). Although caspase-8 processing was detected in control extracts after 130 and 190 minutes of incubation with cytochrome c and dATP (Figure12B, upper panel), the processing of procaspase-8 and the appearance of the active 18-kd subunit of caspase-8 was completely blocked in caspase-3–depleted cell extracts (Figure 12B, lower panel). Thus, both the experiments in intact B cells and in the cell-free system indicate that caspase-8 activation through the mitochondrial pathway is an event occurring downstream of caspase-3 activation.
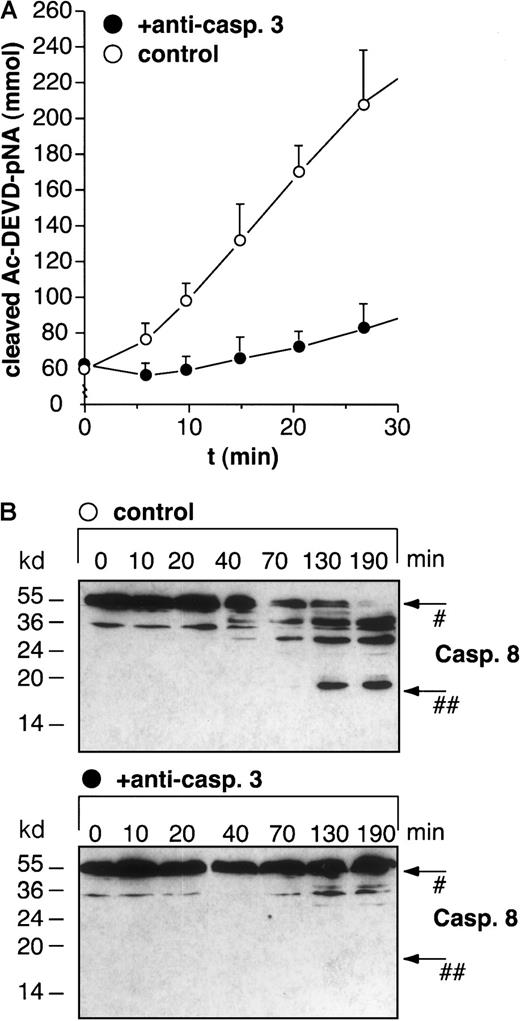
Immunoprecipitation of caspase-3 abolishes in vitro activation of caspase-8 by cytochrome c and dATP in BJAB cell extracts.
Caspase-3 was immunoprecipitated from cellular extracts as described in “Materials and methods.” After in vitro activation with 10 μM cytochrome c and 1 mM dATP for 10 minutes, cleavage of Ac-DEVD-pNA in control (A, open circles) and immunoprecipitated (A, filled circles) extracts was determined after the indicated times. Values are given as mmol cleaved Ac-DEVD-pNA ± SD (n = 3). Furthermore, control extracts (B, upper panel) and caspase-3–depleted extracts (B, lower panel) were activated with 10 μM cytochrome c and 1 mM dATP for different time points and analyzed for caspase-8 processing by Western blot analysis. Positions of molecular mass markers are indicated at the left. Arrows indicate the positions of procaspase-8 (#) and the 18-kd active subunit of caspase-8 (##). The experiments were repeated twice and yielded similar results.
Immunoprecipitation of caspase-3 abolishes in vitro activation of caspase-8 by cytochrome c and dATP in BJAB cell extracts.
Caspase-3 was immunoprecipitated from cellular extracts as described in “Materials and methods.” After in vitro activation with 10 μM cytochrome c and 1 mM dATP for 10 minutes, cleavage of Ac-DEVD-pNA in control (A, open circles) and immunoprecipitated (A, filled circles) extracts was determined after the indicated times. Values are given as mmol cleaved Ac-DEVD-pNA ± SD (n = 3). Furthermore, control extracts (B, upper panel) and caspase-3–depleted extracts (B, lower panel) were activated with 10 μM cytochrome c and 1 mM dATP for different time points and analyzed for caspase-8 processing by Western blot analysis. Positions of molecular mass markers are indicated at the left. Arrows indicate the positions of procaspase-8 (#) and the 18-kd active subunit of caspase-8 (##). The experiments were repeated twice and yielded similar results.
Discussion
Recently, a controversial discussion about the role of the CD95 receptor–ligand system in drug-induced apoptosis in human leukemia T-cell lines was raised, and it is still a matter of debate whether CD95-mediated signaling is functionally involved in the propagation of death signals after drug treatment. Although an involvement of CD95–CD95 ligand was originally demonstrated,11 other groups could not confirm drug-induced CD95 ligand expression and inhibition of drug-induced apoptosis by the use of blocking anti-CD95 antibodies, and they showed CD95-independent death signaling after the exposure of malignant T cells to cytotoxic drugs.13,14,41,42 However, Fulda et al12insisted that a functional CD95 ligand and CD95 death-inducing signaling complex plays an important role in activation-induced and doxorubicin-induced apoptosis in leukemic T cells. These conflicting data might in part be explained by the concept of CD95 type 1 and type 2 cells.43 CD95-mediated apoptosis in type 1 cells is suggested to be initiated by large amounts of caspase-8 formed at the death-inducing signaling complex at the plasma membrane, whereas in type 2 cells small amounts of active caspase-8 are believed to induce the apoptogenic activity of mitochondria. However, many experiments have documented that CD95/Fas-deficient, FADD-deficient, and caspase-8–deficient cells are normally sensitive to chemotherapeutic drugs. Because all these molecules were reported to be essential for CD95-induced apoptosis in type 1 and type 2 cells, even the type 1–type 2 paradigm has recently been questioned.44Nevertheless, the use of different experimental approaches—for example, CD95-resistant cell clones13 or T cells from CD95-deficient lpr mice,14 might be one possible reason for these controversial results. In the current study, we investigated drug-induced death pathways in B-lymphoid cells that, unlike in T cells, have been less intensively characterized. In contrast to T cells, B-lymphoid cells did not express significant amounts of CD95 ligand after challenge with the cytotoxic drug epirubicin. Additionally, epirubicin-induced cell death was not influenced by antagonistic anti-CD95 antibodies, even at low drug concentrations. We therefore conclude that B-lymphoid cells represent a suitable model system to elaborate CD95-independent apoptosis without manipulating cells by means such as the generation of resistant cell clones or the generation of transgenic mice.
In principle, mature B cells can undergo apoptosis by signaling through CD95.18,19,25,45,46 However, most B-lineage ALL cells are negative or only express low amounts of CD95/Fas and are constitutively resistant toward CD95-induced death but can be sensitized for this signaling pathway by pretreatment with chemotherapeutic drugs.47 We report here that epirubicin and Taxol, 2 different kinds of drugs that target the nucleus or the cytoskeleton, respectively, are effective inducers of apoptotic cell death in B-lymphoid cell lines and in primary B-lineage tumor cells. Apoptosis and activation of distinct caspases by these drugs occurred in a CD95-independent manner, as further evidenced by the use of a dominant-negative FADD mutant (FADDdn). Overexpression of FADDdn did not influence caspase-3 or mitochondrial activation as early events nor DNA fragmentation as a late event in drug-induced apoptosis. Interestingly, we also observed processing and activation of procaspase-8 after the addition of cytotoxic drugs to the culture medium of B-lymphoid cells. In all cases, the processing of procaspase-8 was either preceded or paralleled by mitochondrial permeability transition. These results suggest that the mitochondrial apoptosome is a main player in drug-induced apoptosis of B-lymphoid cells, thereby confirming other data that point to the critical role for mitochondria in the apoptotic pathways of other cell types, such as HeLa cells37 and malignant melanoma.48
Caspases with long prodomains, such as caspase-8, are generally considered to be upstream (inducer) caspases because of their ability to associate with cell surface death-receptor molecules. However, it has been shown that the addition of cytochrome c to Jurkat cell-free extracts initiates a cascade of protease activation events in vitro involving caspases-2, -3, -6, -7, -8, -9, and -10.49 We therefore reasoned that the molecular order in our experimental system is represented by the hierarchical activation of mitochondria, caspase-3, and caspase-8. This hypothesis was further substantiated by 2 different experimental approaches: (1) the cell-permeable peptide zDEVD-fmk, which irreversibly inhibits caspase-3–like enzyme activities, blocked processing of procaspase-8 in drug-treated BJAB cells, and (2) the depletion of caspase-3 from BJAB cell extracts abrogated cytochrome c-induced processing of procaspase-8. Thus, unlike its role as an inducer caspase in death receptor-triggered apoptosis, caspase-8 appears to function as a downstream (executioner) caspase during drug-induced apoptosis.
The potential relevance of apoptosis signaling for clinical practice has recently been demonstrated in a patient with B-cell lymphoma. In this study, the protein kinase inhibitor 7-hydroxystaurosporine sensitized the lymphoma cells to the cytotoxic effects of multi-agent chemotherapy, presumably by modulating the threshold for apoptosis.50 Our results, that the drug-induced activation of caspase-8 in B-lymphoid cells occurs independently of the CD95/Fas death-inducing signaling complex and downstream of the main executioner of the mitochondrial apoptosis signaling complex, caspase-3, provide new insights into the molecular cascade of apoptosis signaling in this important cellular system.
We thank Clarissa von Haefen and Verena Lehmann for their expert technical assistance.
Supported by grants from the Deutsche Forschungsgemeinschaft (SFB 273 and SFB 506) (P.T.D.), the European Community TMR Programme (P.T.D., K.S.-O., R.B.), and the Verein zur Förderung der Tagesklinik e. V. (T.W., A.P.). F.E. is a recipient of a fellowship from the DFG Graduiertenkolleg 331 at the University Medical Center Charité.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter T. Daniel, Department of Hematology, Oncology, and Tumor Immunology, University Medical Center Charité, Campus Berlin-Buch, Humboldt University of Berlin, Lindenberger Weg 80, 13125 Berlin, Germany; e-mail:pdaniel@mdc-berlin.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal