Abstract
During mouse embryogenesis, primitive erythropoiesis occurs in blood islands of the yolk sac (YS) on the seventh day of gestation. This study demonstrated for the first time the presence of unique primitive megakaryocytic (Mk) progenitors in the early YS, which disappeared by 13.5 days postcoitum (dpc). When 7.5 dpc YS cells were incubated in the presence of stem cell factor (SCF), interleukin (IL)-3, IL-6, erythropoietin (EPO), thrombopoietin (TPO), and granulocyte colony-stimulating factor in methylcellulose clonal culture, not only erythroid bursts but also megakaryocyte colonies were observed. The megakaryocytes in the colonies matured to proplatelet stages and produced platelets as early as day 3 of culture, much earlier than those from adult bone marrow, although their ploidy class was lower. These megakaryocytes were stained with acetylcholine esterase, and expressed platelet glycoprotein (GP)Ibβ, GPIIIa, and platelet factor 4 by reverse transcription-polymerase chain reaction analysis. The analysis of hemoglobin types in erythrocytes obtained from hematopoietic multilineage colonies containing the megakaryocytes indicated that the Mk progenitors originated from primitive hematopoiesis. The primitive Mk progenitors formed colonies in the absence of any cytokines in fetal bovine serum (FBS)-containing culture, and SCF, IL-3, EPO, and TPO significantly enhanced the Mk colony formation. In FBS-free culture, however, no colony formation was induced without these cytokines. Because megakaryocytes were detected in 8.5-dpc YS, these unique primitive Mk progenitors may rapidly mature and give rise to platelets to prevent hemorrhage in the simultaneously developing blood vessels until definitive hematopoiesis begins to produce platelets.
Introduction
The development of blood cells occurs in 2 waves during mouse embryogenesis. The first and less well-characterized wave, primitive hematopoiesis, occurs in blood islands of the yolk sac (YS) on the seventh day of gestation. Primitive hematopoiesis is followed by a second wave, definitive hematopoiesis, beginning in the aorta-gonad-mesonephros region and fetal liver (FL) at 10 to 11 days postcoitum (dpc).1 At the end of gestation, definitive hematopoiesis shifts to bone marrow (BM) and spleen, where it remains throughout adult life.1-3 Primitive hematopoiesis yields unique erythrocytes, distinguishable from those of definitive hematopoiesis by their morphology and the hemoglobin types they express.4,5 Primitive erythrocytes are nucleated cells containing embryonic as well as adult hemoglobins, whereas definitive erythrocytes are smaller nonnucleated red blood cells committed to only adult hemoglobin synthesis.6-8 Developmental changes in cell populations between embryo/fetus and adult have also been reported for lymphocytes and macrophages. Fetal B- or T-cell populations differ from adult cells in the presence of CD5 antigen and a specific immunoglobulin repertoire or the expression of distinct γδ T-cell receptors, respectively.9-14 Macrophages derived from embryonic tissue express the lysozyme gene at significantly lower levels than those from adult tissue.15 However, there have been few reports on megakaryopoiesis in embryonic hematopoiesis.
This study is the first to demonstrate the presence of embryonic megakaryocytic (Mk) progenitors involved in primitive hematopoiesis. The Mk progenitors were shown to possess characteristics different from the definitive Mk progenitors of adult BM in the kinetics of growth and cytokine sensitivity. These results suggest that primitive hematopoiesis might generate unique Mk progenitors, which rapidly give rise to a great amount of platelets, preventing hemorrhage in the simultaneously developing blood vessels, until definitive hematopoiesis begins to produce platelets.
Materials and methods
Mice and cells
C57BL/6 mice, 8 to 10 weeks old, were purchased from Shizuoka Animal Farm (Shizuoka, Japan) and kept in the Animal Center of the Institute of Medical Science, The University of Tokyo. One or 2 female mice were caged with a male for 2 hours late in the afternoon, and then examined for vaginal plugs. The appearance of the vaginal plug was designated as day 0 of gestation.16 At specified times, pregnant mice were anesthetized and then killed, and embryos were removed and dissected under a dissecting microscope to obtain YS, embryo bodies (EB), and FLs in phosphate-buffered saline (PBS; Nissui, Tokyo, Japan) containing 2% fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT). YS and EB cells were obtained using 0.1% collagenase as previous reported.7 FL cells were obtained by rubbing the excised livers between 2 pieces of glass. Adult BM cells were flushed from femurs and tibiae into α-medium (Flow Laboratories, Rockville, MD). All the cells were passed through metal mesh to delete aggregated cells.
Cytokines
Recombinant mouse interleukin (IL)-3 was from Amgen (Thousand Oaks, CA). Recombinant human IL-6, granulocyte colony-stimulating factor (G-CSF), erythropoietin (EPO), and thrombopoietin (TPO), and mouse stem cell factor (SCF) were generous gifts from Kirin Brewery (Tokyo, Japan). All the cytokines were pure recombinant molecules and used at concentrations that induced optimal response in methylcellulose culture of mouse hematopoietic cells. The concentrations of the cytokines used were 100 ng/mL for SCF, IL-6, and G-CSF; 20 ng/mL for IL-3; 4 U/mL for EPO; and 4 ng/mL for TPO.
Clonalculture
Methylcellulose clonal culture was performed using a standard technique described previously.17,18 Briefly, 1 mL culture mixture containing cells, α-medium, 0.9% methylcellulose (Shinetsu Chemical, Tokyo, Japan), 30% FBS, 1% deionized fraction V bovine serum albumin (BSA; Sigma, St Louis, MO), 5 × 10−5 M mercaptoethanol (Eastman Organic Chemicals, Rochester, NY) and various combinations of cytokines was plated in each 35-mm nontissue culture dish (no. 1008, Falcon, Lincoln Park, NJ) and incubated at 37°C in a humidified atmosphere flushed with 5% CO2 in air. FBS-free methylcellulose culture contained components identical to those in FBS-containing culture except 1% pure BSA (Sigma), 300 μg/mL human transferrin (Sigma), 160 μg/mL soybean lecithin (Sigma), and 96 μg/mL cholesterol (Nacalai Tesque, Kyoto, Japan) replaced fraction V BSA and FBS.19 20 All cultures were performed in triplicate. The number of colonies was counted at days 3 to 7 of culture except the kinetics analysis in which they were counted every day for 14 days.
Determination of colony types
Colony types were determined in situ using an inverted microscope, according to standard criteria.21 22 Briefly, megakaryocyte colonies were scored as such when they had 4 or more megakaryocytes. Except for these megakaryocyte colonies, cell aggregates consisting of more than 50 cells were scored as colonies. The abbreviations used for the colony types were: Mk, megakaryocyte colonies; E, erythroid bursts; EMk, erythrocyte-megakaryocyte colonies; Mk-Mix, hematopoietic mixed colonies containing megakaryocytes; and others means containing granulocyte-macrophage (GM) colonies and mast cell colonies. To assess the accuracy of the in situ identification of colonies, individual colonies were lifted, spread on glass slides, and stained with May-Grunwald Giemsa and acetylcholine esterase (AchE). All of the Mk colonies identified by in situ observation consisted of AchE-positive megakaryocytes, and EMk and Mk-Mix colonies showed the coexistence of both AchE-positive and -negative cells.
Electron microscopic preparations
The Mk colonies derived from 7.5-dpc YS cells and adult BM cells were lifted and gathered in 10% FBS-containing α-medium at days 3 and 5 of clonal culture, respectively. Cells were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2) at 4°C for 30 minutes. The specimens were then rinsed in the buffer, and subjected to ultrastructural studies.23
Measurement of ploidy
The DNA content of megakaryocytes was measured as described previously.21 Briefly, Mk colonies derived from 8.5-dpc YS cells and adult BM cells were lifted and gathered in 10% FBS-containing α-medium at days 3 and 5 of clonal culture, respectively. A small aliquot of these harvested cells was processed for AchE staining, and all the cells were confirmed as AchE-positive megakaryocytes. Subsequently, the remainder of the cells were prepared on glass slides. After the fixation with methanol, the DNA content of the megakaryocytes was measured by staining with 4′, 6-diamidino-2-phenylindole (DAPI, Sigma). The specimens were immersed in a solution consisting of 50 ng/mL DAPI, 10 mM Tris, 10 mM EDTA-2Na, 100 mM NaCl, and 10 mM 2-mercaptoethylamine hydrochloride (pH 7.4), and were placed in a dark moist chamber for 30 minutes. Nuclear DNA was measured under an epifluorescent microfluorometer. Granulocytes in GM colonies grown in the same culture dishes were used as diploid standards.
Determination of size of megakaryocytes
The diameter of megakaryocytes identified as AchE-positive cells on the cytocentrifuged preparation was determined by using a microscope equipped with an ocular micrometer as described previously.24 The mean of 2 perpendicular diameters was calculated.
Reverse transcription-polymerase chain reaction
Messenger RNA (mRNA) was prepared from adult BM cells, individual EMk, Mk-Mix colonies, and E bursts using QuickPrep Micro mRNA Purification Kit (Pharmacia, Piscataway, NJ), and reverse-transcribed using the first strand complementary DNA (cDNA) synthesis kit (Pharmacia). For PCR reaction, specific cDNA product was amplified in a final volume of 25 μL with Taq DNA polymerase, using pairs of oligonucleotide primers as follows25-28: platelet glycoprotein (GP)Ibβ-5′, AGGACAGGACGCGGCATTCA-3′; GPIbβ-3′, AGGCTTCTGGGAGGAAGGCG-3′. GPIIIa-5′, CTGCCGGAAGAGCTGTCACTG-3′; GPIIIa-3′, CATCTCCCCTTTGTAGCGGAC-3′. Platelet factor (PF) 4-5′, CGCTGCGGTGTTTCGAGG-3′; PF4-3′, TCACCTCCAGGCTGGTGA-3′. G-CSF receptor (G-CSFR)-5′, CCACTACACCATCTTCTG-3′; G-CSFR-3′, CCAAGAGGGGCTGAGTGG-3′. hypoxanthine phosphoribosyl transferase (HPRT)-5′, GCTGGTGAAAAGGACCTCT-3′; HPRT-3′, CCACAGGACTAGAACACCTG. α-globin-5′, CTCTCTGGGGAAGACAAAAGCAAC-3′; α-globin-3′, GGTGGCTAGCCAAGGTCACCAGAC-3′. βmajor-globin-5′, CTGACAGATGCTCTCTTGGG-3′; βmajor-globin-3′, CACAACCCCAGAAACAGACA-3′. βH1-globin-5′, AGTCCCCATGGAGTCAAAGA-3′; βH1-globin-3′, CTCAAGGAGACCTTTGCTCA-3′. ε-globin-5′, GGAGAGTCCATTAAGAACCTAGACAA-3′; ε-globin-3′, CTGTGAATTCATTGCCGAAGTGAC-3′. ζ-globin-5′, GCTCAGGCCGAGCCCATTGG-3′; ζ-globin-3′, TAGCGGTACTTCTCAGTCAG-3′. β-actin-5′, GTGGGCCGCTCTAGGCACCAA-3′; β-actin-3′, CTCTTTGATGTCACGCACGATTTC-3′. Samples were denatured at 94°C for 5 minutes, followed by amplification rounds consisting of 94°C for 30 seconds (denaturing), 58 to 65°C for 45 seconds (annealing), and 72°C for 90 seconds (extension) for 40 cycles. Products were separated on 1.0% agarose gels, stained with ethidium bromide, and photographed.
Results
Megakaryocytic progenitors in the early yolk sac
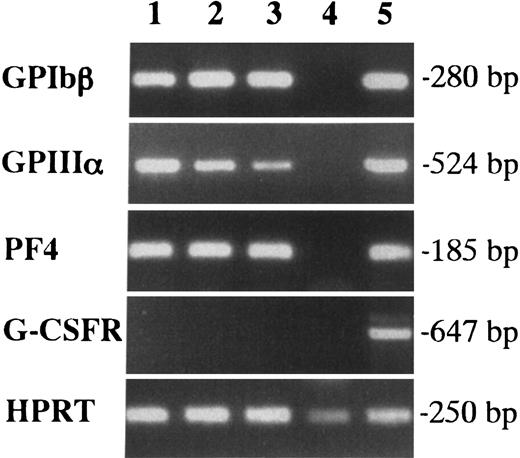
We first examined the colony formation from YS and EB cells at 7.5 dpc in methylcellulose clonal culture (Table1). When 5 × 104 cells were cultured with SCF, IL-3, IL-6, EPO, TPO, and G-CSF, no colonies were detected in the culture of EB cells, whereas YS cells generated a substantial number of colonies containing approximately 50% E bursts. Interestingly, the colonies included Mk colonies, which possessed a unique appearance in situ (primitive-type Mk colonies, Figure1A). The megakaryocytes contained in the colonies exhibited filamentous structures with production of cytoplasmic processes (proplatelet formation). The cytospin preparation showed that AchE-positive megakaryocytes revealed the budding of cell membranes and the proplatelet formation (Figures 1C,E). These magakaryocytes were also detected in EMk and Mk-Mix colonies. To confirm their Mk property, we examined the expression of megakaryocyte-specific markers, GPIbβ, GPIIIa, and PF4 in 3 EMk colonies by reverse transcription-polymerase chain reaction(RT-PCR) analysis (Figure 2). Erythrocytes pooled from 5 E bursts in the same culture dish were used as a negative control (lane 4), and adult BM cells were used as a positive control (lane 5). All the 3 EMk colonies chosen randomly expressed GPIbβ, GPIIIa, and PF4 (lanes 1 to 3) as well as adult BM cells, whereas pooled erythrocytes did not. We also examined G-CSFR expression to demonstrate the specificity of the PCR reactions. Adult BM cells expressed G-CSFR (lanes 4), whereas all the 3 EMk colonies and pooled erythrocytes did not. These data indicated that the primitive-type megakaryocytes derived from 7.5-dpc YS have some properties common to megakaryocytes in adult BM.
Colony formation from various sources
| Cell source . | No. of colonies/5 × 104 cells . | ||||||
|---|---|---|---|---|---|---|---|
| E . | Mk . | EMk . | Mk-Mix . | Others . | Total . | ||
| Pr . | Ad . | ||||||
| 7.5 dpc YS | 27 ± 3 | 7 ± 2 | 0 | 2 ± 1 | 5 ± 2 | 7 ± 2 | 48 ± 4 |
| 7.5 dpc EB | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8.5 dpc YS | 56 ± 5 | 62 ± 6 | 0 | 13 ± 3 | 24 ± 5 | 39 ± 5 | 194 ± 15 |
| 8.5 dpc EB | 21 ± 3 | 4 ± 2 | 0 | 2 ± 1 | 5 ± 3 | 6 ± 2 | 38 ± 5 |
| 10.5 dpc YS | 78 ± 15 | 16 ± 4 | 10 ± 3 | 18 ± 4 | 32 ± 5 | 125 ± 17 | 279 ± 25 |
| 10.5 dpc EB | 8 ± 3 | 1 ± 1 | 1 ± 0 | 1 ± 1 | 3 ± 1 | 11 ± 2 | 25 ± 5 |
| 13.5 dpc FL | 652 ± 64 | 1 ± 1 | 84 ± 9 | 18 ± 6 | 26 ± 6 | 478 ± 35 | 1258 ± 115 |
| Adult BM | 31 ± 4 | 0 | 5 ± 2 | 2 ± 1 | 22 ± 3 | 146 ± 12 | 206 ± 7 |
| Cell source . | No. of colonies/5 × 104 cells . | ||||||
|---|---|---|---|---|---|---|---|
| E . | Mk . | EMk . | Mk-Mix . | Others . | Total . | ||
| Pr . | Ad . | ||||||
| 7.5 dpc YS | 27 ± 3 | 7 ± 2 | 0 | 2 ± 1 | 5 ± 2 | 7 ± 2 | 48 ± 4 |
| 7.5 dpc EB | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8.5 dpc YS | 56 ± 5 | 62 ± 6 | 0 | 13 ± 3 | 24 ± 5 | 39 ± 5 | 194 ± 15 |
| 8.5 dpc EB | 21 ± 3 | 4 ± 2 | 0 | 2 ± 1 | 5 ± 3 | 6 ± 2 | 38 ± 5 |
| 10.5 dpc YS | 78 ± 15 | 16 ± 4 | 10 ± 3 | 18 ± 4 | 32 ± 5 | 125 ± 17 | 279 ± 25 |
| 10.5 dpc EB | 8 ± 3 | 1 ± 1 | 1 ± 0 | 1 ± 1 | 3 ± 1 | 11 ± 2 | 25 ± 5 |
| 13.5 dpc FL | 652 ± 64 | 1 ± 1 | 84 ± 9 | 18 ± 6 | 26 ± 6 | 478 ± 35 | 1258 ± 115 |
| Adult BM | 31 ± 4 | 0 | 5 ± 2 | 2 ± 1 | 22 ± 3 | 146 ± 12 | 206 ± 7 |
5 × 104 cells from various sources were cultured in the presence of 6 factors (SCF, IL-3, IL-6, G-CSF, EPO and TPO). Mk colonies were classified into primitive-type (Pr) and adult-type (Ad) as shown in the text. The number of colonies indicates mean ± SD in triplicate cultures.
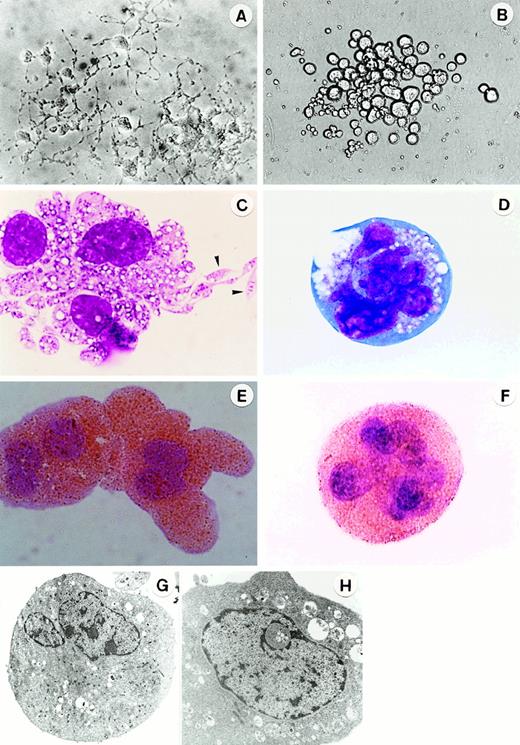
Morphology of megakaryocytes in primitive- and adult-type Mk colonies.
In situ appearance of megakaryocyte colonies cultured from 7.5-dpc YS cells for 3 days (A) (original magnification × 300) and cultured from adult BM cells for 5 days (B) (original magnification × 200) in the presence of SCF, IL-3, IL-6, G-CSF, EPO, and TPO. Appearance of megakaryocytes stained with May-Grunwald Giemsa (C, D; the arrowheads show the proplatelets) and AchE (E,F) in megakaryocyte colonies at day 3 of culture of 7.5 dpc YS cells (C,E) and at day 5 of culture of adult BM cells (D,F) (original magnification × 1000). Ultrastructure of megakaryocytes in megakaryocyte colonies at day 3 of culture of 7.5-dpc YS cells (G) (original magnification × 3200) and at day 5 of culture of adult BM cells (H) (original magnification × 7000).
Morphology of megakaryocytes in primitive- and adult-type Mk colonies.
In situ appearance of megakaryocyte colonies cultured from 7.5-dpc YS cells for 3 days (A) (original magnification × 300) and cultured from adult BM cells for 5 days (B) (original magnification × 200) in the presence of SCF, IL-3, IL-6, G-CSF, EPO, and TPO. Appearance of megakaryocytes stained with May-Grunwald Giemsa (C, D; the arrowheads show the proplatelets) and AchE (E,F) in megakaryocyte colonies at day 3 of culture of 7.5 dpc YS cells (C,E) and at day 5 of culture of adult BM cells (D,F) (original magnification × 1000). Ultrastructure of megakaryocytes in megakaryocyte colonies at day 3 of culture of 7.5-dpc YS cells (G) (original magnification × 3200) and at day 5 of culture of adult BM cells (H) (original magnification × 7000).
Expression of GPIbβ, GPIIIa, and PF4 in megakaryocytes contained in individual EMk colonies from the early YS cells by RT-PCR.
Lanes 1, 2, and 3 showed 3 individual EMk colonies from 7.5-dpc YS. Erythrocytes pooled from 5 E bursts in the same culture were used as a negative control (lane 4), and adult BM cells were as a positive control (lane 5). G-CSFR expression was also examined to demonstrate the specificity of the PCR reactions.
Expression of GPIbβ, GPIIIa, and PF4 in megakaryocytes contained in individual EMk colonies from the early YS cells by RT-PCR.
Lanes 1, 2, and 3 showed 3 individual EMk colonies from 7.5-dpc YS. Erythrocytes pooled from 5 E bursts in the same culture were used as a negative control (lane 4), and adult BM cells were as a positive control (lane 5). G-CSFR expression was also examined to demonstrate the specificity of the PCR reactions.
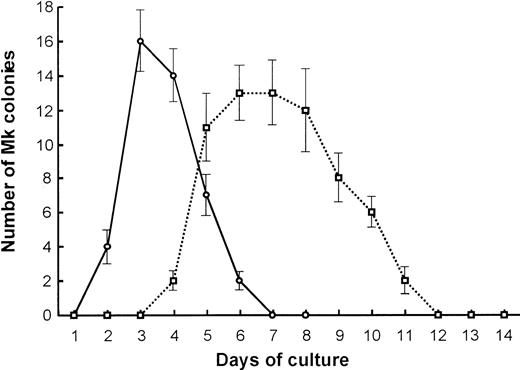
We further compared the Mk colony formation between 7.5-dpc YS and adult BM cells. Sequential observation showed that the kinetics of the colony formation and the feature of megakaryocytes contained in the colonies were quite different from each other (Figure3). The Mk colonies from 7.5-dpc YS cells were first detected as early as at day 2 of culture, and all megakaryocytes in these colonies reached a full maturation stage at day 3 of culture (Figure 1A,C). At day 5 of culture, the megakaryocytes released a large number of fine particles around them. By contrast, the Mk colonies from adult BM cells appeared from day 5 of culture, much later than those from 7.5-dpc YS cells, and the constituent megakaryocytes were large ovoid cells with a translucent cytoplasm and a highly refractile and regular cell membrane20 (Figure1B). Their cytospin preparations showed that these adult-type megakaryocytes were also stained with AchE, but immature (Figure 1D,F), and little platelet formation was found until day 14 of culture, when most of the Mk colonies degenerated. The electron microscopic analysis also showed that the megakaryocytes derived from YS cells revealed mature features. The nuclei were lobulated, and the cytoplasm was wide and contained many small granules (Figure 1G). The megakaryocytes derived from adult BM cells tended to be immature, showing less granule formation in the cytoplasm (Figure 1H). These results indicate that Mk progenitors in the early YS can differentiate and mature to proplatelet megakaryocytes more quickly than those in adult BM.
Kinetics of primitive- and adult-type Mk colony formation.
Comparison in the kinetics of Mk colony formation between 7.5-dpc YS (○) and adult BM cells (■). Cells (1 × 105) from 7.5-dpc YS or adult BM were cultured in the presence of SCF, IL-3, IL-6, G-CSF, EPO, and TPO. The number of Mk colonies were counted every day for 2 weeks. The values shown are the mean ± SD in triplicate cultures.
Kinetics of primitive- and adult-type Mk colony formation.
Comparison in the kinetics of Mk colony formation between 7.5-dpc YS (○) and adult BM cells (■). Cells (1 × 105) from 7.5-dpc YS or adult BM were cultured in the presence of SCF, IL-3, IL-6, G-CSF, EPO, and TPO. The number of Mk colonies were counted every day for 2 weeks. The values shown are the mean ± SD in triplicate cultures.
Developmental change of the generation of megakaryocytic progenitors
We then examined the developmental change of the generation of Mk progenitors in murine embryogenesis. As shown in Table 1, the number of primitive Mk, EMk, and Mk-Mix colonies dramatically increased in 8.5-dpc YS cells compared with 7.5-dpc YS cells. A small number of Mk and Mk-Mix colonies were also detected in the culture of 8.5-dpc EB cells, possibly as a result of circulation in the newly developing blood vessels.1 YS and EB cells at 10.5 dpc generated both primitive- and adult-type colonies; Mk colonies derived from YS and EB cells contained 61% and 50% of the primitive-type colonies, respectively. FLs from 13.5 dpc also produced a large number of Mk and Mk-Mix colonies in the methylcellulose culture, but most of the colonies were adult-type.
Different hemoglobin types of erythrocytes between primitive- and adult-type Mk-Mix colonies
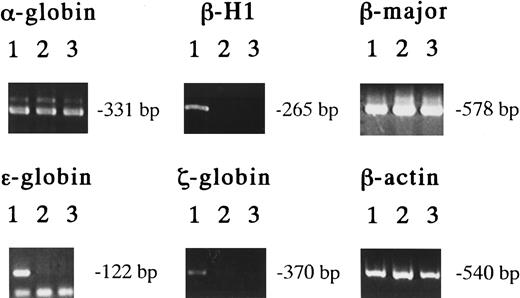
The exclusive expression of these unique Mk progenitors in the early YS suggests that they originate from primitive hematopoiesis. In the erythroid lineage, erythrocytes of primitive and definitive hematopoiesis can be distinguished by the hemoglobin types they contain.4-8 To clarify the origin of the Mk progenitors in the early YS, we examined the hemoglobin types of erythrocytes contained in EMk or Mk-Mix colonies derived from 8.5-dpc YS, 13.5-dpc FL, and adult BM cells using RT-PCR.6 29 The results showed that all the EMk colonies derived from 8.5-dpc YS cells (19 of 19) expressed both the embryonic hemoglobins, such as βH1, ε and ζ globins, and the adult hemoglobins, such as α- and β-major globins (Figure 4, lanes 1). On the other hand, all the EMk or Mk-Mix colonies from 13.5-dpc FL (14 of 14) and adult BM cells (11 of 11) expressed α- and β-major globins, but no detectable level of βH1 and no or low detectable level of ε and ζ globins (Figure 4, lanes 2 and 3). This result indicates that all the EMk or EMk-Mix colonies containing mature megakaryocytes in 8.5-dpc YS originate from primitive hematopoiesis, indicating that megakaryopoiesis is involved in primitive hematopoiesis.
Hemoglobin expression in various EMk or Mk-Mix colonies.
Detection of hemoglobin expression by RT-PCR analysis on individual EMk or Mk-Mix colonies from 8.5 dpc YS (lane 1), 13.5 dpc FL (lane 2), and adult BM cells (lane 3).
Hemoglobin expression in various EMk or Mk-Mix colonies.
Detection of hemoglobin expression by RT-PCR analysis on individual EMk or Mk-Mix colonies from 8.5 dpc YS (lane 1), 13.5 dpc FL (lane 2), and adult BM cells (lane 3).
DNA content of megakaryocytes in primitive- and adult-type Mk colonies
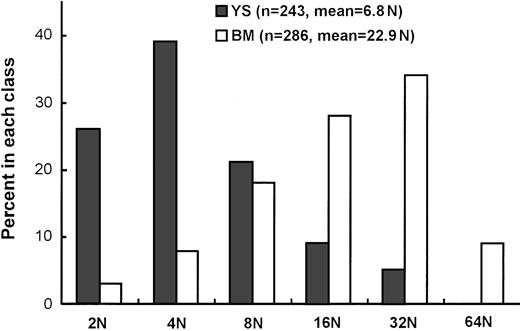
We then examined the maturation state by measuring DNA content of megakaryocytes in Mk colonies derived from 8.5-dpc YS cells and adult BM cells cultured in the presence of SCF, IL-6, IL-3, G-CSF, EPO, and TPO at days 3 and 5 of clonal culture, respectively. Each group of Mk colonies were picked up in 3 separate experiments. They were pooled and were confirmed to be AchE-positive, before being subjected to the measurement of the DNA content by staining with DAPI. As shown in Figure 5, the modal ploidy class of megakaryocytes from adult BM was at 16N, and the geometric mean ploidy was 22.9N. On the other hand, the modal ploidy class of megakaryocytes from 8.5-dpc YS was at 4N, and the geometric mean ploidy was 6.8N, lower than that from adult BM.
Ploidy (N) distribution of megakaryocytes in Mk colonies derived from 8.5 dpc YS and adult BM cells.
The analyzed cell numbers (n) and geometric means of megakaryocyte ploidy are presented in parentheses.
Ploidy (N) distribution of megakaryocytes in Mk colonies derived from 8.5 dpc YS and adult BM cells.
The analyzed cell numbers (n) and geometric means of megakaryocyte ploidy are presented in parentheses.
Different cytokine responsiveness between primitive and definitive megakaryocytic progenitors
Next, the responsiveness of primitive and definitive Mk progenitors to various cytokines was compared. We carried out clonal culture of 8.5-dpc YS and adult BM cells in the presence of various cytokines (Table 2). Mk colonies could be successfully induced from 8.5-dpc YS cells without any additional cytokines in FBS-containing cultures, although the colonies were small in size. Addition of TPO, SCF, IL-3, or EPO increased both the number and size of Mk colonies, with IL-3 being the most potent cytokine. By contrast, no colonies were produced from adult BM cells without cytokine. TPO and IL-3 but neither SCF nor EPO supported Mk or Mk-Mix colony formation.
Colony formation from 8.5-dpc-YS and adult BM cells in the presence of various cytokines
| FBS . | Cytokines . | No. of colonies . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8.5 dpc YS . | Adult BM . | ||||||||||||
| E . | Mk . | EMk . | Mk-Mix . | Others . | Total . | E . | Mk . | EMk . | Mk-Mix . | Others . | Total . | ||
| + | None | 12 ± 2 | 28 ± 4 | 0 | 0 | 10 ± 2 | 50 ± 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| TPO | 13 ± 4 | 72 ± 6* | 0 | 0 | 24 ± 4 | 109 ± 10 | 0 | 4 ± 1 | 0 | 2 ± 1 | 7 ± 2 | 13 ± 3 | |
| SCF | 25 ± 3 | 62 ± 4* | 0 | 0 | 39 ± 3 | 126 ± 13 | 0 | 0 | 0 | 0 | 74 ± 6 | 74 ± 6 | |
| IL-3 | 23 ± 3 | 92 ± 8* | 11 ± 2 | 20 ± 3 | 57 ± 4 | 203 ± 24 | 0 | 5 ± 1 | 0 | 4 ± 1 | 208 ± 14 | 217 ± 14 | |
| EPO | 21 ± 2 | 47 ± 6* | 9 ± 1 | 0 | 12 ± 3 | 89 ± 8 | 4 ± 1 | 0 | 0 | 0 | 0 | 0 | |
| − | None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TPO | 0 | 2 ± 1 | 0 | 0 | 0 | 2 ± 1 | 0 | 2 ± 1 | 0 | 0 | 0 | 2 ± 1 | |
| SCF | 3 ± 1 | 3 ± 1 | 0 | 0 | 9 ± 2 | 15 ± 2 | 0 | 0 | 0 | 0 | 18 ± 2 | 18 ± 2 | |
| IL-3 | 0 | 21 ± 3 | 0 | 6 ± 2 | 31 ± 3 | 58 ± 7 | 0 | 2 ± 1 | 0 | 1 ± 1 | 51 ± 5 | 54 ± 6 | |
| EPO | 9 ± 2 | 3 ± 1 | 2 ± 1 | 0 | 0 | 14 ± 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| FBS . | Cytokines . | No. of colonies . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8.5 dpc YS . | Adult BM . | ||||||||||||
| E . | Mk . | EMk . | Mk-Mix . | Others . | Total . | E . | Mk . | EMk . | Mk-Mix . | Others . | Total . | ||
| + | None | 12 ± 2 | 28 ± 4 | 0 | 0 | 10 ± 2 | 50 ± 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| TPO | 13 ± 4 | 72 ± 6* | 0 | 0 | 24 ± 4 | 109 ± 10 | 0 | 4 ± 1 | 0 | 2 ± 1 | 7 ± 2 | 13 ± 3 | |
| SCF | 25 ± 3 | 62 ± 4* | 0 | 0 | 39 ± 3 | 126 ± 13 | 0 | 0 | 0 | 0 | 74 ± 6 | 74 ± 6 | |
| IL-3 | 23 ± 3 | 92 ± 8* | 11 ± 2 | 20 ± 3 | 57 ± 4 | 203 ± 24 | 0 | 5 ± 1 | 0 | 4 ± 1 | 208 ± 14 | 217 ± 14 | |
| EPO | 21 ± 2 | 47 ± 6* | 9 ± 1 | 0 | 12 ± 3 | 89 ± 8 | 4 ± 1 | 0 | 0 | 0 | 0 | 0 | |
| − | None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TPO | 0 | 2 ± 1 | 0 | 0 | 0 | 2 ± 1 | 0 | 2 ± 1 | 0 | 0 | 0 | 2 ± 1 | |
| SCF | 3 ± 1 | 3 ± 1 | 0 | 0 | 9 ± 2 | 15 ± 2 | 0 | 0 | 0 | 0 | 18 ± 2 | 18 ± 2 | |
| IL-3 | 0 | 21 ± 3 | 0 | 6 ± 2 | 31 ± 3 | 58 ± 7 | 0 | 2 ± 1 | 0 | 1 ± 1 | 51 ± 5 | 54 ± 6 | |
| EPO | 9 ± 2 | 3 ± 1 | 2 ± 1 | 0 | 0 | 14 ± 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
A total of 5 × 104 day 8.5 YS and adult BM cells were plated using the clonal assay methods. The number of colonies indicates mean ± SD of triplicate cultures.
Significantly different from colony formation in the culture without cytokines (P < .001).
To eliminate the effects of factors contained in FBS, we carried out FBS-free culture. Under FBS-free condition, no colonies were induced from 8.5 dpc YS cells without cytokine. TPO, SCF, IL-3, and EPO could support Mk and/or Mk-Mix colony formation, and IL-3 was again the most potent stimulator. However, Mk and Mk-Mix colony formation was significantly reduced as compared with FBS-containing condition. The colony formation from adult BM cells was not affected by the removal of FBS.
Megakaryocytes in the early yolk sac
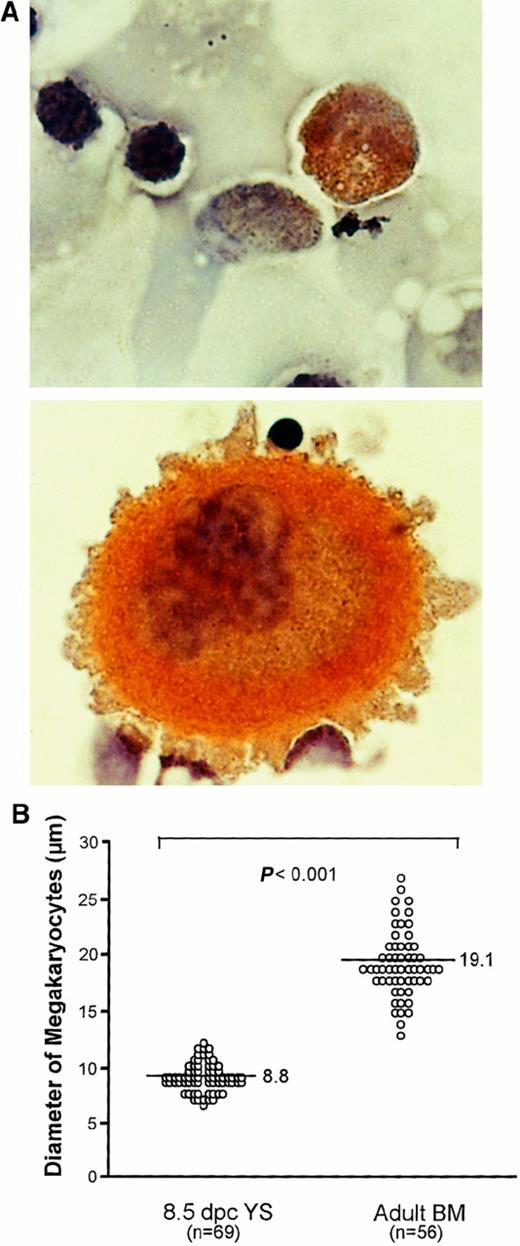
Finally, we searched for megakaryocytes in the early YS to examine whether the primitive Mk progenitors detected in our in vitro culture are functional in vivo. When 8.5-dpc YS cells trypsinized were spread on glass slides and then stained with AchE, a small number of AchE-positive megakaryocytes were found (Figure6A). The megakaryocytes revealed an average cell diameter of 8.8 μm, much smaller than those in adult mouse BM (Figure 6A), which had an average cell diameter of 19.1 μm (Figure 6B). This observation suggests that the primitive Mk progenitors present in the early YS produce megakaryocytes and then may release platelets into the circulation in vivo.
Comparison of megakaryocytes between early YS and adult BM.
(A) Photomicrographs show megakaryocytes stained with AchE in 8.5-dpc YS (upper) and adult BM (lower) (original magnification × 1000). (B) Comparison of size of megakaryocytes in 8.5-dpc YS and adult BM. The horizontal bars in each group represent the mean perpendicular diameters of megakaryocytes. The numbers of measured megakaryocytes are presented in parentheses. The megakaryocytes in 8.5-dpc YS were significantly smaller than those in adult BM (P < .001).
Comparison of megakaryocytes between early YS and adult BM.
(A) Photomicrographs show megakaryocytes stained with AchE in 8.5-dpc YS (upper) and adult BM (lower) (original magnification × 1000). (B) Comparison of size of megakaryocytes in 8.5-dpc YS and adult BM. The horizontal bars in each group represent the mean perpendicular diameters of megakaryocytes. The numbers of measured megakaryocytes are presented in parentheses. The megakaryocytes in 8.5-dpc YS were significantly smaller than those in adult BM (P < .001).
Discussion
Previous reports have shown that 7.5-dpc YS contained progenitor activities for erythroid and myeloid lineages.2 In the present study, we provided the first evidence for the presence of unique Mk progenitors in the early YS, which disappeared by 13.5 dpc. These progenitors produced megakaryocytes whose property was confirmed by the positivity to AchE and the expression of megakaryocyte-specific markers, GPIbβ, GPIIIa, and PF4 by RT-PCR analysis. They were shown to originate from primitive hematopoiesis by the analysis of hemoglobins in erythrocytes from multilineage colonies containing the unique megakaryopoiesis. Although the previous studies reported on the presence of Mk progenitors in 10.5-dpc YS,30 31 our study showed that YS at that time contain both Mk progenitors, which originate from primitive and definitive
hematopoiesis. The detection of megakaryocytes in the early YS suggested that the primitive Mk progenitors, which formed the Mk colonies in in vitro culture, functionally produced megakaryocytes and may further differentiate into platelets in vivo. We then demonstrated that these primitive Mk progenitors possessed characteristics different from definitive Mk progenitors in adult mouse BM.
There were some differences in cytokine sensitivities of Mk progenitors between the early YS and adult BM. In FBS-free culture, TPO and IL-3 stimulated the Mk colony formation from both the early YS and adult BM cells, whereas SCF and EPO stimulated the Mk colony formation from the early YS but not adult BM cells. Interestingly, in FBS-containing culture, the Mk colony formation was induced from the early YS without the addition of cytokines, but not from adult BM. We also observed that the Mk colony formation from 13.5 dpc FL cells were supported in the absence of additional cytokines in FBS-containing cultures (data not shown). Therefore, FBS may contain a factor(s) that stimulates the embryonic/fetal megakaryopoiesis. Because delay of the platelet recovery in the recipients transplanted with cord blood progenitor/stem cells has been reported from several groups,32 33 the identification of such a factor may contribute to further extension of cord blood transplantation, which is now increasingly used as an alternative to BM transplantation.
We also found different kinetics in Mk colony formation between the early YS and adult BM cells. In in vitro culture, the primitive Mk progenitors differentiated and matured earlier than the definitive Mk progenitors. Because the megakaryocytes produced from the primitive Mk progenitors were smaller in size and had a lower ploidy class despite their maturation, the primitive Mk progenitors may produce platelets quickly at the expense of enlargement of the cytoplasm as well as DNA duplication. The simultaneous emergence of murine primitive hematopoietic cells and endothelial cells is observed in the YS blood islands on the seventh day of gestation, and the circulatory system is established within 2 days.34,35 Therefore, the primitive hematopoiesis may generate the unique Mk progenitors, which are sensitive to various cytokines to differentiate and mature rapidly to proplatelet megakaryocytes, and give rise to a number of platelets as soon as possible to prevent the simultaneously developing blood vessels from bleeding, until the definitive hematopoiesis begins to produce platelets. Indeed, AML-1-deficient mouse embryo lacking definitive but not primitive hematopoiesis survives until around 12 dpc, when it dies by hemorrhage in the ventricle of the central nervous system or vertebral canal.36
Although the development of megakaryocytes from Mk progenitors has been well investigated, the mechanism regulating the maturation and platelet production of megakaryocytes remains unclear. Because the primitive Mk progenitors detected in the present study were much easier to mature to proplatelet megakaryocytes and produce platelets than definitive Mk progenitors, clarification of the molecular mechanism that distinguishes biologic activities between the primitive and definitive Mk progenitors should be useful.
Supported by the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Pharmaceutical Safety and Research of Japan. M.-j.X. is supported by a fellowship from the Honjo International Scholarship Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kohichiro Tsuji, Department of Clinical Oncology, The Institute of Medical Science, The University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108-8639, Japan; e-mail: tsujik@ims.u-tokyo.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal