Abstract
Human mast cells in adult tissues have been thought to have limited, if any, proliferative potential. The current study examined mast cells obtained from adult skin and cultured in serum-free medium with recombinant human stem cell factor. During the first 4 weeks of culture, the percentages of mast cells increased from 10 to almost 100. After 8 weeks, a 150-fold increase in the number of mast cells was observed. When freshly dispersed mast cells were individually sorted onto human fibroblast monolayers and cultured for 3 weeks, one or more mast cells were detected in about two thirds of the wells, and in about two thirds of these wells the surviving mast cells showed evidence of proliferation, indicating most mast cells in skin can proliferate. Such mast cells all expressed high surface levels of Kit and FcεRI, each of which were functional, indicating IgE was not required for FcεRI expression on mast cells. Such mast cells also retained the MCTC protease phenotype of mast cells that normally reside in the dermis. After 4 to 8 weeks of culture these mast cells degranulated in response to substance P and compound 48/80, characteristics of skin-derived mast cells that persist outside of the cutaneous microenvironment.
Introduction
Multipotential progenitor cells, capable of becoming mast cells, leave the bone marrow and enter the circulation, but they complete their differentiation into mature mast cells only after arriving in peripheral tissues such as lung, bowel, skin, and nasal and conjunctival mucosa. Mature human mast cells can be distinguished from other cell types by expression of high levels of surface FcεRI and Kit, and of granule tryptase and heparin. Two types of human mast cells have been identified based on their composition of neutral proteases.1-6 MCTC cells contain tryptase, chymase, mast cell carboxypeptidase, and cathepsin G in their secretory granules and are the predominant type of mast cells in normal skin and in intestinal submucosa. MCT cells also contain tryptase in their granules, but lack these other proteases, and are the predominant type of mast cell in normal alveolar wall and in small intestinal mucosa.
In a number of human diseases such as asthma,7,8 allergic rhinitis,9,10 rheumatoid arthritis,11-13 and vernal keratoconjunctivitis,14 significant increases in mast cell density at the local affected sites have been described and mast cells were estimated to play a central role in the pathophysiology of the associated inflammation. The principal mechanisms involved in mast cell hyperplasia were thought to be either the recruitment of progenitor cells and their differentiation to mast cells or the migration of mast cells from other regions of the tissue. Proliferation has been considered less likely in part because cells were considered to be terminally differentiated and also because mitotic mast cells are rarely appreciated at these sites.
The proliferative potential of most myelomonocytic multipotential hematopoietic cells diminishes as they differentiate. However, mature murine mast cells have been reported to proliferate both in vivo and in vitro. About 6% of mouse peritoneal mast cells proliferate after being injected into skin of W/Wv mast cell-deficient mice.15,16 Purified peritoneal mast cells17,18 or dispersed skin mast cells,19when plated in methylcellulose containing pokeweed mitogen-stimulated spleen cell-conditioned medium, produced mast cell colonies. However, in humans, isolated tissue-derived mast cells have shown little capacity for proliferation. For example, dispersed newborn foreskin or adult skin mast cells placed into culture with lymphocyte-conditioned media and various cytokines showed bromodeoxyuridine incorporation in up to 15% mast cells.20
The current study shows that a substantial portion of mast cells dispersed from adult human skin can proliferate under serum-free culture conditions with recombinant human stem cell factor (rhuSCF), while retaining expression of chymase and tryptase in secretory granules, Kit, and FcεRI on their surface and the ability to degranulate in response to IgG anti-FcεRIα, compound 48/80 and substance P.
Materials and methods
Antibodies and reagents
Biotin-conjugated mouse IgG monoclonal antibody (mAb) antichymase, B7-biotin, and IgG mAb antitryptase, G3, were obtained and prepared as described.21 The mAb anti-Kit, YB5.B8, was a gift from Dr L. K. Ashman (Institute of Medical and Veterinary Science, Adelaide, Australia); IgG mAb anti-FcεRIα, 22E7, was obtained and used as described22,23; IgG mAb R24 against disialoganglioside (GD3),24 was a gift from Dr P. O. Livingston (Memorial Sloan-Kettering Cancer Center, New York, NY); and rhuSCF was provided by Amgen (Thousand Oaks, CA).
Dispersal purification and culture of human skin mast cells
Mast cells were dispersed from human skin tissue and enriched essentially as described.25-27 Fresh samples of skin were obtained after breast reduction or mastectomy for breast cancer, or from abdominoplasties, at the Medical College of Virginia Hospital or through the National Disease Research Interchange (Philadelphia, PA) and Cooperative Human Tissue Network of the National Cancer Institute (Columbus, OH) as approved by the Human Studies Internal Review Board at Virginia Commonwealth University. Subcutaneous fat was removed by blunt dissection and residual tissue was cut into 1- to 2-mm fragments, which were incubated in a solution of Hanks balanced salt solution (HBSS) containing 1.5 mg/mL type 2 collagenase, 0.7 mg/mL hyaluronidase (Worthington Biochemical, Lakewood, NJ), 0.3 mg/mL type 1 DNase (Sigma Chemical, St Louis, MO), 1% fetal calf serum (FCS), and 1 mM CaCl2 for 2 hours at 37°C with constant stirring. The dispersed cells were separated from residual tissue by filtration through a no. 80 mesh sieve and suspended in HBSS containing 1% FCS and 10 mM 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid (Hepes) (washing buffer). The remaining tissue was subjected to an additional digestion as above, and combined with the cells from first digestion. Isotonic Percoll was prepared by mixing 9 parts Percoll (Sigma) and 1 part of 10 × HBSS, and further diluted with HBSS to give final concentration of 75%. Cells were resuspend in washing buffer, layered over Percoll cushion, and centrifuged at 700g at room temperature for 20 minutes. Nucleated cells were collected from the buffer/Percoll interface.
Percoll gradient-enriched cells were suspended at the concentration of 1 × 106 cells/mL in serum-free AIM-V medium (Life Technologies, Rockville, MD). Some cells were cultured at the same concentration in complete RPMI 1640 medium supplemented with 10% controlled process serum replacement-3 (CPSR-3; Sigma), 2 mMl-glutamine, 0.1 mM nonessential amino acids, 10 mM Hepes, 50 μM 2-mercaptoethanol, 200 U/mL penicillin, and 100 μg/mL streptomycin. In both cases, 100 ng/mL rhuSCF was included, and half the culture medium was changed every week. When the cell number reached 2 × 106 cells/mL, half the cells were split to another well. Cell numbers per sample were adjusted to account for the cells that were split. Total cell number and viability were assessed by trypan blue staining. The percentages of mast cells were assessed cytochemically by metachromatic staining with toluidine blue, and by flow cytometry with anti-Kit and anti-FcεRI mAbs. The protease phenotype of cultured cells was examined by immunochemistry with antitryptase and antichymase mAbs. For each sample of cytocentrifuged cells, 100 cells or more per field, 4 fields per slide, and 3 slides were examined.
Flow cytometry
Surface Kit and FcεRIα expression were assessed by flow cytometry with mAbs YB5.B8 and 22E7, respectively. Cells were incubated in 10% human AB serum in phosphate-buffered saline (PBS). After washing in PBS containing 1% FCS and 0.1% sodium azide, cells then were incubated with anti-FcεRIα mAb or control mouse IgG for 30 minutes at 4°C. After washing as above, cells were incubated with fluorescein isothiocyanate (FITC)-conjugated goat antimouse immunoglobulins (BD Biosciences, San Jose, CA) for 15 minutes at 4°C, and washed as above. After incubation with normal mouse IgG to saturate antimouse antibody binding sites, cells were incubated with phycoerythrin (PE)-conjugated anti-Kit mAb (BD Pharmingen) or control IgG for 30 minutes at 4°C, resuspended in sheath solution, and analyzed with a FACScan flow cytometer (BD Biosciences). The percentage of positive cells was calculated by subtracting the percentage of cells labeled with a control antibody (< 3% positive cells) from that of cells with experimental mAb. HMC-1 cells, a human mast cell line, and KU812 cells, a human basophil leukemia cell line, were used as positive controls for surface Kit and FcεRIα expression, respectively.
For the detection of cells undergoing apoptosis after withdrawal of rhuSCF, cells were washed in PBS and incubated in the binding buffer as provided and recommended by the manufacturer (R & D Systems, Minneapolis, MN) with FITC-conjugated annexin-V, which binds to phosphatidylserine, a phospholipid that transfers from the inner to the outer side of the lipid bilayer of the plasma membrane during an early stage of apoptosis, and with propidium iodide (PI) for 15 minutes at room temperature to detect dead cells. Samples were directly analyzed by flow cytometry. To estimate the relative DNA content of cultured mast cells, cells were washed with PBS, incubated for 10 minutes on ice in hypotonic fluorochrome solution, which contained 50 μg/mL PI and 0.1% Triton-X100 in 0.1% sodium citrate, and then directly analyzed by flow cytometry.
Immunochemistry
Mast cells were assessed in cytocentrifuge preparations subjected to indirect immunocytochemical labeling as described.23 For chymase detection, B7-biotin–labeled cells were incubated with peroxidase-conjugated streptavidin and visualized with 3-amino-9-ethylcarbazole in 0.01% H2O2 to stain positive cells reddish brown. For tryptase detection, alkaline phosphatase-conjugated goat antimouse IgG (Sigma) was added to G3-labeled cells and visualized with naphthol AS-BI/new fuchsin to stain positive cells red. Cells were lightly counterstained with hematoxylin.
Immunoaffinity magnetic enrichment and individual sorting
Enzymatically dispersed skin cells were further enriched with mAb R24 against GD3, which was recently reported to be selectively expressed on the surface of mast cells in normal skin.28Briefly, cells were incubated in PBS containing 10% human AB serum for 30 minutes, washed with PBS, and incubated with R24 (3 μg/1 × 107 cells) at 4°C for 30 minutes. Cells were washed with PBS to remove unbound mAb, incubated with microbeads conjugated to antimouse IgG (Miltenyi Biotec, Auburn, CA) at 4°C for 15 minutes, washed, and applied to the separation column. The column was exposed to a magnetic field in the magnetic cell separation system (Miltenyi Biotec) and washed to remove cells without attached microbeads. Retained cells were then eluted after the column was withdrawn from the magnetic field. Eluted cells were incubated with normal mouse IgG to saturate the antimouse antibody, and then with PE-conjugated mAb anti-Kit or control antibody for 1 hour at 4°C. Cells were then washed, and resuspended in PBS containing 20% AIM-V medium. Kit+ cells were individually seeded onto confluent 1059SK human fibroblasts (American Type Culture Collection, CRL-2072, Manassas, VA) in 96-well plates using a Coulter Epics Cell Sorting System (Coulter, Miami, FL) at Virginia Commonwealth University's Flow and Imaging Cytometry Facility, which permits single-cell sorting with an accuracy of more than 99%. Sorted cells were then cultured in AIM-V medium supplemented with 100 ng/mL rhuSCF. Half the medium was changed every week. After 3 weeks mast cell numbers were checked by immunocytochemistry for tryptase.
Activation of mast cells and tryptase immunoassay
Cultured mast cells were washed in Tyrode buffer containing 10 mM Hepes, pH 7.4, 0.1% gelatin, and 100 ng/mL DNase (TGD−/−), then suspended in TGD−/− buffer containing 1 mM MgCl2 and 2.5 mM CaCl2(TGD+/+) and preincubated for 5 minutes at 37°C. For stimulation, 5 μL mAb 22E7 or control mouse IgG, or nonimmune reagents such as substance P or compound 48/80 (Sigma) were added to 25 μL cell suspension (1 × 106 cells/mL) in a 96-well plate, and incubated for a further 15 minutes at 37°C. The reaction was stopped by adding 200 μL ice-cold TGD−/− buffer. The cells were separated by centrifugation at 300g for 7 minutes at 4°C, and supernatant was carefully removed and adjusted to 1.2 M NaCl by adding 40 μL 5 M NaCl. The cell pellet was resuspended in 200 μL ice-cold extraction buffer (10 mM MES, pH 6.5, with 2 M NaCl), quickly frozen in liquid nitrogen, and thawed 4 times to release all cellular tryptase. After centrifugation at 12 000g for 15 minutes at 4°C, the soluble extract was collected and stored at −70°C until ready to be assayed. Total immunoreactive tryptase levels were measured by an enzyme-linked immunosorbent assay (ELISA) using mAb B12 to capture tryptase and biotinylated mAb G5 to detect captured tryptase as described29; purified human lung-derived tryptase was used as standard.
Results
Proliferation of human skin–derived mast cells in a serum-free culture
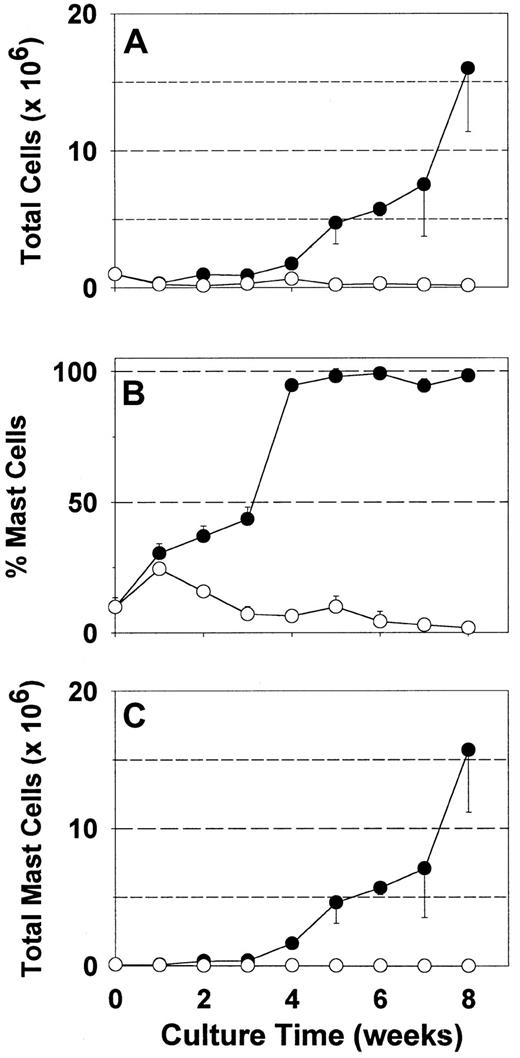
Because serum may contain both beneficial and detrimental factors for mast cell growth in vitro, synthetic media are potentially advantageous. Serum-free AIM-V media permitted an earlier, more selective and greater expansion of fetal liver-derived mast cells than serum-containing media in the presence of rhuSCF.23However, most tissue-derived human mast cells, though probably long-lived, are thought to be terminally differentiated with limited, if any, proliferative capacity. The growth of skin-derived mast cells in the presence of rhuSCF (100 ng/mL) was assessed with this serum-free media compared to serum-containing RPMI 1640 (Figure1). As expected with RPMI 1640 medium with serum, after an 8-week culture period, total cell numbers diminished by 90% (Figure 1A), mast cell percentage by about 80% (Figure 1B), and mast cell number by over 90% (Figure 1C). At 1 week there was a slight increase in the percentage of mast cells due to a more rapid loss of other cell types, but there was no increase in mast cell number. In contrast to serum-containing media, results with AIM-V media were dramatically different. Total cell numbers diminished slightly after the first week, but then increased 16-fold by 8 weeks (Figure 1A). Under these serum-free culture conditions the mast cell percentage increased from about 10% at the initiation of culture to nearly 100% by 4 weeks, and remained so for the remaining 4 weeks (Figure 1B). Mast cell numbers increased progressively from 0.1 × 106 cells on day zero to 15.9 × 106after 8 weeks. A similar growth pattern was recognized in another 5 independent specimens. Of the 4 specimens kept in culture under serum-free conditions beyond 8 weeks, one stopped proliferating at 8 to 9 weeks, 2 stopped at 9 to 10 weeks and one continued until the culture was harvested at 12 weeks.
Expansion of skin-derived mast cell numbers in serum-free media.
Dispersed skin cells, enriched with mast cells by Percoll density-dependent sedimentation, were placed into culture with rhuSCF (100 ng/mL) in either RPMI 1640 supplemented with CPSR-3 (○) or AIM-V (●) as described. Total viable cell numbers (A), percentages of mast cells (metachromatic staining) (B), and numbers of mast cells (product of total cell number and mast cell percentage) (C) are depicted. Each data point is the mean value from 3 separate experiments, each performed in triplicate. Error bars indicate the SD. When the total cell concentration in a well exceeded 2 × 106 cells/mL, half of the cells were either transferred to another well or discarded; cell numbers were adjusted to account for the cells that were discarded as well as those that were retained when cultures were split.
Expansion of skin-derived mast cell numbers in serum-free media.
Dispersed skin cells, enriched with mast cells by Percoll density-dependent sedimentation, were placed into culture with rhuSCF (100 ng/mL) in either RPMI 1640 supplemented with CPSR-3 (○) or AIM-V (●) as described. Total viable cell numbers (A), percentages of mast cells (metachromatic staining) (B), and numbers of mast cells (product of total cell number and mast cell percentage) (C) are depicted. Each data point is the mean value from 3 separate experiments, each performed in triplicate. Error bars indicate the SD. When the total cell concentration in a well exceeded 2 × 106 cells/mL, half of the cells were either transferred to another well or discarded; cell numbers were adjusted to account for the cells that were discarded as well as those that were retained when cultures were split.
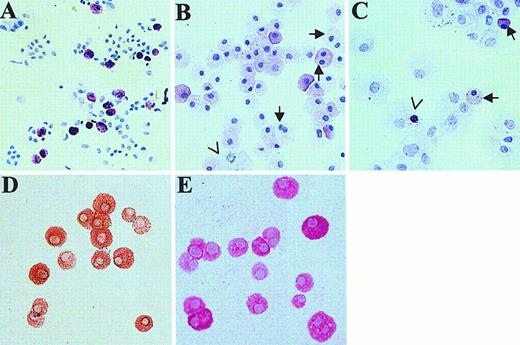
Skin-derived mast cells were examined by light microscopy (Figure2). A cytospin of freshly dispersed skin cells, stained with toluidine blue, shows about 10% of the cells to be metachromatic with oval, nonsegmented nuclei (Figure 2A). After 4 weeks of culture in AIM-V medium, monolayers of tightly adherent cells were not observed. Instead, almost all of the cells were metachromatic (Figure 2B), although the staining intensity appeared to be less than for newly dispersed mast cells. In contrast, after 4 weeks of culture in RPMI 1640 with CPSR-3, the culture well was completely covered by fibroblast-like cells. Toluidine blue staining of cytocentrifuged cell preparations revealed few metachromatic cells, many fibroblast-like cells, and occasional metachromatic bodies in fibroblast- or macrophage-like cells, suggesting that ingestion of mast cells or mast cell granules may have occurred (Figure 2C). Also, morphometric measurements (mean ± SE, n = 4 separate skin samples, 30 mast cells examined per preparation) of mast cell diameter revealed cells cultured for 4 to 8 weeks in AIM-V media (25.7 ± 0.2 μm,) to be larger (P < 0.001, t test) than those prior to culture (13.2 ± 0.2 μm). Although most nuclei were oval and nonsegmented, 4.6% ± 2.1% (n = 5 specimens) of the cultured mast cells were binucleated or showed mitotic figures (Figure 2B), features not observed before culture. Almost no fibroblast-like cells were observed. Essentially all mast cells in normal adult skin contain both tryptase and chymase (MCTC type of mast cell).21 To test whether skin-derived mast cells retained this protease phenotype after 4 to 8 weeks of culture in serum-free AIM-V media containing rhuSCF, cytospins of these cells were stained with antichymase (Figure 2 D) and antitryptase (Figure 2 E) mAbs, and counterstained with hematoxylin. Essentially all cells were labeled with each mAb, indicating the MCTC phenotype was preserved under these culture conditions.
Light microscopic appearance of cytospins of skin-derived mast cells.
Toluidine blue staining dispersed skin cells at the initiation of culture (A) and after 4 weeks of culture in AIM-V (B) and in RPMI 1640 with 10% CPSR-3 (C) as described. In panel B, arrows show 2 cells with dual nuclei; an arrowhead points out a mitotic cell. In panel C, arrows point to mast cells (17% of total cells counted on this slide); the arrowhead shows a metachromatic body inside a nonmast cell. Cells grown for 4 weeks in AIM-V medium (98% mast cells) were stained for chymase (D) and tryptase (E). Essentially all cells contained both chymase and tryptase. The original magnification of all photomicrographs was × 200.
Light microscopic appearance of cytospins of skin-derived mast cells.
Toluidine blue staining dispersed skin cells at the initiation of culture (A) and after 4 weeks of culture in AIM-V (B) and in RPMI 1640 with 10% CPSR-3 (C) as described. In panel B, arrows show 2 cells with dual nuclei; an arrowhead points out a mitotic cell. In panel C, arrows point to mast cells (17% of total cells counted on this slide); the arrowhead shows a metachromatic body inside a nonmast cell. Cells grown for 4 weeks in AIM-V medium (98% mast cells) were stained for chymase (D) and tryptase (E). Essentially all cells contained both chymase and tryptase. The original magnification of all photomicrographs was × 200.
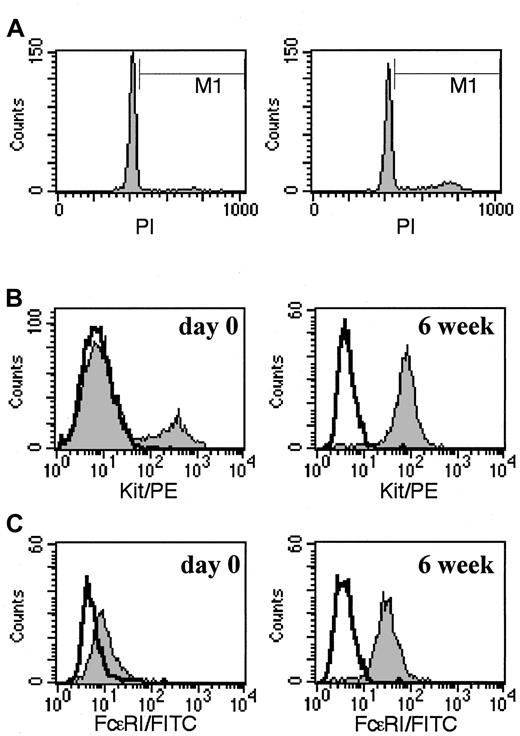
To further evaluate the proliferation activity of 4-week-old cultured mast cells, DNA content was assessed by flow cytometry with PI. In a DNA fluorescence histogram, the cells in S, G2, and M phases are shown in the M1 region of Figure3A, whereas those in G0 and G1 phases reside in the peak that precedes M1. When 4-week-old mast cells grown in AIM-V media were examined before (left panel, 7 days after prior feeding) and 24 hours after rhuSCF feeding (right panel), 3.7% ± 1.8% and 9.4% ± 3.3% of the cells, respectively, were distributed in M1 (n = 3, mean ± SD). Thus proliferation rates appeared to be somewhat greater 24 hours than 7 days after treatment with rhuSCF. Also, to confirm that these cells were mast cells, surface Kit (Figure 3B) and FcεRI (Figure 3C) were evaluated. As shown, after 6 weeks of culture in AIM-V media (right panels), essentially all cells were strongly Kit+ (96%) and FcεRI+ (96%). Similar results were obtained at 4- and 8-week time points. The fluorescence intensity of the Kit+ population within 24 hours after their dispersion from skin (Figure 3B, left panel) was similar to or slightly higher than that of the mast cells after 4 to 8 weeks of culture in AIM-V media. In contrast, FcεRI levels on Kit+ cells appeared to be lower just after dispersion of skin cells (Figure 3C, left panel) than after 4 to 8 weeks of culture.
Cell cycle and surface phenotype of skin-derived mast cells.
(A) Relative DNA content of skin-derived mast cells after about 4 weeks of culture in AIM-V media. Cells were incubated with 50 μg/mL PI for 10 minutes on ice just before (7 days after last rhuSCF feeding, left panel) and 24 hours (right panel) after a rhuSCF feeding, and analyzed by flow cytometry. (B) Surface Kit+ mast cells 1 day after their initial dispersion (left panel) and 7 days after rhuSCF feeding of cells grown in AIM-V suspension culture for 6 weeks (right panel). (C) FcεRI surface expression of Kit+ cells 1 day after their initial dispersion (left panel) and 7 days after rhuSCF feeding of cells grown in AIM-V suspension culture for 6 weeks (right panel). Shaded areas in panels B and C show anti-FcεRI and anti-Kit labeling, respectively; bold lines indicate negative IgG controls. Each panel is representative of 3 separate experiments.
Cell cycle and surface phenotype of skin-derived mast cells.
(A) Relative DNA content of skin-derived mast cells after about 4 weeks of culture in AIM-V media. Cells were incubated with 50 μg/mL PI for 10 minutes on ice just before (7 days after last rhuSCF feeding, left panel) and 24 hours (right panel) after a rhuSCF feeding, and analyzed by flow cytometry. (B) Surface Kit+ mast cells 1 day after their initial dispersion (left panel) and 7 days after rhuSCF feeding of cells grown in AIM-V suspension culture for 6 weeks (right panel). (C) FcεRI surface expression of Kit+ cells 1 day after their initial dispersion (left panel) and 7 days after rhuSCF feeding of cells grown in AIM-V suspension culture for 6 weeks (right panel). Shaded areas in panels B and C show anti-FcεRI and anti-Kit labeling, respectively; bold lines indicate negative IgG controls. Each panel is representative of 3 separate experiments.
Skin-derived mast cells cultured in AIM-V media are dependent on exogenous SCF for survival
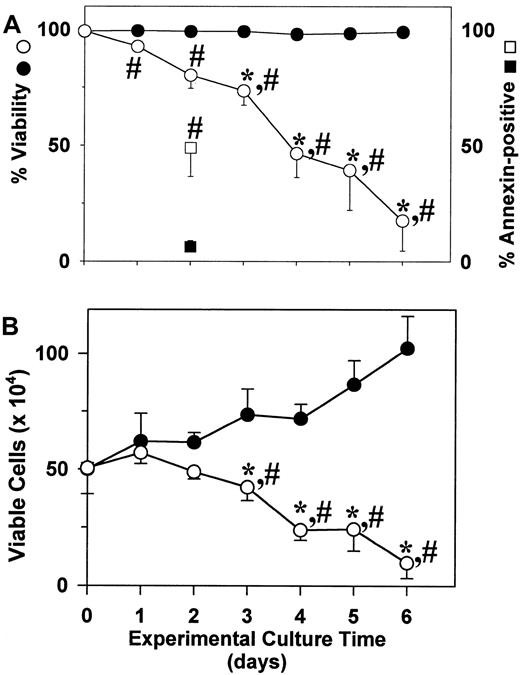
Survival of human mast cells is acknowledged to be primarily dependent on SCF. To test whether proliferating skin-derived mast cells were dependent on this growth factor, 3 separate preparations of 6- to 8-week cultures of mast cells in AIM-V media were washed and placed into culture with or without rhuSCF (100 ng/mL). As shown in Figure4A, viability (trypan blue) remained near 100% in the presence of rhuSCF, but steadily decreased in the absence of rhuSCF. By day 1 and beyond the percent viabilities of cells cultured with rhuSCF were significantly different from those cultured without rhuSCF; by day 3 and beyond the percent viabilities of cells cultured in the absence of rhuSCF were significantly lower than the day 0 cells. The percentages of apoptotic cells on day 2 were evaluated by annexin-V surface and PI labeling. Without rhuSCF 49% of the cells were labeled with annexin V, whereas less than 20% (as with trypan blue) were also labeled with PI. All of the annexin V+cells were smaller than the viable unlabeled cells (forward side scatter by flow cytometry), consistent with apoptosis being their death pathway. With rhuSCF only about 6% of the cells were labeled with annexin V and about 5% with PI. As shown in Figure 4B, with rhuSCF the number of viable cells increased significantly by day 3, whereas without rhuSCF the cell numbers had significantly declined by day 3 when compared to day 0 cells. Also, viable cell numbers in the presence and absence of rhuSCF were significantly different by day 3.
Proliferating skin-derived mast cells require SCF to survive.
After 6 to 8 weeks in culture with rhuSCF (100 ng/mL) in AIM-V medium, cells (95%-99% mast cells) were washed with PBS and placed into culture with (closed circles and square) or without (open circles and square) rhuSCF (100 ng/mL) in AIM-V medium. Cell numbers and viabilities were measured daily for 6 days. (A) Percent viability (circles). Also shown are the annexin V+ cells measured on day 2 (squares). (B) Viable cell numbers. Each data point in panels A and B is the mean ± SD for 3 independent experiments, each performed in duplicate. *, P < .05 for a difference compared to the day 0 value using a one-way repeated measures ANOVA and the Dunnet method for multiple comparisons versus a control. #,P < .05 for a difference between the plus and minus rhuSCF values using a paired t test.
Proliferating skin-derived mast cells require SCF to survive.
After 6 to 8 weeks in culture with rhuSCF (100 ng/mL) in AIM-V medium, cells (95%-99% mast cells) were washed with PBS and placed into culture with (closed circles and square) or without (open circles and square) rhuSCF (100 ng/mL) in AIM-V medium. Cell numbers and viabilities were measured daily for 6 days. (A) Percent viability (circles). Also shown are the annexin V+ cells measured on day 2 (squares). (B) Viable cell numbers. Each data point in panels A and B is the mean ± SD for 3 independent experiments, each performed in duplicate. *, P < .05 for a difference compared to the day 0 value using a one-way repeated measures ANOVA and the Dunnet method for multiple comparisons versus a control. #,P < .05 for a difference between the plus and minus rhuSCF values using a paired t test.
Proliferative potential of human skin–derived mast cells
From the experiments described thus far, it was not clear whether the proliferating mast cells in this serum-free culture condition were derived from mature mast cells or from progenitors that might reside in preparations of dispersed skin cells. To address this, mast cells were immunoaffinity purified with anti-GD3 mAb to at least 95% purity as described,28 and then subjected to cell sorting after labeling the cells with PE-conjugated anti-Kit mAb. A portion of these cells was placed directly onto glass slides that then were stained with toluidine blue. All exhibited metachromasia, indicating this population contained virtually 100% mature mast cells (Figure5). Another portion of the cells was individually sorted into 96-well culture plates, each well containing a confluent monolayer of human fibroblasts to act as feeder cells. Fibroblasts together with rhuSCF were necessary for cell survival; in the absence of either fibroblasts or exogenous rhuSCF, no cells were recovered after 3 weeks. These confluent fibroblasts survived but did not proliferate under the serum-free culture condition used. After 3 weeks of culture with AIM-V medium containing 100 ng/mL rhuSCF, the number of mast cells per well was evaluated by immunocytochemistry with antitryptase mAb. Results are shown in Figure 5B and Table1. Mean data taken from 4 separate experiments indicated only one tryptase-positive cell in 19% of the wells, and 2 or more tryptase-positive cells per well in 44% of the wells (11 cells/well on average). Thus, one or more mast cells were detected in 63% of the wells, and in about two thirds of the mast cell-containing wells there was evidence for mast cell proliferation. In 2 control experiments single Kit-sorted HMC-1 cells yielded detectable cells in 65% (58% and 71%) of the wells, the remainder presumably not surviving the sorting or the culture conditions. The percentages of wells with surviving mast cells are similar for skin-derived mast cells and HMC-1 cells. These results indicate that most mature skin-derived mast cells are capable of proliferating in serum-free media containing rhuSCF.
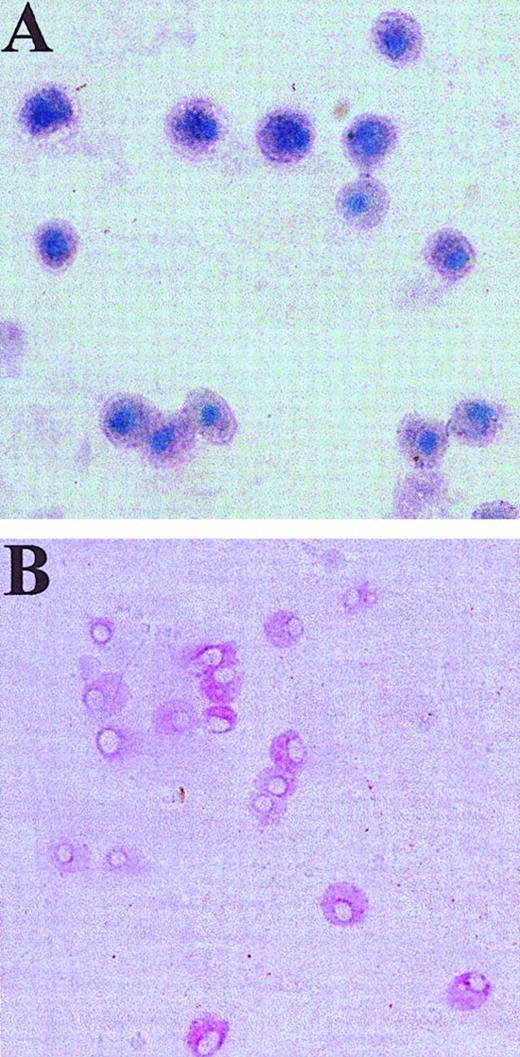
Human skin-derived mast cells after GD3- and Kit-dependent immunoaffinity purification and coculture with fibroblasts.
Dispersed and density-dependent sedimentation-enriched skin-derived mast cells were purified using anti-GD3 mAb and were then labeled with PE-anti-Kit mAb. (A) A portion of these cells was placed on a slide, air dried, and stained with toluidine blue. Mast cells exhibit metachromatic purple cytoplasm and blue nuclei. (B) Another portion of these cells was subjected to sorting of individual cells onto fibroblast monolayers, which were then cultured with rhuSCF (100 ng/mL) for 3 weeks and stained with anti-tryptase mAb (naphthol AS-BI/new fuchsin). Positive cells stain red. Panel B shows a well containing multiple tryptase-positive cells. Original magnifications at the time of photomicroscopy were × 400 for panel A, and × 200 for panel B (inverted microscope).
Human skin-derived mast cells after GD3- and Kit-dependent immunoaffinity purification and coculture with fibroblasts.
Dispersed and density-dependent sedimentation-enriched skin-derived mast cells were purified using anti-GD3 mAb and were then labeled with PE-anti-Kit mAb. (A) A portion of these cells was placed on a slide, air dried, and stained with toluidine blue. Mast cells exhibit metachromatic purple cytoplasm and blue nuclei. (B) Another portion of these cells was subjected to sorting of individual cells onto fibroblast monolayers, which were then cultured with rhuSCF (100 ng/mL) for 3 weeks and stained with anti-tryptase mAb (naphthol AS-BI/new fuchsin). Positive cells stain red. Panel B shows a well containing multiple tryptase-positive cells. Original magnifications at the time of photomicroscopy were × 400 for panel A, and × 200 for panel B (inverted microscope).
Mast cell proliferation of individually sorted skin mast cells
| Plate no. . | No. wells per plate . | Mast cells per well in wells with at least 2 mast cells (mean ± SD, range) . | |
|---|---|---|---|
| 1 mast cell . | At least 2 mast cells . | ||
| 1 | 16 | 39 | 14 ± 12, 2-47 |
| 2 | 9 | 33 | 8.7 ± 6.0, 2-25 |
| 3 | 33 | 47 | 11 ± 11, 2-56 |
| 4 | 15 | 51 | 12 ± 13, 2-56 |
| Mean ± SD | 19 ± 11 | 44 ± 8 | 11 ± 11 |
| Plate no. . | No. wells per plate . | Mast cells per well in wells with at least 2 mast cells (mean ± SD, range) . | |
|---|---|---|---|
| 1 mast cell . | At least 2 mast cells . | ||
| 1 | 16 | 39 | 14 ± 12, 2-47 |
| 2 | 9 | 33 | 8.7 ± 6.0, 2-25 |
| 3 | 33 | 47 | 11 ± 11, 2-56 |
| 4 | 15 | 51 | 12 ± 13, 2-56 |
| Mean ± SD | 19 ± 11 | 44 ± 8 | 11 ± 11 |
Enzymatically dispersed skin cells were immunoaffinity purified with the R24 mAb against GD3, and then sorted with the anti-Kit YB5.B8 mAb using a Coulter Epics Cell Sorting System. Individual mast cells were sorted into each well of 96-well plates containing human fibroblast monolayers, and were then cultured in AIM-V medium containing 100 ng/mL rhuSCF. After 3 weeks of culture, mast cell numbers were determined by immunochemistry for tryptase with the G3 mAb. Results from 4 separate sorts, one plate per sort, are shown.
Proliferating skin-derived mast cells retain their characteristic functional phenotype
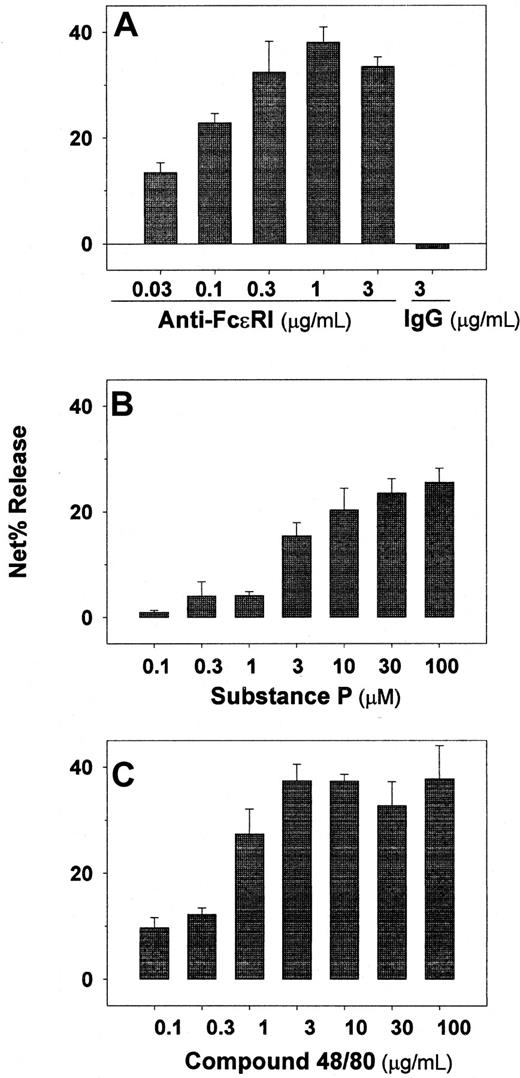
Experiments were performed to determine whether cultured skin-derived mast cells degranulate in response to FcεRI cross-linking and to agents known to stimulate skin but not lung-derived mast cells. As shown in Figure6A, tryptase release occurred in a dose-response fashion to anti-FcεRIα mAb, maximal release being observed at 0.3 to 3 μg/mL. Also, the amount of tryptase per mast cell was calculated to be 22 ± 4 pg/mast cell (n = 5), slightly less than the amount measured per mast cell from skin tissue shortly after their dispersion and density-dependent enrichment, which was 29 ± 3 pg/mast cell (n = 3). Degranulation also was observed in a dose-dependent manner to substance P (Figure 6B) and to compound 48/80 (Figure 6 C).
Skin-derived mast cells retain their capacity to degranulate in response to several agonists after proliferating in vitro.
Anti-FcεRIα mAb (A), substance P (B), and compound 48/80 (C) were assessed as degranulating agents on skin-derived mast cells cultured for 4 to 8 weeks in AIM-V media containing 100 ng/mL rhuSCF. Tryptase release was determined after each agonist was incubated with the mast cells for 15 minutes at 37°C. In each case data are presented as net percent tryptase release. Spontaneous tryptase release values were always less than 5%. Cell viabilities were determined at the high dose of each agonist at the end of the experiment by trypan blue staining, and were observed to be more than 90% in all cases. Bar graphs display mean ± SD values from 3 independent experiments.
Skin-derived mast cells retain their capacity to degranulate in response to several agonists after proliferating in vitro.
Anti-FcεRIα mAb (A), substance P (B), and compound 48/80 (C) were assessed as degranulating agents on skin-derived mast cells cultured for 4 to 8 weeks in AIM-V media containing 100 ng/mL rhuSCF. Tryptase release was determined after each agonist was incubated with the mast cells for 15 minutes at 37°C. In each case data are presented as net percent tryptase release. Spontaneous tryptase release values were always less than 5%. Cell viabilities were determined at the high dose of each agonist at the end of the experiment by trypan blue staining, and were observed to be more than 90% in all cases. Bar graphs display mean ± SD values from 3 independent experiments.
Discussion
The current study shows that skin-derived mast cells are capable of proliferation, which could contribute to mast cell hyperplasia in tissues. However, human mast cells that reside in peripheral tissues of adult hosts have been considered to be long-lived cells with limited, if any, proliferative capacity. At sites where mast cell hyperplasia occurs, such as urticaria pigmentosa in skin,30 rheumatoid synovium,11 bronchopulmonary dysplasia and fibrotic lung disease,31 and atopic keratoconjunctivitis,14,32 the rare presence of mitotic mast cells has been used to argue that proliferation of mature mast cells does not account for their increased presence. Even when mast cell hyperplasia is iatrogenically induced by administration of rhuSCF, mitotic mast cells in skin biopsies were not reported.33Consequently, expansion of mast cell populations at specific tissue sites in humans has been predicted to occur by diverting a greater portion of the pool of hematopoietic progenitor cells to a mast cell lineage, by stimulating proliferation of mast cell progenitors, by increasing recruitment of mast cells and their precursors into tissues, or by increasing survival of mast cells. Recruitment of mast cell from one region of a tissue to another or of precursors from the circulation to tissue sites may be driven by various chemokines,34-37transforming growth factor-β (TGF-β),38SCF,39 and complement anaphylatoxins.40Proliferation of mast cell progenitors may occur before these cells acquire the characteristic phenotypic features of the granules and cell surface of mature mast cells, even in cases of systemic mastocytosis associated with activating somatic mutations ofc-kit.41 However, mitosis should be a relatively brief event in the life of a long-lived cell such as a mast cell. Thus, mitotic figures as a sign of mature mast cell proliferation may lack adequate sensitivity.
The current study demonstrates that most mast cells derived from adult skin can proliferate in serum-free media containing rhuSCF. This suggests that SCF is a proliferation factor as well as a survival42,43 and priming or activation44,45factor for mature human mast cells. Mast cell numbers in suspension cultures increased 150-fold by 8 weeks. At 4 weeks, mitotic or binucleated cells accounted for 4.6% of the mast cells. A DNA fluorescence histogram with PI revealed that 9.4% of the 4-week cells were distributed in the S, G2, and M phases of the cell cycle. To rule out the possibility that this represented expansion of a small progenitor cell population, mature mast cells were purified by density-dependent sedimentation and immunoaffinity purification with anti-GD3 mAb,28 and then were individually sorted using PE-labeled anti-Kit mAb onto fibroblast monolayers in wells of microtiter plates. At least two thirds of the mature mast cells that survived such Kit-dependent sorting had the ability to proliferate. Why this was observed in serum-free but not serum-containing media is not known, but is consistent with previous observations showing survival but not proliferation of mast cells in serum-containing media.26 27 With serum, a substantial increase in fibroblast-like cells and metachromatic bodies in nonmast cells were observed in our experiments. Consequently, the attenuating effects of serum on mast cell growth, in part, could be indirect by stimulating other cell types to grow and to phagocytose mast cells. However, when serum was added to AIM-V–cultured mast cells at 4 weeks, when mast cells predominated and few if any fibroblast-like cells were observed, proliferation of mast cells was curtailed, suggesting direct as well as indirect effects of serum on mast cell growth. Thus, mature mast cells in human skin have the capacity to proliferate when exposed to SCF, but factor(s) in serum or plasma may regulate this response. Whether the extracellular fluid to which tissue mast cells are exposed in vivo acts similarly to serum in vitro remains to be determined.
Proliferating skin-derived mast cells appear to retain several key phenotypic features of freshly dispersed cells. For example, nearly all expressed high levels of cell-surface Kit and FcεRI after 4 weeks of culture. That both receptors were functional can be inferred because survival of these cells was dependent on rhuSCF, and degranulation was induced by cross-linking FcεRI with anti-FcεRIα mAb. Also, given that a substantial amount of proliferation had occurred between day 0 and 4 and 8 weeks of culture, new receptors must have been synthesized. For Kit, similar fluorescence intensities were observed between freshly dispersed mast cells and those cultured for 4 to 8 weeks. In contrast, for FcεRI, higher intensities of surface labeling were observed on mast cells after 4 to 8 weeks of culture than those freshly dispersed from skin. Regardless as to whether the low FcεRI levels on day 1 reflect actual levels in vivo or removal of surface FcεRI during the proteolytic digestion of skin, new receptors must have been synthesized in the absence of IgE to account for the high levels after 4 to 8 weeks of culture. Such persistently high surface levels of FcεRI on skin-derived mast cells in vitro in the absence of IgE contrasts with reports that FcεRI levels dramatically fall in the absence of IgE on human peripheral blood-derived basophils46-49 and in vitro-derived mast cells.50,51 However, FcεRI levels on peripheral blood-derived monocytes and eosinophils do not correlate with serum IgE levels,52 indicating factors other than IgE levels may influence FcεRI levels in some cell types. The current in vitro observation suggests that mast cells in tissues might retain high FcεRI levels after IgE is neutralized with anti-IgE antibody, which in turn may help to explain the persistence of immediate hypersensitivity responses in the skin and airway to allergens in sensitive subjects treated with anti-IgE mAb at a time when basophil FcεRI levels are very low.46 53
The typical protease phenotype of skin-derived mast cells, namely, both tryptase and chymase expression by immunocytochemistry, also was maintained. After 4 to 8 weeks of culture, the tryptase content measured in cell extracts by an ELISA averaged 22 pg/mast cell, slightly less than the 29 pg/mast cell measured after their initial dispersion. These values are comparable to what has been reported for freshly dispersed skin-derived mast cells (35 pg/mast cell),25 and greater than the content of lung-derived mast cells (11 pg/mast cell), and fetal liver-derived mast cells (3.7 ± 0.1 pg/mast cell, n = 323). As for tryptase, essentially all skin-derived mast cells also express chymase when they are dispersed from skin, and then retain their chymase-positive phenotype during culture in serum-free AIM-V medium. In contrast, suspension cultures of fetal liver-derived cells with rhuSCF produce mast cells with abundant chymase messenger RNA, but few mast cells with detectable chymase protein when serum-containing media is used.50About one third of the mast cells express chymase protein when they develop in suspension cultures with serum-free AIM-V medium.23
Although human mast cells from all tissues degranulate in response to FcεRI cross-linking and to calcium ionophores, heterogeneity has been reported for the response to substance P and to compound 48/80. For example, freshly dispersed skin-derived mast cells respond to both agonists; mast cells from most other tissues do not, regardless of their protease phenotype.54-56 The current study showed that proliferating skin-derived mast cells still respond to these agonists after 4 to 8 weeks of culture in serum-free medium. This result suggests that once responsiveness to substance P and compound 48/80 develops, the cutaneous microenvironment is not needed to maintain this functional phenotype.
We are grateful to Frances K. White (Flow and Imaging Cytometry Facility, Virginia Commonwealth University, Richmond, VA) for technical support with cell sorting.
Supported by National Institutes of Health grants AI27517 and AI20487 (to L.B.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Lawrence B. Schwartz, Virginia Commonwealth University, PO Box 980263, Richmond, VA 23298-0263; e-mail:lschwart@hsc.vcu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal