Abstract
Several signaling cascades are engaged by expression of the p210 bcr-abl tyrosine kinase, and evidence suggests that these signals drive leukemogenesis. In this report, signaling pathways were examined and compared between cells derived from leukemic patients and cells expressing a bcr-abl construct (MBA). The effects of acute inhibition of bcr-abl with STI-571 on these signals and the survival of bcr-abl–expressing cells were also evaluated. Expression of bcr-abl in interleukin-3 (IL-3)/granulocyte-macrophage colony-stimulating factor (GM-CSF)–dependent Mo7e cells (MBA) resulted in growth factor independence, constitutive activation of Stat-5 phosphorylation, engagement of mitogen-activated protein (MAP) kinase signals, and increased expression of PTP1B and bcl-xL. STI-571 inhibited cell growth and induced apoptosis in bcr-abl–expressing cells (MBA, K562, BV-173, KBM5) but not in bcr-abl− tumor cells (Mo7e, KG-1, ME-180, Daudi). STI-571–mediated apoptosis correlated with the inhibition of Stat-5 and MAP kinase activation and a reduction in overexpressed bcl-xL but not in PTP1B. Inhibitor had no effect on IL-3/GM-CSF–dependent Mo7e cell signaling and did not prevent activation of the other Jak/Stat pathways (interferon α, IL-3/GM-CSF). However, neither IL-3 nor GM-CSF could reactivate Stat-5 after the STI-571–mediated inhibition of bcr-abl. Expression of the common β-chain of the IL-3/GM-CSF receptor was down-regulated in Stat-5–activated myeloid leukemic cells, suppressing IL-3/GM-CSF signal transduction and the ability of these cytokines to provide apoptotic protection. These studies suggest that bcr-abl activates cytokine-independent mechanisms of survival while inactivating intrinsic cytokine signaling cascades, making bcr-abl+myeloid cells vulnerable to apoptosis after bcr-abl inactivation.
Introduction
Adult-onset leukemia is a heterogeneous hematopoietic stem cell disorder characterized by the overproduction or maturation of hematopoietic cells of several distinct lineages.1 Evidence suggests that most of these tumors arise through the aberrant expression of transcription factors or the engagement of signaling pathways that control their activation.2-4 Specific cytogenetic abnormalities, first described as the Philadelphia chromosome (9:22 translocation) in patients with leukemia, are common in chronic myelogenous and, to a lesser extent, acute lymphocytic leukemia, and much attention has been focused on understanding the consequence and cause of this alteration.5-7
Reciprocal 9:22 chromosomal translocation alters transcriptional control of the c-abl gene, recently shown to play a role in stress-induced signaling.8 Translocation places the c-abl gene under the transcriptional control of the bcr locus, allowing expression of a hybrid protein encoded by 1 to 3 exons of the bcr gene and by all but the first exon of c-abl. This chimeric bcr-abl protein (p190 or p210) expresses intrinsic tyrosine kinase activity with altered compartmentalization and distinctions in substrates when compared to the predominantly nuclear c-abl protein.7,9 Tyrosine kinase activity is essential for the transforming function of bcr-abl, and the expression of bcr-abl in stem cells of immune-deficient mice results in altered hematopoiesis resembling human leukemia-like disorders.10-13 Thus, bcr-abl expression plays a central role in leukemogenesis and provides an appropriate and specific target for therapeutic intervention.
Expression of bcr-abl alters several signaling pathways that deregulate cellular growth and prolong survival.9,13,14 These signals are engaged through adaptor molecule recruitment to the bcr-abl protein or through direct phosphorylation of specific substrates.13-15 Alterations in either the Src homology 2 (SH2) or the SH3 domain affect the transformed phenotype, but tyrosine phosphorylation is critical for leukemogenesis and transformation.13,14 Downstream signals engaged by bcr-abl expression are common to a number of oncogenes (ras,MAPK, PI3K, Akt), but others appear to be shared with growth factor–cytokine signaling cascades involved in normal hematopoiesis and stem cell differentiation (Jak/Stat).13-16 Several points of convergence between cytokine–growth factor signaling and bcr-abl oncogenesis have been described, and cells expressing bcr-abl no longer require exogenous cytokines—interleukin-3 (IL-3) or granulocyte-macrophage colony-stimulating factor (GM-CSF)—for their continual growth or survival.13,15,17-19 These observations predict that the expression of specific signaling molecules that control hematopoiesis (Jak/Stat) may be targets of bcr-abl and critical to the development of leukemia. Recent reports confirm a contribution of activated Stat proteins in the leukemogenic process.13 18-20
Because of the selective expression of bcr-abl tyrosine kinase in leukemic cells but not normal cells, inhibitors that target this kinase have been developed and are under evaluation for clinical safety and efficacy.21-23 A selective inhibitor termed STI-571 (Novartis, Basel, Switzerland) is undergoing clinical trials for bcr-abl+ diseases and has shown antileukemic activity with limited toxicity. However, though the cellular targets of this inhibitor are known, the mechanism of its antileukemic and apoptotic effects are not completely understood. In this study, the effects of bcr-abl expression and STI-571–mediated inhibition on signaling and survival pathways were investigated. Findings suggest that bcr-abl activates Stat-5 and increases bcl-xL expression, but, because of the down-regulation of other key signaling proteins, cytokine-supported cell survival is abrogated, resulting in apoptosis. The state of differentiation/maturation of bcr-abl+leukemic cells and the chronic activation of downstream signaling cascades may determine the efficacy of STI-571 and the ability of exogenous factors to rescue treated cells from bcr-abl inhibition.
Materials and methods
Cell lines, antibodies, growth factors, cytokines, and inhibitors
The IL-3/GM-CSF–dependent Mo7e megakaryocytic cell line and the bcr-abl+ IL-3/GM-CSF–independent Mo7e derivative, MBA (MBA.117) provided by Dr R. Kurzrock (M. D. Anderson Cancer Center) were grown and maintained in RPMI 1640 medium supplemented with 10% fetal calf serum and 200 U/mL GM-CSF (Amgen, Thousand Oaks, CA). Both bcr-abl+ (K562, KBM5, BV-173) and bcr-abl− (KG-1, TF-1) leukemic cell lines, provided by Dr Z. Estrov, were maintained in RPMI 1640 with 10% fetal calf serum, the latter in the presence of GM-CSF, as described above. Cells were maintained at a density of less than 107 cells/mL. A tumor necrosis factor-sensitive ME-180 cervical carcinoma cell line24 was also used as a control in these studies and was grown and maintained as previously described.25
STI-571 was provided by Dr E. Buchdunger (Novartis) and was prepared as a 5-mM stock solution in dimethyl sulfoxide (DMSO). Stock solution was diluted in cell culture media and added directly to cells with no more than 0.2% final DMSO concentration.
Antibodies used in these studies include PARP (Boehringer-Mannheim, Indianapolis, IN), phosphotyrosine, phospho-Jak-2, phospho-Stat-5 (UBI, Lake Placid, NY), Stat-1 and phospho-Stat-1 (Geneka, Montreal, QC, Canada), PTP1B and c-abl (Oncogene Sciences, Boston, MA), bcl-xL (Santa Cruz Biotechnology, Santa Cruz, CA), mitogen-activated protein kinase (MAPK) and phospho-MAPK (Promega, Madison, WI), Akt and phospho-Akt (New England Biolabs, Beverly, MA), and actin (Sigma, St Louis, MO). IL-3/GM-CSF/IL-5 receptor common β-chain antibodies were obtained from UBI and Santa Cruz Biotechnology. Polyclonal anti–Stat-5 (a/b) was kindly provided by Dr Robert Kirken (University of Texas Health Science Center, Houston).
Wortmannin and LY29002 (Calbiochem, San Diego, CA) were also used in these studies and were prepared as stock solutions in DMSO. Recombinant IL-3 was purchased from R&D Systems (Minneapolis, MN). Interferon α (IFN-α) was provided by Hoffman-La Roche (Nutley, NJ). Clinical grade GM-CSF was obtained from Amgen.
Apoptosis and cell survival measurement
Analysis of signal transduction
Cell signal activation was analyzed by immunoblotting equal protein cell lysates (bicinchoninic acid protein assay; Pierce Chemical, Rockford, IL) with antibodies against phosphorylated (activated) forms of signaling intermediates. Blots were subsequently stripped of primary antibody24 and reprobed with antibodies that recognize protein domains independent of the state of activation or phosphorylation. All immunoblots were developed with secondary antibodies and enhanced chemiluminescence (ECL) reagent (Amersham, Piscataway, NJ).
Immunoprecipitation of IL-3/5/GM-CSF receptor common β-chain
Common β-chain was analyzed by immunoprecipitation and immunoblotting as previously described.25 Briefly, equal protein cell lysates (150-400 μg in lysis buffer)25 were subjected to β-chain immunoprecipitation (2 μg; Santa Cruz Biotechnology) with Protein G (Sigma), washed extensively, and resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Resolved precipitate was immunoblotted with anti–β-chain (Santa Cruz) and detected with secondary antibody and ECL reagent.
Detection of Stat-5 activation by electrophoretic mobility shift assay
Consensus Stat-5 DNA binding oligomers (β-casein or mutated sequences) were obtained from Santa Cruz Biotechnology and radiolabeled to 5 × 106 cpm/pmol DNA. Nuclear protein was extracted from MBA cells and incubated with radiolabeled DNA, as previously described.27 DNA–protein complexes were analyzed by electrophoretic mobility shift assay (EMSA) on native polyacrylamide gels. The gels were dried and exposed to x-ray film, and radioactive signals were quantitated by Phosphorimager (Molecular Dynamics, Sunnyvale, CA).
Results
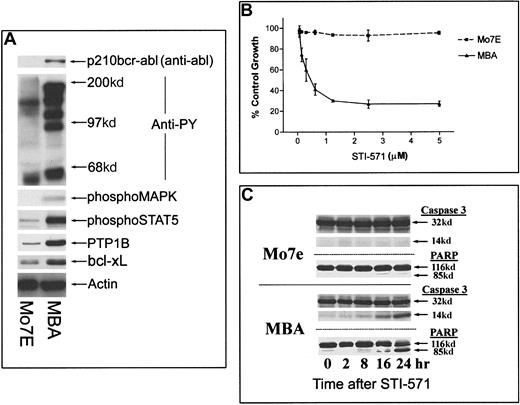
The effect of bcr-abl expression on signaling cascades and cellular sensitivity to STI-571 was examined in Mo7e megakaryocytic cells cultured in the presence of exogenous GM-CSF (500 U/mL). As shown in Figure 1A, bcr-abl expression in Mo7e cells (MBA) correlated with increased tyrosine phosphorylation, detectable activation of the MAP kinase cascade, increased Stat-5 phosphorylation, and increased levels of both bcl-xL and PTP1B. By MTT assay (Figure 1B) and apoptotic characteristics, including caspase 3 activation and PARP cleavage (Figure 1C), bcr-abl+ MBA cells underwent time-dependent apoptosis in the presence of STI-571, whereas cytokine-dependent Mo7e cell survival was not affected by inhibitor. Exogenous GM-CSF (or IL-3) did not affect the apoptotic activity of STI-571 (see below). Parallel studies of other bcr-abl+ (KBM5, BV-173, K562) and bcr-abl− (KG-1, Daudi) leukemic cell lines and epithelial tumor cells (ME-180) demonstrated that STI-571 reduced tyrosine phosphorylation and mediated dose-dependent caspase activation and apoptosis in bcr-abl+ but not bcr-abl− cells (results not shown).
Effect of bcr-abl expression and inhibition on Mo7e cell signaling and apoptosis.
(A) Mo7e cells and Mo7e cells expressing bcr-abl (MBA) were grown in the continual presence of 200 U/mL GM-CSF. Equal-density cultures were harvested, and lysates were examined for distinctions in phosphotyrosine levels, activated signaling cascades, and antiapoptotic proteins. For the detection of bcr-abl, equal-protein aliquots (200 μg) from Mo7e or MBA cell lysates were immunoprecipitated with anti-bcr and immunoblotted with anti-abl. The remaining blots were derived from cell lysates (40 μg), prepared as described in “Materials and methods.” (B) Mo7e or MBA cells were treated with STI-571 (at the indicated concentrations) for 24 hours before cell growth and survival were estimated by MTT staining. Results represent the average ± SEM of 4 determinations. (C) Induction of apoptosis was determined by measuring caspase 3 activation (appearance of a 14-kd band) and PARP cleavage (85-kd band) in Mo7e or MBA cells after incubation with STI-571 for 0 to 24 hours. Similar results were obtained in experiments in which GM-CSF (200 U/mL) or IL-3 (100 ng/mL) was included in the cell culture media (data not shown).
Effect of bcr-abl expression and inhibition on Mo7e cell signaling and apoptosis.
(A) Mo7e cells and Mo7e cells expressing bcr-abl (MBA) were grown in the continual presence of 200 U/mL GM-CSF. Equal-density cultures were harvested, and lysates were examined for distinctions in phosphotyrosine levels, activated signaling cascades, and antiapoptotic proteins. For the detection of bcr-abl, equal-protein aliquots (200 μg) from Mo7e or MBA cell lysates were immunoprecipitated with anti-bcr and immunoblotted with anti-abl. The remaining blots were derived from cell lysates (40 μg), prepared as described in “Materials and methods.” (B) Mo7e or MBA cells were treated with STI-571 (at the indicated concentrations) for 24 hours before cell growth and survival were estimated by MTT staining. Results represent the average ± SEM of 4 determinations. (C) Induction of apoptosis was determined by measuring caspase 3 activation (appearance of a 14-kd band) and PARP cleavage (85-kd band) in Mo7e or MBA cells after incubation with STI-571 for 0 to 24 hours. Similar results were obtained in experiments in which GM-CSF (200 U/mL) or IL-3 (100 ng/mL) was included in the cell culture media (data not shown).
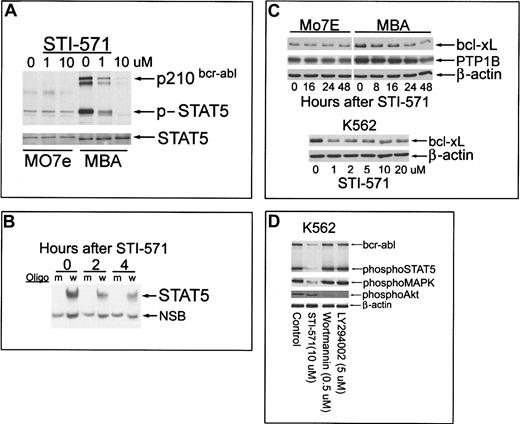
Inhibition of bcr-abl and Stat-5 activation after incubation with STI-571 were examined in bcr-abl+ and bcr-abl−Mo7e cells. For this dual analysis, an antibody directed against activated Stat-5 was used that also detected activated bcr-abl, as previously described by other investigators.20 As shown in Figure 2A, short-term (60-minute) incubation with STI-571 resulted in concomitant reduction in tyrosine phosphorylation of Stat-5 and bcr-abl in MBA cells. Stat-5 activation in Mo7e cells (incubated with GM-CSF or IL-3) was not affected by kinase inhibitor (Figure 4). Although the MAPK cascade was only modestly activated in MBA cells (Figure 1A), kinase inhibitor reduced its activation (data not shown), suggesting that both MAPK and Stat-5 activation reside downstream of bcr-abl. The effect of STI-571 on nuclear translocation/DNA binding of Stat-5 in MBA cells was also examined by EMSA. Stat-5 binding (Figure 2B) was reduced by more than 40% within 2 to 4 hours of STI-571 (1 μM) incubation. These results demonstrate that both Stat-5 phosphorylation and nuclear translocation are inhibited by STI-571 in MBA cells.
Effect of STI-571 on bcr-abl phosphorylation, Stat-5 activation, bcl-xL, and PTP1B expression in Mo7e and MBA cells; comparison with other kinase inhibitors in bcr-abl+K562 cells.
(A) Mo7e or MBA cells were cultured in the presence of GM-CSF (200 U/mL) and treated with 0, 1, or 10 μM STI-571 for 60 minutes before equal-protein (20 μg) cell lysates were analyzed for Stat-5 and bcr-abl tyrosine phosphorylation by immunoblotting (anti-phospho–Stat-5 recognizes tyrosine-phosphorylated bcr-abl). After phosphoprotein detection, the blot was stripped and reprobed with anti–Stat-5 (bottom). (B) MBA cells were incubated with 1 μM STI-571 for 0, 2, or 4 hours before cells were harvested and nuclear proteins extracted (see “Materials and methods”). Consensus Stat-5 DNA binding oligomers (w, wild-type; m, mutant sequence; Santa Cruz Biotechnology) were radiolabeled to 5 × 106 cpm/pmol DNA and incubated with 5 μg nuclear protein from control or STI-571–treated cells. DNA–protein complexes were resolved by EMSA on native polyacrylamide gels (6.5%). Arrows depict Stat-5–complex and nonspecific binding complexes (NSB). Radioactivity in the Stat-5–DNA complex was quantitated by PhosphorImager. (C) Mo7e or MBA cells were treated with 1 μM STI-571 for the interval indicated before cell lysates were harvested and immunoblotted for bcl-xL, PTP1B, or actin as a protein-loading control (top). K562 cells were incubated with the indicated concentration of STI-571 for 16 hours before equal-protein cell lysates were analyzed for bcl-xL levels or β-actin as a protein loading control (bottom). (D) K562 cells were treated as indicated for 60 minutes before cell lysates were analyzed for altered signaling processes by immunoblotting. Equal-protein lysates (20 μg) were sequentially blotted with phospho–Stat-5, phospho-MAPK, and phospho-Akt or β-actin as a protein-loading control.
Effect of STI-571 on bcr-abl phosphorylation, Stat-5 activation, bcl-xL, and PTP1B expression in Mo7e and MBA cells; comparison with other kinase inhibitors in bcr-abl+K562 cells.
(A) Mo7e or MBA cells were cultured in the presence of GM-CSF (200 U/mL) and treated with 0, 1, or 10 μM STI-571 for 60 minutes before equal-protein (20 μg) cell lysates were analyzed for Stat-5 and bcr-abl tyrosine phosphorylation by immunoblotting (anti-phospho–Stat-5 recognizes tyrosine-phosphorylated bcr-abl). After phosphoprotein detection, the blot was stripped and reprobed with anti–Stat-5 (bottom). (B) MBA cells were incubated with 1 μM STI-571 for 0, 2, or 4 hours before cells were harvested and nuclear proteins extracted (see “Materials and methods”). Consensus Stat-5 DNA binding oligomers (w, wild-type; m, mutant sequence; Santa Cruz Biotechnology) were radiolabeled to 5 × 106 cpm/pmol DNA and incubated with 5 μg nuclear protein from control or STI-571–treated cells. DNA–protein complexes were resolved by EMSA on native polyacrylamide gels (6.5%). Arrows depict Stat-5–complex and nonspecific binding complexes (NSB). Radioactivity in the Stat-5–DNA complex was quantitated by PhosphorImager. (C) Mo7e or MBA cells were treated with 1 μM STI-571 for the interval indicated before cell lysates were harvested and immunoblotted for bcl-xL, PTP1B, or actin as a protein-loading control (top). K562 cells were incubated with the indicated concentration of STI-571 for 16 hours before equal-protein cell lysates were analyzed for bcl-xL levels or β-actin as a protein loading control (bottom). (D) K562 cells were treated as indicated for 60 minutes before cell lysates were analyzed for altered signaling processes by immunoblotting. Equal-protein lysates (20 μg) were sequentially blotted with phospho–Stat-5, phospho-MAPK, and phospho-Akt or β-actin as a protein-loading control.
Bcr-abl expression increases the levels of several genes, including antiapoptotic proteins (bcl-xL) and regulators of tyrosine phosphorylation (PTP1B).13,18,19,28-30 Induction of these genes may be dependent on bcr-abl tyrosine kinase activity or Stat-5 activation, as demonstrated in studies of hematopoietic cytokine signaling.31 To determine whether bcr-abl inhibition alters the level of these proteins, STI-571–treated bcr-abl+ and bcr-abl− Mo7e cells were immunoblotted for bcl-xL and PTP1B. As shown in Figure 2C, STI-571 caused a rapid decrease in bcl-xL levels in MBA cells (60% reduced after 8 hours) but did not alter basal expression of this protein in GM-CSF–treated, bcr-abl–independent Mo7e cells. Kinase inhibitor reduced PTP1B levels (approximately 2-fold reduction after 48 hours) in MBA (not Mo7e) cells, but only after the onset of apoptosis. STI-571 had similar effects on bcl-xL in K562 cells, with 1 μM providing effective bcl-xL suppression that was not further reduced by higher STI-571 concentrations (Figure2C). PTP1B was also reduced in K562 cells but, as described above, only after the onset of apoptosis (data not shown). Kinase inhibition reduced antiapoptotic protein expression but had significantly less capacity to reduce PTP1B levels. Because PTP1B overexpression in bcr-abl+ cells has been linked to the inhibition of ras signaling,30 the longstanding expression of PTP1B in STI-571–treated cells may contribute to persistent inhibition of the ras pathway.
In Ph+ K562 cells, STI-571 reduced bcr-abl, Stat-5, and MAPK activation (similar to responses in MBA cells) but did not inhibit Akt phosphorylation (Figure 2D). Both an irreversible (wortmannin) and a reversible (LY294002) inhibitor of phosphatidylinositol-3′-kinase (PI3K) suppressed Akt phosphorylation in K562 cells without affecting Stat-5 or MAPK activation. However, neither PI3K inhibitor induced apoptosis in K562 cells, suggesting only minimal dependence of these cells on the PI3K–Akt axis for survival (data not shown). Based on these observations and on earlier reports of Stat-5 involvement in cytokine signaling,13,18-20,28 29 the results predict a significant role for the interruption of Stat-5 activation (and possibly other pathways) in the apoptotic actions of STI-571 on bcr-abl+ cells.
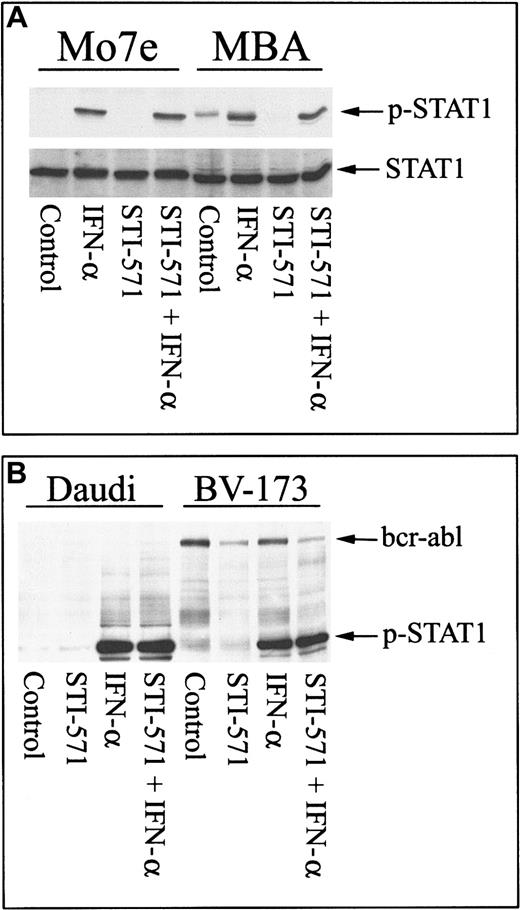
Other cytokines are known to activate Stat proteins and to require tyrosine phosphorylation to initiate signal transduction.31 To determine whether the phosphorylation of other Stat proteins was affected by STI-571, Stat-1 phosphorylation after IFN-α incubation was examined in MBA and Mo7e cells. As shown in Figure 3A, IFN-α induced tyrosine phosphorylation of Stat-1 in Mo7e cells in the presence or absence of STI-571. In MBA cells, bcr-abl caused constitutive phosphorylation of Stat-1 (as previously described13 20), and STI-571 blocked bcr-abl–stimulated Stat-1 activation. As described for Mo7e cells, IFN-α was able to activate Stat-1 in MBA cells in the presence or absence of STI-571. Similar results were obtained in bcr-abl+ BV-173 cells, where STI-571 significantly inhibited bcr-abl tyrosine phosphorylation but did not prohibit IFN-α–mediated activation of Stat-1 (Figure 3B). Similarly, STI-571 did not block IFN-α signaling in Daudi cells. These results suggest that other cytokine signaling modules are not affected by STI-571.
STI-571 does not inhibit other Jak/Stat signaling cascades.
(A) Mo7e or MBA cells were pretreated with nothing or STI-571 (5 μM) for 30 minutes and subsequently treated with IFN-α (500 U/mL; 30 minutes) before cell lysates were collected and analyzed for Stat-1 activation by immunoblotting with phospho–Stat-1–specific antibody. The blot was stripped and reprobed with anti–Stat-1 as a control. (B) Daudi (left) or BV-173 (right) cells were treated with 5 μM STI-571 alone or subsequently treated with IFN-α, as described above, before cell lysates were analyzed for Stat-1 activation with anti–p-Stat-1. Anti–p-Stat-1 also recognizes activated bcr-abl (as described in Figure 2) and can be used as a control to demonstrate that STI-571 reduces bcr-abl activation without affecting IFN-α–mediated Stat-1 phosphorylation in BV-173 cells.
STI-571 does not inhibit other Jak/Stat signaling cascades.
(A) Mo7e or MBA cells were pretreated with nothing or STI-571 (5 μM) for 30 minutes and subsequently treated with IFN-α (500 U/mL; 30 minutes) before cell lysates were collected and analyzed for Stat-1 activation by immunoblotting with phospho–Stat-1–specific antibody. The blot was stripped and reprobed with anti–Stat-1 as a control. (B) Daudi (left) or BV-173 (right) cells were treated with 5 μM STI-571 alone or subsequently treated with IFN-α, as described above, before cell lysates were analyzed for Stat-1 activation with anti–p-Stat-1. Anti–p-Stat-1 also recognizes activated bcr-abl (as described in Figure 2) and can be used as a control to demonstrate that STI-571 reduces bcr-abl activation without affecting IFN-α–mediated Stat-1 phosphorylation in BV-173 cells.
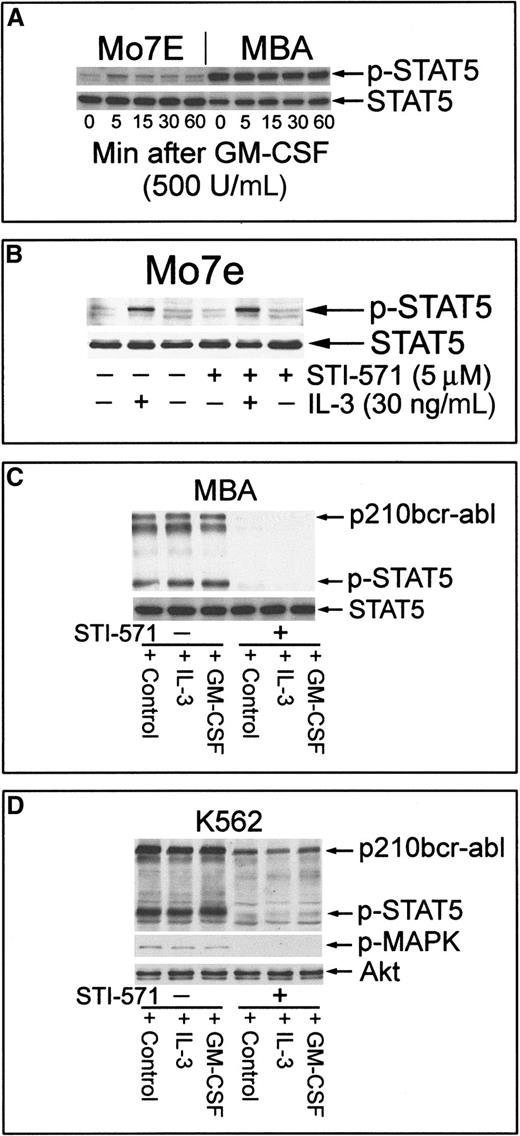
IL-3/GM-CSF plays a major role in the proliferation and sustained survival of hematopoietic stem cells through the activation of receptor-initiated Jak/Stat cascades.17,31 32 Expression of bcr-abl results in growth factor–cytokine independence, suggesting similar downstream effects of bcr-abl expression and IL-3–GM-CSF signaling. As shown in Figure 4A, GM-CSF was able to rapidly activate Stat-5 in Mo7e cells but had no apparent effect on constitutively activated Stat-5 in MBA cells. Further, STI-571 did not affect IL-3–mediated Stat-5 activation in Mo7e cells (Figure 4B), and exogenous IL-3 (or GM-CSF) did not protect MBA cells from STI-571–mediated cell death (see below). To determine whether these cytokines were able to induce phosphorylation of Stat-5 in bcr-abl–inhibited cells, MBA cells were pretreated with kinase inhibitor and subsequently stimulated with IL-3 or GM-CSF before Stat-5 activation was examined. As shown in Figure 4C, the inhibition of bcr-abl reduced Stat-5 activation levels, and the challenge with cytokines failed to reactivate Stat-5 phosphorylation. Similar results were obtained in K562 cells, in which bcr-abl inhibition blocked both Stat-5 and MAPK activation (Figure 4D). Failure of cytokines to reactivate Stat-5 phosphorylation may underlie their inability to provide apoptotic protection after bcr-abl inhibition with STI-571.
Induction of Stat-5 phosphorylation by GM-CSF or IL-3 in bcr-abl− but not bcr-abl+ Mo7e cells.
Exogenous cytokines cannot reactivate Stat-5 in STI-571–treated MBA or K562 cells. (A) Mo7e or MBA cells were cultured in the absence of GM-CSF for 6 hours before the readdition of GM-CSF to their culture media for 0 to 60 minutes. Cells were harvested and equal-protein aliquots (30 μg) were immunoblotted with phospho–Stat-5. After stripping primary antibody, Stat-5 levels were determined by immunoblotting. (B) Mo7e cells were incubated with IL-3 for 30 minutes or pre-incubated with STI-571 for 60 minutes before IL-3. Cell lysates were analyzed for Stat-5 activation and Stat-5 levels by immunoblotting. STI-571 did not affect IL-3–mediated activation of Stat-5 in Mo7e cells. (C) MBA cells were left untreated (lanes 1-3) or were treated with 5 μM STI-571 (lanes 4-6) for 60 minutes before treatment with 500 U/mL GM-CSF or 30 ng/mL recombinant IL-3 (R&D Systems) for 15 minutes. Cell lysates were analyzed for bcr-abl and Stat-5 activation (phospho–Stat-5) and were compared to Stat-5 protein levels by sequential immunoblotting. (D) K562 cells were treated as described above and analyzed for changes in Stat-5, bcr-abl, and MAPK activation by immunoblotting (phospho–Stat-5, phospho-MAPK). Akt protein levels were unchanged by treatment and were used as a protein-loading control.
Induction of Stat-5 phosphorylation by GM-CSF or IL-3 in bcr-abl− but not bcr-abl+ Mo7e cells.
Exogenous cytokines cannot reactivate Stat-5 in STI-571–treated MBA or K562 cells. (A) Mo7e or MBA cells were cultured in the absence of GM-CSF for 6 hours before the readdition of GM-CSF to their culture media for 0 to 60 minutes. Cells were harvested and equal-protein aliquots (30 μg) were immunoblotted with phospho–Stat-5. After stripping primary antibody, Stat-5 levels were determined by immunoblotting. (B) Mo7e cells were incubated with IL-3 for 30 minutes or pre-incubated with STI-571 for 60 minutes before IL-3. Cell lysates were analyzed for Stat-5 activation and Stat-5 levels by immunoblotting. STI-571 did not affect IL-3–mediated activation of Stat-5 in Mo7e cells. (C) MBA cells were left untreated (lanes 1-3) or were treated with 5 μM STI-571 (lanes 4-6) for 60 minutes before treatment with 500 U/mL GM-CSF or 30 ng/mL recombinant IL-3 (R&D Systems) for 15 minutes. Cell lysates were analyzed for bcr-abl and Stat-5 activation (phospho–Stat-5) and were compared to Stat-5 protein levels by sequential immunoblotting. (D) K562 cells were treated as described above and analyzed for changes in Stat-5, bcr-abl, and MAPK activation by immunoblotting (phospho–Stat-5, phospho-MAPK). Akt protein levels were unchanged by treatment and were used as a protein-loading control.
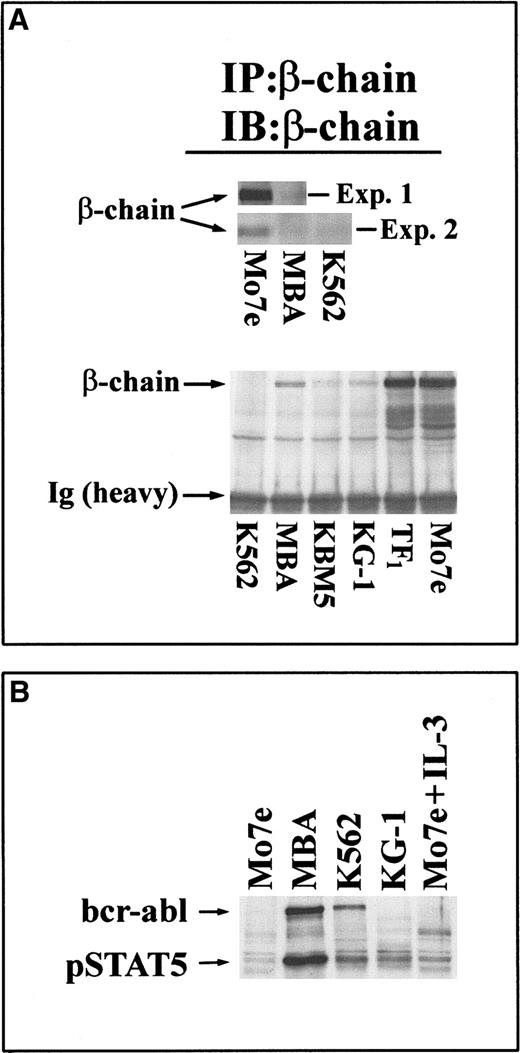
To explore the potential mechanism of altered cytokine signaling in bcr-abl+ Mo7e cells, expression of a key component of IL-3/GM-CSF signaling—the common β-chain of IL-3/GM-CSF receptor—was examined. As shown in Figure5A, expression of the β-chain was significantly lower in bcr-abl+ MBA cells when compared to Mo7e cells (in 2 independent experiments). Similarly, the β-chain was not detected in K562 cells. Analysis of a panel of IL-3/GM-CSF–responsive/dependent cell lines and bcr-abl+or bcr-abl− leukemic cells demonstrated marked distinctions between these cell lines in their β-chain expression levels (Figure 5B). Cells expressing bcr-abl (MBA, K562, KBM5) had significantly lower levels of β-chain than IL-3–dependent TF1 or Mo7e cells. Interestingly, bcr-abl−KG-1 cells also had minimal β-chain expression levels and, though bcr-abl−, had constitutively activated Stat-5 protein (Figure 5B). These results suggest that the down-regulated expression of β-chain in leukemic cells may contribute to their altered cytokine–growth factor responsiveness.
Reduced expression of the IL-3/GM-CSF receptor β-chain subunit in bcr-abl–expressing/Stat-5–activated cells.
(A, top) The β-chain of IL-3/GM-CSF receptor was immunoprecipitated from equal-protein extracts (200 μg) of Mo7e or MBA cells (experiment 1) or from these cell extracts and K562 cells (experiment 2). Immunoprecipitates were immunoblotted with β-chain antibody. The migration of the 130-kd β-chain band is shown. (bottom) Equal-protein lysates (400 μg) from bcr-abl+ (K562, MBA, KBM5) and bcr-abl− (KG-1, TF1, Mo7e) cells were subjected to receptor β-chain immunoprecipitation and immunoblotting with anti–β-chain. Arrows depict the 130-kd β-chain band and the immunoglobulin heavy-chain band. (B) Stat-5 activation in leukemic cells. Leukemic cell lines were examined for activated Stat-5 by immunoblotting equal-protein cell lysates (30 μg) with anti–phospho–Stat-5. All cells were cultured in the absence of exogenous cytokines for 8 hours before extracts were prepared. As a control (last lane), Mo7e cells were treated with 30 ng/mL IL-3 for 15 minutes before harvesting. The relative migrations of p210bcr-abl and 95 kd Stat-5 are depicted.
Reduced expression of the IL-3/GM-CSF receptor β-chain subunit in bcr-abl–expressing/Stat-5–activated cells.
(A, top) The β-chain of IL-3/GM-CSF receptor was immunoprecipitated from equal-protein extracts (200 μg) of Mo7e or MBA cells (experiment 1) or from these cell extracts and K562 cells (experiment 2). Immunoprecipitates were immunoblotted with β-chain antibody. The migration of the 130-kd β-chain band is shown. (bottom) Equal-protein lysates (400 μg) from bcr-abl+ (K562, MBA, KBM5) and bcr-abl− (KG-1, TF1, Mo7e) cells were subjected to receptor β-chain immunoprecipitation and immunoblotting with anti–β-chain. Arrows depict the 130-kd β-chain band and the immunoglobulin heavy-chain band. (B) Stat-5 activation in leukemic cells. Leukemic cell lines were examined for activated Stat-5 by immunoblotting equal-protein cell lysates (30 μg) with anti–phospho–Stat-5. All cells were cultured in the absence of exogenous cytokines for 8 hours before extracts were prepared. As a control (last lane), Mo7e cells were treated with 30 ng/mL IL-3 for 15 minutes before harvesting. The relative migrations of p210bcr-abl and 95 kd Stat-5 are depicted.
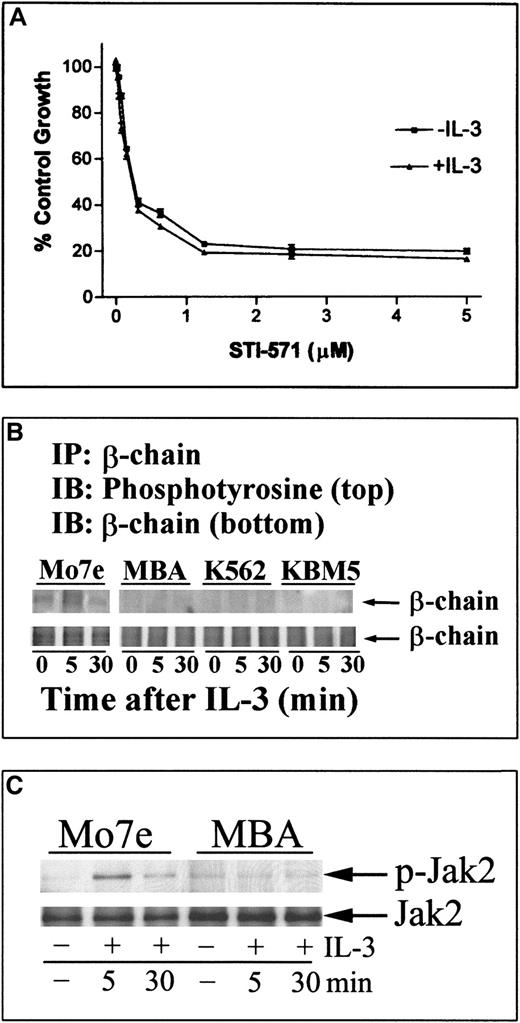
In support of this hypothesis, IL-3 was unable to induce tyrosine phosphorylation of β-chain or to activate downstream signaling (Jak-2) in MBA cells (Figure 6). Further, IL-3 did not reduce STI-571–mediated cell death (Figure 6) in MBA cells. Similar results were obtained in other human leukemic cell lines with constitutively activated Stat-5 cascades (results not shown). Together, these results suggest that bcr-abl (or other mechanisms of constitutive Stat activation) in human leukemic cells reduces responsiveness to exogenous cytokines by down-regulating receptor proximal signaling proteins. Down-regulation of the IL-3/GM-CSF receptor β-chain may be one of the critical events that prevents reactivation of Stat-5 signaling and recovery from STI-571–driven cell death.
IL-3 does not alter cellular sensitivity to STI-571 and fails to activate β-chain or Jak-2 tyrosine phosphorylation in bcr-abl–expressing cells.
(A) MBA cells were incubated with STI-571 in normal growth media at the dose level indicated or were pretreated and maintained in IL-3 (30 ng/mL) before STI-571 incubation. After 24 hours, cells were incubated with MTT reagent to estimate changes in cell growth and survival (Figure 1). Each point represents the average ± SEM of 4 determinations. In apoptosis studies, IL-3 did not alter STI-571–mediated caspase 3 activation in MBA cells (results not shown). Similar results were obtained in K562 cells. (B) Mo7e, MBA, K562, or KBM5 cells were cultured in media without exogenous cytokines for 8 hours and subsequently incubated with IL-3 (30 ng/mL) for 5 or 30 minutes before harvesting. β-Chain was immunoprecipitated from 150 μg protein lysate and immunoblotted for phosphotyrosine (top) or β-chain (bottom). (C) Mo7e (left) or MBA (right) cells were grown in the absence of cytokine (as described above) before the addition of IL-3 for 5 or 30 minutes. Jak-2 activation was determined in equal protein cell lysates (30 μg) by immunoblotting with phosphospecific Jak-2 (p-Jak-2), and relative Jak-2 protein levels were determined in stripped blots with anti–Jak-2. Similar results were obtained in immunoprecipitation/immunoblotting analysis of Jak-2 tyrosine phosphorylation (data not shown). Migration of the 130-kd Jak-2 protein is shown.
IL-3 does not alter cellular sensitivity to STI-571 and fails to activate β-chain or Jak-2 tyrosine phosphorylation in bcr-abl–expressing cells.
(A) MBA cells were incubated with STI-571 in normal growth media at the dose level indicated or were pretreated and maintained in IL-3 (30 ng/mL) before STI-571 incubation. After 24 hours, cells were incubated with MTT reagent to estimate changes in cell growth and survival (Figure 1). Each point represents the average ± SEM of 4 determinations. In apoptosis studies, IL-3 did not alter STI-571–mediated caspase 3 activation in MBA cells (results not shown). Similar results were obtained in K562 cells. (B) Mo7e, MBA, K562, or KBM5 cells were cultured in media without exogenous cytokines for 8 hours and subsequently incubated with IL-3 (30 ng/mL) for 5 or 30 minutes before harvesting. β-Chain was immunoprecipitated from 150 μg protein lysate and immunoblotted for phosphotyrosine (top) or β-chain (bottom). (C) Mo7e (left) or MBA (right) cells were grown in the absence of cytokine (as described above) before the addition of IL-3 for 5 or 30 minutes. Jak-2 activation was determined in equal protein cell lysates (30 μg) by immunoblotting with phosphospecific Jak-2 (p-Jak-2), and relative Jak-2 protein levels were determined in stripped blots with anti–Jak-2. Similar results were obtained in immunoprecipitation/immunoblotting analysis of Jak-2 tyrosine phosphorylation (data not shown). Migration of the 130-kd Jak-2 protein is shown.
Discussion
Several studies have demonstrated the strong influence of bcr-abl expression on signaling events in leukemic and other cells.7,13,14,18,19,28,29 Most recent information suggests that bcr-abl expression results in the activation of similar cascades shared by cytokines that stimulate proliferation or that increase survival in cells of hematopoietic origin.18,19,28 In this regard, the activation of Stat-5 plays a major role in normal hematopoiesis, and aberrant regulation is associated with leukemia and other diseases.33 34 In the present report, bcr-abl expression results in clear alterations in signal transduction and gene expression profiles that are associated with cytokine independence. However, bcr-abl expression also renders these cells vulnerable to apoptosis upon bcr-abl–tyrosine kinase inhibition. Understanding the mechanism of apoptotic vulnerability is essential to the clinical prediction of STI-571 efficacy and is the focus of this report.
Bcr-abl expression in Mo7e cells resulted in increased tyrosine phosphorylation and constitutive activation of Stat-5 tyrosine phosphorylation, whereas only minimal changes in MAP kinase activation were measurable. In addition, when compared to epithelial tumors, PI3′-kinase activity and Akt phosphorylation were only minimally detectable in bcr-abl+ cells (data not shown). These observations are supported by our studies with inhibitors of this survival axis that did not increase cellular sensitivity to STI-571 or have major apoptotic effects on bcr-abl+ cells. These results contrast with those of recent reports describing the involvement of this cascade (PI3K, Akt, BAD) in leukemogenesis and bcr-abl transformation, which predict that inhibition of this axis would increase apoptotic responsiveness to STI-571.14,35,36 Because other leukemic models predict the involvement of both BAD-dependent and -independent survival influences by bcr-abl,14 our results suggest that Akt activation may be independent of direct bcr-abl regulation in K562 and MBA cells and may not be a dominant target for STI-571–mediated inhibition. Other upstream regulatory distinctions (PTEN/MMAC mutation/deletion) and downstream targets of PI3K (mTOR) may exist and influence the contribution of PI3K to bcr-abl signaling and response to STI-571 in leukemic cell targets.37 38
Autophosphorylation of bcr-abl recruits adaptor molecules, such as Grb2, that contribute to the activation of ras and other downstream signals.9,12,15,16 However, tyrosine phosphorylation at this and other sites may be regulated by the induction of PTP1B and other tyrosine phosphatases (SHP-2) that are coexpressed in bcr-abl+ cells.30,39 Our studies confirm the increased expression of PTP1B in bcr-abl–expressing Mo7e cells (and other bcr-abl+ populations), and, though inhibition of bcr-abl blocked Stat-5 activation and reduced bcl-xLlevels, PTP1B levels remained largely unchanged before the onset of apoptosis (Figure 2). This may be due to the relatively long t1/2 of PTP1B, as measured in other cell models.40 However, the sustained expression of PTP1B in STI-571–treated, bcr-abl–expressing cells may prevent the activation of bcr-abl signaling through reduced recruitment of SH2-domain interactive proteins. The role of PTP1B in bcr-abl+ cells and its contribution to STI-571–mediated cell death is under investigation. PTP1B may play a role in regulating other cell-signaling cascades (IGF-1 and insulin).41 42
Bcr-abl promotes the increased expression of bcl-xL through the activation of Stat proteins, as demonstrated in studies of IL-3, GM-CSF, and other cytokine-mediated signaling pathways that regulate Stat phosphorylation.28,29,32 As expected, bcr-abl inhibition reduced bcl-xL expression levels, lowering apoptotic thresholds and engaging caspase 3 activation (Figures 1, 2) in bcr-abl+ cells. Stat-5 activated by mechanisms independent of bcr-abl expression (exogenous growth factors such as GM-CSF or IL-3 [Mo7e] or other leukemogenic mechanisms [KG-1 cells]) were not affected by STI-571, and kinase inhibitor did not induce apoptosis in these cells. These results support earlier observations of a role for bcr-abl–mediated activation of Stat-5 in chronic myelogenous leukemia (CML) cells and the selective inactivation of Stat-5 signaling by STI-571 in bcr-abl+cells.13,19,28,29 The mechanism of bcr-abl–mediated Stat-5 tyrosine phosphorylation is unknown, and, though earlier studies suggested the involvement of Jak-2 in bcr-abl signaling in MBA3.16 cells,43 the coexpression of Jak-2–dominant-negative with bcr-abl did not alter Stat-5 activation in other cell models.44 The MBA cells used in the present study represent a distinct MBA clone (MBA.1) that expresses autocrine growth factors but does not appear to be dependent on these factors for sustained growth or survival.17 In support of cytokine independence in MBA.1 cells, Jak-2 kinase inhibitor (AG490; up to 80 μM) had minimal effects on MBA.1 cells but induced apoptosis in Mo7e cells in the presence of IL-3 or GM-CSF (data not shown). Although earlier reports of constitutive Jak-2/β-chain phosphorylation in MBA3.16 cells suggested their involvement in bcr-abl signaling, the effects of exogenous cytokines on the phosphorylation of these proteins (or on Stat-5 activation) were not examined, and distinctions in β-chain expression levels between Mo7e and MBA3.16 cells were not reported.43 In the present study, cytokines failed to activate Jak-2 (or to induce β-chain phosphorylation) in MBA.1 cells (Figure 6B-C) and other bcr-abl+ cells when analyzed using 2 independent procedures. Dependence of individual clones on autocrine factors for sustained survival may underlie proposed distinctions in the Jak-2/β-chain involvement in bcr-abl signaling reported in MBA cells. In our studies, failure to provide protection from STI-571–mediated apoptosis by IL-3/GM-CSF in MBA.1 and other leukemic cell lines correlates with reduced expression and limited activation of cytokine signaling cascades. Enforced expression of β-chain in MBA cells is being examined to determine its role in the recovery of cytokine-supported survival of STI-571–treated cells.
Recent studies support a role for downstream components of cytokine signaling and transcriptional regulation of bcl-xL and other genes13,18,19,28,29,32 as mediators of survival in STI-571–treated, bcr-abl–expressing cells. Although bcr-abl activates several Stat proteins, there was a distinct defect in the reactivation of some, but not all, of these cascades after STI-571 treatment.44-46 Kinase inhibitor suppressed both Stat-1 and Stat-5 phosphorylation in bcr-abl+ cells. However, only Stat-1 phosphorylation could be reactivated by cytokines (IFN-α) in STI-571–treated cells (Figures 3, 4). Because Stat-1 phosphorylation plays a critical role in IFN-α signaling,45 the cross-inhibition of IFN-α signaling may have restricted therapeutic options in the treatment of CML.47 Therefore, though several Stat proteins can be activated by bcr-abl, inhibition with STI-571 did not induce global restrictions in the reactivation of Stat cascades by exogenous cytokines. This suggests that feedback mechanisms that attenuate global cytokine signaling are not induced in bcr-abl–expressing cells.48,49 However, selective defects in Stat-5 reactivation were evident in several bcr-abl+leukemic or bcr-abl–transduced cells. Persistent Stat-5 activation through bcr-abl expression or other mechanisms (as recently described50) and as detected in KG-1 cells (Figure 5) may be capable of mediated changes in cytokine signaling through β-chain alterations. Constitutive activation of Stat-5 phosphorylation may reduce β-chain expression, or its increased tyrosine phosphorylation43 may target it for degradation, as described for other tyrosine phosphoproteins.51,52 It is important to note that the permanent loss of IL-3 signaling does not occur in murine cells expressing bcr-abl and that inhibition of bcr-abl does not induce cell death in the presence of exogenous IL-3 in murine cell models.13,28,29 Apoptotic protection from STI-571–mediated cell death by IL-3 in murine cells occurs through Stat-5 activation and sustained expression of bcl-xL, results clearly distinct from those provided in this report.28,29 Distinctions between species with regard to protection from STI-571–mediated apoptosis are evident. These distinctions may be related to the presence of 2 murine genes capable of forming a competent IL-3–signaling complex, whereas only a single gene encodes the common β-chain for IL-3/GM-CSF receptor in human cells.53,54 These distinctions may influence the apoptotic response to STI-571 in human versus murine models of leukemia. Because autocrine production of G-CSF and IL-3 varies with the stage or progression of CML,55 changes in β-chain expression may be important in determining the contribution of these cytokines to STI-571 resistance or recovery.
Overall, the studies described here predict that Stat-5 activation and down-regulation of the β-chain are important molecular mediators that regulate in vitro responsiveness to STI-571 and the ability of host cytokines to reduce the antileukemic effects of bcr-abl kinase inhibition. Studies are being conducted on clinical specimens to test this hypothesis.
Supported by National Institutes of Health grant CA-73018.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nicholas J. Donato, Department of Bioimmunotherapy, Box 422, M. D. Anderson Cancer Center, University of Texas, 1515 Holcombe Blvd, Houston, TX 77030; e-mail:ndonato@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal