Abstract
The cyclin-dependent kinase inhibitor p21Waf-1/Cip-1 is expressed at high level during megakaryocyte differentiation, but its precise function remains unknown. In this study, it is confirmed that p21 was expressed at a high level in hypoploid (2N and 4N) and polyploid (at least 8N) human megakaryocytes derived from CD34+ cells. A high expression of p27Kip1, p16, cyclin E, and cyclin D3 was also found in both populations associated with a hypophosphorylated form of retinoblastoma protein, suggesting that the majority of hypoploid and polyploid megakaryocytes are G1-arrested cells. As human megakaryocytes grown in vitro present a defect in their polyploidization, the study switched to the murine model. The modal ploidy of megakaryocytes derived from lineage-negative cells was 32N, and an elevated expression of p21 was found in high-ploidy megakaryocytes. In addition, p21 and p27 were coexpressed in the majority of mature polyploid megakaryocytes. The p21 was detected by immunofluorescence in megakaryocytes derived from p53−/− mice, demonstrating a p53-independent regulation during megakaryocyte differentiation. Megakaryocytopoiesis of p21−/− mice was subsequently studied. No marked abnormality in the ploidy of primary or cultured megakaryocytes was detected. Overexpression of p21 in p21−/− or normal murine megakaryocytes and in human megakaryocytes showed in all these cases a marked inhibition in megakaryocyte polyploidization. In conclusion, while a reciprocal relation is observed between p21 levels in megakaryocytes and the cycling state of the cells, p21 is not essential for the determination of the ploidy profile in normal megakaryocytes in vivo. However, high levels of its expression in cultured megakaryocytes arrest the endomitotic cell cycle.
Introduction
Terminal megakaryocyte differentiation has many original aspects.1,2 When the promegakaryoblast begins to synthesize specific platelet proteins, megakaryocytic cells switch from cell division (mitosis) to polyploidization (endomitosis). At the end of polyploidization, the megakaryocyte increases synthesis of specific platelet proteins and the size of its cytoplasm, leading to enhanced production of platelets.3 Finally, megakaryocytes form proplatelets by extending long-branched pseudopods that will give rise to platelets by fragmentation.4 5
Megakaryocyte polyploidization occurs by an original process called endomitosis, which corresponds to an abortive mitosis in which megakaryocytes skip late mitotic phases, anaphase B, telophase, and cytokinesis.6,7 The endomitotic cell cycle is quite similar to a normal cell cycle but with x successive G1/S/G2/M phases, leading to a 2xN ploidy.6 8 At present, the molecular mechanisms regulating the switch from mitosis to endomitosis are unknown. However, as in all the other cell lineages, cell-cycle exit is associated with terminal differentiation.
The Cip/Kip family of cyclin-dependent kinase (CDK) inhibitors, which includes p21Cip1/Waf1, p27Kip1, and p57Kip2, plays a crucial role in coupling cell-cycle arrest with differentiation.9-14 Members of the Cip/Kip family interact with both cyclins and cyclin-dependent kinases and inhibit all CDK activities. However, their major effects are observed on the cyclin E–CDK2 complex, leading to a block in the G1 phase. Members of this family also have other effects on the cell biology. The p21Cip1/Waf1 molecule, which has been first identified as a p53 target,15,16 has many functions on cell cycle, apoptosis, senescence, and differentiation. The p21 has opposing effects on the cell cycle, being capable of blocking the cell cycle in G1 and also in G2/M.17 More surprisingly, p21 at low concentrations enhances the interaction between cyclin D3 and CDK4 and facilitates G1progression.18,19 The p21 has been also implicated in the polyploidization of different cell models. The p21 induces hepatocyte polyploidization mainly by inhibiting cytokinesis.20 In other cell types, p21 overexpression leads to polyploidization by favoring endoreplication21; however, p21 deficiency may also lead to polyploidy.22
Recently, it has been shown that megakaryocytes express high levels of p21 and p27 during differentiation.23,24 It has been suggested that p21 is implicated in megakaryocyte polyploidization because overexpression of p21 in 2 cell lines with a megakaryocyte phenotype leads to nucleus polylobulation.25,26 In addition, thrombopoietin, the humoral factor that regulates ploidization and platelet production, increases p21 transcription and expression through signal transducer and activator of transcription 5 (Stat5) activation.25
In this study, we have investigated the role of p21 on megakaryocyte polyploidization by studying megakaryocytopoiesis of p21 nullizygote mice (p21−/−). In addition, the effects of p21 overexpression were analyzed in normal and p21−/−megakaryocytes. The results clearly demonstrate that p21 plays an important role in the exit from the endomitotic cell cycle. They suggest that p21 probably couples cell-cycle arrest to terminal differentiation.
Materials and methods
Mice
Homozygote p21−/− mice were a gift from Dr Phil Leder (Boston, MA), and homozygote p53−/− mice were obtained from Dr Françoise Moreau-Gachelin (Paris, France). Because p21−/− mice were backcrossed on a CF1 background, wild-type CF1 mice obtained from Charles River (Saint Aubin les Elbeufs, France) were used as controls. The p53−/− mice were backcrossed on the C57Bl/6 background. Wild-type C57Bl/6 mice were obtained from Iffa Credo (Larbresle, France) and used in indicated experiments.
Cell lines
The UT-7 cell line transduced with c-mpl was grown in alpha–Minimum Eagle Medium (α-ΜΕΜ) (Gibco BRL Life Technologies, Cergy Pontoise, France) with 10% fetal calf serum (Gibco BRL); penicillin/streptomycin/glutamine; and 2 ng/mL human granulocyte-macrophage colony-stimulating factor (GM-CSF) (Immunex, Seattle, WA).27
Antibodies
Phycoerythrin (PE)–murine antihuman CD41, PE–rat antimurine CD62P, anti–CD16/CD32 Fc(III/II), and fluorescein isothiocyanate (FITC)–rat antimurine CD41, all obtained from Pharmingen (San Diego, CA), were used for flow cytometric analysis. Monoclonal antibodies against α, β, and γ tubulins were purchased from Sigma (St Louis, MO). Isotype controls were obtained from Becton Dickinson (Mountain View, CA) and Pharmingen.
The following rat monoclonal antibodies purchased from Pharmingen were used for depletion of lineage-positive cells: TER119, Gr-1, anti-CD11b (or Mac-1), B220, and anti-CD3.
The following antibodies were used for Western blot and immunofluorescence: antihemagglutinin (HA) monoclonal antibody (mAb) (Boehringer Mannheim, Mannheim, Germany); anti–cyclin D3 mAb (Pharmingen); anti–cyclin E mAb (Pharmingen); anti–cyclin E polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA); anti–cyclin A mAb (Pharmingen); anti–cyclin B1 mAb (Pharmingen); anti-p21 mAb (Pharmingen); anti-p21 polyclonal antibody (Calbiochem, Meudon, France); anti-p27 polyclonal antibody (Pharmingen); anti-p16INKb polyclonal antibody (Pharmingen); anti-p57Kip2 polyclonal antibody (Santa Cruz Biotechnology); antiretinoblastoma protein (anti-Rb) mAb (Pharmingen); FITC antihuman Ki-67 mAb (Pharmingen); and an anti–von Willebrand factor (vWF) polyclonal antibody (Dako, Glostrup, Denmark).
For indirect immunofluorescence, donkey tetrahodamine isothiocyanate (TRITC)–labeled antimouse, TRITC-labeled antigoat, or FITC-labeled antirabbit F(ab′)2 fragments (Jackson Immunoresearch, West Grove, PA) were used.
Progenitor cells and platelet isolation
CD34+ cells were obtained either from the bone marrow of healthy patients undergoing hip surgery, with their informed consent, or from aliquots of cytapheresis from the peripheral blood of patients after mobilization. Cells were separated over a Ficoll-metrizoate gradient (Biochrom, Berlin, Germany), and CD34+ cells were purified by immunomagnetic selection (Miltenyi Biotec, Bergisch Gladbach, Germany).
In mice, lineage-negative (lin−) cells were purified after incubation with a mixture of antibodies against lineage antigens (Gr1, TER119, Mac1, B220, and anti-CD3 mAb) and depletion with Dynabeads coupled to a mouse antibody against rat immunoglobulin (Dynal, Oslo, Norway).
Murine platelets were isolated from blood drawn by cardiac puncture and collected into an equal volume of acid dextrose citrate 1/10 diluted in buffered saline-glucose-citrate (BSGC). Plasma rich in platelets was prepared and laid over a 10-mL sepharose 4B column (Pharmacia Biotech, Buckinghamshire, United Kingdom) equilibrated with BSGC, pH 7.3.
In vitro liquid cultures of megakaryocytes
CD34+ cells were grown for 7 to 12 days in Iscoves modified Dulbecco medium (Gibco BRL) containing 1.5% deionized bovine serum albumin (Cohn fraction V; Sigma), iron-saturated human transferrin supplemented with selenium and insulin (Gibco BRL), and a mixture of sonicated lipids (20 μg/mL).28 The medium was supplemented with polyethylene glycol–recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) (10 ng/mL) (Kirin Brewery, Tokyo, Japan), either alone or in combination with 50 ng/mL human stem cell factor (SCF) (Amgen, Thousand Oaks, CA).
Murine lin− cells were grown in similar conditions in the presence of recombinant rat SCF (R&D Systems, Oxon, United Kingdom) and PEG-rHuMGDF for 2 to 5 days.
Immunolabeling for fluorescence microscopy
Cells were cytocentrifuged onto slides at 550 rpm for 4 minutes. Cells were fixed in 0.2% paraformaldehyde (Serva, Heidelberg, Germany) for 5 minutes at room temperature. They were permeabilized with 0.1% Triton X-100 (Sigma) prior to incubation with the antitubulin or the anti-p21, anti-p27, or anti-p57 antibodies. After 3 washes, cells were incubated with the appropriate secondary antibodies. DNA was finally labeled with 4′-6-diamidino-2-phenylindole-2HCl (DAPI) in Vectashield (Vector, Burlingame, CA), and slides were mounted.
Flow cytometric analysis
DNA analysis.
To measure the ploidy of murine marrow megakaryocytes, cells were labeled with FITC–anti-CD41 mAb after preincubation with an anti–CD16/CD32 Fc(III/II), washed, and incubated in a hypotonic citrate solution containing 50 μg/mL propidium iodide (PI) (Sigma) for at least 2 hours (usually overnight) at 4°C.
To measure the ploidy of cultured murine megakaryocytes and sorted human cultured CD41+ cells, cells were incubated in medium containing NP 40 (1/1000) and 50 μg/mL PI for at least 2 hours.
DNA and nuclear protein analysis.
Cultured cells were washed in 1 × phosphate-buffered saline (PBS) prior to fixation in 80% ethanol. Cells were maintained for at least 24 hours at −20°C, washed in PBS containing 1% bovine serum albumin, and permeabilized with 0.25% Tween 20 (Sigma). Cells were subsequently incubated with the anti-Rb mAb or anti-p21 polyclonal antibody or with control antibodies and then with FITC–sheep antimouse or rabbit immunoglobulin G. Finally, PBS containing 50 μg/mL PI (Sigma) and 100 μg/mL RNase A was added to the cell pellet for approximately 2 hours. Cell samples were analyzed on a FACSort (Becton Dickinson).
Activation of platelets by thrombin
Platelet activation by thrombin was studied as previously described.29 Platelets filtrated on a sepharose 4B column were adjusted at a concentration of 1 × 107/mL in BSGC (pH 7.3). Thrombin (Diagnostica Stago, Asnières, France) was serially diluted in BSGC and distributed into round tubes containing the PE–anti-CD62P mAb and FITC anti-CD41 mAbs. Tubes were incubated for 10 minutes at 37°C. The reaction was stopped by the addition of freshly prepared 0.6% paraformaldehyde. After 20 minutes of incubation at room temperature, pellets were suspended in 1 mL PBS and analyzed by flow cytometry.
Cell sorting of megakaryocytes
Cells recovered after 8 to 9 days for human cultures or after 2 days for murine cultures were incubated with the PE–anti-CD41 mAb for human cells or FITC–anti-CD41 mAb for mouse cells for 30 minutes at 4°C and for an additional 2 hours with 0.01 M Hoechst 33342 (Sigma) at 37°C. Megakaryocytes were sorted according to their DNA content by means of a FacsVantage cytometer (Becton Dickinson) equipped with 2 argon lasers (Coherent Radiation, Palo Alto, CA) tuned to 488 and 360 nm, respectively and a 200-μm nozzle. CD41+ cells were sorted into a 2N/4N and an at least 8N cell fraction at 500 cells per second at 4°C for human cells and into an at least 8N cell fraction for mouse cells.
Retroviral vectors, virus production, and cell infection
The following 2 constructs were used: Migr (long terminal repeat–internal ribosome entry site–enhanced green fluorescent protein [LTR-IRES-EGFP]) (a generous gift from J. Miller, Philadelphia, PA) and Migr-p21 (LTR-triple HA-p21-IRES-EGFP). The human p21 complementary DNA (cDNA) was obtained from C. Cayrol (Toulouse, France). A triple HA was inserted at the 5′ end of the p21 cDNA by reverse transcriptase–polymerase chain reaction. The tagged p21 cDNA was sequenced and then inserted into the Migr retroviral vector.
The retrovirus-producing cell line 293 EBNA (Invitrogen, Carlsbad, CA) was maintained in Dulbecco medium containing 10% fetal calf serum (Gibco BRL). Vesicular stomatite virus-G (VSV-G) pseudotyped retroviruses were produced by transient transfection of 293 EBNA cells by means of the Exgen reagent (Euromedex, Mundolsheim, France) and 3 plasmids: pCMV-G (VSV-G coding sequence); pCMV-gag-pol (both kindly supplied by Dr J. Morgenstern, Millenium, Boston, MA); and the Migr vector. Supernatant containing infectious retroviral particles was recovered and concentrated 60-fold by means of an Amicon (Millipore, Bedford, MA). Viral titers were determined by limiting dilution assay on NIH 3T3 cells according to the EGFP fluorescence, and ranged from 1 × 107 to 5 × 108 viral particles per milliliter.
To infect murine cells, an ecotropic retrovirus was prepared. The Phoenix ecotropic packaging cell line obtained from the ATCC (Stanford, CA) was infected with the VSV-G Migr vector, and a stable polyclonal cell line was obtained.
Human cells derived from CD34+ cells were infected at days 5 and 6 of culture by adding 10% viral supernatant. Lin−-derived murine cells were infected after 24 hours of culture. The UT-7 cell line was infected by a 24-hour incubation with the viral supernatant.
Human megakaryocytes were sorted on a FacsVantage after labeling with the PE anti-CD41 mAb. Cells expressing high levels of EGFP and CD41 were sorted. Their ploidy was subsequently measured after staining with PI. Infected UT-7 cells were sorted according to the intensity of EGFP fluorescence into 4 fractions: negative, low/medium, high, and very bright. In the mouse cells, infection was controlled under a fluorescent inverted microscope. Ploidy in the cells of at least 8N was directly measured after PI labeling.
Western blot analysis
Soluble proteins obtained from approximately 500 000 to 5 × 106 cells lysed in Laemmli buffer were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (12%) and then transferred electrophoretically onto a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA). Membranes were incubated for 90 minutes at room temperature with the different primary antibodies. After 2 washes, membranes were incubated with either goat antirabbit or antimouse antibodies conjugated to horseradish peroxidase diluted at 1:5000 and 1:10 000, respectively (Amersham). The bands were developed with an enhanced chemiluminescence system (ECL kit; Amersham).
Results
Expression of cycle-associated proteins in human megakaryocyte differentiation
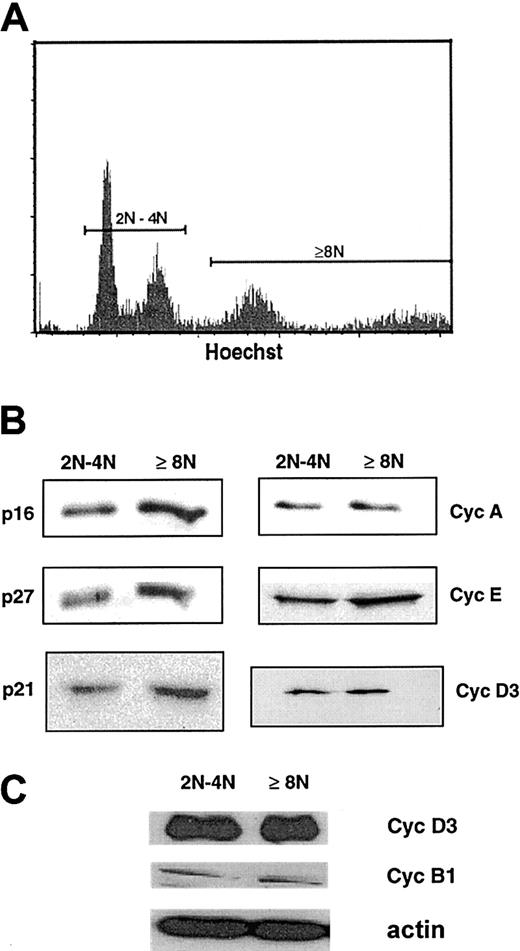
Bone marrow CD34+ cells were cultured in the presence of SCF and PEG-rHuMGDF, and at 8 to 9 days of culture, megakaryocytes were studied. As previously demonstrated,7,30 in most experiments megakaryocyte ploidy was low, with the majority of megakaryocytes with a 2N and 4N content (about 70%). Polyploid megakaryocytes were essentially 8N, and fewer than 10% of the megakaryocytes had a ploidy higher than 8N. In some experiments, ploidy was slightly higher, with up to 15% being 16N cells. Ploidy did not increase with longer times in culture, and a large majority of megakaryocytes were labeled by an anti-vWF antibody. Megakaryocytes were purified by cell sorting, dependent on their ploidy, into 2 cell fractions (2N/4N cells and cells of at least 8N) by double-staining with a FITC anti-CD41 mAb and Hoechst 33342 (Figure1A). Purity of both fractions was greater than 90% after reanalysis as previously reported.7
Presence of cell-cycle proteins in 2N/4N and at least 8N human megakaryocytes.
(A) Flow cytometric analysis of day-8 megakaryocytes derived from G-CSF–mobilized blood CD34+ after labeling with the Hoechst 33342 dye and a PE–anti-CD41 mAb. CD41+ cells were sorted into 2N/4N and into greater than 4N cell fractions. Hoechst staining was analyzed by means of a linear scale. (B) Western blot analysis of cyclin (Cyc) D3, cyclin E, cyclin A, p21, p27, and p16 from the 2 sorted cell populations. The same quantity of proteins was loaded in the 2N/4N and at least 8N lanes. (C) Comparison of the expression of cyclin D3 and cyclin B1 by Western blots from the 2 sorted cell populations. This experiment was perfomed with a pool of sorted cells different from that used to generate the analysis in panel B. The same quantity of protein was loaded in the 2N/4N and at least 8N lanes, as confirmed with the use of actin as a control. In these 2 pools of sorted cells, purity of the cells of at least 8N was greater than 95%, and composed of two thirds of 8N cells and one third 16N cells.
Presence of cell-cycle proteins in 2N/4N and at least 8N human megakaryocytes.
(A) Flow cytometric analysis of day-8 megakaryocytes derived from G-CSF–mobilized blood CD34+ after labeling with the Hoechst 33342 dye and a PE–anti-CD41 mAb. CD41+ cells were sorted into 2N/4N and into greater than 4N cell fractions. Hoechst staining was analyzed by means of a linear scale. (B) Western blot analysis of cyclin (Cyc) D3, cyclin E, cyclin A, p21, p27, and p16 from the 2 sorted cell populations. The same quantity of proteins was loaded in the 2N/4N and at least 8N lanes. (C) Comparison of the expression of cyclin D3 and cyclin B1 by Western blots from the 2 sorted cell populations. This experiment was perfomed with a pool of sorted cells different from that used to generate the analysis in panel B. The same quantity of protein was loaded in the 2N/4N and at least 8N lanes, as confirmed with the use of actin as a control. In these 2 pools of sorted cells, purity of the cells of at least 8N was greater than 95%, and composed of two thirds of 8N cells and one third 16N cells.
Cells sorted at days 8 to 9 from 8 independent experiments were pooled in order to obtain a sufficient number of cells and to diminish the variability among samples. Expression of cell-cycle proteins isolated from both megakaryocyte fractions was studied by Western blots (Figure1B). As previously reported, p21, p27, p16, cyclin E, and cyclin D3 were expressed at high levels in both fractions; in addition, p53 was expressed at high levels in both fractions (data not shown) while cyclin A was also detected but at a lower level in both fractions (Figure 1B). In addition, as we have previously reported,7cyclin B1 was also present but at very low levels in comparison with cyclin D3 (Figure 1C, data obtained from another pool of sorted cells obtained with the same purity). These data show that many cell-cycle proteins associated with the G1 phase were present in the 2 asynchronous megakaryocyte cell populations, suggesting that the majority of cells were in the G1/S phase of the cell cycle. Detection of cyclin B1 in both fractions correlates with the presence of fewer than 1% of cells in mitosis or endomitosis when cells were observed on a slide with a DAPI staining.
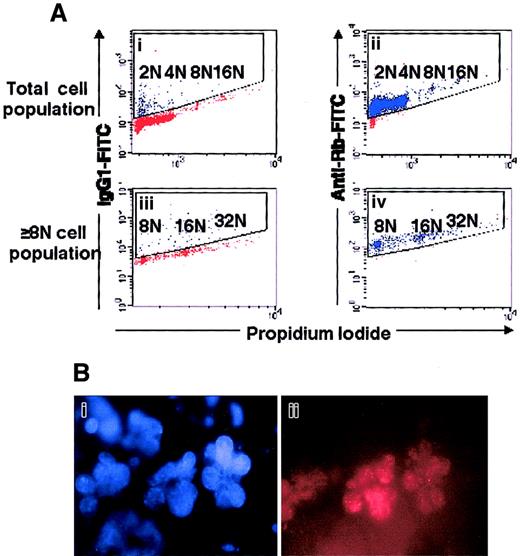
To confirm this hypothesis, we studied the expression and the phosphorylation of Rb (Figure 2). Flow cytometric analysis revealed that Rb was present in megakaryocytes of all ploidy classes, demonstrating that this gene is normally expressed during polyploidization (Figure 2A). In situ immunofluorescence labeling demonstrated a patchy staining of Rb, which was localized to the nucleus (Figure 2B). The phosphorylation status of the Rb protein was studied by the migration pattern in 2N/4N and at least 8N megakaryocytes (Figure 3A), by which we observed a hypophosphorylated form of Rb, which was largely predominant and accumulated in both cell fractions. This result clearly confirms that most hypoploid and polyploid megakaryocytes were blocked at the G1/S transition and have arrested their cell cycle. Then, we studied expression of p21 according to the date of culture and, thus, to megakaryocyte differentiation. At day 7 of culture, a minority of megakaryocytes (17%) expressed p21 with a nuclear localization. At days 9, 10, and 12 of culture, expression of the Ki-67 antigen and p21 protein was studied by double labeling on slides in 2 independent experiments. On average, at day 9, 45% of megakaryocytes expressed p21, and 18.5% the Ki-67 antigen. The percentage of megakaryocytes coexpressing p21 and the Ki-67 antigen was approximately 15%. However, in megakaryocytes expressing a very high level of p21, the Ki-67 antigen was nearly undetectable, confirming the reciprocal expression between p21 and Ki-67.23 At day 10, 65% of megakaryocytes expressed p21 at intermediate or high level with a nuclear localization; 17.5% expressed Ki-67 antigen; and 2.7% coxpressed the 2 markers. We detected p21 both in the nucleus of small megakaryocytes with a round or indented nucleus and in large megakaryocytes. At day 12, Ki-67 antigen was detected in fewer than 5% of the cells. Double labeling was performed between p21 and cyclin D3, and nearly all megakaryocytes (more than 90%) expressed both proteins with a nuclear localization, further suggesting that mature hypoploid and polyploid megakaryocytes are blocked in the G1 phase of the cell cycle. Later in culture, p21 was expressed in a lower number of megakaryocytes because p21 was no longer detected in the nucleus of apoptotic megakaryocytes.
Expression of Rb in polyploid megakaryocytes.
(A) Flow cytometry. Mobilized-blood CD34+ cells, cultured for 8 days in the presence of SCF and PEG-rHuMGDF, were labeled with PI (panels Ai, Aii, Aiii, Aiv) and an anti-Rb mAb (panels Aii, Aiv) or an isotype control (panels Ai, Aiii). The cells were acquired in a gate (not shown) excluding aggregates and cell debris. Analysis was performed either in the total cell population (panels Ai, Aii) or in the polyploid cell population (panels Aiii, Aiv). This is a representative flow cytometric profile of an experiment performed 3 times. (B) Rb has a nuclear localization in megakaryocytes. Human megakaryocytes were labeled by indirect fluorescence by means of an anti-Rb mAb and then a secondary TRITC-labeled antibody (panel Bii), and the nucleus was counterstained with DAPI (panel Bi). A similar localization of Rb was observed on 150 megakaryocytes examined.
Expression of Rb in polyploid megakaryocytes.
(A) Flow cytometry. Mobilized-blood CD34+ cells, cultured for 8 days in the presence of SCF and PEG-rHuMGDF, were labeled with PI (panels Ai, Aii, Aiii, Aiv) and an anti-Rb mAb (panels Aii, Aiv) or an isotype control (panels Ai, Aiii). The cells were acquired in a gate (not shown) excluding aggregates and cell debris. Analysis was performed either in the total cell population (panels Ai, Aii) or in the polyploid cell population (panels Aiii, Aiv). This is a representative flow cytometric profile of an experiment performed 3 times. (B) Rb has a nuclear localization in megakaryocytes. Human megakaryocytes were labeled by indirect fluorescence by means of an anti-Rb mAb and then a secondary TRITC-labeled antibody (panel Bii), and the nucleus was counterstained with DAPI (panel Bi). A similar localization of Rb was observed on 150 megakaryocytes examined.
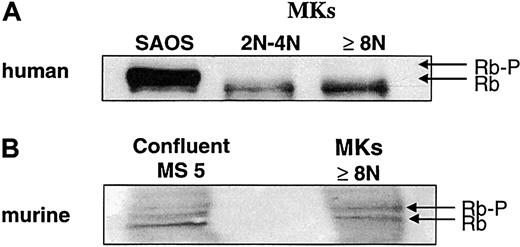
Rb expression during polyploidization.
Human megakaryocytes were sorted as in Figure 1. A similar technique of sorting was used for murine megakaryocytes at day 2 of culture, but only the cell fraction with at least 8N was recovered. Western blot was performed with an anti-Rb mAb, which recognizes both the human (panel A) and murine (panel B) molecules. The human epithelial cell line SAOS (panel A) and the murine stromal cell line MS-5 (panel B) were used as controls. Western blots are from a representative experiment (n = 3).
Rb expression during polyploidization.
Human megakaryocytes were sorted as in Figure 1. A similar technique of sorting was used for murine megakaryocytes at day 2 of culture, but only the cell fraction with at least 8N was recovered. Western blot was performed with an anti-Rb mAb, which recognizes both the human (panel A) and murine (panel B) molecules. The human epithelial cell line SAOS (panel A) and the murine stromal cell line MS-5 (panel B) were used as controls. Western blots are from a representative experiment (n = 3).
Therefore, one limitation of the in vitro human model of megakaryocyte differentiation is related to the fact that there is no correlation between the level of ploidy and the precise stage of maturation. Indeed, terminal differentiation leading to proplatelet formation can occur in low-ploidy megakaryocytes. Thus, to further elucidate the role of p21 during megakaryocyte differentiation, we switched to the murine model of differentiation in which it is known that the majority of megakaryocytes can reach a higher ploidy.31
Expression of Rb and p21 in murine megakaryocytes
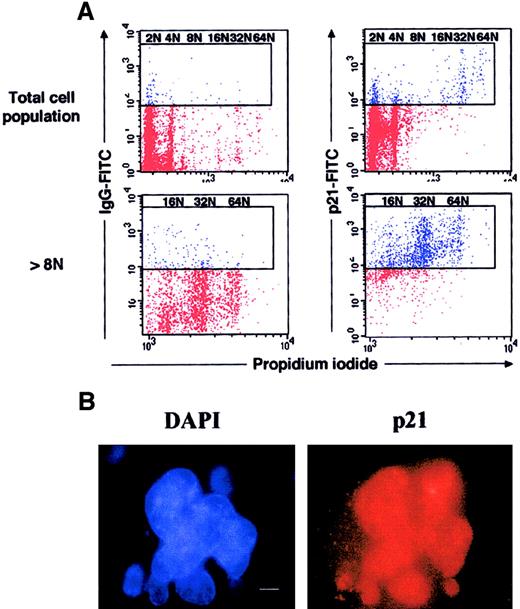
Murine marrow lin− cells were purified and cultured in the presence of SCF and PEG-rHuMGDF. At day 4 of culture, in contrast to human cultures, the modal ploidy of murine megakaryocytes was 32N (see below). Ploidy increased very quickly between day 2 and day 4, suggesting that megakaryocytes were partially synchronous in their cell cycle. This was confirmed by studying expression of the Ki-67 antigen because the large majority of megakaryocytes (94%) express this antigen at day 2 of culture. Thus, megakaryocytes were sorted at this date. However, in 2-day cultures, the number of megakaryocytes was low, and only the cells of at least 8N could be sorted in sufficient number to study the Rb phosphorylation (Figure3B). In contrast to human megakaryocytes, Rb migrated as 2 molecular species corresponding to a hyperphosphorylated and a hypophosphorylated form. The 2 forms were expressed at an equivalent level, which is consistent with the presence of a normal Rb regulation during an endomitotic cell cycle. Expression of p21 was studied by flow cytometry at day 4 of culture (Figure 4A), 2 days later in culture than Rb phosphorylation studies; at this date of culture, megakaryocytes were mature and had stopped their endomitotic process. Expression of p21 was extremely elevated in megakaryocytes with high ploidy. Indeed, by in situ immunofluorescence, we observed that the great majority (greater than 90%) of megakaryocytes with a polylobulated nucleus expressed p21. Labeling was localized in the nucleus with a diffuse staining (Figure 4B). Most megakaryocytes (about 70%) expressed high levels of p21 as illustrated in Figure 4B, and a minor fraction (about 20%) expressed p21 at a lower level. As in humans, a reciprocal expression between p21 and Ki-67 antigen was found. At day 2 of culture, Ki-67 antigen and p21 were detected in 94% and 10% of polyploid murine megakaryocytes, respectively, and 5% coexpressed both markers. The percentage of megakaryocytes positive for Ki-67 antigen decreased the following days (72% and 20% being positive at days 3 and 4, respectively).
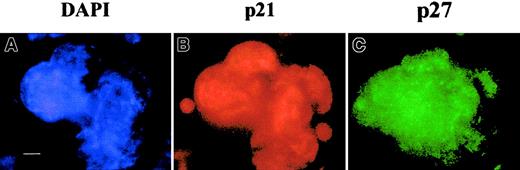
Expression of p21 at high levels in murine polyploid megakaryocytes.
(A) Flow cytometry. Lin− murine marrow cells were grown for 3 days in the presence of SCF and PEG-rHuMGDF, and cells were processed as in Figure 2. Dot plots are from a representative experiment (n = 3). (B) Immunostaining of p21. In parallel, p21 was immunodetected by indirect immunofluorescence on slides, by means of an anti-p21 mAb and a secondary TRITC-conjugated antimouse antibody, while the nuclei were counterstained with DAPI (bar 10 μm). A similar nuclear localization of p21 was observed on 250 megakaryocytes.
Expression of p21 at high levels in murine polyploid megakaryocytes.
(A) Flow cytometry. Lin− murine marrow cells were grown for 3 days in the presence of SCF and PEG-rHuMGDF, and cells were processed as in Figure 2. Dot plots are from a representative experiment (n = 3). (B) Immunostaining of p21. In parallel, p21 was immunodetected by indirect immunofluorescence on slides, by means of an anti-p21 mAb and a secondary TRITC-conjugated antimouse antibody, while the nuclei were counterstained with DAPI (bar 10 μm). A similar nuclear localization of p21 was observed on 250 megakaryocytes.
Expression of p21 in megakaryocytes is not p53 dependent
In human megakaryocytes of 2N/4N and at least 8N, p53 was expressed at high levels. Thus, we next studied whether p21 expression was regulated by p53 during megakaryocyte differentiation. Lin− marrow cells from p53−/− mice were cultured in the presence of SCF and PEG-rHuMGDF for 4 days, and expression of p21 was studied by immunofluorescence labeling on slides. We detected p21 in the nucleus of almost all p53−/−megakaryocytes with a fluorescence intensity quite similar to that observed in wild-type megakaryocytes (Figure5). This result demonstrates that p21 expression is not regulated by p53 during megakaryocyte differentiation.
The p53-independent expression of p21 and p27 in megakaryocytes.
Megakaryocytes were obtained from p53−/−lin− murine cells cultured for 4 days with SCF and PEG-rHu MGDF. Double immunostaining was performed with mouse anti-p21 and rabbit anti-p27 antibodies followed by incubation with donkey TRITC antimouse and FITC antirabbit F(ab′)2 fragments, respectively. Nuclei were counterstained with DAPI. Bar, 10 μm. More than 500 megakaryocytes were examined in 3 experiments.
The p53-independent expression of p21 and p27 in megakaryocytes.
Megakaryocytes were obtained from p53−/−lin− murine cells cultured for 4 days with SCF and PEG-rHu MGDF. Double immunostaining was performed with mouse anti-p21 and rabbit anti-p27 antibodies followed by incubation with donkey TRITC antimouse and FITC antirabbit F(ab′)2 fragments, respectively. Nuclei were counterstained with DAPI. Bar, 10 μm. More than 500 megakaryocytes were examined in 3 experiments.
To more precisely investigate the role of p21 in megakaryocyte differentiation, we studied the megakaryocytopoiesis of p21−/− mice, especially megakaryocyte polyploidization.
p21−/− megakaryocytes have a normal ploidization process
In peripheral blood, no significant difference in platelet number was seen in p21−/− and wild-type CF1 mice (average, 1 270 000 ± 250 000/μL in wild-type and 1 460 000 ± 530 000/μL in p21−/− mice), and no difference was found in the platelet sensitivity to thrombin activation in 4 repeated experiments (data not shown), suggesting that p21−/− platelets have a normal function.
Because it has been reported that p21 plays an important role in the centrosome separation and mitotic spindle organization in several cell types,22 we next studied whether p21−/−megakaryocytes have an identical mitotic spindle organization to wild-type megakaryocytes. Megakaryocytes obtained after 2 days in the presence of PEG-rHuMGDF and SCF were immunostained with anti-α, anti-β, and anti-γ tubulin antibodies. For both genotypes, megakaryocyte spindle organization was multipolar with a spherical structure and a 2X number of asters. At interphase, centrosomes labeled by γ tubulin were either concentrated in the cytoplasm near the nucleus, presumably in the Golgi region, or more dispersed in the cytoplasm (data not shown). Correlating with our previous observation, there was no difference between wild-type and p21−/− megakaryocytes, suggesting that p21−/− megakaryocytes have a normal endomitotic process.
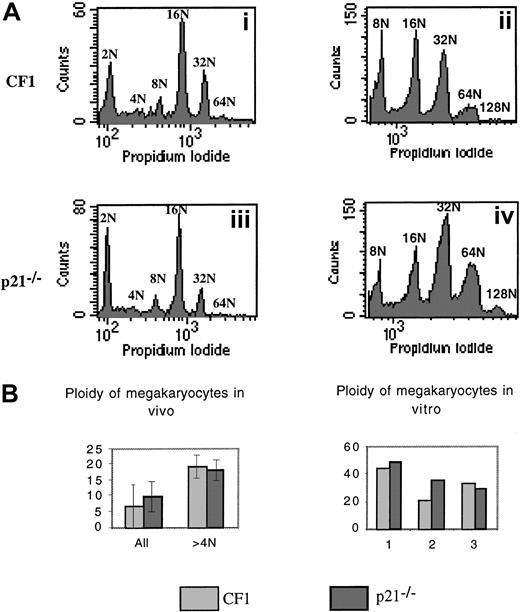
We next analyzed the ploidy of p21−/− versus wild-type CF1 megakaryocytes by 2 approaches (Figure6). First, the ploidy of fresh bone marrow megakaryocytes was measured by flow cytometry using double staining (FITC–anti-CD41 antibody and PI). Experiments were performed in 7 p21−/− and CF1 mice. Ploidy curve, modal ploidy, and mean ploidy of the entire CD41+ cell populations were identical in both mice (Figure 6Ai,Aiii,B). However, in these experiments, a significant 2N peak was found, which raised the possibility that the CD41 antibody binds either to megakaryocyte precursors or to nonmegakaryocytic cells. Thus, ploidy was measured only in polyploid cells (at least 8N). The results did not reveal any difference between p21+/+ and p21−/−megakaryocytes and confirmed our previous data (data not shown). Second, lin− marrow cells were cultured with PEG-rHuMGDF and SCF during 3 days to reveal eventual differences in the polyploidization process after cytokine stimulation. Slight differences in mean ploidy were observed between p21−/− and wild-type CF1 megakaryocytes; in 2 experiments, mean ploidy was higher in p21−/− megakaryocytes whereas in the third it was slightly lower (Figure 6Aii,Aiv,B).
Analysis of the ploidy of p21−/− and p21+/+ CF1 megakaryocytes.
Bone marrow cells were stained with a FITC anti-CD41 mAb and then incubated overnight in a hypotonic solution of PI. Samples were analyzed by flow cytometry. (A) Representative histograms. (i) (iii): Fresh bone marrow. (ii) (iv): Lin− cells stimulated by PEG-rHuMGDF and SCF were cultured for 3 days and ploidy was measured. Histograms are from a representative experiment (n = 7 for fresh marrow cells, n = 3 for cultured cells). (B) Analysis of the mean ploidy. Bone marrow megakaryocyte ploidy of 7 animals in each group was studied. No significant difference was observed between ploidy of p21−/− and CF1 mice.
Analysis of the ploidy of p21−/− and p21+/+ CF1 megakaryocytes.
Bone marrow cells were stained with a FITC anti-CD41 mAb and then incubated overnight in a hypotonic solution of PI. Samples were analyzed by flow cytometry. (A) Representative histograms. (i) (iii): Fresh bone marrow. (ii) (iv): Lin− cells stimulated by PEG-rHuMGDF and SCF were cultured for 3 days and ploidy was measured. Histograms are from a representative experiment (n = 7 for fresh marrow cells, n = 3 for cultured cells). (B) Analysis of the mean ploidy. Bone marrow megakaryocyte ploidy of 7 animals in each group was studied. No significant difference was observed between ploidy of p21−/− and CF1 mice.
Together, these results demonstrated that p21−/−megakaryocytes are normally polyploidized. This absence of abnormality could be related to a p21 redundancy with another member of the Cip/Kip family. In order to study which other members of the Cip/Kip family were expressed by megakaryocytes, murine megakaryocytes were labeled with antibodies against p27Kip1 and p57Kip2. No staining could be detected with the p57Kip2 antibody. In contrast, p27Kip1 was expressed at high levels in most megakaryocytes from wild-type or p53−/− mice (Figure 5), with the same pattern of labeling as p21−/−, correlating with previous reports.23 Nearly all mature megakaryocytes coexpressed these 2 proteins with a heterogeneity that was apparently not related to the ploidy of the cells as evaluated according to the size and polylobulation of the nucleus. In p21−/−megakaryocytes, p27Kip1 was expressed at high levels in all megakaryocytes. However it was not possible to determine by this technique if p27Kip1 was up-regulated in p21−/− megakaryocytes.
Effects on the overexpression of p21 on the ploidy of murine and human megakaryocytes
To further define the role of p21, overexpression in cell lines was performed. A bicistronic retrovirus (Migr) containing HA-tagged p21 cDNA under the control of murine stem cell virus (MSCV) LTR and EGFP was constructed to overexpress p21 in murine and human megakaryocytes. Ecotropic and VSV-G–pseudotyped retrovirus were used for infection of murine and human cells, respectively. Similar retroviruses containing a vector coding for EGFP alone were used as controls.
The UT-7 cell line was first tested to confirm the functionality of the transduced p21. Cells were sorted on the level of EGFP expression into 4 populations (negative, low/intermediate, high, and bright fluorescent intensity). An increase in cells in G1 phase that correlated with the intensity of the EGFP was observed. More than 85% of the UT-7 cells expressing a very high level of EGFP were blocked in G1, impeding cell growth. The level of p21 was increased at least 15-fold in UT-7 cells after retroviral transduction (Figure7B). This result indicates that p21 expression and function correlated with the expression of EGFP. Then, lin− cell fractions obtained from p21−/−, wild-type CF1 and C57/Bl6 bone marrow cells were infected at day 2 of culture for 24 hours, after which, more than 50% of megakaryocytes expressed EGFP as determined by fluorescence microscopy. We demonstrated that p21 was expressed at high levels as shown by Western blot with the use of the HA epitope (Figure 7). Ploidy was measured in all cells with a DNA content higher than 4N by flow cytometry (Figure8). We observed that p21 overexpression significantly decreased the mean ploidy of megakaryocytes (Figure 8B,D) in comparison with cells infected with the control vector (Figure8A,C). Moreover, the effects of this overexpression were similar, being independent of both the p21 status of the mice and the mouse strain. Finally, we examined whether these results could be confirmed in human megakaryocytes. CD34+ cells purified from cytapheresis collections and cultured 5 days in the presence of PEG-rHuMGDF and SCF were infected with the 2 VSV-G retroviruses. Transduction was studied in the CD41+ cell population by means of a PE–anti-CD41 immunostaining and flow cytometry. More than 50% of the CD41bright cells expressed high levels of EGFP and induced about a 4-fold overexpression of p21 in megakaryocytes (Figure 7B). These cells were sorted (Figure 9A), and ploidy was analyzed by means of PI. In 3 repeated experiments, p21 overexpression markedly decreased (about 2-fold) the mean ploidy of human megakaryocytes (Figure 9B). All together, these results indicate that p21 was able to block the endomitotic cell cycle.
Expression of HA-p21 in transduced cells.
(A) Western blot analysis with the anti-HA mAb. UT-7 cells (lane 1) grown with GM-CSF were infected at day 2 of culture with VSV-G–pseudotyped retrovirus containing an EGFP vector alone (lane 2) or an HA-tagged p21 and EGFP (lane 3). CD34+cells, isolated from cytapheresis samples (lane 4) cultured with PEG-rHuMGDF and SCF and were infected with the same supernatants (containing HA-tagged p21 [lane 6] or EGFP alone [lane 5] at days 5 and 6. Murine lin− cells cultured for 2 days with PEG-rHuMGDF and SCF (lane 7) were infected with an ecotropic retrovirus containing the same HA-tagged p21 and EGFP (lane 8). Similarly, lin− marrow cells from p21−/− mice were infected with the ecotropic retrovirus coding for the HA-tagged p21 and EGFP (lane 9). Protein lysates were obtained 48 hours after infection and blotted with the anti-HA mAb. Western blots are from a representative experiment (n = 2). (B) Human CD34+ cells and UT-7 cells were infected with the VSV-G–pseudotyped retrovirus containing the HA-tagged p21 vector (identical to lanes 3 and 6 of panel A). Protein lysates were obtained 48 hours after infection and blotted with an anti-p21 mAb. The wild-type p21 and the HA-tagged p21 could be detected. Overexpression of p21 was about 4-fold in normal megakaryocytes and more than 15-fold in the UT-7 cells when the ratio between the tagged p21 and the wild-type p21 was studied. Western blots are from a representative experiment (n = 2).
Expression of HA-p21 in transduced cells.
(A) Western blot analysis with the anti-HA mAb. UT-7 cells (lane 1) grown with GM-CSF were infected at day 2 of culture with VSV-G–pseudotyped retrovirus containing an EGFP vector alone (lane 2) or an HA-tagged p21 and EGFP (lane 3). CD34+cells, isolated from cytapheresis samples (lane 4) cultured with PEG-rHuMGDF and SCF and were infected with the same supernatants (containing HA-tagged p21 [lane 6] or EGFP alone [lane 5] at days 5 and 6. Murine lin− cells cultured for 2 days with PEG-rHuMGDF and SCF (lane 7) were infected with an ecotropic retrovirus containing the same HA-tagged p21 and EGFP (lane 8). Similarly, lin− marrow cells from p21−/− mice were infected with the ecotropic retrovirus coding for the HA-tagged p21 and EGFP (lane 9). Protein lysates were obtained 48 hours after infection and blotted with the anti-HA mAb. Western blots are from a representative experiment (n = 2). (B) Human CD34+ cells and UT-7 cells were infected with the VSV-G–pseudotyped retrovirus containing the HA-tagged p21 vector (identical to lanes 3 and 6 of panel A). Protein lysates were obtained 48 hours after infection and blotted with an anti-p21 mAb. The wild-type p21 and the HA-tagged p21 could be detected. Overexpression of p21 was about 4-fold in normal megakaryocytes and more than 15-fold in the UT-7 cells when the ratio between the tagged p21 and the wild-type p21 was studied. Western blots are from a representative experiment (n = 2).
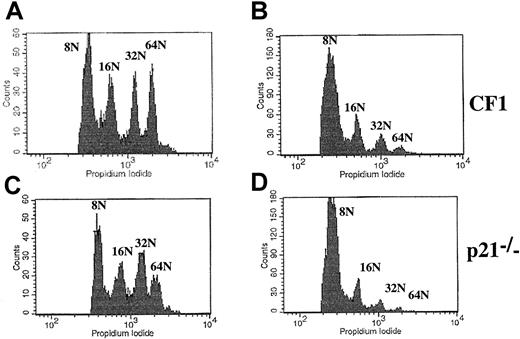
Overexpression of p21 in murine megakaryocytes.
Lin− cells from wild-type CF1 or p21−/− bone marrow were cultured for 2 days in the presence of PEG-rHuMGDF and SCF and infected with an ecotropic retrovirus containing either the HA-tagged p21 and EGFP cDNA (panels B, D) or EGFP cDNA alone vectors (panels A, C) (3 experiments). After infection, more than 50% of the megakaryocytes expressed EGFP as assessed by microscopy. Because the FITC anti-CD41 mAb could not be used for cell sorting, ploidy was measured by flow cytometry in cells with at least 8N. Overexpression of p21 significantly decreased the mean ploidy of megakaryocytes in comparison with cells infected with the control vector independent of p21 status of the mice (CF1, panels A, B; p21−/−, panels C, D). Similar results were obtained in C57Bl/6 mice (data not shown, n = 3).
Overexpression of p21 in murine megakaryocytes.
Lin− cells from wild-type CF1 or p21−/− bone marrow were cultured for 2 days in the presence of PEG-rHuMGDF and SCF and infected with an ecotropic retrovirus containing either the HA-tagged p21 and EGFP cDNA (panels B, D) or EGFP cDNA alone vectors (panels A, C) (3 experiments). After infection, more than 50% of the megakaryocytes expressed EGFP as assessed by microscopy. Because the FITC anti-CD41 mAb could not be used for cell sorting, ploidy was measured by flow cytometry in cells with at least 8N. Overexpression of p21 significantly decreased the mean ploidy of megakaryocytes in comparison with cells infected with the control vector independent of p21 status of the mice (CF1, panels A, B; p21−/−, panels C, D). Similar results were obtained in C57Bl/6 mice (data not shown, n = 3).
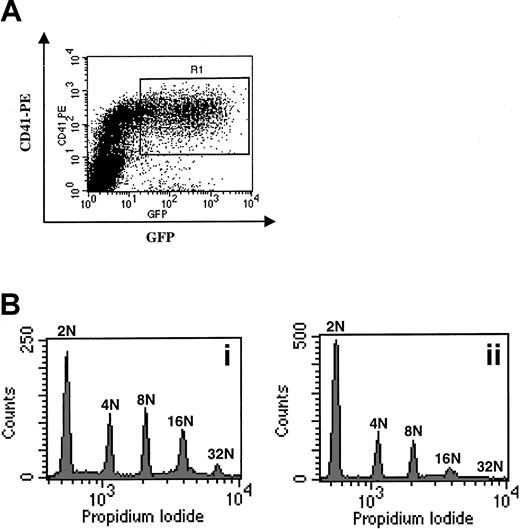
Overexpression of p21 in human megakaryocytes.
(A) Sorting of infected CD41+ cells. Cells were infected at days 5 and 6 of culture and stained at day 7 with a PE–anti-CD41 mAb. Cells expressing high levels of EGFP and CD41, corresponding to infected megakaryocytes, were sorted in the R1 gate. (B) Effects of the p21 overexpression on the ploidy of human megakaryocytes. Cells infected with the VSV-G Migr retrovirus containing EGFP alone (panel Bi) or HA-tagged p21 and EGFP (panel Bii) were sorted as in panel A. Ploidy was then analyzed after PI staining. A typical experiment is illustrated. In this experiment, the ploidy level in the control was higher than in the experiment illustrated in Figure 1A. This difference was related to the variability observed among human samples. In 3 experiments, p21 overexpression markedly decreased (about 2-fold) the mean ploidy of human megakaryocytes.
Overexpression of p21 in human megakaryocytes.
(A) Sorting of infected CD41+ cells. Cells were infected at days 5 and 6 of culture and stained at day 7 with a PE–anti-CD41 mAb. Cells expressing high levels of EGFP and CD41, corresponding to infected megakaryocytes, were sorted in the R1 gate. (B) Effects of the p21 overexpression on the ploidy of human megakaryocytes. Cells infected with the VSV-G Migr retrovirus containing EGFP alone (panel Bi) or HA-tagged p21 and EGFP (panel Bii) were sorted as in panel A. Ploidy was then analyzed after PI staining. A typical experiment is illustrated. In this experiment, the ploidy level in the control was higher than in the experiment illustrated in Figure 1A. This difference was related to the variability observed among human samples. In 3 experiments, p21 overexpression markedly decreased (about 2-fold) the mean ploidy of human megakaryocytes.
Discussion
Here, we sought to determine the precise role of p21 during megakaryocyte differentiation. In previous studies on p21 expression during megakaryocytic differentiation, both p21 messenger RNA and protein were detected at a high level in megakaryocytes. Unlike p27, which was expressed only in Ki67−megakaryocytes,23 p21 was detected in cycling megakaryocytes. We observed a nuclear localization of p21 in a large fraction of small and large human megakaryocytes derived in vitro from CD34+ cells. We also detected p21 and p27 proteins by Western blotting in megakaryocytes with low or high ploidy. This result could indicate that p21 was expressed early and at a high level in diploid cells before endomitosis. However, one limitation of the human in vitro model of megakaryocyte differentiation is the low ploidy of the cultured cells. Indeed, more than 50% of megakaryocytes derived from adult CD34+ cells are 2N and 4N.28,32This low ploidy is even more striking in megakaryocytes derived from cord blood cultures, where up to 85% of the megakaryocytes are diploid or tetraploid.33-35 However, the majority of these hypoploid cells are mature megakaryocytes and not megakaryocytic precursors since they are capable of proplatelet formation.36 Thus, in human megakaryocyte cultures, there is an uncoupling between ploidization and terminal differentiation, as has been observed in some myeloproliferative diseases.37
In contrast, in the mouse system, megakaryocytes can reach a high ploidy level, up to 256N after 3 to 4 days of culture, with a coupling between terminal differentiation and ploidization. In this model, p21 was expressed at a very high level only in polyploid megakaryocytes. A good correlation was observed between p21 and p27 expression, with the majority of megakaryocytes expressing these 2 CDK inhibitors. Therefore, p21 and p27 expression is high only in mature megakaryocytes that have terminated their ploidization. This high expression of the p21 protein in murine megakaryocytes obtained in culture contrasts with previous in vivo studies in which only a minority (fewer than 15%) of megakaryocytes in the spleen synthesized p21 as measured by in situ hybridization.24 Possibly, this can be explained by the differences in the intensity of the cytokine stimulation and by the fact that cultures are nearly synchronized in early days.
The p21 is regulated through p53-dependent and p53-independent mechanisms.9,38 A large part of the p53 effect on cell cycle is mediated by p21, and thus p21 is responsible for the cell-cycle arrest induced by DNA damage.15,16 In contrast, regulation of p21 during differentiation has been shown to be p53 independent in many cellular models39 although it has been recently reported that p21 expression during erythroid differentiation was p53 dependent.40 We observed that p21 was expressed at a similarly high level in wild-type and p53−/−megakaryocytes. Stat5 and HOXA10, which regulate p21 expression,25 41 play an important role in megakaryocyte differentiation and may be involved in this regulation.
The p21 and p27 can induce polyploidy in several cellular models by inducing endoreduplication. The cell-cycle effect of p21 may be related to the down-regulation of proteins involved in cytokinesis and mitosis entry, including CDK1 and cyclin B1.42,43 It has been suggested that overexpression of p21 may transiently block cells at the G2/M transition17,21 and that cells then skip mitosis and endoreplicate DNA. Rb plays a crucial role in this regulation by preventing endoreplication, in which p21 overexpression induces polyploidy only in Rb−/− cells.21 In contrast, polyploidization is favored by the absence of p21 in cells with a functional Rb.14,22 Similarly, endoreplication has been observed in cells lacking SKP2 and expressing high levels of cyclin E and p27.44 In all these cellular models, endoreplication requires a high DNA replication activity with an inhibition of CDK1 kinase activity and a transient arrest in G2/M.45 This mechanism is quite different from the endomitotic process, which requires mitotic entry and consequently a CDK1 activity.6,7,46 For all these reasons, we investigated Rb expression in megakaryocytes. Rb was normally present in low- or high-ploidy human megakaryocytes. A predominantly hypophosphorylated form of Rb was found in hypoploid (2N and 4N) as well as in polyploid megakaryocytes at days 8 to 9 of culture. These asynchronous megakaryocytes also expressed high levels of cyclin D3, p21, and cyclin E. Therefore, it seems likely that most megakaryocytes are in the G1 phase of the cell cycle8,23 47and have ended their endomitotic process. To obtain a more synchronous megakaryocyte population, we purified murine megakaryocytes after 2 days of culture, when the majority of the cells were undergoing endomitosis. In this partially synchronized cell population, the 2 forms of Rb protein (hypophosphorylated and hyperphosphorylated) were detected. This result suggests that Rb is normally regulated during megakaryocyte differentiation and is active at the end of endomitosis.
To more precisely assess the role of p21 on megakaryocyte polyploidization, we studied the effects of its inactivation or overexpression. The p21-deficient mice have a decreased number of hematopoietic progenitors with a defect in their cytokine responsveness.48,49 More recently, it has been shown that p21−/− stem cells have a defect in self-renewal that is probably the consequence of a defect in cell-cycle entry control. This progressively leads to an exhaustion in stem cell activity.50 The p21−/− mice had normal platelet counts with a normal platelet size and function. Bone marrow megakaryocyte ploidy was identical to that of wild-type mice with a 16N modal ploidy. Cultures of lin− cells in the presence of SCF and PEG-rHuMGDF yielded a normal number of megakaryocytes with a normal ploidy. A slight but nonsignificant shift toward classes of higher ploidy was observed in p21−/− megakaryocytes in comparison with wild-type megakaryocytes. Thus, the absence of p21 has no clear effect on megakaryocyte ploidization.
To better understand the role of p21, we subsequently switched to an overexpression strategy. The p21 was transduced into a cell line with a megakaryocyte phenotype (UT-7) by means of a retroviral vector, and as expected, an arrest in G1 was correlated with the level of p21 protein expression. These data are in contrast with results previously obtained studying the transient expression of p21 in UT-7, where p21 induced nuclear polylobulation26; however, ploidy was not measured in these experiments. Re-expression of p21 in p21−/− megakaryocytes markedly inhibited ploidization. A similar result was observed when p21 was overexpressed in human and wild-type murine megakaryocytes. Together, these data suggest that the main role of p21 during megakaryocyte differentiation is to arrest the endomitotic cell cycle. However, we cannot exclude that in the early stages of megakaryocyte differentiation, when p21 is expressed at a low level, it may favor proliferation by increasing the affinity of cyclin D3 for CDK4.18,19 48 The absence of ploidization in p21−/− megakaryocytes may be due to the expression of p27 by megakaryocytes. In preliminary experiments (data not shown), p27 overexpression in human megakaryocytes also induced an endomitotic cell-cycle arrest, suggesting that these 2 Cip/Kip members have the same function during megakaryocytopoiesis.
It is noteworthy that megakaryocyte and myogenic differentiation have several similarities, including polyploidization (by totally different mechanisms) and the same high expression of cyclin D3 and p21.51 In muscle differentiation, cyclin D3 is involved in G1 growth arrest and differentiation by sequestering CDKs and CDK inhibitors in a high molecular complex.51Transgenic mice overexpressing cyclin D3 have megakaryocytes of higher ploidy than wild-type animals, demonstrating that cyclin D3 is involved in the endomitotic process.24 However, treatment of megakaryocyte cultures with a cyclin D3 antisense oligonucleotide inhibited maturation, suggesting that cyclin D3 is also involved in megakaryocyte differentiation.8,47 Similarly, p21 plays an important role in skeletal muscle differentiation, and the molecular mechanisms involved in this process are now well understood. The p21 increases transcriptional activity of MyoD by inhibiting CDK2 kinase activity and consequently MyoD phosphorylation.52,53 Simultaneously, MyoD increases the transcription of p21,54 inducing a loop of activation that favors terminal differentiation. Despite these molecular interactions between p21 and myogenic transcription factors, p21−/−mice have no muscle abnormalities. This is due to the complete redundancy between p21Waf1 and p57Kip2 in skeletal muscle.14 Therefore, it is tempting to speculate that p21 may trigger megakaryocytic terminal differentiation by interfering with basic helix loop helix of the MyoD family expressed in megakaryocytes such as TAL-1 or Lyl-1.55
In conclusion, this study demonstrates that p21 and probably p27 play an important role in endomitotic cell-cycle arrest. It remains to be determined if p21 is implicated in the ontogenic changes occurring in megakaryocyte ploidy and if p21 directly couples cell-cycle arrest with terminal megakaryocyte differentiation and proplatelet formation.
We thank A. Rouchès and P. Ardouin (Institut Gustave Roussy) for taking care of the animals, and the staff of the cell therapy laboratory at the Institut Gustave Roussy for providing cytapheresis samples. We also thank Immunex, Kirin Brewery, Amgen, D. Duménil, C. Cayrol, J. Morgenstern, and J.-L. Villeval for their generous provision of reagents and probes. We are grateful to V. Schiavon, A. Katz, and P. Rameau for cell-sorting experiments and to A. Dugray for assisting in the preparation of viral supernatant.
Supported by grants from the Institut National de la Santé et de la Recherche Médicale (INSERM), the Association contre le Cancer (ARC), and the Ligue Nationale contre le Cancer “équipe labellisée 2000,” and fellowships from the Institut Gustave Roussy (L.R.) and the Research Ministry (N.V. and H.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
William Vainchenker, INSERM U 362, Pavillon de Recherche 1, Institut Gustave Roussy, 39 rue Camille Desmoulins, 94 805 Villejuif Cedex, France; e-mail: verpre@igr.fr.

![Fig. 7. Expression of HA-p21 in transduced cells. / (A) Western blot analysis with the anti-HA mAb. UT-7 cells (lane 1) grown with GM-CSF were infected at day 2 of culture with VSV-G–pseudotyped retrovirus containing an EGFP vector alone (lane 2) or an HA-tagged p21 and EGFP (lane 3). CD34+cells, isolated from cytapheresis samples (lane 4) cultured with PEG-rHuMGDF and SCF and were infected with the same supernatants (containing HA-tagged p21 [lane 6] or EGFP alone [lane 5] at days 5 and 6. Murine lin− cells cultured for 2 days with PEG-rHuMGDF and SCF (lane 7) were infected with an ecotropic retrovirus containing the same HA-tagged p21 and EGFP (lane 8). Similarly, lin− marrow cells from p21−/− mice were infected with the ecotropic retrovirus coding for the HA-tagged p21 and EGFP (lane 9). Protein lysates were obtained 48 hours after infection and blotted with the anti-HA mAb. Western blots are from a representative experiment (n = 2). (B) Human CD34+ cells and UT-7 cells were infected with the VSV-G–pseudotyped retrovirus containing the HA-tagged p21 vector (identical to lanes 3 and 6 of panel A). Protein lysates were obtained 48 hours after infection and blotted with an anti-p21 mAb. The wild-type p21 and the HA-tagged p21 could be detected. Overexpression of p21 was about 4-fold in normal megakaryocytes and more than 15-fold in the UT-7 cells when the ratio between the tagged p21 and the wild-type p21 was studied. Western blots are from a representative experiment (n = 2).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/12/10.1182_blood.v98.12.3274/6/m_h82311837007.jpeg?Expires=1769105563&Signature=yNlqNfDOnAIR8M9mwbtqCvbp0DWzerY7NvDCGaPYAgnWDX1xQTge6Pol1PhyBWdliBHZdfDuPFbZz4AhOHKie7pwtu1am2RGMnO59M4x0oE-U-tbV5qdUKxMX8dHRDCABMYNaJQR4M0t8ITuibawJFvP5diiK4yIa6OVdKe7YqkK91hlFHJoZV55SZAAU-rQfudwudwMeejxjIpOOUFHO2Ro937L39qNlFKUyUqUaCQwAE9SU9h4wX~aCy9elRBidRDlijVCLJ8Gh32tpfusHTwDcv0AYlPkn8NbvBN5WJ3M9MBpW3Kg-x5asH~0CGKdXB4Mb4Szxwh6YUAfxfj0ug__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal