Abstract
The antigen-presenting dendritic cells (DCs) found in mouse lymphoid tissues are heterogeneous. Several types of DCs have been identified on the basis of the expression of different surface molecules, including CD4, CD8α, and DEC-205. Previous studies by the authors showed that the mouse intrathymic lymphoid-restricted precursors (lin−c-kit+Thy-1lowCD4low) can produce DCs in the thymus and spleen upon intravenous transfer, suggesting a lymphoid origin of these DCs. In the current study, the potential for DC production by the newly identified bone marrow (BM) common lymphoid precursors (CLPs), common myeloid precursors (CMPs), and committed granulocyte and macrophage precursors was examined. It was found that both the lymphoid and the myeloid precursors had the potential to produce DCs. All the different DC populations identified in mouse thymus and spleen could be produced by all these precursor populations. However, CLPs produced predominantly the CD4−CD8α+ DCs, whereas CMPs produced similar numbers of CD4−CD8α+ and CD4+CD8α− DCs, although at different peak times. On a per cell basis, the CLPs were more potent than the CMPs at DC production, but this may have been compensated for by an excess of CMPs over CLPs in BM. Overall, this study shows that the expression of CD8α does not delineate the hemopoietic precursor origin of DCs, and the nature of the early precursors may bias but does not dictate the phenotype of the DC product.
Introduction
The antigen-presenting dendritic cells (DCs) constitute a system of hemopoietic cells that are sparsely but widely distributed. Variation in their tissue distribution and in their surface phenotype indicates the existence of distinct subpopulations of DCs.1-3 Several DC subsets differing in surface markers and detailed function have been identified.3-8 It has been suggested that these DC subpopulations are products of precursors of different hemopoietic lineages. We proposed the existence of 2 different DC lineages, namely, the myeloid- and the lymphoid-related lineages. Murine CD8α+ DCs were considered to represent a lymphoid-derived DC lineage, on the basis of our demonstration that thymic and splenic CD8α+ DCs could be produced in recipient mice on transfer of a thymic lymphoid precursor population with little capacity to form myeloid cells.9-11 The DCs that lacked expression of CD8α but expressed the myeloid marker CD11b were considered to represent myeloid-derived DCs. However, since myeloid-restricted precursors were not then available, this hypothesis was untested.
Recently, we have shown that murine DCs are more heterogeneous than just this CD8α+ lymphoid-related, and CD8α− myeloid-related division.3 A total of 5 subpopulations of mature DCs can be identified in mouse lymph nodes.12 Spleen DCs include 3 subpopulations (namely, CD4+CD8α−, CD4−CD8α−, and CD4−CD8α+), and our studies on their developmental kinetics and turnover rate indicate that they represent the products of 3 separate developmental streams rather than different maturation states of one stream.13 However, the ultimate lineage origin of these multiple DC subtypes remains unclear.
The recent identification of murine bone marrow (BM) clonogenic lymphoid- and myeloid-committed progenitors14,15 made it possible to examine the lineage origin of the different DC populations. In this study, we have examined the potential of the committed lymphoid and myeloid progenitors of BM and thymus for their potential to generate different DC populations and have compared the developmental kinetics of each DC population derived from these different precursors. We found that both the common lymphoid precursors (CLPs) and the common myeloid precursors (CMPs) were capable of producing all thymic and splenic DC populations. The more committed precursors, namely, the CD4low lymphoid precursors of thymus16,17 and the granulocyte and macrophage precursors (GMPs) of BM, were also able to produce all DC populations. However, the DC subpopulation balance and the kinetics of development varied with the precursor used. This study demonstrates that DCs can be produced from both lymphoid- and myeloid-committed precursor populations. However, in contrast to our previous assumption that CD8α represents a marker for lymphoid-derived DCs, this study demonstrates that the expression of CD8α does not delineate the ultimate lineage origin of DCs and that the nature of the hemopoietic precursors does not dictate the surface phenotype of the DC product. This basic conclusion is in line with results obtained by the Stanford laboratory.18 19
Materials and methods
Mice
C57BL/Ka-Thy-1.1 (Ly 5.2) mice at 6 to 7 weeks of age were used as donors. C57BL/6 Pep3b Ly 5.1 mice at 8 to 10 weeks of age were used as recipients. Balb/c mice were used as T-cell donors for mixed leukocyte reaction (MLR). All mice were bred in the animal facility of the Walter and Eliza Hall Institute under pathogen-free conditions.
Antibodies
The following antibodies were used as supernatants for immunomagnetic bead depletion of lineage marker–positive BM cells: anti-CD3 (KT-3.1), anti–CD8 (53-6.7), anti-CD2 (RM2-1), anti-B220 (RA3-6B2), anti–Mac-1 (M1/70), anti–Gr-1 (RA6-8C5), and antierythrocyte (TER-119). The following purified antibodies were used as fluorescent conjugates for cell staining and sorting: anti–Ly 5.2 (AL1-4A2) used as a fluorescein isothiocyanate (FITC) conjugate; anti–Thy-1.1 (19XE5) used as a FITC conjugate; anti–Fcγ receptor (anti–FcγR)II/III (2.4G2) used as a FITC conjugate; anti–c-kit (2B8) used as an allophycocyanin (APC) conjugate; anti–Sca-1 (E13-161-7) and anti–interleukin (IL)–7Rα (A7R34) used as Alexa 594 conjugates; and anti–IL-7Rα (A7R34) and anti-CD34 (RAM34) used as biotin conjugates. Antirat immunoglobulin–Texas Red and phycoerythrin (PE)–avidin were used for second-stage staining. The following fluorescent-conjugated antibodies were used for staining the DCs: anti–major histocompatibility complex (MHC) class II–FITC (M5/114), anti-CD11c–Alexa 594 (N418), anti-CD4–PE (GK1.5), anti-CD8–Cy5 (YTS 169.4), and anti-DEC-205–biotin (NLDC-145) with PE-avidin used as the second stage.
Isolation of precursor populations
The early intrathymic lymphoid-precursor population (CD3−CD8−CD4lowc-kit+CD25−Thy-1low) was purified by means of a procedure described previously.20 The BM precursors were isolated by procedures modified from these described by Kondo et al14and Akashi et al.15 The CLP population from mouse BM was purified by immunomagnetic bead depletion of lineage marker–positive cells, followed by enrichment sorting for Sca-1low/+ cells. These enrichment-sorted cells were stained with anti–Thy-1.1–FITC, anti–c-kit–APC, anti–Sca-1–Alexa 594, and anti–IL-7Rα–biotin, followed by PE-avidin as the second stage. CLPs were then sorted as IL-7Rα+Sca-1intc-kitintThy-1.1− cells on a FACstar-Plus instrument (Becton Dickinson, San Jose, CA). The myeloid-committed precursor populations from BM were isolated by first depleting lineage marker–positive cells by means of immunomagnetic beads. The remaining cells were then stained with goat antirat immunoglobulin–Texas Red and anti–c-kit–APC and were then enrichment sorted for lin−c-kit+cells. The enrichment-sorted cells were then stained with anti-FcRγII/III–FITC, anti–c-kit–APC, anti–IL-7Rα–Alexa 594, anti–Sca-1–Alexa 594, and anti-CD34–biotin, followed by PE-avidin. The CMP population was sorted as FcRγII/IIIlowCD34+c-kit+Sca-1−IL-7Rα−cells; the GMP population was sorted as FcRγII/III+CD34+c-kit+Sca-1−IL-7Rα−cells. The purity of sorted cells was determined by reanalyzing a small sample of the collected cells and was usually greater than 97%.
In vivo assays for DC production by different precursor populations
To examine the capacity for DC production, purified precursor cells (1 to 4 × 104) from C57BL Ly 5.2 mice were intravenously (IV) injected into lethally irradiated (5.5 Gy twice with a 3-hour interval) C57BL Ly 5.1 recipients, along with 5 × 104 recipient type Ly 5.1 unfractionated BM cells to ensure mouse survival. At various times after precursor transfer, the thymus and spleen of recipients were collected, and DCs were enriched from these tissues by means of a procedure described elsewhere.3 These DC-enriched preparations were then stained in 4 colors with fluorescent-conjugated antibodies to Ly 5.2 to reveal donor-derived cells, together with conjugated antibodies to other markers expressed by DCs, including the pan-DC markers CD11c and MHC class II and the DC subset–specific markers CD4, CD8α, DEC-205, and CD11b. Donor-derived DCs were revealed by electronic gating for Ly5.2+CD11c+ cells during flow cytometric analysis. The expression of other DC markers by these donor-derived DCs was further examined, and the proportion of the individual donor-derived DC subpopulations was then determined.
Mixed leukocyte cultures for assaying DC function
The culture system has been described in full elsewhere.21 Briefly, various numbers of purified donor-derived splenic DCs were cultured in the presence of IL-2 (20 ng/mL) with 20 000 purified CD4+ or CD8+ T cells from lymph nodes of Balb/c mice. A 6-hour pulse of3H-thymidine (3H-TdR) was given at day 3 of culture and label-incorporated into cellular DNA determined by liquid scintillation counting, as a measurement of CD4 or CD8 T-cell proliferation. Background incorporation in the absence of DCs was always fewer than 1000 cpm.
Analysis of IL-12 production by DCs
The procedures were similar to those described elsewhere.22 Purified splenic DC populations (0.5 × 106/mL) were cultured in duplicate in 96-well round-bottomed plates in a final volume of 100 μL with an IL-12 stimulus (100 U/mL murine recombinant (r) IL-4 (a gift from Immunex, Seattle, WA); 200 U/mL murine rGM-CSF (a gift from Immunex); 20 ng/mL rat recombinant interferon (IFN)–γ (bioactive in mice, Pepro Tech, Rocky Hill, NJ); and 0.5 μM fully phosphorothioated (CpG) CpG1668 oligonucleotide (synthesized by GeneWorks, Adelaide, Australia). After an 18- to 23-hour culture, the supernatant was collected, separated from cells by centrifugation, and stored at −70°C until analysis. Aliquots of the DC culture supernatants were assayed by 2-site enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well polyvinyl chloride microtiter plates (Dynatech Laboratories) were coated with the purified capture monoclonal antibody, namely, R2-9A5 (anti–IL-12 p70) (the hybridoma was obtained from ATCC, Manassas, VA). Cytokine binding was detected with the biotinylated detection monoclonal antibody, namely, C17.8 (anti–IL-12 p40) (hybridoma provided by L. Schofield, The Walter and Eliza Hall Institute of Medical Research). The readout was then obtained by using streptavidin–horseradish peroxidase conjugate (Amersham Pharmacia–Biotech, Buckinghamshire, United Kingdom) and a substrate solution containing 548 μg/mL 2,2′-azino-bis (3-ethylbenz-thiazoline-6-sulfonic acid) (ABTS) (Sigma-Aldrich, St Louis, MO) and 0.001% hydrogen peroxide (Ajax Chemicals, Auburn, Australia) in 0.1 M citric acid, pH 4.2, followed by scanning the optical density at 405 to 490 nm.
Results
BM-derived precursors generate all DC populations in thymus and in spleen
Our previous studies demonstrated the existence of different DC populations in mouse thymus and spleen.3 We have identified at least 2 thymic DC populations, namely, CD4−CD8α+ and CD4−CD8α−; and 3 splenic DC populations, namely CD4+CD8α−, CD4−CD8α−, and CD4−CD8α+. We have also shown that all 3 splenic DC populations could be generated on transfer of BM cells into irradiated recipients.13 We now extend this to show that all DC populations present in normal mouse thymus and spleen can be generated by precursors in BM (Figure 1). However, at the earlier times (2 weeks) after BM transfer, the highest level and proportion of CD4−CD8α+ DCs were generated in spleen, whereas the generation of CD4+CD8α− DCs, the major DC population in normal mouse spleen, reached its peak much later (4 weeks). The proportion of each splenic DC population generated by BM did not match the proportions found in the normal mouse spleen until 4 to 8 weeks after BM transfer13(Table 1; Kamath et al13(Fig 8)).
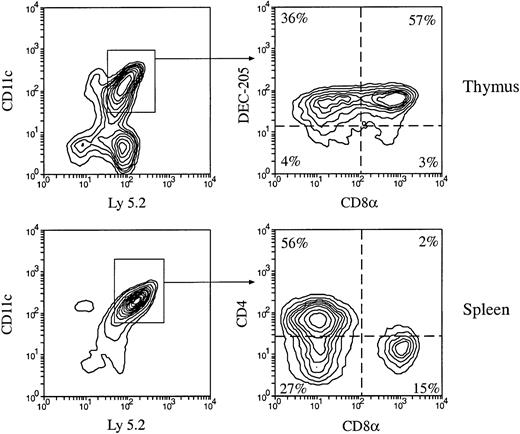
Production of thymic and splenic DC populations by mouse BM cells.
Total BM cells (3 × 106) from C57BL/6 Ly 5.2 mice were IV injected into lethally irradiated Ly 5.1 recipient mice. At various times after BM cell transfer, DCs were enriched from the recipient thymus and spleen, then stained with fluorescent-conjugated antibodies to Ly 5.2 and to DC markers CD11c, CD4, CD8α, and DEC-205. The phenotype of BM-derived DCs was determined by examining the CD4, CD8, and DEC-205 expression on the gated donor type Ly 5.2+CD11c+ cells. Examples of the phenotypes of BM-derived DCs in the recipient thymus and spleen 4 weeks after BM transfer are shown.
Production of thymic and splenic DC populations by mouse BM cells.
Total BM cells (3 × 106) from C57BL/6 Ly 5.2 mice were IV injected into lethally irradiated Ly 5.1 recipient mice. At various times after BM cell transfer, DCs were enriched from the recipient thymus and spleen, then stained with fluorescent-conjugated antibodies to Ly 5.2 and to DC markers CD11c, CD4, CD8α, and DEC-205. The phenotype of BM-derived DCs was determined by examining the CD4, CD8, and DEC-205 expression on the gated donor type Ly 5.2+CD11c+ cells. Examples of the phenotypes of BM-derived DCs in the recipient thymus and spleen 4 weeks after BM transfer are shown.
Thymic and splenic dendritic cell reconstitution by total unseparated bone marrow cells
| Weeks posttransfer . | Total DCs per thymus (× 10−3, per 106 BM transferred) . | Thymic DCs (× 10−3) . | Total DCs per spleen (× 10−3, per 106 BM transferred) . | Splenic DCs (× 10−3) . | |||
|---|---|---|---|---|---|---|---|
| CD8α+ . | CD8α− . | CD4+8α− . | CD4−8α− . | CD4−8α+ . | |||
| 1 | 0.7 | 0.1 | 0.6 | 43 | 6 | 14 | 23 |
| 2 | 40.3 | 25.4 | 14.9 | 1556 | 293 | 330 | 933 |
| 4 | 25.9 | 11.4 | 14.5 | 703 | 426 | 174 | 103 |
| 8 | 64.0 | 38.1 | 25.9 | 636 | 357 | 170 | 108 |
| Weeks posttransfer . | Total DCs per thymus (× 10−3, per 106 BM transferred) . | Thymic DCs (× 10−3) . | Total DCs per spleen (× 10−3, per 106 BM transferred) . | Splenic DCs (× 10−3) . | |||
|---|---|---|---|---|---|---|---|
| CD8α+ . | CD8α− . | CD4+8α− . | CD4−8α− . | CD4−8α+ . | |||
| 1 | 0.7 | 0.1 | 0.6 | 43 | 6 | 14 | 23 |
| 2 | 40.3 | 25.4 | 14.9 | 1556 | 293 | 330 | 933 |
| 4 | 25.9 | 11.4 | 14.5 | 703 | 426 | 174 | 103 |
| 8 | 64.0 | 38.1 | 25.9 | 636 | 357 | 170 | 108 |
DCs indicate dendritic cells; BM, bone marrow.
Total BM cells (3 to 5 × 106) from C57BL/6 Ly 5.2 mice were intravenously injected into lethally irradiated Ly 5.1 recipient mice. At 1 to 8 weeks after BM cell transfer, DCs were enriched from the recipient thymus and spleen and then stained with fluorescent-conjugated antibodies to Ly 5.2, and DC markers CD11c, CD4, CD8α, and DEC-205. The phenotype of BM-derived DCs was determined by examining the expression of CD4 and CD8α on the donor type Ly 5.2+CD11c+ cells. The absolute number of total BM-derived DCs, as well the number of each BM-derived DC population per thymus and per spleen, was calculated at each time point and expressed per 106 BM cells transferred. The data are the means of 2 to 3 such experiments at each time point. Each experimental group contained 3 mice.
The BM-derived splenic DCs can function as mature antigen-presenting cells
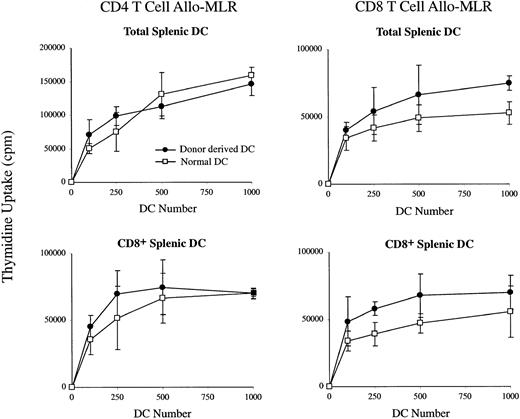
To determine whether the DCs produced in the irradiated recipients by the transferred BM precursors were mature and functional antigen-presenting cells, we examined these BM-derived DCs for their ability to stimulate T-cell proliferation in an allo-MLR assay. As shown in Figure 2, when compared with normal splenic DCs, the BM-derived total splenic DCs and CD8α+ DC subset, isolated after BM transfer, stimulated proliferation of both CD4+ and CD8+ allogeneic T-cells with similar, if not better, efficiency compared with normal total splenic DCs and CD8α+ DCs. This indicated that these BM-derived splenic DC populations were mature and did not require further maturation to become functional antigen-presenting cells.
The stimulation of allogeneic T cells by BM-donor–derived DCs in the spleens of irradiated recipients.
Ly 5.2+ C57BL/6 BM-derived total DCs or CD8α+DCs purified from the spleen of recipient mice were cultured in the presence of IL-2 with purified CD4+ or CD8+ T cells from Balb/c mice. Uptake of 3H-TdR was measured at day 3 of culture. Freshly isolated total splenic DCs or CD8α+ DCs from normal mice were used as controls. Background incorporation in the absence of DCs was always fewer than 1000 cpm. Error bars represent the range of triplicate samples.
The stimulation of allogeneic T cells by BM-donor–derived DCs in the spleens of irradiated recipients.
Ly 5.2+ C57BL/6 BM-derived total DCs or CD8α+DCs purified from the spleen of recipient mice were cultured in the presence of IL-2 with purified CD4+ or CD8+ T cells from Balb/c mice. Uptake of 3H-TdR was measured at day 3 of culture. Freshly isolated total splenic DCs or CD8α+ DCs from normal mice were used as controls. Background incorporation in the absence of DCs was always fewer than 1000 cpm. Error bars represent the range of triplicate samples.
The splenic CD4−CD8α+ DC population generated at the early stages of reconstitution by BM cells are potent IL-12 producers
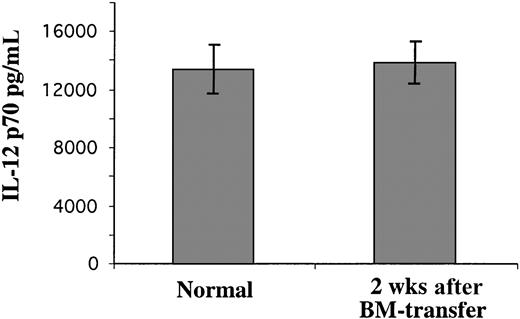
It was not clear whether the high levels of CD4−CD8α+ DCs generated at early time points (2 weeks) after BM transfer were identical with normal steady-state splenic CD4−CD8α+ DCs. To determine whether these early CD4−CD8α+ DC products of BM were functional, we used an ELISA assay to determine their ability to produce IL-12, as normal splenic CD4−CD8α+DCs have a high capacity to produce IL-12 p70 (the bioactive form).4,5 7 As shown in Figure3, when activated by a combination of stimuli including IL-4, GM-CSF, IFN-γ, and CpG, the BM-derived splenic CD4−CD8α+ DCs formed early after BM transfer produced an amount of IL-12 p70 similar to that produced by normal splenic CD4−CD8α+ DCs. By this criterion, the splenic CD4−CD8α+ DCs produced in large numbers soon after BM transfer were functional DCs.
The production of IL-12 p70 by BM-donor–derived DCs isolated from irradiated recipient mouse spleen.
The Ly 5.2+ donor BM-derived CD8α+ DCs from BM reconstituted irradiated Ly 5.1 recipient mice 2 weeks after BM transfer, or CD8α+ DCs from normal mice, were purified from the spleens. The purified CD8α+ DCs (105) were cultured with an optimized IL-12 stimulus; the supernatant was collected after overnight culture and assayed for IL-12 p70 content by ELISA. The result is representative of 2 such experiments. Error bars represent the range of duplicate samples.
The production of IL-12 p70 by BM-donor–derived DCs isolated from irradiated recipient mouse spleen.
The Ly 5.2+ donor BM-derived CD8α+ DCs from BM reconstituted irradiated Ly 5.1 recipient mice 2 weeks after BM transfer, or CD8α+ DCs from normal mice, were purified from the spleens. The purified CD8α+ DCs (105) were cultured with an optimized IL-12 stimulus; the supernatant was collected after overnight culture and assayed for IL-12 p70 content by ELISA. The result is representative of 2 such experiments. Error bars represent the range of duplicate samples.
DC production by lymphoid-restricted precursors
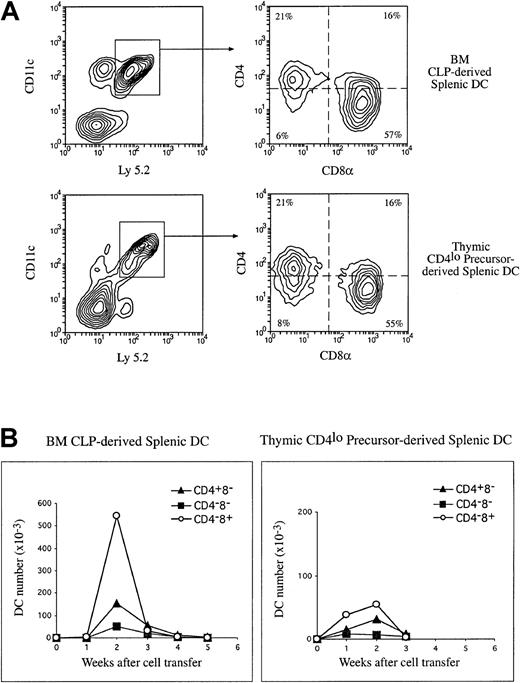
Our previous studies have shown that the early intrathymic lymphoid precursors (CD4low precursors) have the potential to generate DCs.9,11,23 To extend this to BM, we compared the capacity for DC production by the intrathymic CD4lowprecursors with that of the BM CLPs. Purified CD4lowprecursors (2 to 3 × 104) and BM CLPs (1 to 2 × 104) from C57BL Ly 5.2 mice were IV injected into irradiated C57BL Ly 5.1 recipients. At 1 to 4 weeks after transfer, DCs were enriched from the thymus and spleen of the recipients and stained for Ly 5.2, together with the DC markers CD11c, CD4, CD8, and DEC-205. Donor-derived DCs were revealed by gating for Ly 5.2+CD11c+ cells during flow-cytometric analysis, and the donor-derived DC subpopulations were determined on the basis of their expression of CD4, CD8, and DEC-205, as shown in Figure 1. The total number of donor-derived DCs and the total number of each donor-derived DC subpopulation in thymus and spleen were calculated and are shown in Table 2. Both the intrathymic CD4low and BM CLP precursor populations were capable of repopulating thymic and splenic DC populations, as shown in Table 2 and Figure 4. On a per cell basis, the BM CLPs were much more potent than the intrathymic CD4lowprecursors in DC production. However, the production of DCs by both these lymphoid precursors was transient, with a maximum in both thymus and spleen at 2 weeks after precursor transfer (Table 2; Figure 4B). The DC production then declined rapidly and was almost undetectable by 4 weeks (Table 2; Figure 4B). In contrast, after the initial 2-week peak and partial decline, DC production by BM cells13(Table 1) was maintained at a constant level for at least 8 weeks after BM cell transfer, along with the maintenance of other hemopoietic lineages (data not shown). These results were expected, since the lymphoid-restricted precursors have very little self-renewal capacity and therefore gave a transient DC reconstitution, whereas the multipotent hemopoietic stem cells (HSCs) contained in the BM have self-renewal capacity and therefore gave a long-term repopulation of DCs.
Thymic and splenic dendritic cells produced by lymphoid-restricted precursor populations
| Weeks posttransfer of precursors . | Total DCs per thymus (× 10−3, per 104 precursors transferred) . | Thymic DCs (× 10−3) . | Total DCs per spleen (× 10−3, per 104 precursors transferred) . | Splenic DCs (× 10−3) . | |||
|---|---|---|---|---|---|---|---|
| CD8α+ . | CD8α− . | CD4+8α− . | CD4−8α− . | CD4−8α+ . | |||
| Thymic CD4low | |||||||
| 1 | 2.8 | 1.7 | 1.1 | 60.3 | 15.0 | 7.5 | 38.0 |
| 2 | 3.6 | 2.7 | 0.9 | 92.5 | 31.0 | 6.5 | 55.0 |
| 3 | 0.4 | 0.3 | 0.1 | 15.0 | 8.1 | 3.5 | 3.4 |
| BM CLPs | |||||||
| 1 | 0.7 | 0.3 | 0.4 | 6.9 | 1.4 | 1.3 | 4.2 |
| 2 | 15.0 | 11.3 | 3.7 | 747.0 | 152.0 | 50.0 | 545.0 |
| 3 | 5.1 | 3.0 | 2.1 | 107.0 | 55.0 | 21.0 | 31.0 |
| 4 | 2.5 | 1.0 | 1.5 | 18.9 | 5.0 | 3.9 | 10.0 |
| 5 | 2.6 | 1.5 | 1.1 | 6.7 | 4.5 | 0.6 | 1.4 |
| Weeks posttransfer of precursors . | Total DCs per thymus (× 10−3, per 104 precursors transferred) . | Thymic DCs (× 10−3) . | Total DCs per spleen (× 10−3, per 104 precursors transferred) . | Splenic DCs (× 10−3) . | |||
|---|---|---|---|---|---|---|---|
| CD8α+ . | CD8α− . | CD4+8α− . | CD4−8α− . | CD4−8α+ . | |||
| Thymic CD4low | |||||||
| 1 | 2.8 | 1.7 | 1.1 | 60.3 | 15.0 | 7.5 | 38.0 |
| 2 | 3.6 | 2.7 | 0.9 | 92.5 | 31.0 | 6.5 | 55.0 |
| 3 | 0.4 | 0.3 | 0.1 | 15.0 | 8.1 | 3.5 | 3.4 |
| BM CLPs | |||||||
| 1 | 0.7 | 0.3 | 0.4 | 6.9 | 1.4 | 1.3 | 4.2 |
| 2 | 15.0 | 11.3 | 3.7 | 747.0 | 152.0 | 50.0 | 545.0 |
| 3 | 5.1 | 3.0 | 2.1 | 107.0 | 55.0 | 21.0 | 31.0 |
| 4 | 2.5 | 1.0 | 1.5 | 18.9 | 5.0 | 3.9 | 10.0 |
| 5 | 2.6 | 1.5 | 1.1 | 6.7 | 4.5 | 0.6 | 1.4 |
CLPs indicate common lymphoid precursors. See Table 1 footnote for other abbreviations.
Purified BM CLPs or the thymic CD4low precursors (1 to 2 × 104) were injected intravenously into lethally irradiated Ly 5.1 recipients. At various times after precursor cell transfer, donor-derived DC populations were analyzed, and the number of total donor-derived DCs and the number of each donor-derived DC population were calculated as described in Table 1 and expressed per 104 cells transferred. The data presented are means of 2 to 3 such experiments at each time point. Each experimental group included 3 mice.
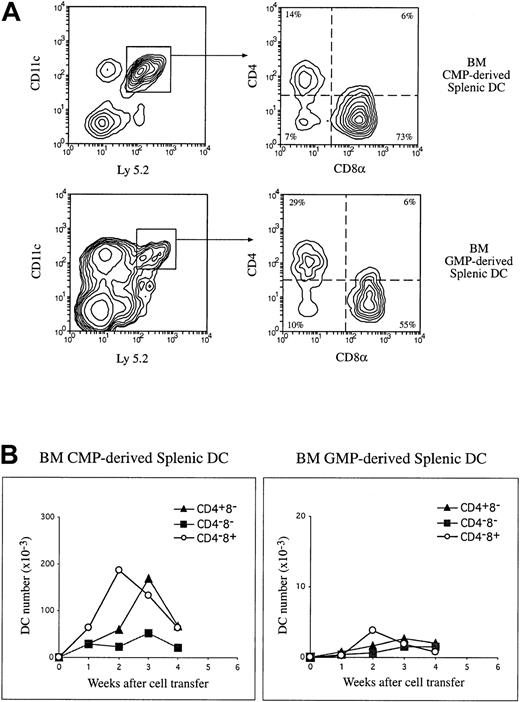
The production of splenic DC populations by lymphoid-restricted precursors.
Purified Ly 5.2+ BM CLPs or the thymic CD4lowprecursors (1 to 2 × 104) were injected IV into lethally irradiated Ly 5.1 recipients. At various times after precursor cell transfer, DCs were enriched from the recipient spleen and stained with fluorescent-conjugated antibodies to Ly 5.2 and to DC markers CD11c, CD4, and CD8α. Donor-derived DCs were revealed by the expression of Ly 5.2 and CD11c. The phenotype of donor-derived DCs were determined on the basis of the CD4 and CD8α expression on the Ly 5.2+CD11c+ cells. (A) Examples of the phenotype of precursor-derived DCs 2 weeks after IV transfer of CLPs or the CD4low precursors. (B) The absolute number of each donor-derived DC population was calculated and expressed per 104 cells transferred for each time point, and these are presented here in a kinetic manner. The data presented here are the means of 2 to 3 such experiments at each time point, and each experimental group included 3 mice.
The production of splenic DC populations by lymphoid-restricted precursors.
Purified Ly 5.2+ BM CLPs or the thymic CD4lowprecursors (1 to 2 × 104) were injected IV into lethally irradiated Ly 5.1 recipients. At various times after precursor cell transfer, DCs were enriched from the recipient spleen and stained with fluorescent-conjugated antibodies to Ly 5.2 and to DC markers CD11c, CD4, and CD8α. Donor-derived DCs were revealed by the expression of Ly 5.2 and CD11c. The phenotype of donor-derived DCs were determined on the basis of the CD4 and CD8α expression on the Ly 5.2+CD11c+ cells. (A) Examples of the phenotype of precursor-derived DCs 2 weeks after IV transfer of CLPs or the CD4low precursors. (B) The absolute number of each donor-derived DC population was calculated and expressed per 104 cells transferred for each time point, and these are presented here in a kinetic manner. The data presented here are the means of 2 to 3 such experiments at each time point, and each experimental group included 3 mice.
The major DC product of both lymphoid-restricted precursors was CD4−CD8α+ DCs, both in the thymus and in the spleen, in line with our previous studies on the thymic CD4low precursor.9-11 However, it was also clear that as well as this dominant product, some CD4+CD8α− DCs and a few CD4−CD8α− DC progeny were detected in the spleen. These had not been evident in our earlier studies using a different DC isolation procedure.11 Additional staining with monoclonal antibody against CD11b revealed that these CD8α− DC progeny were also CD11b+ (data not shown). All DC progeny declined after the sharp 2-week peak, but transiently at 3 weeks the proportions of the populations resembled those in normal spleen, with CD4+CD8α− DCs dominant. However, in contrast to what occurred in whole-BM reconstitution, all DC subtypes then declined further rather than being sustained.
DC production by myeloid-committed precursors
Although we had considered the CD8α− DC populations myeloid derived, direct evidence had not been available. A recent study by Traver et al18 reported that both CD8α+and CD8α− DCs could be generated by BM CMPs. However, it was not clear whether all 3 splenic DC populations could be generated from CMPs and whether these DCs were generated with similar kinetics. Accordingly, we examined the capacity for DC production by the BM CMPs and their more restricted downstream product, the GMPs. Purified CMPs and GMPs from C57BL/6 Ly 5.2 mice were injected IV into lethally irradiated Ly 5.1 recipients. DC production in the thymus and spleen by these precursors was analyzed 1 to 4 weeks after precursor transfer. As shown in Figure 5 and Table 3, both CMPs and GMPs were capable of producing all thymic and splenic DC subpopulations, including the CD8α+ DCs. And as expected, the splenic CD4+CD8α− and CD4−CD8α− DCs derived from both precursor populations also expressed the myeloid marker CD11b, but the CD4−CD8α+ DC progeny did not (data not shown). However, the capacity for DC production by GMPs was rather poor compared with CMPs, and that of CMPs was substantially less than for CLPs (Tables2-3). The DC production by both myeloid precursor populations was transient, indicating a lack of self-renewal capacity. Similarly to what occurs in the lymphoid precursors, the production of the CD4−CD8α+ DC population in the spleen by these myeloid precursors reached a peak 2 weeks after cell transfer (Figure 5 and Table 3). However, unlike the lymphoid precursors, the production of the CD4+CD8α− DCs reached a peak level at 3 weeks after precursor transfer, when the number of the CD4+CD8α− DCs was almost the same as that of CD4−CD8α+ DCs at their peaks. These results indicated that the committed myeloid precursors were capable of generating all thymic and splenic DC populations. However, on a cell-for-cell basis, the myeloid precursors were less efficient at DC production than the lymphoid-restricted precursors, and they produced a different balance of DC subtypes.
The production of splenic DC populations by BM myeloid-committed precursors.
Purified Ly 5.2+ BM CMPs and GMPs were IV injected into lethally irradiated Ly 5.1 recipients. At various times after precursor cell transfer, DCs were enriched from the recipient spleen, and donor-derived DC populations were analyzed as described in Figure 4. (A) Examples of the phenotype of precursor-derived DCs 2 weeks after IV transfer of CMPs or GMPs are shown. (B) The absolute number of each donor-derived DC population was calculated at each time point, and these are presented in a kinetic manner. The data presented here are the means of 2 to 3 such experiments for each time point, and each experimental group included 3 mice.
The production of splenic DC populations by BM myeloid-committed precursors.
Purified Ly 5.2+ BM CMPs and GMPs were IV injected into lethally irradiated Ly 5.1 recipients. At various times after precursor cell transfer, DCs were enriched from the recipient spleen, and donor-derived DC populations were analyzed as described in Figure 4. (A) Examples of the phenotype of precursor-derived DCs 2 weeks after IV transfer of CMPs or GMPs are shown. (B) The absolute number of each donor-derived DC population was calculated at each time point, and these are presented in a kinetic manner. The data presented here are the means of 2 to 3 such experiments for each time point, and each experimental group included 3 mice.
Thymic and splenic denritic cells produced by myeloid-committed precursor populations
| Weeks posttransfer of precursors . | Total DCs per thymus (× 10−3, per 104 precursors transferred) . | Thymic DCs (× 10−3) . | Total DCs per spleen (× 10−3, per 104 precursors transferred) . | Splenic DCs (× 10−3) . | |||
|---|---|---|---|---|---|---|---|
| CD8α+ . | CD8α− . | CD4+8α− . | CD4−8α− . | CD4−8α+ . | |||
| BM GMPs | |||||||
| 1 | < 0.1 | < 0.1 | < 0.1 | 1.4 | 0.7 | 0.4 | 0.3 |
| 2 | 1.0 | 0.8 | 0.2 | 8.1 | 2.2 | 0.8 | 5.1 |
| 3 | 0.5 | 0.3 | 0.2 | 6.1 | 2.7 | 1.5 | 1.9 |
| 4 | 0.4 | 0.3 | 0.1 | 4.3 | 2.0 | 1.5 | 0.8 |
| BM CMPs | |||||||
| 1 | 1.2 | 0.8 | 0.4 | 169.6 | 48.6 | 43.5 | 77.5 |
| 2 | 8.8 | 6.2 | 2.6 | 270.1 | 59.4 | 23.7 | 187.0 |
| 3 | 4.0 | 2.6 | 1.4 | 354.8 | 170.1 | 52.2 | 132.5 |
| 4 | 1.5 | 1.2 | 0.3 | 154.0 | 68.0 | 22.0 | 64.0 |
| Weeks posttransfer of precursors . | Total DCs per thymus (× 10−3, per 104 precursors transferred) . | Thymic DCs (× 10−3) . | Total DCs per spleen (× 10−3, per 104 precursors transferred) . | Splenic DCs (× 10−3) . | |||
|---|---|---|---|---|---|---|---|
| CD8α+ . | CD8α− . | CD4+8α− . | CD4−8α− . | CD4−8α+ . | |||
| BM GMPs | |||||||
| 1 | < 0.1 | < 0.1 | < 0.1 | 1.4 | 0.7 | 0.4 | 0.3 |
| 2 | 1.0 | 0.8 | 0.2 | 8.1 | 2.2 | 0.8 | 5.1 |
| 3 | 0.5 | 0.3 | 0.2 | 6.1 | 2.7 | 1.5 | 1.9 |
| 4 | 0.4 | 0.3 | 0.1 | 4.3 | 2.0 | 1.5 | 0.8 |
| BM CMPs | |||||||
| 1 | 1.2 | 0.8 | 0.4 | 169.6 | 48.6 | 43.5 | 77.5 |
| 2 | 8.8 | 6.2 | 2.6 | 270.1 | 59.4 | 23.7 | 187.0 |
| 3 | 4.0 | 2.6 | 1.4 | 354.8 | 170.1 | 52.2 | 132.5 |
| 4 | 1.5 | 1.2 | 0.3 | 154.0 | 68.0 | 22.0 | 64.0 |
GMPs indicate granulocyte and macrophage precursors; CMPs, common myeloid precursors. See Table 1 footnote for other abbreviations.
Purified BM CMPs or GMPs (1 to 2 × 104) were injected intravenously into lethally irradiated Ly 5.1 recipients. At various times after precursor cell transfer, donor-derived DC populations were analyzed, and the number of total donor-derived DCs and the number of each donor-derived DC population were calculated as described in Table1 and expressed per 104 cells transferred. The data presented are means of 2 to 3 such experiments at each time point. Each experimental group included 3 mice.
Production of IL-12 p70 by CD8α+ DCs of lymphoid and myeloid precursor origin
The CD8α+ DCs of mouse spleen have been shown to have the greatest potential to produce the bioactive p70 form of IL-12.4,5,7 To determine if the CD8α+ DCs derived from CLPs and CMPs were equivalent in this respect, the CD8α+ DC progeny of the isolated precursors were sorted from separate groups of recipient mice and assayed for IL-12 p70 production side by side under optimized culture conditions.7 In 3 separate experiments, the production of IL-12 p70 by CD8α+ DCs (0.5 × 106/mL) was 42.3 to 49.3 ng/mL for CLP-derived DCs, and 43.8 to 53.1 ng/mL for CMP-derived DCs, compared with 26.0 to 33.1 ng/mL for CD8α+ DCs isolated from normal mouse spleen. Thus both myeloid- and lymphoid-derived CD8α+ DCs share this common function, with the conditions in the irradiated recipient promoting the potential for IL-12 production.
Discussion
DCs are specialized for the collection, transport, processing, and presentation of antigen to T lymphocytes. Although all DCs share these biological features, DCs have been found to be heterogeneous in surface phenotype and in biological functions.1,2,24,25Particularly important is the ability of different subtypes to produce different cytokines and so induce different T-cell responses.4,5,7,26-28 Are these functionally distinct DC subtypes simply activation or maturation states of a single DC lineage, or are they products of quite separate developmental lineages? Our recent studies on the relative maturation state and developmental kinetics of the 3 splenic DC populations, CD4+CD8α−, CD4−CD8α−, and CD4−CD8α+, indicated these are products of 3 separate developmental streams, at least as far back as their immediate dividing precursors.13 Can these sublineages be tracked back to committed precursors in the BM? In the present study, we re-examined our earlier hypothesis that the separate DC lineages are already determined at the level of the lymphoid- or myeloid-committed early hemopoietic precursors in BM, the former producing CD8α+ DCs, the latter CD8α− DCs. This hypothesis is found to be incorrect in this extreme form, although many of the original findings are confirmed.
The starting point of the lymphoid DC versus myeloid DC concept was our finding that an early lymphoid-restricted T-cell precursor in mouse thymus was able to produce CD8α+ DCs.9-11However, this link between DC and T-cell development has been challenged by 2 recent studies.29,30 In the study by Radtke et al30 using hemopoietic precursor cells deficient in the Notch-1 protein, it was found that a blockage in T-cell development at an early stage with an absence of detectable T precursors still allowed normal development of thymic CD8α+ DCs. This suggested either that the supposed lymphoid-restricted precursor population included separate DC precursors of identical surface phenotype or that CD8α+DC development was more clearly linked to other lymphoid cells, particularly B cells. Our current results now introduce a third possibility of developmental flexibility: that in the absence of lymphoid-restricted T precursors, myeloid precursors can form the thymic CD8α+ DCs.
Our results agree with the recent studies of Manz et al19in reinforcing the originally surprising concept that a lymphoid-restricted precursor population still has the capacity to produce DCs; this is now extended to include the lymphoid-restricted precursors of BM, which are even more efficient at DC production in our adoptive transfer system. We have found that these lymphoid-restricted precursors can also generate DCs in culture (unpublished data, March 2000). Since these lymphoid precursors are more efficient at DC production than myeloid-restricted precursors, trace contamination with the latter cannot explain our results. Since these lymphoid precursors give a fast and transient repopulation of DCs along with the lymphoid cells, a characteristic of committed precursors, rather than the prolonged reconstitution of all lineages characteristic of multipotent stem cells, trace contamination with the latter cannot explain our results. Separate committed DC precursors and lymphoid precursors with identical surface markers within our lymphoid-restricted population remains a possibility, but then these DC precursors would have to be separate specialized subsets of lymphoidlike DC precursors with reconstitution characteristics different from those of myeloidlike DC precursors. The simplest hypothesis at this stage is that individual lymphoid-restricted precursor cells retain a capacity to form DCs, even though they have lost the capacity to form most myeloid cells.
Our original hypothesis was that lymphoid-precursors would produce only CD8α+ DCs. This indeed is the dominant DC type that they produce. However, it has been clear for some time from our own work that such precursors could also form DCs lacking CD8α, both in culture23 and in lymph nodes after IV transfer.31 Our present results, and those of Martin et al,32 now clearly show that the lymphoid precursors also give rise to some CD8α− DCs in recipient mouse spleen, and these are predominantly the CD4+CD8α− DC subtype. These would have been selectively lost in the depletion procedures used in our earlier studies to avoid any macrophage or lymphocyte contamination.3 Despite this lack of determination by the early precursor population, CD8α remains a useful marker for a functionally distinct subclass of murine DCs.4,5,7,27,28 33
The key piece of evidence missing at the time of our original hypothesis was the nature of the DCs produced by the myeloid-restricted precursors; they were assumed to generate only CD8α−DCs. The availability of methods to isolate myeloid-restricted precursors15 led to a direct test of the DC products, first by Traver et al18 and now by us. Both the CMPs and the more restricted GMPs are clearly able to produce both CD8α+ DCs and CD8α− DCs, including both the CD4+CD8α− and the CD4−CD8α− DC types. Both appear less efficient at DC production than the CLPs, and the downstream GMPs are the least efficient of all. While there is some bias, with the CMPs showing greater ability to produce CD4+CDα−DCs at later time points, it is now clear that the surface phenotype of the DCs is not predetermined at the hemopoietic precursor stage.
Our results are therefore in accordance with the Stanford laboratory18 19 in indicating that all DC subtypes as determined by surface phenotype can be of lymphoid- or myeloid-precursor origin. As assessed by IL-12 p70 production, they are also equivalent in function. Although in our studies the lymphoid precursors are more efficient at DC production on a per cell basis, the large excess of myeloid precursors in BM makes it likely that most splenic DCs are of myeloid-precursor origin. However, local environmental influences could greatly alter this calculated balance. In addition, it is not clear that all immediate DC precursors are accounted for by the CLP, CMP, and GMP BM populations, since these precursors were selected and purified on the basis of assays for production of hemopoietic lineages other than DCs. Our data and our preliminary studies suggest that DC precursors other than CLPs and CMPs may exist in the BM. The total number of CD8α+ DCs produced by these precursors 2 weeks post–precursor transfer would not make up the peak of CD8α+ DCs produced by 1 × 106 total BM cells at this time point (Table 1), given their low incidence in BM. The DCs generated from BM HSCs could account for only a small proportion of DCs (less than 10%) generated by total BM (data not shown). Our preliminary studies showed that some lineage marker–positive BM cells could also produce DCs upon IV transfer. Therefore, DCs generated from total BM will include DCs derived from HSCs, CLPs, CMPs, and GMPs, and from DC precursors yet to be identified.
Our results extend the data from our previous studies and from the recent studies of the Stanford laboratory by following the kinetics of development of each DC subtype, particularly the development of the major CD4+CD8α− spleen subset we recently identified.3,13 This subset can be produced by both lymphoid- and myeloid-precursor populations, but the myeloid precursors have a relatively greater potential for generating them. This is not apparent unless the kinetics of DC development are considered, since the CD4−CD8α+ DCs are generated early after transfer (2 weeks), whereas the CD4+CD8α−DCs are generated later (3 to 4 weeks). This sequence is completely in accordance with the generation rate and lifespan of splenic DCs as determined by bromodeoxyuridine (BrDu)–labeling techniques, which demonstrate a much faster turnover of CD4−CD8α+ DCs than of CD4+CD8α− DCs.13 Although with a slower turnover rate and being generated later than the CD4−CD8α+ DCs following BM transfer, the CD4+CD8α− DCs are not progeny of the CD4−CD8α+ DCs, which has been demonstrated by our previous studies on the kinetics of BrDu labeling of the 3 DC subtypes from mouse spleen.13
Overall, our results point to a substantial developmental flexibility, in terms of DC subtype production, at the level of the early hemopoietic precursors. The points downstream of these precursors where the DC sublineages diverge and become more fixed, and the factors determining this divergence, now need to be elucidated.
The authors wish to thank Dr F. Battye, D. Kaminaris, V. Lapatis, and J. Parker for their assistance with flow cytometry.
Supported by the National Health and Medical Research Council, Australia, and by a Deutsche Krebshilfe fellowship (H.H.). L.W. is a Clinical Investigator of Cancer Research Institute (New York, NY). The Wellcome Trust provided funds for cell sorting instruments.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Li Wu, The Walter and Eliza Hall Institute of Medical Research, P.O. Royal Melbourne Hospital, Victoria. 3050, Australia; e-mail: wu@wehi.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal