Abstract
Complement-dependent cytotoxicity is thought to be an important mechanism of action of the anti-CD20 monoclonal antibody rituximab. This study investigates the sensitivity of freshly isolated cells obtained from 33 patients with B-cell chronic lymphocytic leukemia (B-CLL), 5 patients with prolymphocytic leukemia (PLL), and 6 patients with mantle cell lymphoma (MCL) to be lysed by rituximab and complement in vitro. The results showed that in B-CLL and PLL, the levels of CD20, measured by standard immunofluorescence or using calibrated beads, correlated linearly with the lytic response (coefficient greater than or equal to 0.9;P < .0001). Furthermore, the correlation remained highly significant when the 6 patients with MCL were included in the analysis (coefficient 0.91; P < .0001), which suggests that CD20 levels primarily determine lysis regardless of diagnostic group. The role of the complement inhibitors CD46, CD55, and CD59 was also investigated. All B-CLL and PLL cells expressed these molecules, but at different levels. CD46 was relatively weak on all samples (mean fluorescence intensity less than 100), whereas CD55 and CD59 showed variability of expression (mean fluorescence intensity 20-1200 and 20-250, respectively). Although CD55 and CD59 levels did not permit prediction of complement susceptibility, the functional block of these inhibitors demonstrated that they play an important role in regulating complement-dependent cytotoxicity. Thus, lysis of poorly responding B-CLL samples was increased 5- to 6-fold after blocking both CD55 and CD59, whereas that of high responders was essentially complete in the presence of a single blocking antibody. These data demonstrate that CD20, CD55, and CD59 are important factors determining the in vitro response to rituximab and complement and indicate potential strategies to improve the clinical response to this biologic therapy.
Introduction
The use of therapeutic monoclonal antibodies (MAbs) for the treatment of cancer has become a promising approach over the last few years, as exemplified by the success of the anti-CD20 chimeric MAb rituximab, used for the treatment of B-cell non-Hodgkin lymphoma (B-NHL).1-4 Other promising MAbs are emerging, such as Campath-1H (anti-CD52) for the treatment of B-cell chronic lymphocytic leukemia (B-CLL),5 anti-CD33 for acute myelocytic leukemia,6 anti-p185HER2/neu for breast cancer,7 and some others.8 The unconjugated forms of these antibodies are thought to act in vivo mostly through activation of antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC),9-12 although direct growth inhibition and/or induction of apoptosis may also take place.11,13-15The relative contributions of these different mechanisms of action are still a matter of debate. The available evidence suggests that activation of ADCC and CDC by rituximab is crucial for in vivo response because an IgG4 version of the antibody does not deplete normal B cells in primates.9 Furthermore, complement consumption has been observed in vivo after rituximab administration.16 We and others have shown previously that follicular lymphoma (FL) cells in vitro can be lysed effectively by rituximab and human complement, although a high degree of heterogeneity in the response was observed in different cell lines.11,12 Furthermore, we have shown in 4 FL cell lines that the levels of the complement inhibitors CD55 and CD59 are at least in part responsible for this heterogeneity.11 Thus, these data using FL cells suggested that complement-mediated lysis is likely to be an important mechanism of action of rituximab in vivo. Furthermore, the data suggested that this mechanism could be the basis for the heterogeneity of the response of different patients to rituximab in vivo.
Rituximab was originally approved for the treatment of low-grade B-NHL, in particular FL, but several trials are in progress testing its activity in mantle cell lymphoma (MCL), hairy cell leukemia (HCL), and chronic lymphocytic leukemia (CLL).16-19 It is therefore of particular interest to determine the capacity of freshly isolated B cells obtained from patients with different types of leukemias or lymphomas to be lysed by rituximab and complement and to determine the relative roles of CD20 expression levels as well as CD55 and CD59 complement inhibitors in these cells. In the longer term, these studies should help answer the question whether the in vitro response of leukemic cells to rituximab can be predictive of in vivo response and whether easily measurable parameters, such as CD20, CD55, and CD59 expression levels, may predict such a response. Such studies may permit us in the future to select patients who will benefit from rituximab therapy and to design new therapeutic protocols, for example blocking of complement inhibitors, to increase significantly rituximab activity in vivo.
Materials and methods
Cells
Heparinized peripheral blood was obtained after informed consent from patients with B-CLL, prolymphocytic leukemia (PLL), and MCL with significant circulating disease (at least 50% of neoplastic cells in the mononuclear cell fraction). Except for some B-CLL, all patients' samples were taken at diagnosis. All patients were diagnosed by routine immunophenotypic, morphologic, and clinical criteria. In all cases, double staining with CD19 and sIgκ or sIgλ was performed, allowing us to establish monoclonality and to determine the percentage of neoplastic versus normal B cells present in the sample. In addition, all patients with MCL were checked for the BCL1 translocation by standard polymerase chain reaction analysis. The cells were separated on a Ficoll Hypaque gradient (Seromed, Berlin, Germany). As controls, mononuclear cell fractions from healthy volunteers were also obtained. In some cases, B cells were purified using the B Cell Isolation Kit, MACS LS separation columns, and a MidiMACS magnetic cell separator (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Peripheral blood T lymphocytes were purified by Ficoll-Hypaque gradient centrifugation and rosetting with aminoethylisothiouronium-treated sheep red blood cells using standard procedures. The resulting lymphocytes were 85% CD3+. DHL-4 cells have been described previously.11
Immunofluorescence
Blood samples from healthy individuals or neoplastic peripheral blood mononuclear cells (PBMCs) were stained with fluorescein isothiocyanate (FITC)–conjugated anti-CD20 antibody (BD Biosciences, San Diego, CA) or phycoerythrin (PE)–conjugated anti-CD20 (BD Biosciences) together with FITC-conjugated anti-CD46 (BD Biosciences), anti-CD55, or anti-CD59 antibody (Caltag Laboratories, Burlingame, CA) or fluorochrome-labeled control antibodies. Samples were analyzed in single or double immunofluorescence on a FACScan instrument or on a FACS Calibur (BD Biosciences). To compare staining intensities for different patients on different days, the negative control curves for all patients were set between the first and 10th channel of fluorescence, and the gate for positive cells was set at or near channel 10. Furthermore, for a number of samples, the absolute number of CD20 molecules was measured using calibrated Quantibrite beads (BD Biosciences) and PE-labeled anti-CD20, following the manufacturer's instructions.
For complement deposition, the anti-C9 MAb aE11 and an anti-C3 goat polyclonal antiserum were used (kindly provided by Dr F. Tedesco, University of Trieste, Italy), together with the appropriate FITC-conjugated secondary antibodies (Jackson Immunoresearch Laboratories, West Grove, PA; and Sigma, St Louis, MO, respectively).
Complement-mediated lysis
Complement cytotoxicity assays were performed essentially as described,11,20 with some modifications. Briefly, 50 000 cells/well were plated in 60 μL in triplicate in 96-well plates in the presence of 10 μg/mL rituximab and/or 10 μg/mL functionally blocking anti-CD55 (BRIC216; International Blood Group Reference Laboratory (IBGRL), Bristol, United Kingdom) or anti-CD59 (BRIC229; IBGRL) antibodies and in the presence or absence of pooled human serum to a final concentration of 25%. The cells were incubated for 4 hours at 37°C, then diluted with medium to 270 μL, and 1/10 volume of Alamar blue solution was added (Biosource International, Camarillo, CA). Incubation was performed overnight at 37°C, and the plates were read in a fluorimeter (Cytofluor 2300; Millipore, Bedford, MA) with excitation at 530 nm and emission at 590 nm.20 In all cases, the effect of rituximab alone in the absence of human serum was determined. The samples with human serum alone were used as negative controls to normalize for the quenching of fluorescence by serum proteins and for the presence of dead cells in the samples in the absence of any treatment. According to the manufacturer's instructions (Biosource), 0.25% Triton-X100 (Sigma) was added to the wells used to set up the background fluorescence (all cells lysed). To confirm the validity of the data, we performed the same assay on at least 10 samples with 106 cells/mL and analyzed the percentage of dead cells using acridine orange staining and fluorescence-activated cell sorter (FACS) analysis, as described previously.11 Furthermore, the linearity of the Alamar blue assay was verified using serial dilutions of cells. Relative lysis was obtained using the following formula: percentage lysed cells/percentage CD20+ cells × 100. In some experiments, cells were stained with 0.5 μg/mL propidium iodide (PI; Sigma) to detect dead cells by FACS analysis.
Statistical analysis
Statistical analysis was performed using the Stat4.5 program on a Macintosh power computer. The Pearson correlation coefficient was obtained for all patients, either individually or grouped together. In the CD55 and CD59 functional assays, 2-way analysis of variance (ANOVA) was performed. To analyze the effects of blocking antibodies against their controls, we performed a t test for paired data. One-way ANOVA was performed to evaluate the differences between the different antibody treatments.
Results
Specificity of CDC assays
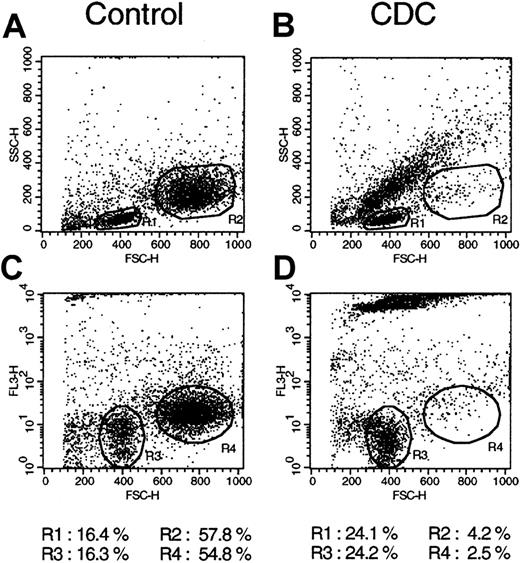
Because we planned to analyze the complement-mediated lysis (CDC) of a large panel of freshly isolated mononuclear cell samples that contained different percentages of CD20+ cells, we first investigated the specificity of lysis in heterogeneous samples. The DHL-4 cell line, which expresses high levels of CD20 and is lysed extremely rapidly (within 15 minutes) after the addition of rituximab and complement,11 was mixed with freshly isolated purified peripheral blood T lymphocytes from healthy donors. Lysis was then performed on this mixed population using rituximab and human complement at standard concentrations. As control, the same cell mix was incubated with human serum alone. Because phenotypic analysis of lysed cells was not possible as a result of high nonspecific staining of dead cells, cell death was analyzed using FACS scatter analysis (Figure1A-B) as well as PI staining (Figure 1C-D). The 2 cell populations (DHL-4 and T lymphocytes) could be easily distinguished by scatter analysis (gate R1 for lymphocytes and gate R2 for DHL-4; Figure 1A). Scatter analysis was confirmed by staining with anti-CD3 and anti-CD19 antibodies, which showed that R1 cells were 85% CD3+ and R2 cells were 90% CD19+ (data not shown). Addition of rituximab and complement led to nearly complete lysis of the DHL-4 cells, as shown by a change in scatter of R2 cells, which passed from 54.8% before lysis to 4.2% of the total cell population (Figure 1B). Rituximab and complement did not, however, affect T cells in gate R1, which increased from 16.4% to 24.1% (Figure 1B). As expected, R1 cells remained 87% CD3+ after lysis (data not shown). A small increase in the percentage of R1 cells was observed in 2 different experiments and was probably due to a decrease in total cell number resulting from complete rupture in small fragments of some DHL-4 cells by complement. Red fluorescence analysis confirmed that a change in scatter was accompanied by PI uptake above control levels in DHL-4 cells (Figure 1C-D; gate R4), but not in T lymphocytes (gate R3). This demonstrates the extreme specificity of lysis for CD20+ cells, even in the presence of strong and rapid complement activation.
Specificity of rituximab and complement-mediated lysis.
Peripheral blood T lymphocytes were mixed with DHL-4 B cells and incubated for 3 hours at 37°C with 25% human serum alone (A,C; control) or in the presence of 10 μg/mL rituximab (B,D; CDC). Cells were then washed, stained with propidium iodide, and analyzed on the FACS. (A,B) Results of scatter analysis. (C,D) Results of dot plots of the red fluorescence. The percentages of cells in both the control and CDC panels are shown at the bottom. The results are representative of 2 separate experiments.
Specificity of rituximab and complement-mediated lysis.
Peripheral blood T lymphocytes were mixed with DHL-4 B cells and incubated for 3 hours at 37°C with 25% human serum alone (A,C; control) or in the presence of 10 μg/mL rituximab (B,D; CDC). Cells were then washed, stained with propidium iodide, and analyzed on the FACS. (A,B) Results of scatter analysis. (C,D) Results of dot plots of the red fluorescence. The percentages of cells in both the control and CDC panels are shown at the bottom. The results are representative of 2 separate experiments.
Complement-mediated lysis of B-CLL and PLL is determined by CD20 levels
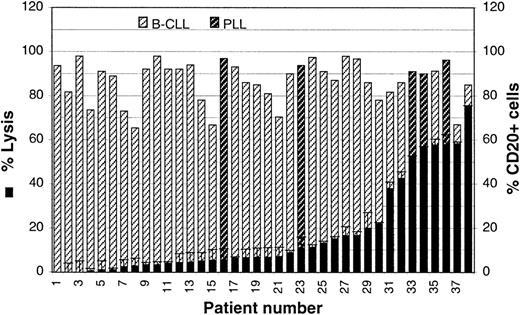
We have shown previously that complement-mediated lysis of FL cell lines is highly heterogeneous and is regulated by the CD55 and CD59 complement inhibitors. To determine whether rituximab and complement can efficiently kill other subtypes of leukemias or lymphomas, we analyzed a large panel of freshly isolated B-CLL and PLL samples. These pathologic entities were grouped in the analysis because the latter represents a clinical and morphologic variant of B-CLL, the 2 show an overlapping phenotype, and the results obtained with the 2 groups of patients were found to be overlapping (see below). Mononuclear cells were isolated from the peripheral blood of 33 patients with B-CLL and 5 with PLL. The percentage of CD20+ cells for each of these cell populations was determined by immunofluorescence and ranged from 65% to 98%, with a mean of 82% (Figure2, hatched bars). The 5 PLL samples are identified with darker hatched bars. Double staining with CD19 and sIgκ or sIgλ showed that normal B cells were present at less than 3% in all cases, assuming a normal κ to λ ratio of 2:1 (data not shown).
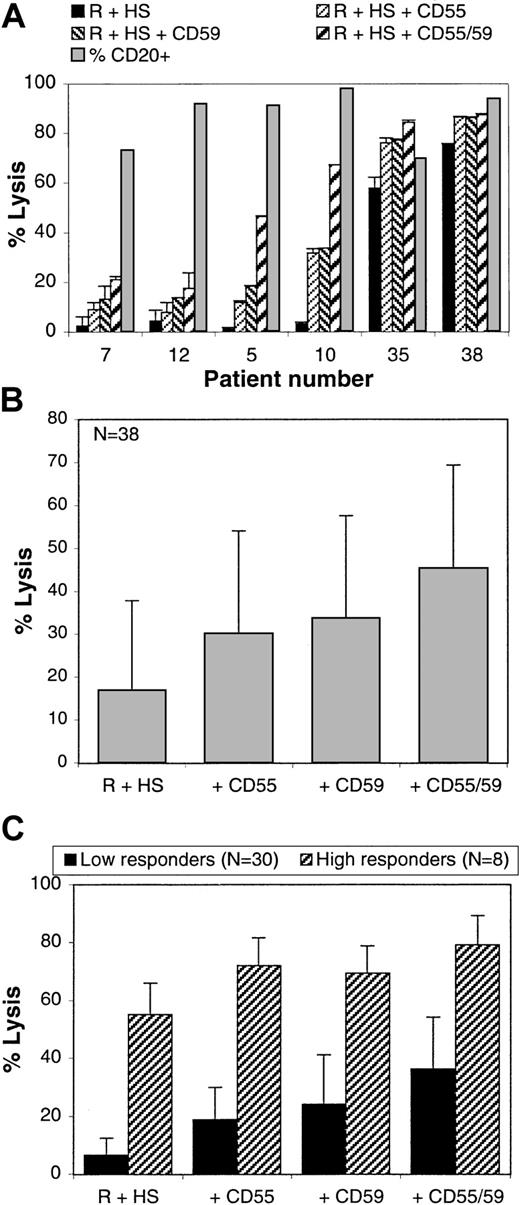
Complement-mediated lysis of B-CLL cells.
CDC assays were performed on the PBMCs isolated from 33 patients with B-CLL and 5 with PLL. Percentage lysis in the presence of 10 μg/mL rituximab and 25% human serum is shown (black bars). The percentage of cells expressing CD20 was also evaluated by standard direct immunofluorescence (hatched bars). The PLL samples are identified by darker hatched bars.
Complement-mediated lysis of B-CLL cells.
CDC assays were performed on the PBMCs isolated from 33 patients with B-CLL and 5 with PLL. Percentage lysis in the presence of 10 μg/mL rituximab and 25% human serum is shown (black bars). The percentage of cells expressing CD20 was also evaluated by standard direct immunofluorescence (hatched bars). The PLL samples are identified by darker hatched bars.
The patients' cells were then incubated in the presence of rituximab and human complement for 4 hours, and cell death was determined using the Alamar blue assay.20 The samples have been ordered according to their susceptibility to CDC (Figure 2). As shown in Figure2 (black bars), most samples were lysed poorly with rituximab and complement. Indeed, 22 of 38 samples (58%) showed lysis of less than 10%, and another 8 cases (21%) were below 25%. Only 8 samples showed lysis of greater than 30%, which in fact corresponded to lysis of 50% or more of CD20+ cells (Figure 2). Among cases with efficient lysis, 3 were PLL and 5 were B-CLL.
The effect of rituximab alone in the absence of human serum was also analyzed in 21 cases of B-CLL and PLL, including 7 high responders. The measured cell death ranged from 0% to 6% in different samples, with a mean of 3.1%, suggesting little effect of rituximab alone under these experimental conditions (data not shown).
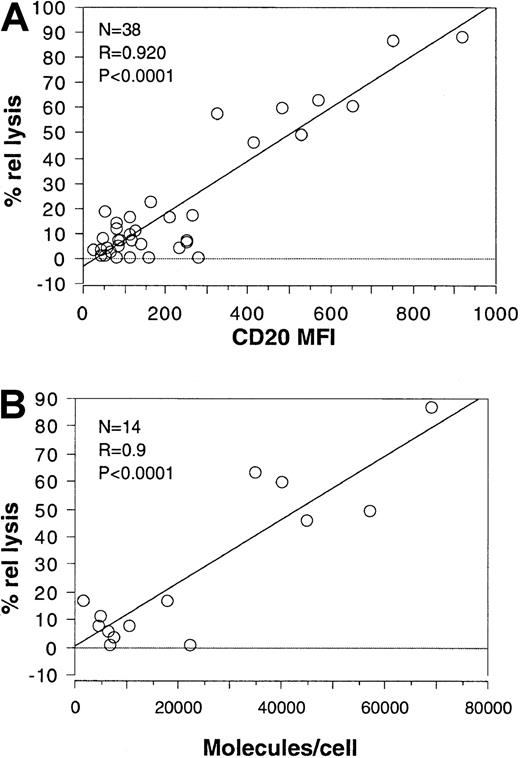
Because B-CLL cells are generally known to express relatively low levels of CD2021 22 and also show little susceptibility to rituximab and complement (Figure 2), we wondered whether the extent of lysis was determined by the levels of expression of CD20 itself. Thus, the mean fluorescence intensity (MFI) for CD20 for each sample was plotted against the relative lysis (ie, the percentage lysis normalized for the total percentage of CD20+ cells in the sample). As shown in Figure 3A, the relative lysis correlated highly significantly with the MFI of CD20 (correlation coefficient 0.92; P < .0001). Interestingly, the correlation was high even though standard FACS analysis was performed using 2 different FACS apparatus and FACS operators (who analyzed 18 and 20 samples, respectively). Furthermore, the correlation was significant for the B-CLL and PLL samples also when analyzed separately (coefficients 0.92 and 0.87, respectively), although significance was relatively low for PLL (P = .05) because only 5 samples could be analyzed in this case. A high significance (P < .0001) remained, however, for the 33 B-CLL cases when analyzed separately from the PLL cases. These results justify the grouping of B-CLL and PLL in the analysis.
Complement-mediated lysis correlates with the intensity of CD20 expression.
(A) The mean fluorescence intensity of CD20 was plotted against the relative lysis obtained in the presence of rituximab and complement for each of the 33 B-CLL and 5 PLL patients shown in Figure 2. (B) The number of molecules per cell measured for 14 B-CLL/PLL patients was plotted against the percentage relative lysis for the same patients. The results of the statistical analysis are shown in each plot.
Complement-mediated lysis correlates with the intensity of CD20 expression.
(A) The mean fluorescence intensity of CD20 was plotted against the relative lysis obtained in the presence of rituximab and complement for each of the 33 B-CLL and 5 PLL patients shown in Figure 2. (B) The number of molecules per cell measured for 14 B-CLL/PLL patients was plotted against the percentage relative lysis for the same patients. The results of the statistical analysis are shown in each plot.
To verify that standard immunofluorescence analysis gives results that correspond to the number of molecules actually expressed, we analyzed 14 B-CLL cases that covered the whole spectrum of CD20 intensities for CD20 expression with the use of calibrated beads. As shown in Figure3B, CD20 expression ranged from 1500 to 70 000 molecules per cell. Furthermore, rituximab and complement-mediated lysis also significantly correlated with the number of CD20 molecules per cell (coefficient 0.9;P < .0001), even though the number of samples analyzed was relatively small.
These data show that in CLL/PLL samples that express variable levels of CD20, rituximab- and complement-mediated lysis depends primarily on the levels of expression of CD20 itself.
Expression and role of the CD55 and CD59 complement inhibitors in B-CLL and PLL
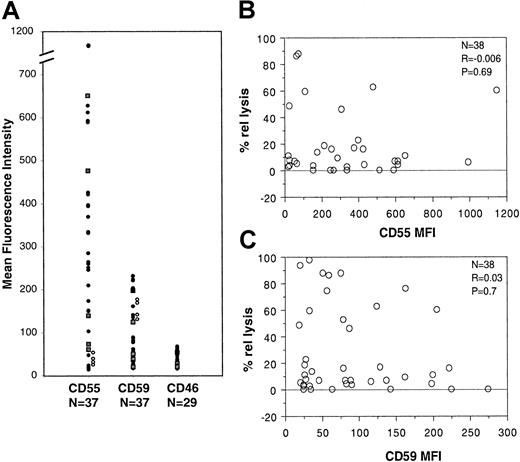
Because surface-associated complement inhibitors have been shown previously to regulate CDC in FL cells,11 we also investigated in B-CLL and PLL the patterns of expression of the CD46, CD55, and CD59 complement inhibitors on CD20+ cells by double immunofluorescence analysis. All B-CLL and PLL cells were 95% to 100% positive for all 3 complement inhibitors (data not shown). However, the intensity of expression varied among samples. In particular, CD55 was expressed at different levels in different samples, with an MFI ranging from 20 to 1150 in 37 patients analyzed (Figure 4A, closed circles for B-CLL and squares for PLL). CD59 showed some variability in expression, although less than that of CD55; the CD59 MFI ranged from 20 to 224 in 37 samples tested (Figure 4A). Finally, CD46 expression was quite constant, with an MFI ranging from 17 to 68 in 29 patients analyzed (Figure 4A). The 5 PLL samples analyzed did not differ from the B-CLL samples for complement inhibitor expression, again justifying their inclusion within the same group.
Analysis of CD55 and CD59 expression and function in B-CLL/PLL.
(A) PBMCs from 33 patients with B-CLL (closed circles) and 4 patients with PLL (closed squares) as well as 4 healthy volunteers (open circles) were double stained with anti–CD20-PE and anti–CD55-FITC, anti–CD59-FITC, or anti–CD46-FITC antibodies and analyzed on the FACS. (B) The CD55 MFI was plotted against the percentage relative lysis for the same patients. (C) The CD59 MFI was plotted against the relative lysis. The results of the statistical analysis are shown in each plot.
Analysis of CD55 and CD59 expression and function in B-CLL/PLL.
(A) PBMCs from 33 patients with B-CLL (closed circles) and 4 patients with PLL (closed squares) as well as 4 healthy volunteers (open circles) were double stained with anti–CD20-PE and anti–CD55-FITC, anti–CD59-FITC, or anti–CD46-FITC antibodies and analyzed on the FACS. (B) The CD55 MFI was plotted against the percentage relative lysis for the same patients. (C) The CD59 MFI was plotted against the relative lysis. The results of the statistical analysis are shown in each plot.
To determine complement inhibitor expression in normal B lymphocytes, we also analyzed peripheral blood B cells from 4 healthy volunteers by double immunofluorescence. As shown in Figure 4A (open circles), CD55 and CD59 were expressed at similar levels in all 4 samples. Interestingly, CD55 was low (MFI 26-54) in all 4 samples, whereas CD59 was higher in all cases. Indeed, the ratio of CD55 to CD59 for normal B cells ranged from 0.2 to 0.3 (mean, 0.23), whereas it was 1 to 15 (mean, 4.4) for all B-CLL and PLL patients. Thus, B-CLL and PLL have complement inhibitor expression skewed toward higher CD55 relative to CD59.
The relatively high and variable expression of CD55 and CD59 in patients led us to investigate whether there was any inverse correlation between complement lysis and CD55 or CD59 expression. As shown in Figure 4B and C, no significant correlation could be found because the coefficient was close to 0 in all cases (37 cases analyzed). Again, performing the analysis for B-CLL or PLL separately did not change the result (data not shown). Thus, the levels of expression of CD55 or CD59, which are expressed on all B-CLL cells, are not predictive of lysis in these pathologies.
The functional role of CD55 and CD59 can be investigated more directly by performing complement lysis assays in the presence of antibodies that functionally block these molecules but are unable by themselves to activate complement23 (also, data not shown). This assay was performed on all 38 B-CLL and PLL samples. Figure5A shows the results obtained with 6 representative B-CLL cases. The results show that anti-CD55 or anti-CD59 and, more effectively, both antibodies together increased the lytic response, although to a variable degree in different patients. In some patients showing basal CDC of less than 10% (eg, cases 5 and 10), lysis was increased 2- to 3-fold with a single blocking antibody and up to 10-fold with both anti-CD55 and anti-CD59. In cases showing high basal CDC (more than 50%; eg, cases 35 and 38), lysis was complete by adding either CD55 or CD59 blocking antibodies singly. Figure 5B shows the mean percentage lysis with or without blocking the CD55 and/or CD59 inhibitors calculated from all samples. The data demonstrate that anti-CD55 or anti-CD59 increased the mean lysis by an average of 2-fold, whereas the combination of antibodies augmented it by nearly 3-fold (Figure 5B). These increases are statistically significant (P < .0001). The effect of the combined relative to single antibodies is also statistically significant (P < .0001). Because the basal CDC response (rituximab plus complement alone) was highly variable among different samples (ranging from 0% to 75%), the effect of blocking CD55 and/or CD59 could be different in patients who show a low or high basal CDC. The mean levels of lysis were therefore split in Figure 5C between low responders (30 samples with basal lysis less than 25%) and high responders (8 samples with basal lysis greater than 30%). The results show that the effect of the blocking antibodies was proportionally greater in low responders, with a 2.8-, 3.6-, and 5.5-fold increase for CD55, CD59, and both antibodies together, respectively. In high responders, lysis was nearly complete after adding only a single blocking antibody because the mean percentage of CD20+cells for this group was 87%.
CD55 and CD59 block rituximab and complement-mediated lysis.
PBMCs from 33 patients with B-CLL and 5 patients with PLL were lysed with rituximab and 25% human serum in the presence or absence of blocking anti-CD55 and/or anti-CD59 antibodies (10 μg/mL). Lysis was measured after 4 hours with the Alamar blue assay. (A) The mean and SDs obtained with 6 different representative patients are shown. (B) The mean lysis and SDs for all 38 patients are shown. (C) The mean lysis of the 30 low responders (basal lysis less than 25%; black bars) and of the 8 high responders (basal lysis greater than 30%; hatched bars) and SDs are shown.
CD55 and CD59 block rituximab and complement-mediated lysis.
PBMCs from 33 patients with B-CLL and 5 patients with PLL were lysed with rituximab and 25% human serum in the presence or absence of blocking anti-CD55 and/or anti-CD59 antibodies (10 μg/mL). Lysis was measured after 4 hours with the Alamar blue assay. (A) The mean and SDs obtained with 6 different representative patients are shown. (B) The mean lysis and SDs for all 38 patients are shown. (C) The mean lysis of the 30 low responders (basal lysis less than 25%; black bars) and of the 8 high responders (basal lysis greater than 30%; hatched bars) and SDs are shown.
We conclude that in B-CLL and PLL, all of which express detectable levels of CD55 and CD59, both complement inhibitors effectively block complement activation. However, CD55 and CD59 levels of expression do not allow prediction of the extent of lysis.
Complement-mediated lysis of MCLs
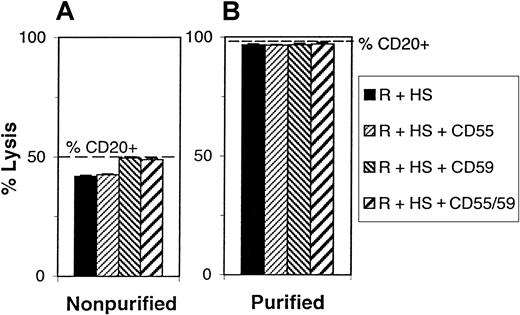
Because clinical trials are being conducted to test the activity of rituximab in MCL,17,24 25 we extended our analysis of CDC to a smaller number of fresh MCL samples. Because the percentage of CD20+ cells in the peripheral blood of these patients is in some cases relatively low (45% to 50%), we first verified that the Alamar blue CDC assay was sufficiently specific in such samples. CDC was performed on a sample containing 50% CD20+ cells, and the same sample was then subjected to negative selection to purify B cells (to 98% purity), and CDC was performed on these cells in parallel. In addition, CDC was performed in the presence or absence of the anti-CD55 or anti-CD59 blocking antibodies. As shown in Figure 6, lysis of unpurified and purified samples with rituximab and complement was 42% and 96%, which corresponded to lysis of 84% and 98% of CD20+ cells, respectively, after adjustment for the percentage of CD20+cells in the sample. Addition of both CD55 and CD59 blocking antibodies increased lysis to 49% for unpurified cells and 98% for purified cells, which corresponded in both cases to greater than 98% of CD20+ cells. These data demonstrate that the assay can accurately measure CDC even in samples containing relatively low numbers of CD20+ cells. Although the assay could not distinguish between neoplastic and contaminating normal B cells, double immunofluorescence analysis of CD19 and sIgκ/λ showed that contaminating normal B cells were present at less than 3% in all cases (data not shown). Such a small component in the sample cannot influence significantly the results obtained.
Specificity of lysis of MCL samples.
(A) PBMCs from a patient with MCL containing 50% CD20+cells were lysed with rituximab and 25% human complement in the absence (black bars) or presence (hatched bars) of anti-CD55 and or anti-CD59 antibodies (10 μg/mL). The percentage of CD20+cells was determined and is indicated by the broken line. (B) The MCL B cells were purified by negative selection, reaching 98% CD20+, and lysis was repeated as in (A).
Specificity of lysis of MCL samples.
(A) PBMCs from a patient with MCL containing 50% CD20+cells were lysed with rituximab and 25% human complement in the absence (black bars) or presence (hatched bars) of anti-CD55 and or anti-CD59 antibodies (10 μg/mL). The percentage of CD20+cells was determined and is indicated by the broken line. (B) The MCL B cells were purified by negative selection, reaching 98% CD20+, and lysis was repeated as in (A).
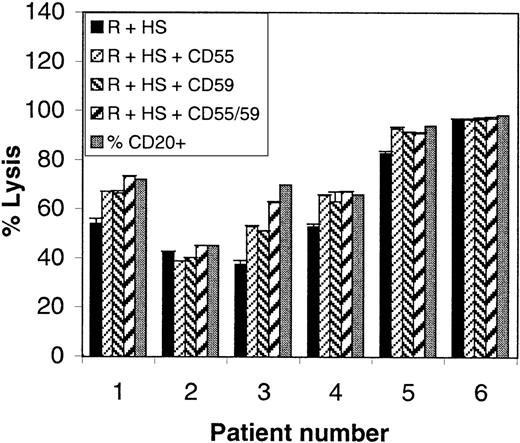
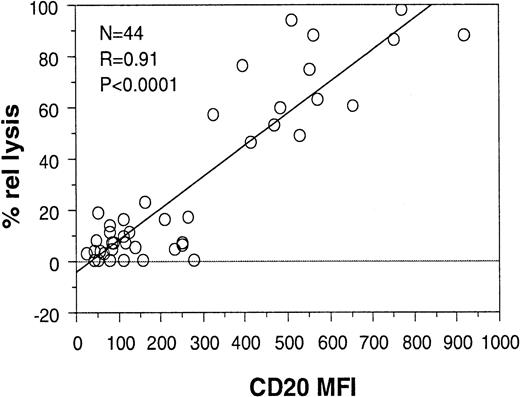
Peripheral blood samples from 6 patients with MCL, which contained 45% to 98% CD20+ B cells (mean, 74%), were then analyzed for CDC in the presence or absence of CD55 and/or CD59. As shown in Figure7 (black bars), lysis with rituximab and complement alone was high in all cases (54% to 98% of CD20+ cells). These data are in agreement with those obtained earlier for B-CLL and PLL because MCLs express relatively high levels of CD20 (MFI greater than or equal to 400). Although the MCL patients were too few and too homogeneous to allow an analysis of correlation between CD20 expression and CDC on their own, they were analyzed together with the 33 B-CLL and 5 PLL samples. The data shown in Figure 8 demonstrate that a high correlation was still observed between relative lysis and CD20 expression (coefficient 0.91; P < .0001) when all 44 patients were grouped together. These data suggest that CD20 levels can predict sensitivity to rituximab regardless of diagnostic group, at least within the pathologic entities examined (B-CLL, PLL, and MCL).
Rituximab and complement-mediated lysis of MCLs.
PBMCs from 6 cases of MCL were lysed with rituximab and 25% human serum (black bars) in the presence or absence of blocking anti-CD55 and/or anti-CD59 antibodies (10 μg/mL; hatched bars). Lysis was measured with the Alamar blue assay. The percentage CD20+cells in each sample is also shown (gray bars).
Rituximab and complement-mediated lysis of MCLs.
PBMCs from 6 cases of MCL were lysed with rituximab and 25% human serum (black bars) in the presence or absence of blocking anti-CD55 and/or anti-CD59 antibodies (10 μg/mL; hatched bars). Lysis was measured with the Alamar blue assay. The percentage CD20+cells in each sample is also shown (gray bars).
CDC correlates with CD20 expression levels in all patients (CLL, PLL, and MCL).
The CD20 MFIs of the 33 B-CLL, 5 PLL, and 6 MCL patients were plotted against the relative lysis obtained with rituximab and complement for the same patients. The results of the statistical analysis are shown.
CDC correlates with CD20 expression levels in all patients (CLL, PLL, and MCL).
The CD20 MFIs of the 33 B-CLL, 5 PLL, and 6 MCL patients were plotted against the relative lysis obtained with rituximab and complement for the same patients. The results of the statistical analysis are shown.
With regard to CD55 and CD59 function in MCL, Figure 7 shows that blocking either molecule significantly increased lysis of those samples that were not lysed completely with rituximab alone (cases 1, 3, 4, and 5). Anti-CD55 and anti-CD59 were equally effective and in several cases (nos. 1, 4, and 5) one blocking antibody alone was sufficient to induce complete lysis (Figure 7).
Complement deposition
To analyze in more detail the complement cascade on fresh neoplastic B cells, we selected 4 cases with sufficient cell numbers that differed in CD20 expression. We then measured the deposition of C3 and C9 complement fragments on the cell surface after exposure to rituximab and complement in vitro. As shown in Table1, the percentage of cells positive for C3 was high in all cases (greater than 80%), showing that C3 deposition occurred on all B cells. However, the intensity of C3 varied significantly in the different samples, and this correlated with CD20 expression. PLL 2 cells, which expressed little CD20 (MFI 80), also showed the lowest level of C3 deposition (MFI 294). As expected, the other cases, which showed CD20 expression ranging from 480 to 530, also had higher C3 deposition (MFI 599-875). The results with C9 were somewhat different in that, in this case, the percentage of cells that became C9 positive correlated approximately with the percentage of cells killed by rituximab, whereas C9 intensity was similar between samples. Although this analysis was performed on few samples, the data show in a direct way that the complement cascade is triggered more efficiently by rituximab in the presence of a higher CD20 density on the cell surface. Furthermore, the data show that in cells that are lysed poorly, the completion of the complement cascade is efficiently counteracted by complement inhibitors, which are likely to include CD55 and CD59.
C3 and C9 complement fragment deposition in vitro
| Patient . | Percentage C3-positive cells (MFI) . | Percentage C9-positive cells (MFI) . | Lysis (%) . | ||
|---|---|---|---|---|---|
| R . | R + HS . | R . | R + HS . | ||
| PLL 2 | 25 (23) | 88 (294) | 1 | 3 (46) | 11 |
| PLL 4 | 2 (108) | 86 (875) | 1 | 43 (21) | 58 |
| CLL 32 | 23 (95) | 81 (599) | 0 | 50 (20) | 42 |
| MCL 1 | 22 (127) | 83 (799) | 0 | 59 (17) | 54 |
| Patient . | Percentage C3-positive cells (MFI) . | Percentage C9-positive cells (MFI) . | Lysis (%) . | ||
|---|---|---|---|---|---|
| R . | R + HS . | R . | R + HS . | ||
| PLL 2 | 25 (23) | 88 (294) | 1 | 3 (46) | 11 |
| PLL 4 | 2 (108) | 86 (875) | 1 | 43 (21) | 58 |
| CLL 32 | 23 (95) | 81 (599) | 0 | 50 (20) | 42 |
| MCL 1 | 22 (127) | 83 (799) | 0 | 59 (17) | 54 |
PBMCs from the indicated patients were incubated with rituximab in the presence or absence of 25% human serum for 3 hours, washed, and stained with antibodies specific for the indicated complement fragments by indirect immunofluorescence. MFI indicates mean fluorescence intensity; R, rituximab only; R + HS, rituximab and human serum; PLL, prolymphocytic leukemia; CLL, chronic lymphocytic leukemia; MCL, mantle cell lymphoma.
Discussion
Several lines of evidence suggest that the mechanism of action of rituximab in vivo may include complement-mediated cell lysis.9,11,12 16 These considerations have led us to investigate the response to rituximab and complement of several subtypes of fresh B-cell leukemias and lymphomas in vitro. In addition, we determined whether the levels of expression of CD20 and the complement inhibitors CD55 and CD59 could predict killing by complement in vitro. Finally, we analyzed directly the role of the CD55 and CD59 inhibitors using MAbs that functionally block these molecules.
Our data demonstrate that CDC triggered by rituximab is extremely specific to CD20+ cells in a mixed population. Analysis of 33 B-CLL and 5 PLL freshly isolated samples showed that they are heterogeneous in their susceptibility to rituximab and complement, with lysis ranging from 0% to 90% of CD20+ cells. Analysis of CD20, CD55, and CD59 expression levels by standard immunofluorescence showed unambiguously that lysis correlated closely with CD20 MFI, but not with either CD55 or CD59. The correlation with CD20 expression level was highly significant statistically, and the same results were obtained when analyzing the individual diagnostic groups either separately or together, further confirming the validity of the results. A number of samples were also analyzed using calibrated fluorescent beads to quantify the number of CD20 molecules per cell. These data showed that CD20 expression in B-CLL/PLL varied from 2000 to 70 000. The data also showed that lysis correlated highly significantly with the number of CD20 molecules per cell, as expected, confirming the validity of the standard immunofluorescence measurements. These results suggest that standard immunofluorescence analysis is sufficient to predict CDC in vitro and could be applied to analyze the role of CD20 expression in the in vivo response of different patients to rituximab.
The analysis of sensitivity to rituximab and complement was also extended to 6 cases of MCL. All MCLs tested showed relatively high lysis (54% to 98%) and also high CD20 expression levels (MFI greater than or equal to 400). Thus, a highly significant correlation between lysis and CD20 expression levels was observed when analyzing all 44 B-CLL, PLL, and MCL patients together. These data show that the CDC response is primarily determined by the levels of CD20, regardless of diagnostic group, at least within the pathologies examined. In this regard, it is worth noting that 2 HCL cases were also analyzed for CD20 expression and CDC. Both cases showed high CD20 levels (MFI greater than 1000) and also complete lysis in the presence of rituximab and complement, suggesting that the correlation may apply to other B-cell neoplasias as well (data not shown).
As expected from the correlation analysis, CD20 levels determined the extent to which the complement cascade was triggered by surface-bound antibody. Indeed, C3, which reflects the initiation of the cascade, was deposited on all cells, but to a different extent depending on the levels of expression of CD20 itself.26 In contrast, C9 deposition was detected only on cells undergoing lysis, demonstrating that complement inhibitors are present that inhibit completion of the complement cascade.26
Both the expression and functional roles of the CD55, CD59, and CD46 complement inhibitors were investigated. Expression of CD55 was found to be heterogeneous in B-CLL/PLL, varying up to 100-fold in MFI. CD59 expression also showed some variability, with approximately a 10-fold difference in MFI. Interestingly, the mean ratio of CD55 to CD59 was reversed in B-CLL/PLL patients relative to that observed in peripheral blood B cells from 4 healthy donors. Although the number of normal B cells was small, the data suggest that in B-CLL/PLL, complement inhibitor expression is skewed toward a relatively high CD55 expression. Further work will be required to determine whether high CD55 is characteristic also of normal CD5+ B cells and is beyond the scope of this article.
Although we found no correlation between levels of CD55 or CD59 expression and lysis, direct analysis of the effect of CD55 and CD59 using functionally blocking MAbs demonstrated that these are important inhibitors of the complement cascade in neoplastic B cells. Both anti-CD55 and anti-CD59 antibodies were effective to a similar extent, and the effect was most dramatic after adding both blocking antibodies together. The effect was most notable in cells having a low basal response, in which a mean 6-fold increase in lysis could be obtained with both blocking antibodies. On the other hand, in many cases showing relatively high basal lysis, addition of only one blocking antibody was sufficient to lead to complete lysis. This finding may be important in the context of resistance to rituximab in vivo, where increasing rituximab activity may allow elimination of residual resistant cells.4 The fact that lysis did not correlate with CD55 or CD59 expression suggests that even low levels of CD55 or CD59 are sufficient to efficiently inhibit the complement cascade, because all patient samples tested expressed the inhibitors, albeit at relatively low levels in some cases. Thus, increasing CD55 and/or CD59 expression above that level does not appear to increase their capacity to inhibit complement.
Although several lines of evidence suggest that CDC is one of the mechanisms of action of rituximab in vivo,9,11,12 16 it is likely that other mechanisms operate, such as ADCC and apoptosis. Dissecting the contribution of CD20 or of other factors for each of these mechanisms will be obviously important. Under the experimental conditions used here (4 hours' incubation with rituximab without cross-linking), we were unable to detect significant cell death in the absence of complement. We have therefore no evidence that significant apoptosis can take place in the different neoplastic B cells examined here. We cannot exclude, however, that cross-linking and/or longer incubation times or other factors could allow induction of apoptosis in these same cells. Such study is beyond the scope of this article.
The variability in the response of fresh leukemic cells to complement in vitro may reflect the heterogeneity in the response of leukemic patients to this drug in vivo. Furthermore, particularly for B-CLL, patients showing stronger lysis in vitro may be those more at risk of developing infusion-related side effects.19,27Indeed, a relatively poor response of B-CLL patients to rituximab has been reported, as well as some cases of life-threatening tumor lysis syndrome,16,19,27 which would correlate with the data presented here. Even though it is evident that other factors, such as tumor mass and systemic disease, influence the overall response in vivo,28 we propose that the simple, reproducible, inexpensive, and rapid quantitative assay of CDC on fresh leukemic samples, such as described here, should be a valuable tool to predict the in vivo response of different patients. The need for a relatively small quantity of cells for this assay should allow its applicability also to neoplastic cells isolated from lymph node biopsies. Finally, our data strongly suggest that inhibiting the CD55 and/or CD59 antigens in vivo could markedly improve the biologic activity of rituximab.
We thank Dr F. Tedesco for kindly providing anti-C3 and C9 antibodies and Dr L. Chatenoud for her assistance in the statistical analysis.
Supported by Roche Italia (Monza, Italy), the Associazione Italiana Ricerca sul Cancro (AIRC), the Associazione Paolo Belli-Lotta alla Leucemia, the Istituto Superiore di Sanità (ISS Rome, project 30C.40), the Ministero dell' Universitá e della Ricerca Scientifica e Technologica (MURST) (Rome, project 2000-2001), and by MURST–Consiglio Nazionale delle Ricerche (CNR) (project I. 27.12.1997, no. 449).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Martino Introna, Istituto Ricerche Farmacologiche Mario Negri, via Eritrea 62, 20157 Milano, Italy; e-mail:martino@marionegri.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal