The clinical benefit of intravenous immunoglobulin (IVIG) preparations in the treatment of immune thrombocytopenic purpura (ITP) is supposed to be mediated by blockade of Fcγ receptor–bearing phagocytes. In 2 experimental models for ITP, it is shown that the therapeutic efficacy of IVIG preparations is related to the IgG dimer content present in these preparations. A rat monoclonal antibody (mAb; MWReg30) directed to the murine platelet-specific integrin αIIbβ3 (gpIIb/IIIa) was administered intraperitoneally either as bolus injection or continuous infusion. With bolus injection, the circulating platelet count dropped to almost zero within 3 hours. Pretreatment with cobra venom factor did not affect platelet depletion, whereas pretreatment with anti-FcγRII/III mAb 2.4G2 or IVIG greatly reduced platelet clearance. With continuous infusion, platelet numbers reached a steady state after 4 days, at approximately 25% of control. This reduction in platelets was, however, not observed in mice deficient for the FcRγ-chain, lacking FcγRI, FcγRIII, and FcγRIII−/− mice. Infusion of a single dose of IVIG with a high IgG dimer content on the 4th day—ie, mimicking therapeutic administration—resulted in a platelet increase for several days. IVIG predominantly consisting of monomeric IgG had no effect on platelet numbers. In conclusion, continuous infusion of MWReg30 induces thrombocytopenia in mice by enhancing Fcγ receptor–mediated clearance of platelets. In this model, it is shown that IgG dimers present in IVIG preparations are responsible for the increase in platelet counts.
Introduction
Immune thrombocytopenic purpura (ITP) is an autoimmune disorder in which antibody-sensitized platelets are prematurely destroyed by phagocytic cells in the reticuloendothelial system (RES).1 Therapeutic interventions in ITP are often directed toward reducing antibody-mediated clearance by either decreasing the production of pathogenic platelet autoantibodies or impairing RES function. Imbach et al2 was the first to report that intravenous immunoglobulin (IVIG) preparations were effective in children with acute and chronic ITP. Administration of IVIG leads to rapid, yet often transient, reversal of thrombocytopenia in most patients with ITP.3
In the treatment of ITP, there may be multiple mechanisms of action of IVIG. It has been proposed that IVIG exerts its function though blockade of Fcγ receptors or neutralization of autoantibodies by idiotype–anti-idiotype interaction. However, clinical observations sometimes show prolonged effects of IVIG that cannot be explained by the short-term neutralization of pathologic antibodies or Fcγ receptor blockade. Therefore, other mechanisms, such as modulation of cytokine synthesis or interference with T- and B-cell function, have been suggested to explain long-term effects in the treatment of ITP by IVIG.4
Fcγ receptors play an important role in antibody-dependent clearance, and many studies have provided evidence that reversal of thrombocytopenia can be induced by (non)specific blockade of Fcγ receptors on phagocytes in the RES.5 A predominant role for low-affinity Fcγ receptors in antibody-mediated thrombocytopenia has been shown in animal studies6,7 and mice deficient in Fcγ receptors8 or transgenic in mice overexpressing human FcγRIIA.9 Treatment of a patient with chronic ITP with a monoclonal antibody against FcγRIII was reported to result in impressive, albeit transient, increases in platelet counts, supposing that the platelets in human ITP are also destroyed by binding to low-affinity Fcγ receptors.10
Three different types of murine FcγR exist: a high-affinity receptor, FcγRI (CD64), capable of affinity-binding monomeric IgG, and 2 low-affinity receptors, FcγRII (CD32) and FcγRIII (CD16), preferentially binding polymeric IgG or immune complexes. FcγRII is widely distributed on lymphoid and myeloid cells and functions as a negative regulator of immunity. FcγRIII is restricted to macrophages, neutrophils, natural killer cells, and mast cells. Interaction of IgG with FcγRI or FcγRIII leads to cellular activation, which requires interaction of these receptors with a homodimer of the so-called FcRγ-chain. The situation for human Fcγ receptors is more complex than the mouse system. Like mice, humans express 3 types of Fcγ receptors—a high-affinity receptor, FcγRI (CD64), and the low-affinity receptors FcγRII (CD32) and FcγRIII (CD16). However, different forms of these low-affinity receptors exist—FcγRIIA, which functions as an activating Fcγ receptor, and FcγRIIB, which is a regulatory Fcγ receptor that has an inhibitory function. In addition, different forms of the FcγRIII receptor exist in humans—FcγRIIIb, which is exclusively expressed on neutrophils, and FcγRIIIA, which is expressed on natural killer cells and macrophages. An important difference in Fcγ receptor expression between humans and mice is the expression of FcγRIIA, which is expressed on human but not on mouse platelets.11,12 Recently, McKenzie et al9demonstrated an important role for this Fcγ receptor in the induction of experimental ITP using mice overexpressing human FcRIIA.
IVIG preparations contain variable amounts of monomers and dimers and small amounts of aggregated IgG; it is unknown which of these fractions constitutes the active component, making standardization of IVIG preparations for the treatment of ITP tedious. The aim of this study was to develop a clinically relevant murine model for ITP. The usefulness of the experimental model was demonstrated by assessing the component in IVIG-mediating therapeutic efficacy, which was shown to represent the IgG dimeric fraction.
Materials and methods
Animals
IVIG immunoglobulin preparations
Human γ-globulin for IVIG use was obtained from the CLB (immunoglobulin IV, 6% wt/vol; Amsterdam, The Netherlands). This is a freeze-dried product prepared from pooled plasma from at least 1000 donors by Cohn fractionation followed by pepsin incubation, pH 4. Fresh IVIG was immediately frozen after reconstitution and kept at −80°C until further use. To obtain an IVIG preparation with a high IgG dimer content, we reconstituted a freeze-dried IVIG preparation and stored it at 4°C (further referred to as aged IVIG). A gradual increase in dimer content was observed from less than 5% to values greater than 10% after 1 week of storage. The percentage of IgG dimers remained at this level when analyzed after several months or even after 1 year of storage. Monomeric IgG was prepared by gel filtration of IVIG on a TSK4000 column. Fractions corresponding to the monomeric peak were collected and concentrated to a concentration of 50 mg/mL using Centriplus concentrators according to the manufacturer's instructions (Amicon, Beverly, MA). IVIG preparations used in the experiments were analyzed for actual monomer, dimer, and polymer contents on a calibrated Superose 12 gel filtration column connected to an FPLC system (Pharmacia, Uppsala, Sweden). A computer program (Ezchrom Chromatography Data System, version 6.5) was applied to determine the peak areas of chromatograms.
Reagents
Cobra venom factor (CoVF) was kindly provided by Dr J. S. Verbeek (LUMC, Leiden, The Netherlands). Rat antimouse platelet monoclonal antibody (mAb; MWReg30, IgG1) and rat anti–mouse FcγRII/III mAb (2.4G2, IgG2b) were obtained from Pharmingen (San Diego, CA). Fluorescein isothiocyanate-conjugated F(ab)2fragments of goat anti–rat IgG were obtained from Caltag (Burlingame, CA).
Induction of thrombocytopenia
Mice were rendered thrombocytopenic by administration of the rat mAb MWReg30, directed to the platelet-specific integrin αIIbβ3 (gpIIb/IIIa). In an acute model, a bolus of 5 μg mAb was injected in the peritoneal cavity in 200 μL saline. The effect of IVIG was determined in mice by giving IVIG (1 g/kg) or saline intravenously 1 hour before injection of MWReg30. Blood was obtained from the retro-orbital plexus of anesthetized mice using heparinized hematocrit tubes (Clinitubes; Radiometer, Copenhagen, Denmark). Blood samples were taken before IVIG or saline treatment (t = 0), 5 minutes before injection of MWReg30 and 10, 60, and 180 minutes after injection of MWReg30. For determination of platelet numbers, blood (20 μL) was diluted in isotone and centrifuged at 300g for 10 minutes at room temperature. Numbers of platelets were counted using an electronic cell counter (model 2F and Channelyser model 256; Coulter Electronics, Dunstable, United Kingdom).
To obtain a clinically more relevant model for testing IVIG, MWReg30 was continuously infused using an osmotic pump implanted in the peritoneal cavity (Alzet micro-osmotic pump, model 1002; Alza, Palo Alto, CA). Pumps with a pumping rate of 0.25 μL/h for a duration of 14 days were filled with 100 μL mixture containing MWReg30 (165 μg/mL), human serum albumin (1.5 mg/mL to prevent absorption of proteins to the device), and an irrelevant mouse mAb directed against human CRP to check for the infusion rate. Blood samples were taken under anesthesia at t = 0, 4, 5, and 6 days to determine numbers of platelets in peripheral blood. At day 4, aged IVIG, monomeric IgG, or saline was given intravenously at a dose of 1 g/kg.
Complement-depleted mice
Complement-depleted mice were prepared by intraperitoneal injection of 2 doses of 500 U CoVF in 200 μL saline at a 6-hour interval. Sera were analyzed on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) to confirm complement depletion.
Detection of anti-idiotype antibodies directed against MWReg30
To detect antibodies in the IVIG preparations with affinity to MWReg30, IVIG was added to 96-well microtiter plates (Maxisorb: Nunc, Roskilde, Denmark) coated with MWReg30 (0.8 μg/mL), human serum albumin (HSA; 1 μg/mL), or buffer. Bound antibodies were detected by an alkaline phosphatase–labeled monoclonal antibody to human IgG (clone GG-5; Sigma Chemical, St Louis, MO). After the addition of p-nitrophenyl phosphatase as substrate, the optical density was measured with Titertek Multiscan (Flow Labs, McLean, VA)
Detection of free circulating MWReg30 in plasma and platelet-bound MWReg30
For the determination of membrane binding of MWReg30 to platelets, whole blood from mice with implanted pumps was stained at a final concentration of 5 μg/mL fluorescein isothiocyanate-conjugated F(ab)2 fragments of goat anti–rat IgG mAb for 30 minutes (4°C in the dark) and analyzed with a FACScan flow cytometer (Becton Dickinson, San Jose, CA). To detect free circulating MWReg30 in plasma from thrombocytopenic mice, plasma from mice with implanted pumps was incubated for 1 hour at room temperature with whole blood from control mice. Binding of MWReg30 to platelets was assessed as described above.
Statistical analyses
Results are expressed as mean ± SEM. Statistical analyses were performed using Student t tests. P < .05 was considered significant.
Results
MWReg30 bolus injection model for ITP
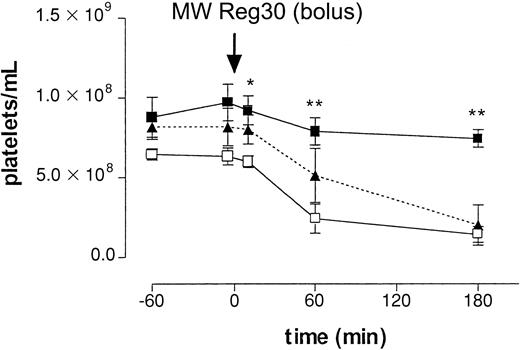
First, we determined the clearance of platelets after intraperitoneal injection of a rat mAb directed against mouse platelet-specific integrin αIIbβ3(gpIIb/IIIa; MWReg30), at a dose of 5 μg per mouse. After injection, platelet numbers decreased over several hours, reaching platelet counts of 1.43 ± 0.49 × 108/mL (n = 5, 22% of control,P < .01) at 3 hours (Figure1). To determine the mechanism of antibody-induced thrombocytopenia, mice were pretreated with an mAb against FcγRII/III or, alternatively, with CoVF to deplete complement. Figure 1 shows that in vivo blocking of FcγRII/III by pretreatment with the 2.4G2 mAb completely prevented the MWReg30-induced clearance of platelets, whereas complement depletion did not have an effect. Analysis of sera on SDS-PAGE confirmed complement depletion. These results indicated the enhanced platelet clearance in this model to be mediated by Fcγ receptors.
Intraperitoneal injection antiplatelet mAb.
The role of Fcγ receptors in antibody-induced thrombocytopenia after intraperitoneal injection of 5 μg of antiplatelet mAb MWReg30. Time-course of mean platelet counts (± SEM, n = 6) is shown after injection of MWReg30 at t = 0 minute. Mice were pretreated with 2.4G2 (▪, 5 μg/g intravenously), CoVF(▴), or saline (■), before MWReg30. Baseline platelet numbers were 6.37 ± 0.32 × 108/mL blood. Results are expressed as the mean of 3 experiments ± SEM. Asterisks indicate statistically significant differences compared to saline control (*P < .05; **P < .01).
Intraperitoneal injection antiplatelet mAb.
The role of Fcγ receptors in antibody-induced thrombocytopenia after intraperitoneal injection of 5 μg of antiplatelet mAb MWReg30. Time-course of mean platelet counts (± SEM, n = 6) is shown after injection of MWReg30 at t = 0 minute. Mice were pretreated with 2.4G2 (▪, 5 μg/g intravenously), CoVF(▴), or saline (■), before MWReg30. Baseline platelet numbers were 6.37 ± 0.32 × 108/mL blood. Results are expressed as the mean of 3 experiments ± SEM. Asterisks indicate statistically significant differences compared to saline control (*P < .05; **P < .01).
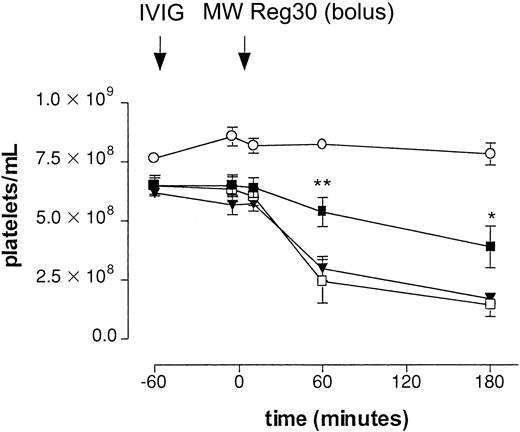
Different IVIG preparations were evaluated for their effect on platelet clearance in the model. Table 1 shows the actual amounts of monomeric, dimeric, and polymeric IgG in the IVIG preparations evaluated in the ITP model. To evaluate the effect of these IgG preparations in our model, mice were pretreated with either fresh IVIG or aged IVIG at a dose of 1 g/kg, 1 hour before injection of MWReg30 (Figure 2). With intraperitoneal injection of MWReg30, the drop in platelet counts was significantly smaller in mice pretreated with aged IVIG. The final count was 3.88 ± 0.89 × 108/mL (60% of baseline, n = 5,P < .05) as measured at 3 hours. Significant differences were already observed 1 hour after the injection of MWReg30. In contrast, pretreatment of mice with fresh IVIG did not significantly decrease the effect on platelet numbers; the final count was 1.69 ± 0.23 × 108/mL (27% of baseline, n = 5), suggesting an important role for IgG dimers in the therapeutic effect in this model. Administration of aged IVIG in the absence of MWReg30 did not result in a change of platelet counts.
Percentages of monomeric, dimeric, and polymeric immunoglobulin G in intravenous immunoglobulin preparations
| . | Monomeric IgG (%) . | Dimeric IgG (%) . | Polymeric IgG (%) . |
|---|---|---|---|
| Fresh IVIG | 97.6 | 2.7 | 0.5 |
| Aged IVIG | 87.2 | 12.0 | 0.8 |
| Monomeric IgG | 99.5 | 0.4 | < 0.1 |
| . | Monomeric IgG (%) . | Dimeric IgG (%) . | Polymeric IgG (%) . |
|---|---|---|---|
| Fresh IVIG | 97.6 | 2.7 | 0.5 |
| Aged IVIG | 87.2 | 12.0 | 0.8 |
| Monomeric IgG | 99.5 | 0.4 | < 0.1 |
Analysis was performed by size exclusion chromatography using a calibrated Superose 12 gel filtration column connected to an FPLC system, as described in “Material and methods.”
IgG indicates immunoglobulin G; IVIG, intravenous immunoglobulin.
Effect of IVIG in antibody-mediated thrombocytopenia after intraperitoneal injection.
Immune thrombocytopenia after intraperitoneal injection of antiplatelet mAb MWReg30. Time-course of mean platelet counts (± SEM) is shown after injection of MWReg30 at t = 0 minute. Mice were pretreated with aged IVIG (▪), fresh IVIG (▾), or saline (■) 1 hour before MWReg30 at a dose of 1 g/kg. Injection of aged IVIG alone, without subsequent injection of the antiplatelet mAb (○), was used as a control. Results are expressed as mean of 6 experiments ± SEM on different occasions. The aged IVIG preparation used in the experiment was stored at 4°C for 1 year. Asterisks indicate statistically significant differences compared to saline control (*P < .05; **P < .01).
Effect of IVIG in antibody-mediated thrombocytopenia after intraperitoneal injection.
Immune thrombocytopenia after intraperitoneal injection of antiplatelet mAb MWReg30. Time-course of mean platelet counts (± SEM) is shown after injection of MWReg30 at t = 0 minute. Mice were pretreated with aged IVIG (▪), fresh IVIG (▾), or saline (■) 1 hour before MWReg30 at a dose of 1 g/kg. Injection of aged IVIG alone, without subsequent injection of the antiplatelet mAb (○), was used as a control. Results are expressed as mean of 6 experiments ± SEM on different occasions. The aged IVIG preparation used in the experiment was stored at 4°C for 1 year. Asterisks indicate statistically significant differences compared to saline control (*P < .05; **P < .01).
It has been reported that in vivo application of high doses of MWReg30 (greater than 30 μg intravenously) in mice induces acute hyperthermia and pathologic changes, especially in the lung, probably caused by macrophage activation leading to PAF production.16,17However, a recent study shows that lower doses of MWReg30 (less than 7.5 μg) do not induce pathologic changes in mice.17 In accordance with the latter findings, we did not find any macroscopic pathologic changes in lungs at the low dose (5 μg intraperitoneally) we used to induce thrombocytopenia. Furthermore, microscopic examination of the lungs did not show signs of lung damage, such as enhanced thickness of alveolar septa, because of edema and increased cellularity (data not shown).
Continuous MWReg30 infusion model
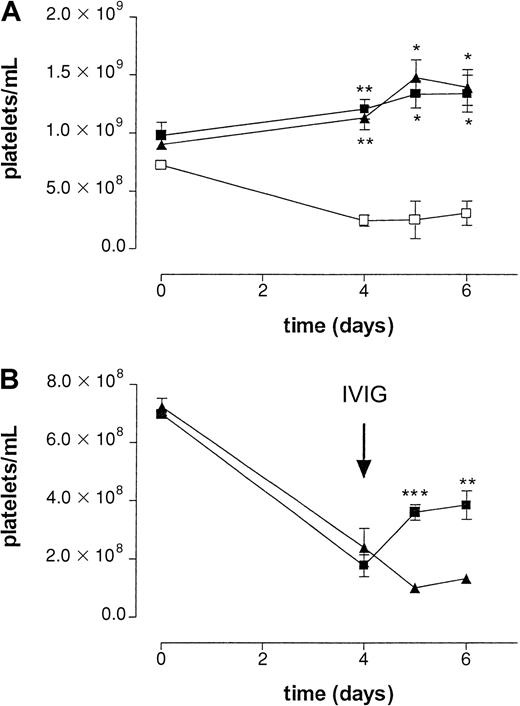
To determine the effect of IVIG in a setting better paralleling the clinical situation, MWReg30 was continuously infused using an implanted osmotic pump. After 4 days, platelet numbers reached a steady state of approximately 25% of control (Figure3). To check the efficacy of the osmotic pumps, an irrelevant mAb, directed against human CRP, was added to the mixture of MWReg30 and HSA. The amount of this antibody was determined using an enzyme-linked immunosorbent assay in plasma samples taken at different time points during the experiment (data not shown). All mice analyzed were found to have the same amount of circulating antibody until 6 days after implantation of the pumps, indicating pumps to be equally efficient in releasing antibodies.
Effect of IVIG in antibody-mediated thrombocytopenia after continuous infusion of antiplatelet mAb.
(A) Effect on platelet counts by continuous infusion of antiplatelet mAb MWReg30 by an implanted osmotic pump in wild-type (■), FcγRIII−/− (▪), and FcR γ-chain−/−(▴) mice. Time course of mean platelet counts of 3 experiments (± SEM) is shown except for the wild-type mice (n = 2). Asterisks indicate statistically significant differences compared to saline control (*P < .05; **P < .01). B) Effect on platelet counts after intravenous injection of aged IVIG (▪) or monomeric IgG (▴) at t = 4 days. Aged IVIG preparations used in the experiment was stored at 4°C for 1 year. Results are expressed as mean of 5 experiments ± SEM on different occasions. Asterisks indicate statistically significant differences between the 2 groups (**P < .01; ***P < .001).
Effect of IVIG in antibody-mediated thrombocytopenia after continuous infusion of antiplatelet mAb.
(A) Effect on platelet counts by continuous infusion of antiplatelet mAb MWReg30 by an implanted osmotic pump in wild-type (■), FcγRIII−/− (▪), and FcR γ-chain−/−(▴) mice. Time course of mean platelet counts of 3 experiments (± SEM) is shown except for the wild-type mice (n = 2). Asterisks indicate statistically significant differences compared to saline control (*P < .05; **P < .01). B) Effect on platelet counts after intravenous injection of aged IVIG (▪) or monomeric IgG (▴) at t = 4 days. Aged IVIG preparations used in the experiment was stored at 4°C for 1 year. Results are expressed as mean of 5 experiments ± SEM on different occasions. Asterisks indicate statistically significant differences between the 2 groups (**P < .01; ***P < .001).
In the acute bolus model, we used 2.4G2 to block Fc receptors. It is known that infusion of this antibody efficiently blocks Fc receptor–mediated immune clearance; however, the maximal inhibition by this antibody only persists for 24 hours.6 The continuous infusion model takes much longer; therefore, we decided to use Fc receptor knockout mice to avoid repeated injection of 2.4G2 in these mice. Mice deficient in the common FcRγ chain (FcRγ−/−), lacking FcγRI and FcγRIII, and mice deficient in the FcγRIII alone were used to determine the role of the different Fcγ receptors in this model. No MWReg30-induced thrombocytopenia was observed in either FcRγ chain−/−or FcγRIII−/− mice, suggesting an important role for FcγRIII in the clearance of antibody-sensitized platelets (Figure3A).
Administration of IVIG in patients with ITP is often associated with a rapid increase in platelet counts. To determine whether administration of IVIG shows a similar effect on platelet counts in our model, mice were treated with aged IVIG or monomeric IgG 4 days after implantation of the pumps. To better determine the role of IgG dimers, we used in this series a monomeric IgG fraction isolated from IVIG instead of fresh IVIG because the latter still contains a significant amount of IgG dimers (Table 1). Infusion of a single dose of aged IVIG resulted in a transient increase in platelets for several days (Figure 3B), reaching platelet counts of approximately 52% of baseline values. No increase in platelet counts was observed after infusion of monomeric IgG or saline.
Table 2 shows the results of FACS analysis for detecting MWReg30 bound to platelets (membrane bound) and circulating amounts of MWReg30 in plasma expressed as mean fluorescence intensity. We tested whether circulating MWReg30 could be detected in plasma with continuous infusion and after bolus injection. Serial dilution of MWReg30 incubated with control platelets was used as a reference to estimate the amount of MWReg30 in plasma with continuous infusion. At day 4, 0.89 μg/mL anti-CRP was present in the plasma, indicating a 125-fold dilution of the input (112 μg/mL). Based on the amount of anti-CRP mAb measured in the enzyme-linked immunosorbent assay, we also expected a 1:125 dilution of MWReg30 to be present in plasma at day 4 if no antibody bound to membrane surfaces. Table 2shows that binding of antibody in plasma was similar to a 1:2500 dilution of the infused MWReg30 solution, suggesting that approximately 5% of MWReg30 was not bound to platelets. Furthermore, membrane binding of MWReg30 in vivo was shown to be one third the maximal value for each type of administration.
Platelet-bound and free circulating MWReg30 in the thrombocytopenic mice
| Platelet-bound MWReg30 (MFI) . | |
|---|---|
| Circulating platelets | |
| Osmotic pump (n = 3) | 700 ± 150 |
| Bolus (n = 3) | 683 ± 111 |
| Normal platelets incubated with plasma | |
| Osmotic pump (n = 3) | 51 ± 13 |
| Bolus (n = 3) | 27 ± 1 |
| Normal platelets incubated with MWReg30 | |
| 1:100 | 2266 ± 634 |
| 1:500 | 501 ± 299 |
| 1:2500 | 58 ± 10 |
| control | 18 ± 9 |
| Platelet-bound MWReg30 (MFI) . | |
|---|---|
| Circulating platelets | |
| Osmotic pump (n = 3) | 700 ± 150 |
| Bolus (n = 3) | 683 ± 111 |
| Normal platelets incubated with plasma | |
| Osmotic pump (n = 3) | 51 ± 13 |
| Bolus (n = 3) | 27 ± 1 |
| Normal platelets incubated with MWReg30 | |
| 1:100 | 2266 ± 634 |
| 1:500 | 501 ± 299 |
| 1:2500 | 58 ± 10 |
| control | 18 ± 9 |
MFI indicates mean fluorescence intensity.
Anti-idiotypic antibodies against MWReg30 in IVIG
IVIG efficacy in ITP could also result from the neutralization of platelet antibodies by anti-idiotypic antibodies in IVIG. Hence, aged IVIG and fresh IVIG were evaluated for the presence of anti-idiotypic antibodies with binding capacity to MWReg30. As a control, we tested possible binding capacity of components present in the IVIG preparations to human serum albumin. Binding of IgG to different coatings was similar: no specific binding to MWReg30 was observed when testing the various preparations (data not shown). We also evaluated whether IVIG could interfere with the binding of MWReg30 to platelets. Aged IVIG, at a concentration of 5 mg/mL, did not reduce MWReg30 binding of murine platelets in vitro.
Discussion
In the present study, we used 2 murine models for ITP to evaluate the effect of IVIG preparations that differed in the content of IgG dimers. We demonstrated that clearance of platelets sensitized with an anti-platelet mAb could only be reduced by administration of an IVIG preparation containing significant amounts of IgG dimers. An IVIG preparation without IgG dimers hardly showed an effect. The effect of IgG dimers was observed in both murine models for ITP, that is, a single intraperitoneal injection or continuous infusion of antiplatelet antibody. We recently described the hypotensive effects of IgG dimers in IVIG preparations, in which we compared 16 different IVIG preparations of 11 different manufacturers.18 This study revealed that commercially available IVIG preparations contain variable amounts of IgG dimers (range, 5%-15%). This is consistent with the observations of Tankersley et al19 that IgG dimers are a normal constituent of IVIG prepared from a large donor pool. The amount of dimers in the preparations mainly depends on the pool size and the storage conditions. Our present findings strongly suggest that because of differences in dimer content, preparations also will differ in therapeutic efficacy in the treatment of patients with ITP. They also suggest that therapeutic efficacy in ITP entails the risk for side effects, probably also related to the presence of IgG dimers through the activation of macrophages and neutrophils.18 20
Low-affinity Fcγ receptors apparently were involved in the antibody-dependent clearance of platelets in our model because in vivo blocking of FcγRII/III or the absence of these receptors in knock-out mice completely prevented the effect of antiplatelet mAb. The importance of low-affinity Fcγ receptors in the clearance of immune complexes has been shown in several human and animal studies.6 Furthermore, infusion of mAbs against FcγRIII caused a significant, transient increase in platelet counts in a patient with ITP.10 Infusion of an mAb directed against FcγRI did not change the platelet count over a 5-day course of treatment, whereas the administration of IVIG in this patient improved platelet count.21 Our experiments with blocking mAb (2.4G2) or in mice deficient for Fcγ receptors were compatible herewith and in particular pointed to a role for FcγRIII in the clearance of antibody-sensitized platelets, emphasizing the relevance of these models for human ITP. However, others have demonstrated involvement of the platelet Fcγ receptor in human immune thrombocytopenias accompanied by thrombosis, such as heparin-induced thrombocytopenia,9 and it cannot be precluded that this receptor also plays a pathophysiologic role in other forms of ITP. Because mice do not express this Fcγ receptor on platelets, our model is unable to address beneficial or detrimental effects of IVIG on platelet Fcγ receptors. Hence, one should be careful to extrapolate the results obtained in this model to human ITP. Interference with FcγR expression level and FcγR-mediated functions (such as phagocytosis) are well-established characteristics of polymeric IgG and immune complexes.22,23 Furthermore, human IgG aggregates and IgG dimers bind more efficiently to low-affinity Fcγ receptors than do IgG monomers. Dimeric IgG may, therefore, have a stronger effect than monomeric IgG. It has been suggested that in IVIG, small amounts of aggregated IgG can form spontaneously and that these aggregates, rather than IgG monomer itself, constitute the active component of IVIG mediating Fcγ-receptor blockade in ITP.7,19 Augener24 reported that the efficacy of IVIG treatment of a patient with ITP was not associated with monomeric IgG levels but with the presence of IgG aggregates in the preparation. This patient showed normal levels of IgG complexes before treatment with IVIG and showed stepwise increases in total IgG levels, including aggregates, during therapy. When IVIG administration was terminated, the concentration of complexed IgG rapidly dropped to a normal level, and this was paralleled by a drastic decrease of platelet numbers even though IgG plasma levels were still high.24In another study it was shown that the use of intramuscular preparations of anti-D that contained aggregated IgG resulted in small platelet increases in several Rh-negative recipients.1These observations agree well with the results of our study.
Acute systemic response seen on a bolus injection of a relatively high dose of MWReg30 is a process that requires a threshold-dose of antibody to occur. The density of antibodies bound to the platelets can explain these differences. In patients with ITP, platelet-specific antibodies are primarily directed to gpIIb/IIIa—as is MWReg30. However, specific antiplatelet antibodies account for only a small fraction of the platelet-associated IgG, with the major fraction attributed to nonspecific IgG associated with platelets.25 26 This suggests that in a model with clinical relevance, the amount of antibody used to induce thrombocytopenia should be low, so that only the antibody occupies a few epitopes on the platelet surface. We showed that in our models only some of the MWReg30 binding sites were occupied.
An alternative mechanism for the reversal of thrombocytopenia by IVIG involves idiotype–anti-idiotype interaction with autoantibodies and anti-idiotype antibodies in the IVIG preparations.5 This mechanism is independent of RES blockade, but it postulates the neutralization of antiplatelet antibody by IVIG. However, it was shown that Fcγ fragments have the same therapeutic effects as IVIG in patients with ITP.27 Thus, anti-idiotypic effects of IVIG are not likely a major mechanism of action in ITP. In our study, binding of MWReg30 to platelets was not influenced by IVIG, nor did we find (anti-idiotypic) antibodies with binding capacity to MWReg30.
In conclusion, in a clinically relevant mouse model for ITP, we show the efficacy of IgG preparations containing high amounts of IgG dimers. The experimental model applied may not only help to define the mechanisms causing ITP but may also add to the evaluation of novel therapeutic strategies to treat this disease. We suggest that therapeutic efficacy of different IVIG preparations used to treat human ITP depends on the presence of IgG dimers, with preparations containing relatively high amounts of IgG dimers having better therapeutic efficacy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Wim K. Bleeker, CLB, Department of Immunopathology, Academical Medical Center, University of Amsterdam Plesmanlaan 125, 1066 CX, Amsterdam, The Netherlands; e-mail:w_bleeker@clb.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal