Congenital dyserythropoietic anemia type II (CDA-II) is an autosomal recessive disease characterized by anemia, jaundice, splenomegaly, and erythroblast multinuclearity. The natural history of the disease is unknown. The frequency, the relevance of complications, and the use of splenectomy are poorly defined. This study examined 98 patients from unrelated families enrolled in the International Registry of CDA-II. Retrospective data were obtained using an appropriate questionnaire. The mean age at presentation was 5.2 ± 6.1 years. Anemia was present in 66% and jaundice in 53.4% of cases. The mean age at correct diagnosis was 15.9 ± 11.8 years. Twenty-three percent of patients for whom data were available developed anemia during the neonatal period, and 10 of these individuals required transfusions. Splenectomy produced an increased hemoglobin (P < .001) and a reduced bilirubin level (P = .007) in comparison with values before splenectomy. Preliminary data indicate that iron overload occurs irrespective of the hemochromatosis genotype.
Introduction
Congenital dyserythropoietic anemia type II (CDA-II) is an autosomal recessive disorder affecting the normal differentiation-proliferation pathway of the erythroid lineage.1-4 It comprises an anemia of variable severity, jaundice, and variable splenomegaly. Erythroid hyperplasia with binuclearity or multinuclearity involving late erythroblasts in the bone marrow (BM) is a key feature of the diagnosis. In addition, on electron microscopy, vesicles of endoplasmic reticulum (ER) appear to be running beneath the plasma membrane in this disorder.5
Polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate (SDS-PAGE) of erythrocyte membrane proteins shows that band 3, the anion exchanger, is narrower and has an accelerated migration rate,6,7 because of abnormal glycosylation. Underglycosylated band 3 aggregates in solution and clusters in membranes, resulting in membrane disorganization.8,9Western blotting following SDS-PAGE shows the presence of calreticulin, the glucose-regulated protein 78 (GRP 78) and the protein disulfide isomerase,5 which belongs in the ER and corresponds to the above-mentioned vesicles. The view of CDA-II as a primary genetic defect of glycosylation8 has been discounted because of the lack of linkage with the genes encodingN-acetylglucosaminyltransferaseII or α-mannosidaseII,10 2 enzymes deficient in CDA-II. We obtained evidence for linkage of CDA-II to the long arm of chromosome 20,11 but at least 5% to 10% of cases failed to map to 20q,12 stressing genetic heterogeneity.
Despite the progress in genetics and pathophysiology, the primary molecular lesion remains speculative. No information exists on the frequency and the clinical relevance of complications. Treatment is largely supportive and the benefit of splenectomy is uncertain. Based on long-term follow-up of a large group of patients, we here report the natural history of CDA-II.
Study design
We investigated 98 CDA-II patients (39 males and 59 females; mean age, 17.6 years; range 1 month to 67 years) from 78 unrelated families enrolled in the CDA-II International Registry, which has been collecting all newly notified cases since 1996. Patients originated predominantly from Italy or from other European countries.13
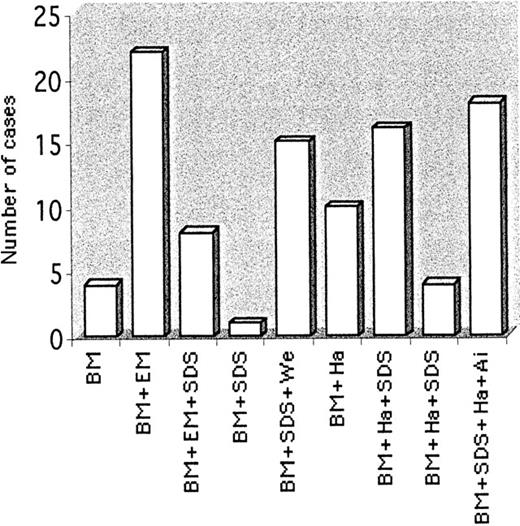
The enrollment questionnaire contained information on age and symptoms at discovery, age at diagnosis, diagnostic criteria, complications, and therapy. The tests concerning erythrocyte membrane protein analysis used to confirm the diagnosis in some cases were performed as reported1,3 and are shown in Figure1. C282Y and H63D mutations in the hemochromatosis (HFE) gene were analyzed as described.14
Diagnostic criteria for patients enrolled in the International Registry of CDA-II.
Overall determinations: bone marrow (BM), 98; electronic microscopy (EM), 30; Ham-test (Ha), 48; Ag-i (Ai), 22; SDS-PAGE (SDS), 58; Western blots for ER proteins (We), 15.
Diagnostic criteria for patients enrolled in the International Registry of CDA-II.
Overall determinations: bone marrow (BM), 98; electronic microscopy (EM), 30; Ham-test (Ha), 48; Ag-i (Ai), 22; SDS-PAGE (SDS), 58; Western blots for ER proteins (We), 15.
Approval for these studies was obtained from the University of Bari Institutional Review Board. Informed consent was provided according to the Declaration of Helsinki.
Results and discussion
Of the 98 patients enrolled in the registry, 88 questionnaires were fully evaluable. Diagnostic criteria were based on BM examination and on further tests (Figure 1).
Age at presentation
The age at presentation ranged from 1 month to 25 years (mean, 5.2 ± 6.1 years). The symptoms included anemia (66%) and jaundice (53.4%). The age at diagnosis ranged from 4 months to 65 years (mean, 15.9 ± 11.8 years).
Extent of anemia
By the time of diagnosis, the basic parameters were hemoglobin (Hb), 8.3 ± 1.2 g/dL; mean corpuscular volume (MCV), 86 ± 10 fL; reticulocytes, 103.5 ± 45.6 × 109/L; total biliribin, 4.6 ± 2.3 mg/dL. In the 26 patients who were diagnosed after the age of 16, Hb level was 10.1 ± 1.8 g/dL.
Clinical course during infancy
Information on neonatal period was available for 64 subjects. Fifteen had a Hb level less than 8.0 g/dL on the third postpartum day, with 10 requiring transfusions. Seven of these subjects had transfusions in adult life and 5 became dependent on transfusions. Sixteen had jaundice that required phototherapy and 10 underwent exchange transfusion. A 2-month-old anemic girl died of pulmonary complications. Considering both newborns and infants, 66% of patients were anemic (Hb level 7.9 ± 1.4 g/dL). During infancy spleen enlargement was documented in 68 of 88 cases, jaundice in 48, and gallstones in 14 cases.
Transfusion requirements
Thirty-three of 88 patients with a Hb value below 6 g/dL had transfusions during the first year of life. In the following years the transfusion requirements decreased; only 5 subjects (3 with associated β-thalassemia trait) remained dependent on transfusions during adulthood.
A patient aged 15 received a BM transplant from an identical sibling and was disease-free after 2 years.15
Splenomegaly and effect of splenectomy
Splenectomy was performed in 26 patients at a mean age of 12.6 years (range, 3.5-40 years). In 4 cases it was performed prior to the diagnosis of CDA-II. In 19 patients, the Hb level was 8.05 ± 1.62 g/dL before and 9.75 ± 1.03 g/dL after splenectomy (P < .001) and the serum bilirubin level was 5.07 ± 1.22 mg/dL before and 2.50 ± 1.42 mg/dL after splenectomy (P = .007). Three transfusion-dependent patients became transfusion-independent after splenectomy. Twenty subjects had cholecystectomy, usually at the time of splenectomy.
Iron overload
Forty-seven transfusion-independent patients were evaluable for iron studies. One died from severe heart failure at age 15. No other clinical complications related to iron overload were recorded. Serum ferritin level gradually increased from the time of diagnosis onward. There were no significant differences between patients with or without H63D mutation. Both subgroups showed an age-dependent increase in ferritin (Table 1). In 21 splenectomized patients ferritin levels were 299 ± 219 μg/L versus 220 ± 114 μg/L in 39 nonsplenectomized subjects, matched for age (P = .070).
Iron parameters (±1 SD) in 47 transfusion-independent patients
| . | No.cases . | Mean age (y) . | Transferrin SI (%) . | Serum ferritin (μg/L) . |
|---|---|---|---|---|
| At diagnosis | 47 | 15.6 | 63 ± 27 | 128 ± 122 |
| During follow-up | 47 | 18.9 | 68 ± 30 | 254 ± 182 |
| P < .001 | ||||
| H63D+ | ||||
| At diagnosis | 8 | 11.5 | 77.7 ± 29 | 169.25 ± 134 |
| During follow-up | 8 | 19.6 | 79.9 ± 28 | 303 ± 106 |
| P = .044 | ||||
| H63D− | ||||
| At diagnosis | 39 | 12.3 | 61.8 ± 35 | 121 ± 97 |
| During follow-up | 39 | 19.1 | 66.2 ± 34 | 220 ± 114 |
| P < .001 |
| . | No.cases . | Mean age (y) . | Transferrin SI (%) . | Serum ferritin (μg/L) . |
|---|---|---|---|---|
| At diagnosis | 47 | 15.6 | 63 ± 27 | 128 ± 122 |
| During follow-up | 47 | 18.9 | 68 ± 30 | 254 ± 182 |
| P < .001 | ||||
| H63D+ | ||||
| At diagnosis | 8 | 11.5 | 77.7 ± 29 | 169.25 ± 134 |
| During follow-up | 8 | 19.6 | 79.9 ± 28 | 303 ± 106 |
| P = .044 | ||||
| H63D− | ||||
| At diagnosis | 39 | 12.3 | 61.8 ± 35 | 121 ± 97 |
| During follow-up | 39 | 19.1 | 66.2 ± 34 | 220 ± 114 |
| P < .001 |
Data are presented at diagnosis and during follow-up in the whole series studied and in the two subgroups according to HFE genotype. Statistical evaluation of the difference between the ferritin levels at diagnosis status versus at follow-up status are provided.
Discussion
The remarkable difference between age at presentation and age at diagnosis points to the difficulty in recognizing CDA-II. Because of hemolytic symptoms, CDA-II is not infrequently misdiagnosed as hereditary spherocytosis (HS). Only a suboptimal reticulocyte count for the degree of anemia may hint at dyserythropoietic anemia. BM examination remains the “gold standard” for diagnosis (Figure 1). SDS-PAGE and Western blotting are straightforward and reliable and should gain wider use in the future. The other investigations are either less sensitive or not widely available.
No information was previously available on neonatal CDA-II. We show that 25% of affected newborns were anemic, that transfusions were required in two thirds of them, and that jaundice was common and required treatment. Neonatal CDA-II appears less severe than HS, in which up to 65% of newborns have disease-related symptoms.16 However, whereas clinical symptoms during the neonatal period are not predictive of the outcome in HS,16they may anticipate the disease evolution in CDA-II.
Anemia, jaundice, and splenomegaly were present in 66% of patients during infancy and childhood. Although CDA-II is considered a benign condition, 2 patients died and 33 required transfusions during the first year of life. Anemia during this period has been attributed to a low physiologic reserve of the BM with a weak response to erythropoietin.16,17 Ten patients required transfusions after the first year and only 5 became dependent on transfusions. A small percentage of cases had late diagnosis, reflecting a milder disorder. The phenotypic heterogeneity could reflect both heterogeneous molecular lesions and different acquired factors. In our experience 20q unlinked disorders seem to be more severe (data not shown). Other modifier genes may play a role; β-thalassemia, adding a further cause (globin chain imbalance) of ineffective erythropoiesis, aggravates the severity of the anemia.18
Although splenomegaly is common, the recommendation regarding splenectomy is not uniform among hematologists. Thirty percent of the patients had splenectomy when last observed. From our analysis, splenectomy is sometimes of benefit because anemia improves and hemolysis decreases.
One complication of dyserythropoiesis is iron overload, because iron absorption increases secondary to expanded erythropoiesis. In a limited follow-up, serum ferritin level increases with age, irrespective of the HFE H63D genotype, suggesting that the erythropoietic expansion is the major determinant of iron loading. Because our series did not include any C282Y mutations, no conclusions could be drawn on the effect of C282Y heterozygosity. A single case of iron loading due to C282Y homozygosity is known in CDA-II.19 Prolonged follow-up of patients in the registry will provide additional data on the evolution of iron overload.
The authors thank all the colleagues for referring the investigated families. The authors are particularly grateful to the chairmen of the centers participating in the recruitment of patients: U. Ramenghi (Torino), M. D. Cappellini (Milano), P. Scartezzini (Genova), G. Barosi (Pavia), R. Galanello (Cagliari), D. Gallisai (Sassari), P. Rigano (Palermo), L. Felici (Pesaro), P. Izzo (Bari), G. Schilirò(Catania), P. Sticca (Como), B. Nobili and E. Miraglia del Giudice (Napoli), J. Vives-Corrons (Barcelona), G. Tchernia (Paris), S. Eber (Zurich), F. Gilsanz (Madrid), V. Premetis (Athens), A. Colita (Bucharest), H. Toriello and S. Mckanzie (United States), S. Aftimos (New Zealand), and H. Heimpel (Germany). The authors also thank the pediatricians affiliated with the AIEOP and ESPHI.
Supported by grants from the Italian Ministry of Health (A.I.), MURST 40% and 60% University of Bari (A.I.), and Telethon project E-645 (A.I.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Achille Iolascon, Dipartimento di Biomedicina dell'Età Evolutiva, Piazza G. Cesare 11, 70124 Bari, Italy; e-mail: a.iolascon@bioetaev.uniba.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal