The anthracycline daunorubicin is widely used in the treatment of acute nonlymphocytic leukemia. The drug has, of course, been the object of intense basic research, as well as preclinical and clinical study. As reviewed in this article, evidence stemming from this research clearly demonstrates that cell response to daunorubicin is highly regulated by multiple signaling events, including a sphingomyelinase-initiated sphingomyelin-ceramide pathway, mitogen-activated kinase and stress-activated protein/c-Jun N-terminal kinase activation, transcription factors such as nuclear factor κB, as well as the Fas/Fas-ligand system. These pathways are themselves influenced by a number of lipid products (diacylglycerol, sphingosine-1 phosphate, and glucosyl ceramide), reactive oxygen species, oncogenes (such as the tumor suppressor gene p53), protein kinases (protein kinase C and phosphoinositide-3 kinase), and external stimuli (hematopoietic growth factors and the extracellular matrix). In light of the complexity and diversity of these observations, a comprehensive review has been attempted toward the understanding of their individual implication (and regulation) in daunorubicin-induced signaling.
Introduction
The anthracycline daunorubicin (DNR) is one of the major antitumor agents widely used in the treatment of acute myeloid leukemias (AMLs). Cytotoxicity mediated by DNR is generally thought to be the result of drug-induced damage to DNA. This damage is mediated by quinone-generated redox activity, intercalation-induced distortion of the double helix, or stabilization of the cleavable complex formed between DNA and topoisomerase II.1 However, how and why such events should bring about cell death remains unclear, especially when one considers that DNA interaction may not be a prerequisite for anthracycline cytotoxicity.2-5 Hence, the exploration and understanding of the process of apoptosis has forced a reconsideration of the mechanisms whereby myeloid leukemia cells respond to DNR. In this context, we have shown that, within a narrow concentration range (0.2-1 μM), DNR can trigger apoptosis in the monocytic U937 or the myelocytic HL-60 AML cells but not in the immature (CD 34+) KG1a, KG1, or HEL cells,6the latter being more resistant to DNR than the former.7These results suggested that DNR triggers apoptotic signals in drug-sensitive AML cells and that inhibition of these signals may contribute to drug resistance and, for example, to the inherent resistance of immature AML cells. For this reason, others and we have investigated the mechanism by which DNR activates apoptosis in DNR-sensitive AML cells.
Implication of sphingomyelin metabolism in DNR-induced apoptosis
DNR activates the sphingomyelin cycle in sensitive leukemic cells
In the early 1990s, a number of studies provided growing evidence that sphingomyelin breakdown products (ie, ceramide [CER] and phosphorylcholine) could play an important role in mediating the action of cytokines, including tumor necrosis factor α (TNFα) and interferon. For example, Obeid et al8 were the first to report that TNFα could stimulate a neutral sphingomyelinase (N-SMase) responsible for sphingomyelin hydrolysis and subsequent generation of CER in U937 cells and that C2-CER, a synthetic cell-permeable CER analog, induced apoptosis in these cells. These results strongly supported the implication of the sphingomyelin-CER pathway in apoptosis induced by TNFα and, perhaps, by other cytotoxic molecules. In further studies, Haimovitz-Friedmann et al9proposed the involvement of the sphingomyelin-CER pathway in apoptosis induced by γ-radiation in bovine endothelial cells.
On the basis of those studies, our group investigated the role of the sphingomyelin-CER pathway in DNR-induced apoptosis. DNR exposure at concentrations that induced apoptosis (0.5-1 μM) stimulated early (5-10 minutes) sphingomyelin cycle (hydrolysis and resynthesis) and subsequent CER generation in both U937 and HL-60 cells. The concentration of endogenous CER generated (∼10 μM, estimated by cell volume) is clearly sufficient to induce apoptosis.8Because these cells exhibit a mutated or deleted form of p53, it appears that CER operated through a p53-independant mechanism. Further studies have shown that DNR induced sphingomyelin hydrolysis associated with the stimulation of N-SMase, whereas acidic SMase did not appear to be involved.10 Moreover, we have recently reported that DNR induced sphingomyelin cycle, CER generation, and apoptosis in Epstein-Barr virus–transformed lymphoblastoid cell lines established from patients with Niemann-Pick disease, a genetic disorder characterized by the lack of acidic SMase activity.11These results suggest that acidic SMase is dispensable for DNR-induced sphingomyelin hydrolysis.
In another study, Bose et al12 have proposed that DNR may induce CER accumulation because of enhanced de novo synthesis through CER synthase stimulation, a mechanism that has also been suggested for TNFα-induced cell death in nonhematopoietic cell death.13,14 However, it should be noted that, in the study by Bose et al,12 cells were treated with very high DNR doses, whereas, at clinically relevant drug concentrations, we were unable to detect CER synthase stimulation.10 The fact that sphingomyelin hydrolysis occurred within 5 to 10 minutes after drug exposure and apoptosis was not detected before 4 to 6 hours raised some controversies about the implication of the sphingomyelin cycle in DNR apoptosis. Therefore, we have carefully examined the influence of DNR on sphingomyelin metabolism over a longer period of time (0-12 hours).
In this study, we have found that DNR exposure resulted in at least 4 sphingomyelin cycles (hydrolysis and resynthesis) with concomitant (4 cycles) CER production within the first 4 hours, after which poly(ADP-ribose) polymerase cleavage, DNA laddering, and apoptosis-associated morphologic changes occurred. Moreover, the successive waves of CER production were not influenced by fumonisin B1, a potent and specific CER synthase inhibitor.15 These results confirmed that de novo CER synthase plays little if any role in DNR-induced CER signaling. The fact that DNR induced several peaks of CER production suggested that sphingomyelin hydrolysis products (ie, CER or phosphorylcholine) might reactivate N-SMase. In fact, we have found that cell-permeant CER may indeed stimulate N-SMase, sphingomyelin hydrolysis, and endogenous CER production, suggesting that CER may enhance its own production through feedback control of N-SMase.15 Therefore, one can speculate that DNR activates a single sphingomyelin cycle that is sufficient for inducing CER autoproduction and that these repeated CER-mediated apoptotic signals are perhaps needed for maintaining an apoptotic (cell suicide) signaling pressure overcoming latent cell survival mechanisms. Finally, it is noteworthy that DNR-induced topoisomerase II cleavable complexes have not been described in the literature under these conditions (1 μM DNR treatment for 4 hours); of course this does not demonstrate unambiguously that DNR interaction with DNA, or more specifically with topoisomerase II, is not requisite for apoptosis signaling because it can be argued that present experimental procedures lack sensitivity in detecting minute amounts of DNR-topoisomerase-DNA complexes.
CER targets
The emergence of CER as an important mediator of cell death induced by cytotoxic molecules has stimulated an extensive search for downstream targets involved in CER-mediated apoptosis. In fact, CER was found to activate a large variety of signaling proteins, including Raf-1, extracellular-regulated kinases (ERKs), a CER-activated protein phosphatase, as well as stress-activated protein/c-Jun N-terminal kinase (SAPK/JNK). By using dominant- negative mutants, Verheij et al16 elegantly demonstrated that the MEKK1-SEK1-JNK cascade is one of the major components of CER-mediated apoptosis. This finding is consistent with another study which showed that c-Jun activation is required for CER-mediated apoptosis.17Further studies have supported the function of JNK in the initiation of programmed cell death. For example, it was shown that overexpression of MEKK1 or ASK1, 2 potent and specific in situ activators of SEK1 and JNKs, had lethal effect on fibroblasts.18-20 Because DNR triggers both CER production and apoptosis, it was expected that this drug may also activate JNK signaling. In fact, we and others have reported that both DNR and doxorubicin, in a dose-dependent manner, increased JNK1 tyrosine phosphorylation, stimulated JNK1 enzymatic activity, and enhanced DNA binding activity of the transcription factor activated protein-1 (AP-1).21-23 Anthracyclines are not the unique antitumor agents that activate the JNK/c-Jun pathway. Indeed, other genotoxic agents such as UV,24 ionizing radiation,25-27 alkylating agents,25etoposide,21 or cytosine arabinoside28 as well as nongenotoxic agents such as microtubule poisons29,30 also activate JNK. These observations suggest a general function for JNK in mediating cell death induced by antitumor agents (see Kyriakis and Avruch for review31). Therefore, it appears that, in leukemic-sensitive cells, DNR activates a complex signaling cascade that consists of repeated sphingomyelin cycles followed by several waves of CER production; this second messenger, in turn, activates a common downstream cell death effector-mediated pathway that involves JNK stimulation and c-Jun/AP-1 activation.
Regulation of CER production
CER production is limited by both N-SMase activity and the magnitude of sphingomyelin pool disposable for hydrolysis. Previous studies have shown that N-SMase activity is strongly influenced by protein kinase C (PKC) activity. For example, we have reported that phorbol ester– or phosphatidylserine-induced PKC stimulation resulted in the inhibition of N-SMase stimulation, sphingomyelin hydrolysis, CER production, and apoptosis induced by DNR in U937 cells.32Conversely, PKC inhibitors were found to stimulate N-SMase.33 The amount of hydrolyzable sphingomyelin is another candidate for regulating CER production. Indeed, it has been reported that, whereas sphingomyelin is preferentially distributed within the outer leaflet of the plasma membrane (sphingomyelin transverse asymmetry), the sphingomyelin pool disposable for hydrolysis consists primarily in the sphingomyelin component that is associated to the plasma membrane inner leaflet.34 Thus, it is conceivable that reduction of hydrolyzable sphingomyelin pool because of altered transverse asymmetry may result in reduced CER production. In 2 studies, we have reported that, in KG1a AML cells, which are naturally resistant to DNR,7 mitoxantrone,35and TNFα,36 the inner leaflet-associated sphingomyelin pool was greatly reduced, compared with the sensitive U937 cells, whereas KG1a and U937 cells exhibited similar total sphingomyelin amount. Interestingly, in the resistant cells, cytotoxic effectors such as TNFα,36 DNR, and mitoxantrone (A Bettaiëb et al, unpublished results, July 1997) failed to induce sphingomyelin hydrolysis, CER generation, and apoptosis. Thus, it is possible that, in some AML cells, the activation of yet undefined sphingomyelin translocases results not only in modification of sphingomyelin transverse asymmetry but also in reduced sphingomyelin hydrolyzable pool, decreased CER production, and inhibition of apoptosis.37 Confirming this hypothesis, it has recently been shown that the CER generated from the plasma membrane sphingomyelin gains access to a SMase because of phospholipid scrambling.38
Regulation of CER metabolism
Intracellular CER concentration results from the equilibrium between CER production and CER metabolism. CER metabolism also plays an important role in regulating intracellular CER concentration and therefore DNR cytotoxicity. Theoretically, CER produced by DNR may enter into 3 distinct metabolic pathways that all result in decreasing intracellular CER levels.
First, CER can be transferred to a phosphorylcholine group to generate sphingomyelin (and diacylglycerol [DAG]) on sphingomyelin synthase stimulation. It is likely that this metabolic pathway is activated for sphingomyelin resynthesis after sphingomyelin hydrolysis during the sphingomyelin cycle. This enzyme therefore has the important ability to directly regulate, in opposite directions, CER and DAG levels within the cells.39 However, despite the great biological potential of sphingomyelin synthase, very little is known about location, distribution, and regulation of this enzyme.40Whether sphingomyelin synthase plays a role in DNR-induced cytotoxicity remains to be investigated.
Second, on ceramidase stimulation, CER can be catabolized into sphingosine that, in turn, can be converted into sphingosine-1-phosphate through sphingosine-1-kinase. In a recent study, we have reported that DNR induced CER production and apoptosis in cells derived from Farber disease, which are genetically deficient for lysosomal ceramidase, the major component of cellular ceramidase activity.41 Although we cannot totally exclude the implication of extralysosomal ceramidases,42 this result suggests that sphingosine plays little if any role in DNR-induced apoptosis. However, from studies performed by Cuvillier et al,43,44 sphingosine-1-phosphate has emerged as one of the most potent regulators of apoptosis. Indeed, sphingosine-1-phosphate inhibits apoptosis induced by CER and other effectors.43,44 The mechanism by which sphingosine-1-phosphate interferes with CER-induced apoptotic signaling is not fully understood. However, it has been reported that sphingosine-1-phosphate inhibits CER-induced JNK stimulation and caspase activities.44,45 Therefore, sphingosine-1-phosphate and sphingosine-1-kinase might play an important role in regulating DNR-induced apoptosis. In fact, it has been demonstrated that sphingosine-1-phosphate inhibits doxorubicin-induced apoptosis.46 From these studies, one can speculate that sphingosine-1-kinase stimulation may contribute to DNR resistance. Because sphingosine-1-kinase is potently stimulated by PKC, it is possible that sphingosine-1-phosphate overproduction represents another mechanism by which PKC exerts its protective function in DNR-treated cells.
Third, CER can be transformed to glucosylceramide by a glucosylceramide synthase. Previous reports have indicated that glucosylceramide has no cytotoxic property or may even stimulate cell proliferation47 and that glucosylceramide synthase inhibitors have been found to display some antitumor activity.48,49 These results suggest that glucosylceramide synthase plays an important role in cellular protection. Indeed, it has been described that cells transduced by glucosylceramide synthase gene were highly resistant to anthracyclines50 and that enzyme activity was significantly boosted in multidrug-resistant (MDR) cells.51 Conversely, transfection of glucosylceramide synthase antisense reverses adriamycin resistance.52 DNR triggers the sphingomyelin cycle in MDR cells when used at high doses.53 Therefore, one can speculate that in DNR-treated MDR cells CER originated from sphingomyelin hydrolysis is rapidly converted to glucosylceramide because of glucosylceramide synthase overactivity.54,55 If this speculation is the case, glucosylceramide synthase appears as an attractive target for MDR reversal. In fact, inhibition of CER glycosylation pathway increases MDR cell sensitivity to cytotoxics.56 The same group has reported that most MDR modulators, including cyclosporin A, tamoxifen, and verapamil, are potent glucosylceramide synthase inhibitors, whereas the cyclosporin A analogue PSC 833 (Valspodar), a clinically used potent MDR reversal agent, increases intracellular CER concentration by stimulating CER synthase.51,57,58 These results suggest that those agents may exert their chemosensitizing effect not only through their P-glycoprotein binding capacity, as previously postulated, but also by facilitating CER accumulation. However, the mechanism by which CER modulates anthracycline-induced cytotoxicity in MDR cells remains to be determined. For example, there is no evidence that CER interferes with P-glycoprotein function or intracellular drug distribution in MDR cells.59
Regulation of CER apoptotic signaling pathway
The sphingomyelin-CER pathway appears to be efficiently regulated downstream of CER generation. Bcl-2 inhibits apoptosis induced by DNR without interfering with DNR-induced sphingomyelin cycle activation.60 This result is consistent with previous studies which have shown that Bcl-2 inhibits apoptosis induced by cell-permeant CER.61 PKC is also a potent regulator of CER-induced apoptosis. Indeed, previous studies not only showed that PKC activators, including phorbol esters and DAG, could inhibit the ability of cell-permeant CER to induce apoptosis but also that PKC inhibitors enhanced CER-induced apoptosis.9,32,62,63Therefore, PKC appears as a critical regulator of the sphingomyelin-CER pathway because it operates both upstream and downstream of CER production. This finding may have important implications in our understanding of AML-cell drug resistance. Indeed, a large variety of hematopoietic growth factors (HGFs), including interleukin 3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), or basic fibroblast growth factor, were found to stimulate PKC activity through phosphatidylinositol or phosphatidylcholine hydrolysis and subsequent DAG production.64 Thus, it is conceivable that autocrine production of HGFs by leukemic cells may result in DNR-induced sphingomyelin-CER pathway inhibition and thereby contribute to reduce DNR cytotoxicity in AML. The fact that HGFs were found to inhibit DNR-induced apoptosis,65,66 as well as the chemosensitizing effect of PKC inhibitors on fresh AML cell progenitors cultured in the presence of HGFs,67 supports this hypothesis. Moreover, we have recently found that Kit signaling, activated either by its natural ligand (stem cell factor) or by modification of its intracellular domain, inhibits DNR-induced SMase stimulation, CER production, and apoptosis through a PKC-dependent mechanism.68
Implication of radical oxygen species in DNR-induced apoptosis
During the treatment of the cells with anthracyclines, nicotinamide adenine dinucleotide phosphate (NADPH)–dependent flavin reductase reduces the drug to a semiquinone radical, which can donate its free electron to molecular oxygen and generate the superoxide radical (O). At neutral pH, the main reaction of O is a relatively slow spontaneous dismutation to H2O2 and O2, but this reaction can be accelerated by superoxide dismutase. Superoxide anion and hydrogen peroxide may interact (with metal ions such as iron or copper as catalyst), by the Haber-Weiss reaction to generate hydroxyl radicals (•OH). By employing electron spin resonance together with a spin-trap such as DMPO (5,5-dimethyl-1-pyrroline N-oxide), anthracycline-induced •OH has been detected in several types of cancer cells (see Sinha and Mimmaugh for review69). Electron spin resonance radicals have also been detected in intact erythrocytes treated with DNR.70 This result suggests that anthracycline-induced free radical production may occur independently from the interaction of the drug with internal membrane or even DNA. In addition, it has been demonstrated that all forms of radical oxygen species (ROS) may also be generated through the direct interaction of anthracyclines with cell membranes and subsequent lipid peroxidation.71 Although there is strong evidence that ROS plays a role in the cytotoxicity of anthracyclines, the mechanism by which ROS influences cell viability remains unclear.
Previous studies have shown that DNR-induced apoptosis was inhibited by ROS scavengers such as pyrrolidine dithiocarbamate and N-acetylcystein, a thiol antioxidant and a glutathione precursor, and was enhanced by buthionine-sulfoximine, which depletes the glutathione store.6 These results encouraged us to investigate the role of ROS in the DNR-stimulated sphingomyelin-CER-JNK apoptotic pathway. Indeed, the level of endogenous H2O2generated is comparable to that of a cell treated with 12 μM H2O2. In fact, further studies showed that pretreatment with N-acetylcystein abrogated DNR-induced N-SMase stimulation, sphingomyelin hydrolysis, and CER generation induced by DNR, evoking a role for ROS in N-SMase regulation.23 This hypothesis is supported by 2 other studies which showed that H2O2 stimulates sphingomyelin hydrolysis and CER generation and that N-acetylcystein and pyrrolidine dithiocarbamate were potent inhibitors of TNFα-induced sphingomyelin degradation to CER.72 73 Thus, it is conceivable that ROS originated from the NADPH-dependent DNR reduction contributes to N-SMase stimulation and subsequent CER generation. However, the mechanism by which ROS regulates N-SMase activity is not known.
Other studies have demonstrated that ROS also plays a role in CER-induced apoptosis. Indeed, permeant CER induced early and transient (5-10 minutes) H2O2 production, followed by a second wave of H2O2 detected at 1 to 3 hours that originated from mitochondrial oxidative metabolism disturbances. Furthermore, N-acetylcystein inhibited H2O2production, JNK activation, AP-1 activation, and apoptosis induced by CER.23,74 75 These results suggest that ROS plays an important role in the sphingomyelin-CER apoptotic pathway triggered by DNR at 2 different levels: upstream CER generation by stimulating N-SMase activity and downstream CER activity by mediating CER-induced JNK activation. The results also suggest a novel function of cytosolic-originated ROS in the cytotoxicity mechanism of anthracyclines. Thus, antioxidants, including superoxide dismutase, catalase, glutathione peroxidase, or thioredoxin, may influence DNR-induced cytotoxicity.
However, the fact that antioxidants inhibit apoptosis induced by chemotherapeutic drugs that have not been documented to stimulate the sphingomyelin cycle (ie, actinomycin D, camptothecin, etoposide, and melphalan),76 raises the possibility that ROS interferes with other apoptotic pathways in DNR-treated cells. Furthermore, there is mounting evidence that ROS activates or mediates many other signaling pathways, which can contribute more generally to the cellular response to DNR. For example, ROS modulates both PKC77-79 and tyrosine kinase activities,80-85contributes to cell cycle block,86 stimulates Raf-1/ERK mitogen-activated protein (MAP) kinases,84,87 and triggers activation of critical transcription factors, including nuclear factor-κB (NF-κB) (see Baeuerle and Henkel for review88), a negative regulator of DNR-induced apoptosis,89 which we will discuss later. These results suggest a complex role for ROS, which may mediate both cell death and survival signaling pathways, and thereby could play a major role in orientating the cellular response to genotoxic insult.
Implication of phosphatidylcholine metabolism in DNR-induced apoptosis
Anthracyclines, as other genotoxic agents including alkylating agents and ionizing radiation, have been shown to increase cellular DAG levels and PKC activity.90 However, the source of DAG as well as its functional role in the cellular response to the drug were not determined. In another study, we have reported that, in U937 cells, DNR (and mitoxantrone) transiently stimulated concurrently with CER generation both DAG and phosphorylcholine production by phospholipase C hydrolysis of phosphatidylcholine. Moreover, pretreatment of cells with the xanthogenate compound D609, a potent inhibitor of phosphatidylcholine hydrolysis, led to a sustained increase in CER levels. This result suggests that DNR may trigger in parallel both cell death (CER) and survival (DAG and phosphorylcholine) mediators and that, in sensitive leukemic cells, CER overrides the protective function of DAG and phosphorylcholine.91 These results support the notion that reciprocal regulation through DAG and CER may be implicated in the regulation of apoptosis.92 The mechanism by which phosphatidylcholine-derived DAG influenced intracellular CER concentration in DNR-treated cells is not yet characterized; however, because phosphatidylcholine-derived DAG did not appear to influence N-SMase stimulation, it is conceivable that DAG enhanced CER metabolism by facilitating sphingomyelin synthesis. Furthermore, it has been documented that phosphatidylcholine-derived DAG binds and stimulates Raf-1 kinase activity93,94 and, through Raf-1, may activate the MEK1/ERK classical MAP kinase module, a negative regulator of apoptosis induced by various stresses.84,87,95-97 Phosphatidylcholine-derived DAG has been found to stimulate some PKC isoforms such as PKC ζ or ι “atypical” isoforms that have been involved in cell proliferation and/or survival.98-100 In fact, we have recently observed that DNR stimulates Raf-1, ERK1, and PKC ζ activities through a D609-inhibitable mechanism (V Mansat-De Mas, I Plo, C Bezombes, et al; unpublished results, November 2000). Altogether these findings suggest that, in DNR-treated cells, phosphatidylcholine-derived DAG potentially operates by modulating the activity of diverse serine kinases and thereby contributes to reduce drug cytotoxicity. Regardless of the mechanism by which phosphatidylcholine-derived DAG exerts its protective function, this result may have some clinical implications. Indeed, it has been demonstrated that p21Ras or src oncogenes, as well as cytokines, including IL-3, GM-CSF, and TNFα, are potent activators of phosphatidylcholine hydrolysis and thereby may induce sustained DAG production.101-104 Therefore, it is conceivable that, in some AML cells, expression of some oncogenic products, as well as stimulation by cytokines, may severely impair DNR-induced apoptosis through a phosphatidylcholine-derived DAG-dependent mechanism.
Implication of the phosphoinositide 3-kinase in DNR-induced apoptosis
Among other enzymes that are involved in signal transduction pathways, phosphoinositide 3-kinase (PI3K) plays an important role. PI3Ks are a family of enzymes that catalyze the phosphorylation of inositol lipids at the D3 position of the inositol ring, generating new intracellular second messengers (see Franke et al105 for review). The lipid products of PI3K are phosphatidylinositol-3-phosphate (PtdIns-3-P), phosphatidylinositol-3,4-biphosphate (PtdIns-3,4-P2), and phosphatidylinositol-3,4,5-triphosphate (PtdIns-3,4,5-P3). PtdIns-3,4-P2 and PtdIns-3,4,5-P3 have been demonstrated to interact with a large variety of downstream effectors, including serine-threonine kinase Akt,106 calcium-insensitive PKCδ, ε, and η isoforms,107 as well as atypical PKC ζ isoform108 or phospholipase C.109 The use of PI3K inhibitors, wortmannin and LY294002, and PI3K mutants has demonstrated a role for PI3K in cell survival after various stresses, including γ or UV irradiation.110,111 For this reason, we have investigated the implication of PI3K in the cellular response to DNR in U937 cells. In fact, DNR induced a 2-fold increase in PI3K activity with transient accumulation of PtdIns-3,4-P2 and PtdIns-3,4,5-P3, and Akt activation. Wortmannin and LY294002 also accelerated DNR-induced apoptosis, suggesting that PI3K stimulation may contribute to cell survival.112 However, as already described earlier for phospholipase C activation by DNR, the stimulation of PI3K integrates into a futile response of the cells to the drug and only slightly affect DNR cytotoxicity. However, according to a recent report, it appears that PI3K may play a much more prominent protective effect against doxorubicin-induced apoptosis.113 Moreover, these results suggest that potent and sustained PI3K activation induced by oncogenic products, such as Ras114 or BCR-ABL115-117, or by mutated forms of HGF receptors, such as Flt3118, may result in significant protection toward DNR and perhaps other antileukemic agents. The mechanism by which PI3K or PI3K products interfere with DNR-induced apoptosis is still under investigation. However, from other studies, one can speculate that the PI3K protective function involves Akt-mediated phosphorylation of Bcl-2 family,119inhibition of caspase activity,120,121 or stimulation of PKC isoform activities.107,108 Moreover, it has been reported that cell-permeant CER decreases Akt activity,122suggesting possible cross-talk between sphingomyelin-CER and PI3K-Akt pathways in DNR-treated cells.
Implication of NF-κB activation in DNR-induced apoptosis
NF-κB is a member of the mammalian Rel family of transcriptional activators that plays a central role in the regulation of immune responses, embryogenesis, and hematopoiesis. NF-κB is a heterodimer consisting of the 50-kD (p50) and 65-kD (p65) subunits that, in its inactive state, is located in the cytoplasm bound to an inhibitory protein (IκBα). On activation, NF-κB disassociates from IκBα, translocates to the nucleus, and binds to DNA to regulate the expression of many genes.88 By using electrophoretic mobility shift assays, it has been shown that NF-κB DNA binding activity is stimulated by cytotoxic effectors, including DNA-damaging agents, such as ionizing radiation, alkylating agents, anthracyclines, topoisomerase inhibitors, and cytosine arabinoside.89,123-130 However, the fact that nongenotoxic anticancer compounds such as vinca alkaloids and taxanes125 may also activate NF-κB suggests that DNA damage is not necessarily needed for drug-induced NF-κB activation. This finding could mean that these drugs share some NF-κB–activating signaling pathways that can be generated in the cytosol but not in the nucleus, similar to that observed in the UV response in which NF-κB activation was clearly demonstrated to be initiated not in the nucleus but at or near the plasma membrane through a Src- and Ras-dependent mechanism.80 DNR-induced NF-κB activation does not require de novo protein synthesis and appears to be specific.125
Despite many efforts, the mechanism by which DNR activates NF-κB is not totally understood. Because, on one hand, NF-κB is considered to be an oxidative stress-responsive transcription factor,88and, on the other hand, DNR is known to generate ROS, one could speculate that ROS could be involved in DNR-induced NF-κB activation. However, the role of ROS was not firmly established. Indeed, whereas the antioxidant pyrrolidine dithiocarbamate was found to inhibit DNR-induced NF-κB activation in HL-60 and Jurkat T cells,124 opposite results have been reported in a human colon carcinoma cellular model.126 Moreover, mitoxantrone, which is unable to undergo redox cycling, was also found to activate NF-κB in a pyrrolidine dithiocarbamate–insensitive manner, suggesting that the inhibitory effect of pyrrolidine dithiocarbamate could not be related to its antioxidant property.129 CER was also a plausible candidate. Indeed, previous studies have shown that the addition of CER induced NF-κB activation.130 In fact, we found that the inhibition of DNR-induced SMase stimulation and subsequent CER production by serine-protease inhibitors reduces the capacity of the drug to activate NF-κB.131However, because of the broad specificity of these agents, the role of CER was not totally established, and one could not exclude a CER-independent mechanism as it has been proposed for TNFα-induced NF-κB activation.132,133 On the basis of previous studies which have shown that phosphorylation events are critical for NF-κB activation,88 the role of PKC has been investigated. Also, PKC inhibitors were found to block DNR-induced NF-κB activation,125 suggesting that DNR-triggered PKC stimulation plays an important role in NF-κB activation. In fact, previous studies have already suggested that PKC may act indirectly on phosphorylation and subsequent degradation of IκB.88More recent studies have indicated that IκB phosphorylation is under control of 2 IκB kinases (IKKα and ΙΚΚβ) which phosphorylate residues 32 and 36 of IκBα and that atypical PKC isoforms are critical regulators of the IKK activity.134Altogether these results render those PKC isoforms plausible candidates for mediating anthracycline-induced NF-κB activation. Finally, one cannot rule out the role of PI3K/Akt pathway. Indeed, it has been shown that activated Akt directly interacts and phosphorylates IκB-kinase (IKK).135 136 Whether or not DNR-induced PI3K/Akt pathway is involved in DNR-induced NF-κB activation should be examined.
Previous studies have shown that NF-κB inhibits apoptosis and/or enhances survival in a large variety of hematopoietic cells, including B lymphocytes,137 Hodgkin disease cells,138and CD34+ bone marrow cells.139 Therefore, it was tempting to speculate that this transcription factor provides significant protection against DNR-induced cytotoxicity. Such a hypothesis was supported by the fact that NF-κB was found to play an essential role in preventing TNFα-induced cell death.89,140,141 To address this question more directly for DNR, Wang et al89 have used a gene construct coding for a super-repressor form of the NF-κB inhibitor IκBα. This IκB repressor contains mutations, which inhibit signal-induced phosphorylation and subsequent proteasome-mediated degradation of IκB. This mutant protein binds to NF-κB and prevents nuclear translocation as well as DNA binding.142,143 Using this methodology, those investigators have shown that NF-κB inhibition resulted in increased cytotoxic effect of DNR (and ionizing radiation) in vitro.89 Although other studies suggest that the role of NF-κB in drug resistance may be a function of the cellular model,127,128 these results may have important clinical implications. Indeed, some pharmacologic agents are known to inhibit cytokine-induced NF-κB activation, including glucocorticoids, which act through induction of IκB synthesis.144,145Glucocorticoids are largely used in association with anthracyclines as part of front-line therapy of myeloma, acute lymphoid leukemia, and lymphoma. Whether or not glucocorticoids act in synergy with anthracyclines in lymphoid cells through a NF-κB–dependent mechanism has not been evaluated. Altogether these findings provide new insights in the understanding of synergistic drug combinations currently used in the clinical setting. These findings also provide a rationale for novel chemosensitization strategies based on NF-κB pathway inhibition. For example, it is possible that liposomal or viral delivery of an IκB super-repressor could result in sensitizing tumor cells in vivo to DNR, as it has been shown for the topoisomerase I inhibitor CPT-11.146 It is also conceivable to interfere with proximal upstream regulators of NF-κB activation (ie, Akt or PKCζ by inhibiting IKK activation147).
The mechanism by which NF-κB contributes to apoptosis inhibition remains uncertain. It has been suggested that NF-κB is a negative regulator of p53 gene expression, and it has also been reported that p53-activating signal is partially blocked by inhibition of NF-κB activation in DNR-treated cells.126
Role of death receptors/death ligand and caspases
Doxorubicin, as other antitumor compounds such as methotrexate, cisplatin, fludarabine, or bleomycin as well as ionizing radiation, was shown to enhance the expression of Fas and Fas-L on the surface of certain leukemic or epithelial malignant cells. For this reason, it has been proposed that doxorubicin-induced apoptosis occurs through autocrine or paracrine induction of the Fas-dependent pathway.148-151 In these studies, this hypothesis was supported by 2 lines of evidence: (1) cell lines resistant to Fas were found insensitive to anticancer drug-induced apoptosis, and (2) doxorubicin-induced apoptosis was prevented by CD95-neutralizing antibodies. However, this issue remains highly controversial mainly because antagonistic anti-Fas antibodies do not always inhibit drug-induced cell death.152-154 Moreover, many cells either naturally resistant to Fas (ie, HL-60 cells) or selected for Fas resistance152-154 remain sensitive to doxorubicin-induced apoptosis. Therefore, convincing evidence now exists showing that Fas/Fas-L pathway is not a principal and necessary mechanism of anthracycline-induced apoptosis.154
Other investigators have reported that in carcinoma cells doxorubicin induced the clustering of Fas receptor and its interaction with Fas-associated death domain-containing protein (FADD) in a Fas-L–independent fashion.155 This has also been described for UV and ionizing radiation.156,157 FADD is an adapter molecule, which is recruited to Fas cell death domain, and then binds to and activates procaspase-8; active caspase-8, in turn, triggers activation of a proteolytic cascade that leads through caspase-7, caspase-3, and caspase-6 to apoptosis. Moreover, these investigators showed that FADD overexpression facilitated drug-induced apoptosis, whereas down-regulation of FADD by transient transfection of an antisense construct decreased tumor cell sensitivity to the drug.155 For this reason, it has been proposed that doxorubicin-induced cell death involves the Fas/FADD pathway without interfering with Fas-L production and that FADD expression may significantly influence the cellular response to this drug. Fas clustering by cytotoxic agents was also described for VP-16, cisplatinum, and vinblastin155 as well as for UV radiation.157 However, the role of FADD in doxorubicin-induced caspase activation and apoptosis may be a function of the cellular model. Indeed, in doxorubicin-treated Jurkat leukemic T cells, Wesselborg et al154 have shown a correlation between doxorubicin-apoptosis and cleavage of procaspase-8 to its active p18 subunit; however, the expression of a dominant-negative FADD construct selectively abrogated Fas but not drug-induced effects. This result suggests that caspase-8 can be activated by doxorubicin in the absence of Fas receptor signaling.154 Fas- and FADD-independent caspase-8 activation have been observed in lymphoid cells treated with ionizing radiation.158 Altogether these results suggest that, whatever the role of FADD, caspase-8 activation represents a critical step in the cascade resulting in terminal proteolytic events, including caspase-3 and caspase-6 activation responsible for the cleavage of poly(ADP-ribose) polymerase and nuclear lamins, respectively. However, other investigators have questioned the role of caspase-8. For example, Villunger et al153 have reported that expression of cowpox virus cytokine response modifier A, a potent inhibitor of distinct members of the caspase-protease family, including caspase-8,159 did not influence doxorubicin-induced apoptosis in T-acute lymphatic leukemia CEM cells, whereas it prevented Fas-mediated apoptosis. Moreover, expression of FLIP (FLICE-inhibitory protein), which binds to caspase-8 and interferes with its function, was found to have no influence on apoptosis induced by doxorubicin or by other antileukemic compounds or by γ irradiation, whereas it abrogated Fas-mediated apoptosis in Jurkat T cells.160 Although the implication of Fas system and/or Fas signaling proximal adapter molecules including FADD or caspase-8 remains a controversial issue, the role of caspase-3 in doxorubicin-induced apoptosis was firmly established through different approaches, including peptide inhibitor and antisens.161However, the fact that these inhibitors were effective, even if added several hours after drug treatment, indicates that this caspase is involved in the execution and not in the triggering phase of drug action.161 Finally, it should be pointed out that the link, if any, between caspase signaling and CER pathway activated by anthracyclines is not yet determined. Indeed, previous studies have established that cytokine response modifier A–induced caspase inhibition resulted in the inhibition of CER generation and cell death induced by cytotoxic molecules such as TNFα162 or Fas agonist,163 suggesting a role for caspase in controlling SMase stimulation. Whether or not caspase-mediated proteolytic events take place upstream, CER generation in anthracycline-treated cells has not been yet investigated.
Whatever the intimate mechanism by which Fas and caspases are involved in the cellular response to anthracyclines, all these results have important clinical implications. First, on the basis of the stimulatory effect of anthracyclines on Fas-L production in some tumor cells and with the demonstration that Fas-L produced by tumor cells can kill the specific effector CTL and other activated T cells that express Fas,164 it can be speculated that chemotherapy may decrease cellular cytotoxicity of natural effectors and, therefore, may facilitate immune escape.165 Conversely, it has been proposed that drug-induced Fas expression may result in sensitization toward Fas-dependent cytotoxicity of cellular immune effectors.166-169 Second, previous studies have shown that, whereas short-term culture with doxorubicin enhanced Fas expression level, prolonged exposure to the drug may result in Fas reduction or even lack of expression, establishing an intriguing link between MDR phenotype and Fas resistance.170 Similar results have been obtained with mitoxantrone.171 Third, if Fas signaling molecules (or Fas itself) play an important role in anthracycline-induced apoptosis, it is conceivable that diminution of their expression and/or altered capacity to form the multimolecular death-initiating signaling complex constituted by Fas death domain, FADD, and procaspase-8 may impair drug cytotoxicity. In this perspective, it is important to note that AML cells (as well as immature progenitor cells) are generally insensitive to Fas commitment although most of them do express Fas, suggesting a disruption in Fas-mediated death signaling.172-174 Therefore, it is tempting to speculate that negative control of Fas signaling contributes not only to immune escape but also to decreased drug cytotoxicity in leukemia cells.
Finally, one should not disregard the emerging evidence that genotoxic stress, such as anthracycline treatment, has been described to increase death receptors such as DR5 for TRAIL/Apo-2 ligand in leukemia cells lines such as U937 and HL-60.175 176 These observations strongly suggest that one may optimize the antileukemic activity of anthracyclines by combining them with Apo-2 ligand treatment.
Role of p53 and cyclin kinases in anthracycline-induced cytotoxicity
Previous studies have shown that doxorubicin, as many other DNA-damaging agents, activates p53-DNA binding.177 On the basis of the crucial role of p53 in the execution of some forms of apoptosis, it has been therefore speculated that p53 could play an important function in anthracycline cytotoxicity. In fact, the requirement for wild-type p53 for apoptosis after doxorubicin exposure has been demonstrated in rodent normal or minimally transformed fibroblasts and lymphocytes.178,179 However, the role of p53 mutations in drug-induced apoptosis and cytotoxicity in human tumor cells is much less clear (see Brown and Wouters for review180). As far as leukemic cells are concerned, it should be noted that most AML cell lines displayed mutated or deleted forms of p53, including U937 or HL-60 cells, which exhibit high sensitivity to DNR. This observation suggests that p53 plays a minor role in anthracycline-induced cytotoxicity in AML cells. However, some clinical studies have shown that p53 mutations predict poor clinical outcome in patients treated with anthracycline-containing regimens for hematologic neoplasias such as AML or large cell non-Hodgkin malignant lymphomas.181,182 It is therefore possible that loss of p53 function correlates with other drug resistance mechanisms or interferes with other apoptotic pathways. In this perspective, it is important to note that wild-type p53 is necessary for c-Myc–mediated facilitation of doxorubicin-induced apoptosis.183
Besides its role in apoptosis, p53 plays an important function in regulating cell cycle transition in doxorubicin-treated cells. Indeed, it has been shown that doxorubicin-induced p53 activation contributes to the induction of the WAF1/CIP1 p21 gene product, which is a strong inhibitor of cyclin-dependent kinases involved in G1 to S transition.184 Although p53-independent doxorubicin-induced WAF1/CIP1 has been described,185,186this mechanism may account for G1 block after anthracycline exposure in p53 proficient cells. It has been suggested that WAF1 expression protects cells from doxorubicin-induced cytotoxicity because G1 block facilitates complete repair of DNA damage before the cells undergo DNA replication. In fact, it has been shown that high levels of constitutive WAF1/CIP1 protein are associated with chemoresistance in AML.187
Other cell cycle alterations have been described in doxorubicin-treated cells. Indeed, depending on the dose and cellular model, anthracyclines as many other genotoxic agents may also induce G2-M block because of p34cdc2 kinase inhibition. Indeed, previous studies have shown that doxorubicin prevented p34cdc2 dephosphorylation through a cdc25-independent mechanism, resulting in alteration of p34cdc2/cyclin B1-mediated complex.188 The mechanism by which anthracyclines interfere with p34cdc2 function has not been carefully examined. However, Kharbanda and coworkers189 190 have shown that most DNA damaging agents, including cytosine arabinoside, mitomycin C, and ionizing radiation, induce activation of p56/p53lyn, a tyrosine kinase of the Src family, which, in turn, phosphorylates and inactivates p34Cdc2. Whether or not Lyn is involved in anthracycline-induced p34cdc2 kinase inhibition and subsequent G2-M block should be confirmed.
Role of extracellular matrix proteins in anthracycline-induced cytotoxicity
It is now admitted that the extracellular matrix (ECM) plays an important role in cell proliferation and survival through a complex network of signals regulating cytoplasmic kinases, growth factor receptors, and ion channels.191 In another study, it has been demonstrated that β1-integrin–mediated adhesion to ECM proteins protects tumor epithelial cells from doxorubicin-induced apoptosis.192 This study also provides strong evidence that ECM-activated outside-in signals involve caspase inhibition through a protein tyrosine kinase mechanism, whereas adhesion to ECM does not influence DNA damage.192 In another study, it has been reported that myeloma cells adhered to fibronectin, a predominant component of ECM, have a survival advantage over nonadhered cells after doxorubicin exposure.193 The fact that ECM adhesion offers protection against anthracyclines may have important implications in other clinical settings. Indeed, it has been documented that both normal and leukemic myeloid progenitors strongly interact with ECM in the bone marrow and that β1-integrins play a predominant role in this process.194 Therefore, it is possible that contact with ECM contributes significantly to the natural chemoresistance of immature hematopoietic cells. Moreover, it cannot be excluded that anthracyclines activate inside-out signals, which modulate stem cell/ECM interactions, and, for example, facilitate the mobilization of progenitor subsets from the bone marrow to the peripheral circulation. This possibility could explain the efficacy of anthracycline-contained regimens used for CD34+ cell mobilization before peripheral stem cell transplantation. Of course, the role of ECM relative to intracellular antiapoptotic pathways is at present unknown.
Summary and conclusion
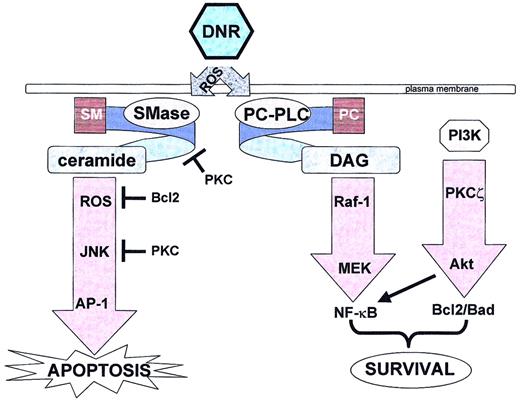
It is evident from these investigations that apoptosis induced by DNR brings into play a complex network of coordinated and highly controlled events. Although the characterizations of these different signaling pathways are still incomplete, we can at present attempt to concisely recapitulate (with a little speculation) as to the individual implication of these signaling pathways and mediators in DNR-induced cell signaling (Figure 1).
Daunorubicin triggers both apoptotic and survival pathways.
DNR indicates daunorubicin; ROS, reactive oxygen species; SM, sphingomyelin; PC, phosphatidylcholine; SMase, sphingomyelinase; PC-PLC, phosphatidylcholine-specific phospholipase C; DAG, diacylglycerol; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; JNK, c-Jun N-terminal kinase; AP-1, activated protein-1; MEK, mitogen-activated protein kinase kinase 1; NF-κB, nuclear factor-κB.
Daunorubicin triggers both apoptotic and survival pathways.
DNR indicates daunorubicin; ROS, reactive oxygen species; SM, sphingomyelin; PC, phosphatidylcholine; SMase, sphingomyelinase; PC-PLC, phosphatidylcholine-specific phospholipase C; DAG, diacylglycerol; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; JNK, c-Jun N-terminal kinase; AP-1, activated protein-1; MEK, mitogen-activated protein kinase kinase 1; NF-κB, nuclear factor-κB.
Present knowledge suggests that in drug-sensitive cells clinically relevant concentrations of DNR trigger within minutes the generation of ROS that leads to a first wave of N-SMase activation, sphingomyelin hydrolysis, and consequently CER generation. This process makes the initial ROS burst the rate-limiting step in DNR-induced apoptosis signaling, and one can consider these early events (within the first 5 minutes) as the primary apoptotic initiation step. Then follows a cyclical cascade of ROS-dependent N-SMase activation and CER generation that perpetuates for about 4 hours until the apoptotic execution steps (caspase activation, mitochondrial depolarization, etc) come into play. It remains to be clearly determined, however, whether these cyclical events (which are p53-independent) are critical in the apoptotic signaling process. For example, does the inhibition of continual CER or ROS production significantly affect DNR-induced apoptosis (as is suggested by studies in which the overexpression of Bcl2 [thereby inhibiting DNR-induced apoptosis] failed to block initial CER generation)? The ROS-dependent sphingomyelin-CER pathway has been shown to be responsible for rapid activation of the MEKK1-SEK1-JNK cascade leading to enhanced DNA binding activity of the transcription factor AP-1. This latter pathway appears essential in DNR-triggered apoptosis. Of course, all of these events are absent in drug-resistant cells, and the literature proposes several potential mechanisms such as rapid CER metabolism into other lipid products (such as sphingosine-1-phosphate or glucosylceramide) that possess survival/proliferative properties. However, it remains to be determined to what extent (eg, which is dominant?) these metabolic events play a role in DNR resistance of myeloid leukemic cells.
Remarkably, in drug-sensitive cells, DNR also triggers pathways that should negatively regulate apoptosis. In parallel, a phospholipase C–dependent DAG/raf-1/MEK cascade leading to activation of the transcription factor NF-κB and a DAG-independent PI3K/PKCζ cascade play a significant role in cellular protection. Inhibition (pharmacologically or genetically) of one or more of these mediators significantly increases the apoptotic response to DNR. One can, of course, speculate that these “survival” pathways are overridden by the CER pathway in sensitive cells but are predominant in resistant cells because the CER pathway is effectively down-regulated. But it appears obvious that more research is needed to better understand the relationship between both proapoptotic and antiapoptotic pathways triggered by DNR (interdependency, cross-talk, degree to which they occur in dose-dependent studies, and, of course, their relevance in normal myeloid cells). Finally, the emerging data on gene induction by anthracyclines, based on cDNA microarray technology, raise the question of not only the role of these genes (such as DNA-damage-response genes, eg, GADD) in cell resistance but also the link between such gene induction and the above-mentioned drug-triggered signaling pathways.195 196
In conclusion, we now know that DNR triggers both positive and negative regulatory pathways of apoptosis; therefore, it is now essential to discriminate among resistance mechanisms of AML cells: those that lead to decreased drug-target interactions and those that interfere with cell death commitment. It will also most certainly be of great importance to delineate the role of effector-induced cell damage in programmed cell death signaling, and it will also be necessary to determine if the discrimination between apoptosis and necrosis is an essential phenomenon for in vivo therapeutics. Finally, this review incites for a reinterpretation of the actions of well-established therapeutic agents in the light of recent advances in the basic sciences that should allow cellular pharmacology of antineoplastic agents to continue gathering momentum in the perspective of overcoming drug resistance by defining pharmacologic strategies capable of sensitizing resistant tumor cells and/or protecting normal physiologic cells to DNR.197
We apologize to colleagues whose works were not cited because of the size restrictions of the review.
Supported by la Ligue Nationale Contre le Cancer and les Comités Départementaux du Gers, de l'Aveyron, du Lot et de la Haute-Garonne (J.P.J.), in part by l'Association pour la Recherche sur le Cancer grant 5526 (G.L.), by La Faculté de Médecine Toulouse-Rangueil (G.L.), and by le Centre National d'Etudes Spaciales (J.P.J.).
References
Author notes
Guy Laurent, INSERM E9910, Institut Claudius Régaud, 20 rue du Pont St Pierre, 31052 Toulouse, France; e-mail:laurent@icr.fnclcc.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal